Abstract
Background and objectives
We investigated the association of urinary potassium and sodium excretion with the incidence of renal failure and cardiovascular disease in patients with type 2 diabetes.
Design, setting, participants, & measurements
A total of 623 Japanese type 2 diabetic patients with eGFR≥60 ml/min per 1.73 m2 were enrolled in this observational follow-up study between 1996 and 2003 and followed-up until 2013. At baseline, a 24-hour urine sample was collected to estimate urinary potassium and sodium excretion. The primary end point was renal and cardiovascular events (RRT, myocardial infarction, angina pectoris, stroke, and peripheral vascular disease). The secondary renal end points were the incidence of a 50% decline in eGFR, progression to CKD stage 4 (eGFR<30 ml/min per 1.73 m2), and the annual decline rate in eGFR.
Results
During the 11-year median follow-up period, 134 primary end points occurred. Higher urinary potassium excretion was associated with lower risk of the primary end point, whereas urinary sodium excretion was not. The adjusted hazard ratios for the primary end point in Cox proportional hazards analysis were 0.56 (95% confidence interval [95% CI], 0.33 to 0.95) in the third quartile of urinary potassium excretion (2.33–2.90 g/d) and 0.33 (95% CI, 0.18 to 0.62) in the fourth quartile (>2.90 g/d) compared with the lowest quartile (<1.72 g/d). Similar associations were observed for the secondary renal end points. The annual decline rate in eGFR in the fourth quartile of urinary potassium excretion (–1.3 ml/min per 1.73 m2/y; 95% CI, –1.5 to –1.0) was significantly slower than those in the first quartile (–2.2; 95% CI, –2.4 to –1.8).
Conclusions
Higher urinary potassium excretion was associated with the slower decline of renal function and the lower incidence of cardiovascular complications in type 2 diabetic patients with normal renal function. Interventional trials are necessary to determine whether increasing dietary potassium is beneficial.
Keywords: cardiovascular disease; electrolytes; nutrition; diabetes mellitus, type 2; follow-up studies; myocardial infarction; peripheral vascular diseases; potassium; potassium, dietary; renal insufficiency
Introduction
Patients with type 2 diabetes mellitus are at a high risk for progression to ESRD and incidence of CVD, both of which are life-threatening complications of diabetes (1). Hyperglycemia, hypertension, and dyslipidemia are well recognized as conventional risk factors for ESRD and CVD, and their intensive management could reduce the risk for these complications in patients with type 2 diabetes (2,3). Despite these efforts, however, numerous patients still suffer from these disorders, which emphasizes that additional therapeutic targets should urgently be explored.
In terms of lifestyle interventions of diabetes care, clinical guidelines recommend a restriction in total energy intake and an appropriate intake of specific nutrients (1,4). In particular, reducing dietary sodium intake for patients with diabetes, and the general population, has been recommended to prevent renal dysfunction, CVD onset, and premature death (5–7). However, findings from observational studies evaluating the association between sodium intake and mortality have been conflicting (8,9). High potassium intake is also recommended for most of the population who do not have impaired renal handling of potassium as a measure to prevent and control hypertension and stroke (10). However, there were few reports regarding the effect of potassium intake on preventing renal function and CVD onset in patients with diabetes. Therefore, we explored the association of urinary sodium and potassium excretion, which closely correlates with their intake amounts, with renal dysfunction and CVD onset in patients with type 2 diabetes mellitus.
Materials and Methods
Study Population
This study is part of the ongoing Shiga Prospective Observational Follow-up Study, with the aims to explore novel biomarkers and genetic and clinical risk factors for diabetic complication in Japanese patients with diabetes (11–13). Participants in this study were enrolled from among those with type 2 diabetes who registered in our prospective cohort between 1996 and 2003. Patients with an apparent history of CVD and those using any diuretics were excluded from this study. After obtaining written informed consent, each individual provided 24-hour urine and fasting blood samples at baseline. The serum and urine samples were immediately used to measure all laboratory variables at the Shiga University of Medical Science Hospital. Hemoglobin A1c (HbA1c) levels were presented as National Glycohemoglobin Standardization Program values, according to the recommendations of the Japanese Diabetes Society (14). Serum and urinary concentrations of creatinine were measured via an enzymatic method. eGFR was calculated using the simplified prediction equation proposed by the Japanese Society of Nephrology (15). In this study, patients with eGFR≥60 ml/min per 1.73 m2 were eligible because patients with eGFR<60 ml/min per 1.73 m2 may have already received education about dietary therapy, including restricting potassium and sodium intake. Finally, 623 patients with eGFR≥60 ml/min per 1.73 m2 were enrolled and followed-up until the end of 2013 or the occurrence of the primary end point. During the follow-up, the participants had an annual medical examination, and we checked their medical records to identify the onset of primary end points at each year. All patients continuously received appropriate diabetes care and education, including diabetes dietary advice during the follow-up period. This study was conducted with adherence to the Declaration of Helsinki. The study protocol and informed consent procedure were approved by the Ethics Committee of Shiga University of Medical Science.
