Abstract
Background and objectives
Definition of individual risk profile is the first step to implement strategies to keep the delicate balance between under- and overimmunosuppression after kidney transplantation.
Design, setting, participants, & measurements
We used data from the Efficacy Limiting Toxicity Elimination Symphony Study (1190 patients between 2002 and 2004) to model risk of rejection and infection in the first year after kidney transplantation. External validation was performed in a study population from the Fixed-Dose Concentration-Controlled Trial (630 patients between 2003 and 2006).
Results
Despite different temporal dynamics, rejections and severe infections had similar overall incidences in the first year after transplantation (23.4% and 25.5%, respectively), and infections were the principal cause of death (43.2% of all deaths). Recipient older age, deceased donor, higher number of HLA mismatches, and high risk for cytomegalovirus disease were associated with infection; deceased donor, higher number of HLA mismatches, and immunosuppressive therapy including cyclosporin A (compared with tacrolimus), with rejection. These factors were integrated into a two–dimensional risk stratification model, which defined four risk groups: low risk for infection and rejection (30.8%), isolated risk for rejection (36.1%), isolated risk for infection (7.0%), and high risk for infection and rejection (26.1%). In internal validation, this model significantly discriminated the subgroups in terms of composite end point (low risk for infection/rejection, 24.4%; isolated risk for rejection and isolated risk for infection, 31.3%; high risk for infection/rejection, 54.4%; P<0.001), rejection episodes (isolated risk for infection and low risk for infection/rejection, 13.0%; isolated risk for rejection and high risk for infection/rejection, 24.2%; P=0.001), and infection episodes (low risk for infection/rejection and isolated risk for rejection, 12.0%; isolated risk for infection and high risk for infection/rejection, 37.6%; P<0.001). External validation confirmed the applicability of the model to an independent cohort.
Conclusions
We propose a two–dimensional risk stratification model able to disentangle the individual risk for rejection and infection in the first year after kidney transplantation. This concept can be applied to implement a personalized immunosuppressive and antimicrobial treatment approach.
Keywords: transplant outcomes, transplant infectious disease, cause of death, cyclosporin, death, humans, immunosuppression, kidney transplantation, tacrolimus, transplant recipients
Introduction
Rejection and infection are the most important complications after solid organ transplantation. Preventing acute rejection has been the principal aim in transplantation medicine for several decades, and the success of transplantation was, to a significant extent, related to the development of effective immunosuppressive therapy (1). However, intense immunosuppression inevitably increases susceptibility to infections and malignancies, some of which are infection triggered (2–4). As a result, the relevance of infection episodes and infection–associated allograft failures progressively increased in the last decades, and balancing the beneficial and deleterious effects of immunosuppressive therapy emerged as a critical aim in the modern transplantation era (5–8).
Recipients of kidney transplants are a very heterogeneous patient population, and the risks for rejection and infection markedly vary among different patient subgroups (9,10). Although it seems obvious that immunosuppression should be adapted according to the individual patient risk profile, there is still no established approach to assess it. Although risk factors for rejection have been investigated in previous studies (11), an integrated risk stratification approach including relevant side effects of immunosuppressive therapy is currently not available. The definition of an individual risk profile separating rejection and infection would not only help the clinician with the challenging task of personalizing immunosuppression but also, help to improve the design of clinical trials in transplantation.
Here, we investigated the incidence of rejection and infection in the first year after kidney transplantation in the study population of the largest randomized controlled trial performed in de novo kidney transplantation (the Efficacy Limiting Toxicity Elimination [ELITE] Symphony Study) (12), and we developed an integrated approach to help balance immunosuppression in recipients of transplant.