Follow-Up Evaluation
The primary end point was the first occurrence of any of the renal and cardiovascular events, which were as follows: initiation of RRT for chronic renal failure and the occurrence of myocardial infarction, angina pectoris, stroke, peripheral vascular disease (PAD), and death from cardiovascular causes (13). Myocardial infarction was defined as a clinical presentation characterized by angiographic evidence of coronary thrombosis. Angina pectoris was defined as the presence of responsible lesions detected by imaging studies. Stroke, including ischemic stroke and cerebral hemorrhage, was defined as a persistent focal neurologic symptom, not caused by trauma or a tumor, and where the responsible lesion was detected by imaging studies. PAD was defined as revascularization with typical symptoms, such as cold feet or intermittent claudication. In fatalities, the medical record was reviewed to identify the cause of death. If the cause of death was unclear, it was not counted as a death as caused by cardiovascular events.
In evaluating the secondary outcomes, we separately assessed CVD events and renal secondary outcomes. In regard to secondary renal outcomes, we assessed two categorical outcomes, a 50% decline in eGFR from baseline and the progression to CKD stage 4 (eGFR<30 ml/min/1.73 m2), and one outcome as a continuous variable, the annual rate of decline in eGFR over the study period. In the analysis of secondary renal outcomes, only data measured at annual medical examination were used, but the data after the onset of the primary end point were excluded because the onset of these disorders may influence renal function.
Statistical Analysis
Data are expressed as mean±SD or median (interquartile range), where appropriate. In comparing the two groups, the chi-squared test was applied for categorical variables, whereas the unpaired t test was used for normally distributed variables and the Mann–Whitney U test was used for variables with skewed distributions. Statistical significance of the differences among quartile subgroups was determined using a chi-squared test for categorical variables and ANOVA followed by the Tukey–Kramer test or the Kruskal–Wallis test. The incidence rate per 1000 person years for each outcome was calculated. In this study, we analyzed urinary sodium and potassium excretion at baseline as both quartile categories and continuous variables (grams per day). The hazard ratio (HR) for each outcome was evaluated by using a Cox proportional hazards regression model. The follow-up time was censored if any primary end point occurred or if the patient was unavailable for follow-up. In this analysis, the adjusted cardiovascular risk factors were age, sex, body mass index (BMI), HbA1c, total cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol, systolic BP, use of renin-angiotensin system (RAS) inhibitors, hypertension, current smoking, urinary albumin excretion rate (UAER), and eGFR at baseline. Non-normally distributed variables were log-transformed and used in the analysis. Hypertension was defined as BP≥140/90 mmHg or on the use of antihypertensive drugs. Obesity was defined as BMI≥30 kg/m2. The cumulative incidences were estimated by using the Kaplan–Meier method and were compared with the log-rank test. The linear mixed models were used to estimate the annual rate of decline in eGFR and the change in urinary potassium and sodium excretions over time and to compare each difference between groups.
In the sensitivity analysis, we evaluated the association of urinary potassium excretion with the outcomes, including the primary end points and all-cause mortality, to examine the competing risk of urinary potassium excretion with mortality versus the primary end points. In addition, estimating the amount of sodium and potassium intake from a single 24-hour urine collection has limitations. During the follow-up, the data of urinary potassium and sodium excretions in the 24-hour urine collection from a median of 6 samples per participant (interquartile range: 3–9) were available. In addition, the data of age, BMI, HbA1c, total cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol, systolic BP, use of RAS inhibitors, hypertension, UAER, and eGFR were also available at the same annual medical examination that 24-hour urinary samples were measured (Supplemental Table 1). Using these variables measured during the follow-up, the associations between the primary end points and urinary potassium excretion as the time-dependent covariate were investigated with the use of the time-dependent Cox proportional hazards model. This model was adjusted for baseline sex, current smoking and time-dependent covariates during the follow-up, including age, BMI, HbA1c, total cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol, systolic BP, use of RAS inhibitors, hypertension, UAER, eGFR, and urinary sodium excretion. In this model, the last observation carried forward was used when there were missing covariates in the annual data. Pearson correlation coefficient was used to analyze the correlation between the mean levels during the follow-up and baseline levels of urinary potassium and sodium excretion.
All analyses were performed with IBM SPSS Statistics (version 22; IBM, Armonk, NY) or SAS (version 9.4; SAS Institute, Cary, NC) for the time-dependent Cox proportional hazards model. A two-sided P value <0.05 was considered statistically significant.
Results
The baseline characteristics of 623 patients, and two groups stratified by the occurrence of any of the primary end points, are presented in Table 1. During a 11-year median follow-up (interquartile range: 8–16 years), 134 primary end points (19 patients presented with chronic hemodialysis, 48 patients presented with myocardial infarction, 25 patients presented with angina pectoris, 36 patients presented with stroke, and six patients presented with PAD) occurred. The incidence rate per 1000 person years of the primary end point was 19.1 in all participants (total: 7024 person years). Interestingly, urinary potassium excretion in patients with the occurrence of any primary end points was significantly lower than in those without it, whereas urinary sodium excretion levels were not different between them.
Table 1.
Baseline clinical characteristics of all patients with type 2 diabetes and of the two subgroups stratified according to the occurrence of primary outcomes
| Variable | All | Primary End Point | P Valuea | |
|---|---|---|---|---|
| None | Occurrence | |||
| n | 623 | 489 | 134 | |
| Male (%) | 57.8 | 54.2 | 70.9 | 0.001 |
| Age (yr) | 59±10 | 58±10 | 62±10 | <0.001 |
| Body mass index (kg/m2) | 23.5±3.3 | 23.4±3.3 | 23.9±3.3 | 0.07 |
| Obesity, ≥30 kg/m2 (%) | 4.5 | 4.1 | 6.0 | 0.35 |
| Duration of diabetes (yr) | 10 (5–16) | 9 (4–16) | 11 (7–18) | 0.001 |
| Diet/OHA/insulin (%) | 23/54/23 | 26/54/20 | 10/57/33 | <0.001 |
| HbA1c (%) | 7.6±1.1 | 7.5±1.0 | 7.9±1.3 | <0.001 |
| Total cholesterol (mg/dl) | 214±35 | 214±36 | 213±30 | 0.78 |
| HDL-cholesterol (mg/dl) | 55 (46–65) | 56 (47–66) | 52 (43–62) | 0.03 |
| LDL-cholesterol (mg/dl) | 132±31 | 133±32 | 132±29 | 0.65 |
| Triglycerides (mg/dl) | 100 (72–151) | 96 (70–147) | 112 (81–155) | 0.02 |
| Systolic BP (mmHg) | 134±18 | 132±18 | 139±18 | <0.001 |
| Diastolic BP (mmHg) | 77±10 | 76±10 | 78±10 | 0.11 |
| Hypertension (%) | 46.9 | 41.7 | 65.7 | <0.001 |
| Using RAS inhibitors (%) | 15.4 | 13.7 | 21.6 | 0.02 |
| Current smoking (%) | 28.3 | 27.2 | 32.1 | 0.27 |
| Urinary AER (μg/min) | 12 (6–29) | 10 (6–23) | 23 (10–89) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 89±19 | 89±19 | 84±18 | 0.010 |
| Urinary sodium excretion (g/d) | 5.3±2.2 | 5.3±2.2 | 5.3±2.3 | 0.96 |
| Urinary potassium excretion (g/d) | 2.4±0.9 | 2.4±0.9 | 2.2±1.0 | 0.02 |
| Urinary sodium to potassium ratio | 4.1±1.6 | 3.9±1.6 | 4.4±1.7 | 0.006 |
| Serum sodium (mEq/L) | 141±2 | 141±2 | 141±2 | 0.83 |
| Serum potassium (mEq/L) | 4.4±0.3 | 4.4±0.3 | 4.4±0.4 | 0.19 |
Data are expressed as mean±SD for normally distributed continuous variables, median (interquartile range) for skewed continuous variables, or as otherwise indicated. OHA, oral hypoglycemic agent; HbA1c, hemoglobin A1c; RAS, renin-angiotensin system; AER, albumin excretion rate.