Materials and Methods
Study Population
For this exploratory post hoc analysis, we considered all patients included in the ELITE Symphony Trial treated with a calcineurin inhibitor/mycophenolate mofetil (MMF) –based immunosuppression regimen (n=1190). The ELITE Symphony Study is the largest randomized, controlled trial performed in de novo kidney transplantation. It was a 12-month, prospective, open–label, multicenter study including 1645 adult recipients of renal transplants recruited in 83 sites in 15 countries from November of 2002 to November of 2004, and randomly assigned to four immunosuppression regimens (A, cyclosporin, MMF, and corticosteroids; B, daclizumab, low-dose cyclosporin, MMF, and corticosteroids; C, daclizumab, low-dose tacrolimus, MMF, and corticosteroids; and D, daclizumab, low-dose sirolimus, MMF, and corticosteroids) (12). Because the tested sirolimus–based regimen is not recommended as a starting treatment after kidney transplantation and because a calcineurin inhibitor/MMF–based regimen is the most common standard immunosuppressive regimen in the majority of transplant centers, we excluded study arm D from this analysis. Prophylactic or preemptive cytomegalovirus (CMV) treatment and Pneumocystis jiroveci prophylaxis were administered according to center practice.
We also included an external validation cohort comprising patients of the Fixed-Dose Concentration-Controlled (FDCC) Trial (13). This study was performed between May of 2003 and April of 2006 and compared a concentration–controlled and a fixed–dose MMF therapy in 901 recipients of renal transplants. All patients were additionally treated with a calcineurin inhibitor (54% received cyclosporin and 46% received tacrolimus). Because the ELITE Symphony Study did not include a similar treatment regimen, we excluded 254 patients from the FDCC Trial who received tacrolimus without induction therapy from the validation cohort.
Analyses of End Points
We defined two composite end points. The infection end point included all serious or severe infections defined as infections fulfilling the serious adverse event criteria or reported as being of severe intensity by the investigator according to the original study protocol. The rejection end point included all biopsy–proven acute rejections according to the Banff criteria (the first Banff classification was applied for the ELITE Symphony Study [14], and the Banff 1997 classification was applied for the FDCC Trial [15]) and graft losses. No protocol biopsies were routinely performed in the ELITE Symphony Study. For patients with more than one event during the study period, only the first rejection and the first infection were considered.
Statistical Analyses
We used all available data from the intent to treat study population and did not impute missing data. We compared means and proportions using Wilcoxon’s rank sum tests and Fisher’s exact tests, respectively. Freedom from specific events (infection end point and rejection end point) was analyzed descriptively using the Kaplan–Meier method and modeled by means of multiple Cox regression, including a set of baseline characteristics using study arms A–C of the ELITE Symphony Study population. The models were then submitted to a backward variable selection procedure on the basis of Akaike’s information criterion. Predictors from the two final models were then used to classify patients as being at high or low risk for the rejection and infection end points depending on whether the individual risk for the specific end point (computed on the basis of the model) was higher or lower, respectively, than the risk for virtual patients with average covariates. These tests and all other statistical tests were two sided, and P values <0.05 were considered to indicate statistical significance without correction for multiplicity.
Validation
Two evaluations of predictive performance were performed. In the first internal validation, the models were recalculated in a randomly selected sample including 50% of the ELITE Symphony Study population (study arms A–C) and validated in the remaining patients. For an additional external validation, the original models obtained using all patients in study arms A–C of the ELITE Symphony Study were tested in the independent patient cohort from the FDCC Study.
Results
Patients
In total, 1190 patients of the ELITE Symphony Study received a calcineurin inhibitor/MMF–based therapy (study arms A–C). The characteristics of the cohort are summarized in Table 1. The average age at the time of transplantation was 47.3 years old, 64.9% were men, and 92.8% were white. Mean donor age was 47 years, 29.0% of the patients received the organ from a living related donor, and 6.6% of the patients received the organ from a living unrelated donor. Among the transplantations from deceased donors, 556 were nonextended criteria and 209 were extended criteria donations. A high–risk CMV constellation (donor positive/recipient negative) was present in 13.3% of the patients. The patients were equally distributed in the three arms of the study.
Table 1.