Differences between the two subgroups were compared with a chi-squared test for categorical variables, t test for normally distributed continuous variables, and Mann–Whitney U test for skewed continuous variables.
Primary End Points
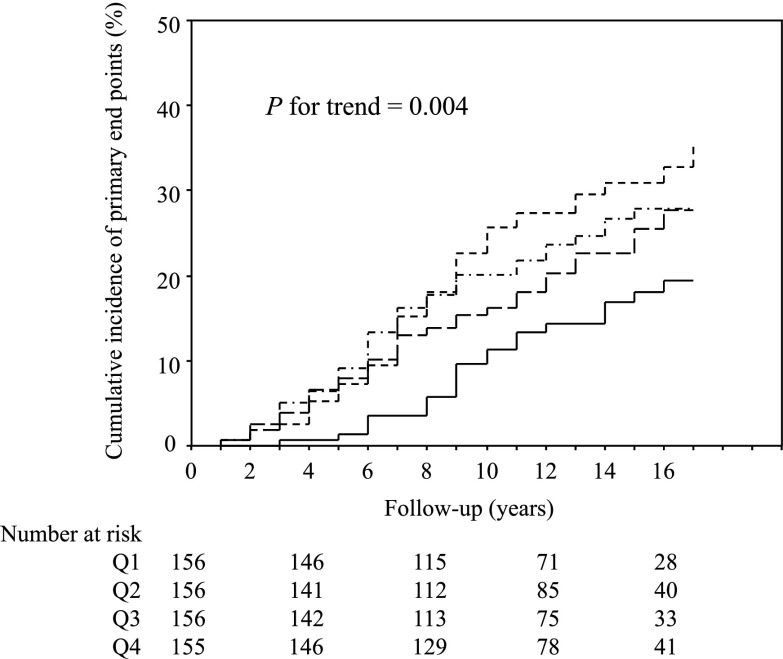
The clinical characteristics in patients stratified by the quartile categories of urinary potassium excretion at baseline are shown in Table 2. There was a gradient of higher incidence of the primary end points among patients in the lower quartiles of urinary potassium excretion (Figure 1) (P for trend=0.004). The adjusted risk for the primary end point was 0.56 (95% confidence interval [CI], 0.33 to 0.95) in the third quartile and 0.33 (95% CI, 0.18 to 0.62) in the fourth quartile when compared with the first quartile (Table 3), respectively. On the other hand, the cumulative incidence (P for trend=0.98) and adjusted HRs (Table 3) of the primary end points were not different among the quartile subgroups stratified by urinary sodium excretion levels. There was no interaction between urinary potassium and sodium excretions (P=0.42).
Table 2.
Baseline clinical characteristics of quartile subgroups stratified according to the levels of urinary potassium excretion
| Variable | Quartiles of Urinary Potassium Excretion (g/d) | P Valuea | |||
|---|---|---|---|---|---|
| Q1 (<1.72) | Q2 (1.72–2.32) | Q3 (2.33–2.90) | Q4 (>2.90) | ||
| n | 156 | 156 | 156 | 155 | |
| Male (%) | 46.2 | 51.3 | 64.1 | 69.7 | <0.001 |
| Age (yr) | 59±12 | 59±11 | 59±10 | 59±8 | 0.98 |
| Body mass index (kg/m2) | 23.4±3.5 | 23.6±3.6 | 23.2±3.0 | 23.7±3.1 | 0.58 |
| Duration of diabetes (yr) | 9 (4–16) | 10 (6–16) | 11 (5–18) | 10 (5–17) | 0.52 |
| Diet/OHA/insulin (%) | 24/50/26 | 21/58/21 | 20/56/24 | 25/54/21 | 0.25 |
| HbA1c (%) | 7.7±1.3 | 7.6±1.0 | 7.6±1.2 | 7.4±1.0 | 0.29 |
| Total cholesterol (mg/dl) | 211±34 | 219±32 | 216±38 | 209±34 | 0.04 |
| HDL-cholesterol (mg/dl) | 55 (47–65) | 54 (47–64) | 56 (46–66) | 54 (45–66) | 0.88 |
| LDL-cholesterol (mg/dl) | 129±30 | 137±30 | 134±35 | 129±29 | 0.07 |
| Triglycerides (mg/dl) | 106 (73–153) | 103 (78–147) | 101 (75–160) | 93 (66–144) | 0.41 |
| Systolic BP (mmHg) | 138±19 | 135±18 | 130±18 | 132±18 | 0.001 |
| Diastolic BP (mmHg) | 78±10 | 77±10 | 75±11 | 77±10 | 0.07 |
| Hypertension (%) | 58.3 | 51.9 | 37.2 | 40.0 | <0.001 |
| Using RAS inhibitors (%) | 20.0 | 17.3 | 11.5 | 19.9 | 0.15 |
| Current smoking (%) | 26.9 | 28.2 | 30.1 | 27.7 | 0.94 |
| Urinary AER (μg/min) | 11 (5–28) | 11 (6–24) | 12 (6–35) | 13 (7–37) | 0.17 |
| eGFR (ml/min per 1.73 m2) | 89±22 | 89±19 | 87±16 | 87±16 | 0.44 |
| Urinary sodium excretion (g/d) | 3.7±1.6 | 5.0±1.7 | 5.7±1.8 | 6.9±2.3 | <0.001 |
| Urinary potassium excretion (g/d) | 1.4±0.3 | 2.0±0.2 | 2.6±0.2 | 3.6±0.6 | <0.001 |
| Urinary sodium to potassium ratio | 4.8±2.1 | 4.2±1.5 | 3.8±1.2 | 3.3±1.1 | <0.001 |
| Serum sodium (mEq/L) | 141±2 | 141±2 | 141±2 | 140±2 | 0.54 |
| Serum potassium (mEq/L) | 4.3±0.4 | 4.3±0.3 | 4.4±0.3 | 4.4±0.4 | 0.001 |
Data are expressed as mean±SD for normally distributed continuous variables, median (interquartile range) for skewed continuous variables, or as otherwise indicated. Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile; OHA, oral hypoglycemic agent; HbA1c, hemoglobin A1c; RAS, renin-angiotensin system; AER, albumin excretion rate.
Differences between quartile subgroups were compared with a chi-squared test for categorical variables and ANOVA for continuous variables.
Figure 1.
Kaplan–Meier curves for cumulative incidences of primary end points in the quartile subgroups stratified by urinary potassium excretion. Short dashed line represents Q1 (<1.72 g/d); short dashed/dotted line represents Q2 (1.72–2.32 g/d); long dashed line represents Q3 (2.33–2.90 g/d); and solid line represents Q4 (>2.90 g/d). Differences among groups were compared by a log-rank test. Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile.
Table 3.
Incidence rates and hazard ratios (95% confidence intervals) for primary end points in quartile subgroups stratified according to the levels of urinary potassium excretion
| Primary End Point | Urinary Potassium Excretion (g/d) | Urinary Sodium Excretion (g/d) | ||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (<1.72) | Q2 (1.72–2.32) | Q3 (2.33–2.90) | Q4 (>2.90) | Q1 (<3.81) | Q2 (3.81–5.07) | Q3 (5.08–6.50) | Q4 (>6.50) | |
| Incidence (n) | 43 | 37 | 32 | 22 | 33 | 35 | 33 | 33 |
| Incidence rate (per 1000 person years) | 25.3 | 20.9 | 18.7 | 11.9 | 18.7 | 19.8 | 19.4 | 18.2 |
| Crude HR | 1 (Reference) | 0.84 (0.54 to 1.31) | 0.76 (0.48 to 1.20) | 0.48 (0.29 to 0.80) | 1 (Reference) | 1.05 (0.65 to 1.69) | 1.06 (0.65 to 1.72) | 0.98 (0.60 to 1.59) |
| Age- and sex-adjusted HR | 1 (Reference) | 0.79 (0.51 to 1.22) | 0.61 (0.38 to 0.97) | 0.38 (0.23 to 0.65) | 1 (Reference) | 1.14 (0.70 to 1.84) | 1.01 (0.62 to 1.66) | 0.86 (0.51 to 1.43) |
| All adjusted HRa | 1 (Reference) | 0.70 (0.44 to 1.13) | 0.56 (0.33 to 0.95) | 0.33 (0.18 to 0.62) | 1 (Reference) | 1.54 (0.91 to 2.59) | 1.37 (0.78 to 2.41) | 1.30 (0.71 to 2.38) |
Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile; HR, hazard ratio.