Baseline characteristics of the Efficacy Limiting Toxicity Elimination Symphony Study and the Fixed-Dose Concentration-Controlled Study populations
| Variable | ELITE Symphony (n=1190) | FDCC (n=630) | P Value |
|---|---|---|---|
| Recipient age (yr) | 47.3 (35.4–57.0) | 43.9 (33.0–57.0) | 0.07 |
| Men, no. (%) | 772 (64.9) | 383 (60.8) | 0.09 |
| Race, no. (%) | <0.001 | ||
| White | 1104 (92.8) | 503 (79.8) | |
| Black | 21 (1.8) | 23 (3.7) | |
| Asian | 11 (0.9) | 35 (5.6) | |
| Other | 54 (4.5) | 69 (11.0%) | |
| Pretransplant diabetes mellitus, no. (%) | 127 (10.7) | 37 (5.9) | 0.001 |
| Donor type, no. (%) | <0.001 | ||
| Living | 424 (35.6) | 149 (23.7) | |
| Deceased nonextended criteria donor | 556 (46.8) | 397 (63.0) | |
| Deceased extended criteria donor | 209 (17.6) | 84 (13.3) | |
| Donor age (yr) | 47 (35.8–57.0) | 43.3 (30.3–56.0) | 0.07 |
| Cold ischemia time (h) | 11.3 (1.4–18.0) | 12.5 (4.0–18.0) | <0.001 |
| Cytomegalovirus status, no. (%) | |||
| D+/R− | 158 (13.3) | 98 (15.6) | 0.20 |
| HLA mismatches, no. (%) | 0.02 | ||
| 0 | 102 (8.9) | 29 (4.6) | |
| 1 | 83 (7.3) | 48 (7.6) | |
| 2 | 193 (16.9) | 102 (16.2) | |
| 3 | 370 (32.3) | 197 (31.3) | |
| 4 | 208 (18.2) | 135 (21.4) | |
| 5 | 132 (11.5) | 88 (14.0) | |
| 6 | 56 (4.9) | 31 (4.9) | |
| Immunosuppression, no. (%) | NA | ||
| Standard-dose CsA, MMF, steroids | 390 (32.8) | ||
| Daclizumab, low-dose CsA, MMF, steroids | 399 (33.5) | ||
| Daclizumab, low-dose Tac, MMF, steroids | 401 (33.7) |
Recipient and donor ages and cold ischemia time are reported as median (25th–75th percentiles). ELITE Symphony, Efficacy Limiting Toxicity Elimination Symphony Study; FDCC, Fixed-Dose Concentration-Controlled Study; D+/R–, donor seropositive and recipient seronegative; NA, not applicable; CsA, cyclosporin A; MMF, mycophenolate mofetil; Tac, tacrolimus.
Infection and Rejection Episodes after Transplantation
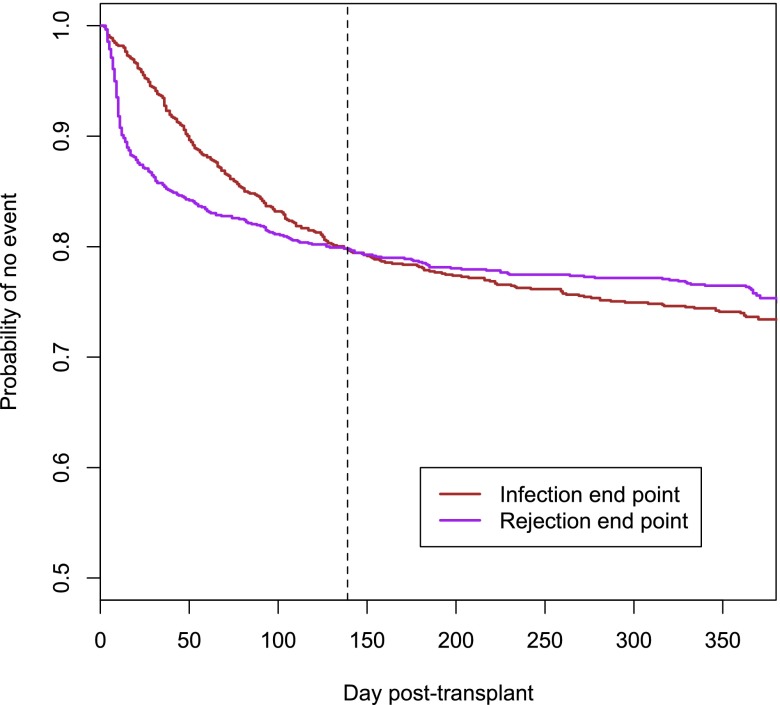
In the first year after transplantation, 292 (25.5%) patients reached the infection end point, 268 (23.4%) patients reached the rejection end point, and 89 patients reached both the infection and the rejection end points (7.8% of the whole study population, 30.5% of patients with an infection episode, and 33.2% of patients with a rejection episode). Most rejections occurred within the first 2 months after transplantation, whereas the infection rate was more uniform during the study period. As a result, the initially higher probability of rejection was overcome by the probability of infection 139 days after transplantation (Figure 1). Among 89 patients with both an infection episode and a rejection episode, 52 (58%) patients reached the infection end point first, 33 (37%) patients reached the rejection end point first, and in 4 patients, infection and rejection were registered on the same day. Infections were the most important cause of death in the first year after transplantation. Among 37 deaths (3.1% of patients) reported during the study period, 16 (43.2%) were associated with infections, and 9 (24.3%) were caused by cardiovascular events; additionally, three (8.1%) patients died from a pulmonary embolism, three (8.1%) patients died from uncontrolled bleeding, and six patients died for other reasons.
Figure 1.
Rejections and severe infections have similar incidences in the first year after transplantation. Kaplan–Meier estimate of freedom from the rejection end point and the infection end point. Kaplan–Meier curves in consideration of the complete study population of the Efficacy Limiting Toxicity Elimination Symphony Study (study arms A–C; n=1190). The curves for infection (red) and rejection (purple) end points intersect at day 139 after transplantation.
Risk Factors for Infection and Rejection
We investigated parameters associated with infection and rejection end points by multiple Cox regression analysis. Because the aim of the study was to establish a generally applicable approach to guide the choice of immunosuppression, only parameters universally available at the time of transplantation were considered. Panel-reactive antibodies were not included in our analysis, because they were not determined with a standardized method across the different study centers. We found that the risk to reach the infection end point was associated with recipient older age, deceased donor, higher number of HLA mismatches, and high risk for CMV disease (i.e., donor positive and recipient negative). In the final models (including relevant factors from larger models for infections and rejections), the rejection end point was significantly associated with deceased donor, higher number of HLA mismatches, and immunosuppressive therapy including cyclosporin A (compared with tacrolimus) (Table 2). Notably, older patients had a higher risk for infection, but in contrast to general assumptions, age was not associated with the risk for rejection (16,17).
Table 2.
Cox regression analysis of predictors of the infection end point and the rejection end point
| Variable | Infection End Point | Rejection End Point | ||
|---|---|---|---|---|
| Overall Relative Risk (95% CI) | P Value | Overall Relative Risk (95% CI) | P Value | |
| Recipient age (yr) | 1.01 (1.00 to 1.02) | 0.01 | 0.99 (0.98 to 1.00) | 0.20 |
| Women | 1.08 (0.85 to 1.38) | 0.51 | 0.92 (0.71 to 1.19) | 0.42 |
| Pretransplant diabetes mellitus | 1.12 (0.86 to 1.71) | 0.27 | 1.40 (0.97 to 2.03) | 0.07 |
| Donor type | ||||
| Living | 1.00 (reference group) | 1.00 (reference group) | ||
| Deceased not ECD | 2.15 (1.41 to 3.26) | <0.001 | 1.57 (1.05 to 2.35) | 0.03 |
| Deceased ECD | 1.69 (1.20 to 2.38) | 0.003 | 1.17 (0.84 to 1.61) | 0.35 |
| Donor age (yr) | 1.00 (0.99 to 1.02) | 0.27 | 1.00 (0.99 to 1.01) | 0.24 |
| No. of HLA mismatches | 1.08 (1.01 to 1.17) | 0.05 | 1.18 (1.08 to 1.28) | <0.001 |
| Cytomegalovirus status | ||||
| Other | 1.00 (reference group) | 1.00 (reference group) | ||
| D+/R− | 1.45 (1.08 to 1.94) | 0.01 | 0.99 (0.70 to 1.41) | 0.96 |
| Immunosuppression | ||||
| Group A | 1.00 (reference group) | 1.00 (reference group) | ||
| Group B | 0.91 (0.69 to 1.20) | 0.51 | 0.88 (0.67 to 1.15) | 0.34 |
| Group C | 0.82 (0.62 to 1.09) | 0.17 | 0.46 (0.33 to 0.63) | <0.001 |
95% CI, 95% confidence interval; ECD, extended criteria donor; D+/R−, donor seropositive and recipient seronegative.