Adjusted by the baseline data, including age, sex, body mass index, hemoglobin A1c, total cholesterol, log triglyceride, log HDL-cholesterol, LDL-cholesterol, systolic BP, renin-angiotensin system inhibitor, hypertension, log urinary albumin excretion rate, eGFR, current smoking, and urinary sodium excretion (or urinary potassium excretion), in the Cox proportional regression analysis.
In the Cox proportional hazards model using continuous variables, instead of quartile categories, there was a gradient of lower incidence of the primary end point in patients with higher urinary potassium excretion (grams per day) (adjusted HR, 0.69; 95% CI, 0.53 to 0.90), whereas urinary sodium excretion (gram/day) was not associated (adjusted HR, 1.01; 95% CI, 0.92 to 1.12). Higher urinary sodium to potassium ratio was also significantly associated with the higher risk for the primary end point (adjusted HR, 1.11; 95% CI, 1.01 to 1.22).
Secondary Renal End Points
The adjusted HRs for the secondary renal categorical outcomes were significantly lower in the highest quartile subgroup (fourth quartile) of urinary potassium excretion (Table 4). As well, the annual rate of decline in eGFR was significantly different among the quartiles stratified by urinary potassium excretion levels (Table 4).
Table 4.
Adjusted hazard ratios for secondary end points and annual decline rate in eGFR in quartile subgroups stratified according to the levels of urinary potassium excretion
| Secondary End Points | Quartiles of Urinary Potassium Excretion (g/d) | |||
|---|---|---|---|---|
| Q1 (<1.72) | Q2 (1.72–2.32) | Q3 (2.33–2.90) | Q4 (>2.90) | |
| CVD events (n=115)a | 1 (Reference) | 0.82 (0.50 to 1.34) | 0.69 (0.40 to 1.19) | 0.42 (0.22 to 0.81) |
| 50% decline in eGFR (n=68)a | 1 (Reference) | 0.78 (0.40 to 1.51) | 0.71 (0.35 to 1.43) | 0.24 (0.08 to 0.70) |
| Progression to CKD stage 4 (n=32)a | 1 (Reference) | 0.69 (0.25 to 1.92) | 0.50 (0.18 to 1.40) | 0.08 (0.01 to 0.50) |
| Annual decline rate in eGFRb | −2.2 (−2.4 to −1.8) | −1.9 (−2.0 to −1.8) | −1.7 (−2.0 to −1.5) | −1.3c (−1.5 to −1.0) |
CVD, cardiovascular disease; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile.
Values are adjusted hazard ratios (95% confidence intervals). Hazard ratios are adjusted for the baseline data, including age, sex, body mass index, hemoglobin A1c, total cholesterol, log triglyceride, log HDL-cholesterol, LDL-cholesterol, systolic BP, renin-angiotensin system inhibitor, hypertension, log urinary albumin excretion rate, eGFR, current smoking, and urinary sodium excretion, in the Cox proportional regression analysis.
Data are expressed as ml/min per 1.73 m2 per year (95% confidence intervals).
P<0.01 versus Q1 and Q2.
Sensitivity Analysis
During follow-up, 26 patients without the primary end points died. Higher urinary potassium excretion (grams per day) was associated with a lower risk for the outcomes, including the primary end points and all-cause mortality (adjusted HR, 0.71; 95% CI, 0.56 to 0.90).
The urinary excretion levels of potassium (–0.02 g/d per y; 95% CI, –0.04 to –0.01) and sodium (–0.09 g/d per y; 95% CI, –0.11 to –0.07) gradually decreased over time; however, the mean levels of urinary potassium or sodium excretion during the follow-up were tightly correlated with the respective baseline data (r=0.80, P<0.001 for urinary potassium excretion and r=0.80, P<0.001 for urinary sodium excretion). However, higher urinary potassium excretion (grams per day) as the time-dependent covariate was similarly associated with the lower risk for the primary end point (adjusted HR, 0.64; 95% CI, 0.49 to 0.84) in the time-dependent Cox proportional hazards model, whereas the urinary sodium excretion (grams per day) was not (adjusted HR, 1.01; 95% CI, 0.89 to 1.13).