Risk Stratification for Infection and Rejection
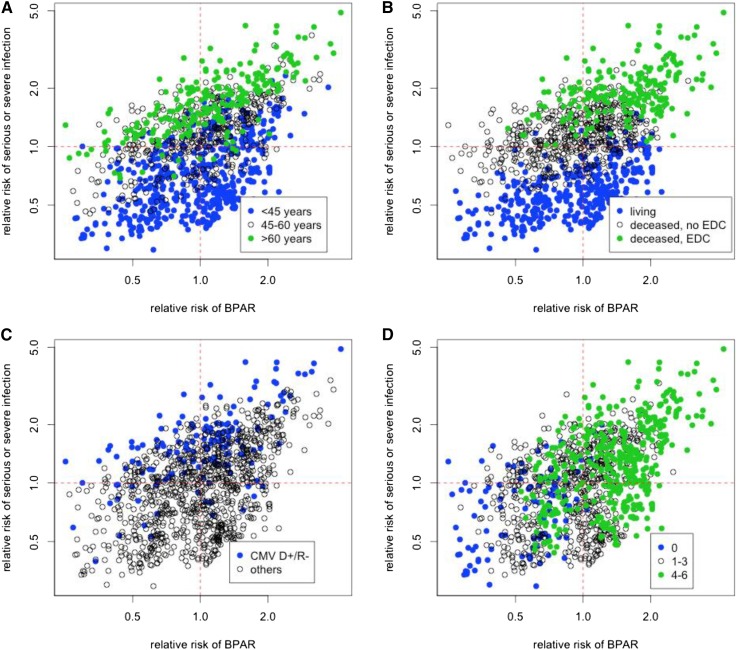
Using the model coefficients presented above, we calculated the risks for infection and rejection for each patient. To highlight divergences in the two types of risks, we plotted the predicted relative risk for rejection against that for infection. The two–dimensional risk space was divided in four quadrants on the basis of the risk for the two end points being higher or lower than the respective average risk (Figure 2 shows the effect of some of the predictors). This average patient was computed on the basis of average values for the model covariates from the population used to fit the model, and the relative risk for a given patient was determined by the hazard function from the Cox model divided by the hazard function for the average patient. Patients in the four-risk quadrants are termed low risk for both infection and rejection (ir), high risk for infection and low risk for rejection (Ir), low risk for infection and high risk for rejection (iR), and high risk for both infection and rejection (IR) according to their predicted relative risk for the infection end point and the rejection end point.
Figure 2.
Two–dimensional risk stratification for serious infection and rejection. The risk for rejection and infection was calculated for each patient in the Efficacy Limiting Toxicity Elimination Symphony Study (study arms A–C; n=1190) and displayed in a two-dimensional plot (x axis, rejection; y axis, infection). The two–dimensional risk space was divided in four quadrants by an average patient. The panels indicate the influences of (A) age, (B) donor type, (C) cytomegalovirus (CMV) status, and (D) HLA mismatches on the individual risk profile. BPAR, biopsy–proven acute rejection; CMV D+/R−, high risk for CMV disease with a seropositive donor and a seronegative recipient; EDC, extended criteria donor.