Discussion
This study demonstrated that the higher urinary potassium excretion, but not urinary sodium excretion, was associated with the lower risk for renal dysfunction and incidence of cardiovascular complications in type 2 diabetic patients with normal renal function. In clinical practice of diabetes care, reducing energy intake while maintaining a healthful eating pattern is recommended. This may result in reduced potassium intake. Therefore, our study suggests an increase of potassium intake, with restricting total energy, may be recommended to prevent renal and cardiovascular complications in patients with diabetes.
Recently, urinary potassium excretion and the urinary sodium to potassium ratio, rather than urinary sodium excretion, have been reported to have an association with mortality and CVD onset in the nondiabetic population (16–18). More recently, a large, international, prospective cohort study including >100,000 participants, only 9.1% of whom were diabetes, reported an estimated high potassium intake was associated with a lower risk of death and cardiovascular outcomes (19). Smyth et al. also reported urinary potassium excretion, but not urinary sodium excretion, predicted the progression to CKD stage 4 or chronic dialysis in a post hoc analysis of the ongoing telmisartan alone and in combination with ramipril global endpoint trial and telmistartan randomized assessment study in ACE intolerant subjects with cardiovascular disease studies (20). Our findings in patients with diabetes are consistent with these previous results. Taken together, these results suggest the high potassium intake may be a clinically beneficial measure for the decline of renal function and the incidence of CVD in individuals with normal renal function, regardless of the coexistence of diabetes.
In diabetes care, the restriction of total energy intake receives the most attention. In addition, restricting salt intake is also recommended in patients with diabetes, especially for individuals with hypertension (1,4,21). With these dietary restrictions for patients with diabetes, the intake of other nutrients, including potassium, which has a beneficial effect against renal and cardiovascular risk, tends to reduce in parallel. In fact, potassium and sodium urinary excretion levels correlated with each other in this study. This may be a plausible explanation for why some cohort studies, especially in patients with diabetes, have not shown the renal and cardiovascular benefits of low sodium intake (7,8).
What might be the underlying mechanism by which the high urinary potassium excretion is associated with the low risk for renal and cardiovascular outcomes in patients with type 2 diabetes mellitus? High urinary potassium excretion is reported to be associated with low BP (22,23). In this study, the higher quartile subgroups showed lower systolic BP than the lower quartile subgroup. This result suggests that the beneficial effect of higher urinary potassium excretion on renal and cardiovascular outcomes is attributed to the BP-lowering effect of potassium. Additionally, the high urinary potassium excretion may be associated with other factors or direct effects on the renal and cardiovascular systems, which may be independent of, but additive to, its effect on BP (24) because this association was observed even after adjusting systolic BP and hypertension. High urinary potassium excretion is generally recognized as correlating with consuming high amounts of potassium-rich food items, such as fresh vegetables and fruits. These foods are known to have antioxidant and anti-inflammatory effects (25–27). In addition, a high potassium intake is reported to increase endothelium-dependent nitric oxide production and decrease salt-induced TGF-β production (28). These high potassium intake effects may encourage vascular protection against atherosclerosis, which might result in preventing renal and cardiovascular complications. However, we did not have any information regarding other dietary and lifestyle factors, which may influence urinary potassium excretion. In addition, our study design was not adequate to address the possibilities as to whether this association is independent of its effect on BP. Further studies are required to elucidate the mechanisms by which the higher urinary potassium excretion is associated with the lower risk for renal dysfunction and cardiovascular disease.
There are some limitations to this study that must be addressed. Our study was designed as an observational follow-up study and not an intervention trial; therefore, we were not able to assess causality. Further, the main data in this study were from a single urine collection at baseline; however, all data on urinary potassium excretion measured during the follow-up as the time-dependent covariate were also analyzed with the use of the time-dependent Cox proportional hazards model, and its beneficial association was similar with the two methods. During the long-term follow-up period, the therapeutic strategy in diabetes management changed, and several new drugs were introduced in this field. For example, the prescription rate of RAS inhibitors at baseline, mainly in the late 1990s, was only 15%, but it is now >40%. These drugs may have reduced the number of patients that progress to ESRD and experience the onset of CVD. In addition, some patients have newly added some medicines, including diuretics, during the follow-up; this would affect the levels of urinary potassium and sodium excretion. The dietary therapy used, including the restriction of potassium and salt intake, could be changed according to the progression of diabetic nephropathy or increased risk for CVD. However, these were not reflected in this analysis. Further, this study enrolled only Japanese patients from a single center, who tend to have a higher salt intake than whites (29). Some clinical characteristics in our population were somewhat different from typical white patients with type 2 diabetes, such as lower prevalence of obesity and hypertension. Therefore, we need to confirm the findings of this study in other ethnic groups.