Internal Validation
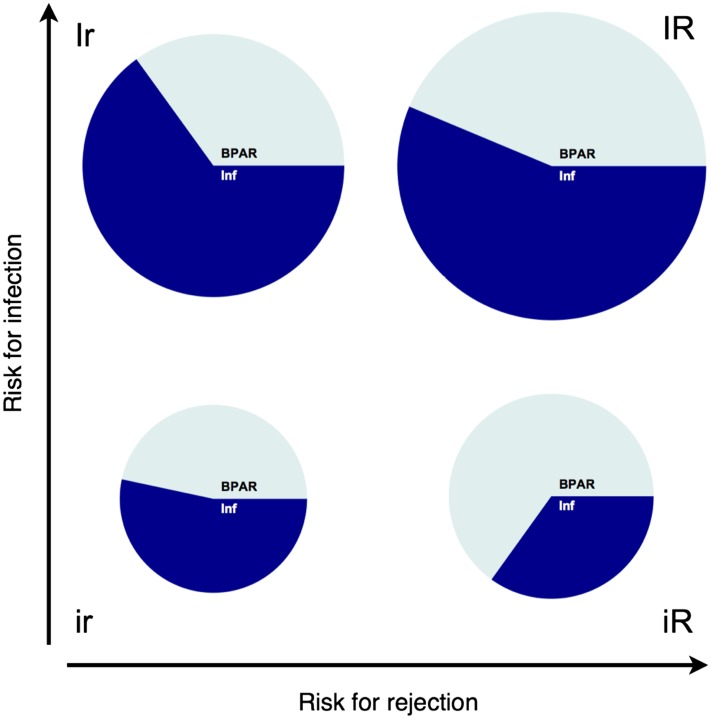
For internal validation, the model was refitted using a derivation cohort of 572 patients (50% of the patients randomly selected) and applied to a validation cohort including the remaining 571 patients. The distribution of the validation cohort in the four subgroups defined by the two–dimensional risk stratification plot was ir, 176 (30.8%); iR, 206 (36.1%); Ir, 40 (7.0%); and IR, 149 (26.1%). The incidence of the composite end point (infection or rejection) (proportional to the area of the pie plot in Figure 3) substantially varied among the different subgroups: 54.4% of patients in the IR group but only 24.4% of patients in the ir group reached the rejection or infection end point. Among the intermediate groups, the incidence of the composite end point was higher in the Ir (47.5%) compared with the iR (28.2%) group.
Figure 3.
The two-dimensional risk stratification model discriminated the subgroups in terms of composite end point, rejection and infection in the internal validation. Patients in the validation cohort were classified in four risk groups according to the two–dimensional stratification model. The registered incidences of infection and rejection end points in the four subgroups are shown. The area of the pie plot is proportional to the event frequency (proportion of patients with an event of any type in the respective quadrant). The compartments of the pie plots indicate the incidence of rejection and infection in patients with at least one episode. BPAR, biopsy–proven acute rejection; Inf, infection; ir, low risk for both infection and rejection; iR, low risk for infection and high risk for rejection; Ir, high risk for infection and low risk for rejection; IR, high risk for both infection and rejection.
The specific analysis for the rejection end point confirmed that patients predicted to have a high risk for rejection reached the rejection end point more frequently (IR, 30.2%; iR, 19.9%; ir, 11.9%; and Ir, 17.5%). The model correctly predicted an isolated risk for rejection because in the iR group 70.7% of patients with an event reached the rejection end point (compared with ir, 48.8%; Ir, 36.8%; and IR, 55.6%). Furthermore, the model correctly predicted the risk for infection: the infection end point was reached by 38.9% of patients in the IR group and 32.5% of patients in the Ir group but only 13.6% of patients in the ir group and 10.7% of patients in the iR group. Patients with an isolated risk for infections (Ir) were more likely to achieve the infection end point (68.4% of patients with an event had an infection) compared with the groups with a low risk for infection (ir, 55.8% and iR, 37.9%) but not compared with the IR group (71.6%). Thus, the model significantly discriminated four subgroups in terms of composite end points (P<0.001), rejection episodes (P<0.01), and infection episodes (P<0.001).
External Validation
To assess the general applicability of this approach and the specific model, we applied the model calculated with the population of the ELITE Symphony Study to the independent population of the FDCC Trial. The baseline characteristics of the two trial populations were only partly overlapping: patients in the ELITE Symphony Study more frequently had a living donor, and on average, they had fewer HLA mismatches and more frequent pretransplant diabetes mellitus (Table 1). The outcomes of the two studies in terms of rejection and infection were different: the rejection rate was lower in the FDCC Trial compared with the ELITE Symphony Study (20.6% versus 23.4%), but the infection rate was higher (28.3% versus 25.5%) (Supplemental Figure 1).
The risk stratification model classified 630 evaluable patients as follows: 163 patients (25.9%) in the ir group, 146 (23.2%) patients in the iR group, 98 (15.6%) patients in the Ir group, and 223 (35.4%) patients in the IR group. Because of the relevant discrepancies between the two studies, the ELITE Symphony Study–based risk model may have been less accurate when applied to the FDCC Trial. To avoid an incorrect classification of patients with an intermediate risk score, for the comparison of the outcomes in four groups in the FDCC Trial, we excluded about 10% of patients whose risk score minimally differed from the average patient (<0.2 log units).
Among the patients classified in the iR and the IR groups, 30 (23.4%) and 52 (25.4%) reached the rejection end point, respectively. This was the case for smaller proportions of patients from the ir and the Ir groups: 23 (15.6%) and 14 (16.1%), respectively. The infection end point was reached by 32 (36.8%) patients in the Ir group and 63 (30.7%) patients in the IR group but only 40 (27.2%) patients in the ir group and 30 (23.4%) patients in the iR group.
Furthermore, compared with the ELITE Symphony Study cohort, the risk stratification model identified a higher fraction of patients at high risk for infection (Ir + IR; validation cohort of the ELITE Symphony Study versus the FDCC Trial, 33.1% versus 51.0%), which is reflected in a slightly higher incidence of infection in the latter trial. A correspondence with the assessed incidence was also present for the lower number of patients predicted to be at risk for rejection (iR + IR) in the FDCC Trial (58.6%) compared with the ELITE Symphony Study (62.2%).
Discussion
The delicate balance between under- and overimmunosuppression emerged as a critical issue in transplantation medicine. The need for effective strategies to avoid overimmunosuppression was pointed out by the high incidence and potential fatal outcomes of infection episodes in the first year after transplantation in both the ELITE Symphony Study and the FDCC Trial. Moreover, in consideration of the slope of the curves presented in Figure 1 and according to previous reports (18) and preliminary data on infectious diseases end points in the Swiss Transplant Cohort Study (19), infections are likely to assume even a more pronounced relevance in the long term.
The first step toward personalized medical management after transplantation is to map out the individual risk profile for the effects of under- und overimmunosuppression. To our knowledge, this is the first multifactorial model that combined risk stratification for rejection (linked to underimmunosuppression) and infection (linked to overimmunosuppression). The model was calculated using parameters universally available at the time point of transplantation. The validation process provided support for the robustness of the risk assessment principle. The potential usefulness of this strategy is broad and ranges from the definition of personalized target levels of immunosuppressants, the duration of antibiotic/antiviral prophylaxis, and the identification of patients who might benefit from immunosuppression minimization or tolerance induction protocols to the optimization of organ allocation systems (2,20–24). Depending on the preferred application, different end points should be considered. We decided to restrict our analysis to severe infections and biopsy–proven acute rejections to increase the clinical relevance of the study. Expanding the end points by consideration of additional clinical, ethical, or economic factors would lead to a different model but would not change the principle of the study.
The applicability of our model is limited by the particular composition of the study population in terms of ethnicity and immunologic risk profile. Our choice of the ELITE Symphony Study cohort for our modeling work was on the basis of the fact that it is a well characterized and representative group of patients with tight and complete follow-up. Moreover, a precisely defined study protocol in terms of immunosuppression management was pivotal to minimize the risk for bias related to a change in medical therapy on the basis of clinical factors. However, this predominantly European low– to medium–risk transplant population treated with standard immunosuppression but without lymphocyte depletion induction therapy might not be representative of the local transplant recipient population in many centers worldwide. Thus, to implement personalized immunosuppression on the basis of this concept, the model presented here should first be recalculated using data from a relevant local patient population. Then, the accuracy of the risk stratification principle needs to be tested in prospective studies. Finally, for the application of this approach in interventional studies, the precise position of an individual patient in the two-dimensional plot might be more important than its classification in one of four groups, and adapting immunosuppression strategy might be appropriate only for patients with extreme risk profiles. Notably, this approach might assume a particular relevance for the significant number of patients (about one half of the validation sample) showing a discordant risk profile (i.e., Ir or iR) who might benefit from a tailored therapy.