In conclusion, higher levels of urinary potassium excretion are associated with a lower risk for renal and cardiovascular complications in type 2 diabetic patients with normal renal function. Interventional clinical trials are necessary to determine whether increasing dietary potassium is beneficial for these complications.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported in part by a Grant-in-Aid for Diabetic Nephropathy and Nephrosclerosis Research from the Ministry of Health, Labour and Welfare of Japan (S.A.); a Grant-in-Aid for Research on Diabetic Kidney Disease from the Japan Agency for Medical Research and Development (S.A.); the Salt Science Research Foundation, Grant No. 1317 (U.T.); and research grants from AstraZeneca and MSD K.K. (for this prospective observational follow-up study).
S.A. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00980115/-/DCSupplemental.
References
- 1.American Diabetes Association : Standards of medical care in diabetes--2014. Diabetes Care 37[Suppl 1]: S14–S80, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A: Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 56: 1727–1730, 2007 [DOI] [PubMed] [Google Scholar]
- 4.The Japan Diabetes Society: Treatment Guide for Diabetes 2012-2013. Available at: http://www.jds.or.jp/modules/en/index.php?content_id=1. Accessed August 28, 2015
- 5.Dodson PM, Beevers M, Hallworth R, Weberley MJ, Fletcher RF, Taylor KG: Sodium restriction and blood pressure in hypertensive type II diabetics: Randomised blind controlled and crossover studies of moderate sodium restriction and sodium supplementation. BMJ 298: 227–230, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suckling RJ, He FJ, Macgregor GA: Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev (12): CD006763, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, Wadén J, Tolonen N, Saraheimo M, Gordin D, Groop PH, FinnDiane Study Group : The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 34: 861–866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, Jerums G: Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 34: 703–709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA, European Project on Genes in Hypertension (EPOGH) Investigators : Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 305: 1777–1785, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP: Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 346: f1378, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hidaka H, Terada M, Maegawa H, Kojima H, Koya D, Nishio Y, Haneda M, Yasuda H, Kashiwagi A, Kikkawa R: Evaluation of a new care system provided to diabetic patients in the outpatient clinic. Intern Med 39: 783–787, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D: Polymorphisms of the protein kinase C-beta gene (PRKCB1) accelerate kidney disease in type 2 diabetes without overt proteinuria. Diabetes Care 29: 864–868, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H: Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care 36: 1248–1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K, Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus : Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 1: 212–228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK, Trials of Hypertension Prevention Collaborative Research Group : Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The Trials of Hypertension Prevention follow-up study. Arch Intern Med 169: 32–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB: Sodium and potassium intake and mortality among US adults: Prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 171: 1183–1191, 2011 [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE: Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 306: 2229–2238, 2011 [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S, PURE Investigators : Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 371: 612–623, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM, ONTARGET and TRANSCEND investigators : The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int 86: 1205–1212, 2014 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization: Guideline: Sodium Intake for Adults and Children, Geneva, Switzerland, 2012 [PubMed] [Google Scholar]
- 22.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S, PURE Investigators : Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371: 601–611, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL: Association of urinary sodium/potassium ratio with blood pressure: Sex and racial differences. Clin J Am Soc Nephrol 7: 315–322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He FJ, MacGregor GA: Beneficial effects of potassium on human health. Physiol Plant 133: 725–735, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Sia CL, Dandona P: Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care 30: 1406–1411, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, Verga S, Rini G: Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr 95: 1089–1095, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Dunkler D, Dehghan M, Teo KK, Heinze G, Gao P, Kohl M, Clase CM, Mann JF, Yusuf S, Oberbauer R, ONTARGET Investigators : Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Intern Med 173: 1682–1692, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Ying WZ, Aaron K, Wang PX, Sanders PW: Potassium inhibits dietary salt-induced transforming growth factor-beta production. Hypertension 54: 1159–1163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intersalt Cooperative Research Group : Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.