Furthermore, we observed that infection and rejection were linked in a relevant number of patients (i.e., in about 30% of patients who reached the infection or the rejection end point). The partial overlap among risk factors for infection and rejection (as reported in Table 2) might explain this link. Alternatively, the combined incidence of rejection and infection might be the consequence of changes in medical therapy in response to the first event (e.g., intensification of immunosuppression because of an acute rejection followed by a severe infection or reduction of immunosuppression because of a severe infection followed by a rejection episode). The equal distribution in the sequence of events in patients who reached both end points suggests that both scenarios might have clinical relevance. Our model can be used to identify patients with IR. These patients are at high risk for complications and require special attention in the management of immunosuppression. Additional studies are needed for a better characterization of this high-risk population.
This study presents several limitations. To assess the general applicability of the proposed concept, we directly applied the model derived from the ELITE Symphony Study cohort to the independent population of the FDCC Trial. This approach might lead to an incorrect classification of patients with an intermediate risk profile and is appropriate for the validation process (which allowed us to exclude a small fraction of patients) but not suitable in clinical practice. Moreover, the risk prediction model may be improved and the overlap in outcomes among the groups may be reduced by integrating additional biomarkers, particularly donor-specific antibodies at time point of transplantation (25–29), which were not available in the ELITE Symphony Study, or considering additional parameters during follow-up (i.e., drug levels or de novo donor–specific antibodies).
In conclusion, the prevalence of clinically relevant infections after kidney transplantation is high and represents the major threat for recipients of kidneys in the first year after transplantation. We identified risk factors for infection and rejection that were able to stratify patients according to their relative risk for these two types of events. Our approach may provide a means to tailor immunosuppressive and anti–infective regimens according to the patient’s individual risk. This model might find broader clinical application and help improve survival and quality of life of the thousands of patients receiving solid organ transplantation each year worldwide (30).
Disclosures
None.
Supplementary Material
Acknowledgments
This article is dedicated to H.E., who sadly passed away on December 29, 2012.
This work was supported by unrestricted grants from Roche Pharma (Schweiz) AG and Astellas and the matching fund program of the University Hospital of Zurich.
This study was presented as an abstract at the 46th Annual Meeting of the Swiss Society of Nephrology, Interlaken, Switzerland, December 3 to December 5, 2014 and in part, a poster at the 14th Congress of the European Society of Organ Transplantation, Paris, France, August 30 to September 2, 2009.
Footnotes
Deceased.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01790215/-/DCSupplemental.
References
- 1.Sayegh MH, Carpenter CB: Transplantation 50 years later--progress, challenges, and promises. N Engl J Med 351: 2761–2766, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB: Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 346: 580–590, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA, Issa NC: Infection in organ transplantation: Risk factors and evolving patterns of infection. Infect Dis Clin North Am 24: 273–283, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Karuthu S, Blumberg EA: Common infections in kidney transplant recipients. Clin J Am Soc Nephrol 7: 2058–2070, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Fishman JA: Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Yabu JM, Vincenti F: Kidney transplantation: The ideal immunosuppression regimen. Adv Chronic Kidney Dis 16: 226–233, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Kuypers DR: Diagnosis and prevention of chronic kidney allograft loss. Lancet 378: 1428–1437, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Wagner M, Balk EM, Kent DM, Kasiske BL, Ekberg H: Subgroup analyses in randomized controlled trials: The need for risk stratification in kidney transplantation. Am J Transplant 9: 2217–2222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia D, Cheshire J, Begaj I, Khosla S, Ray D, Sharif A. Death within the first year after kidney transplantation--an observational cohort study. Transpl Int 27: 262–270, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Sanfilippo F, Vaughn WK, LeFor WM, Spees EK: Multivariate analysis of risk factors in cadaver donor kidney transplantation. Transplantation 42: 28–34, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF, ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 13.van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, Lohmus A, Sommerer C, Hartmann A, Le Meur Y, Oellerich M, Holt DW, Tönshoff B, Keown P, Campbell S, Mamelok RD: Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: The fixed-dose concentration-controlled trial. Transplantation 86: 1043–1051, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF, Häyry P, Jennette JC, Keown PA, Marcussen N, Mihatsch MJ, Morozumi K, Myers BD, Nast CC, Olsen S, Racusen LC, Ramos EL, Rosen S, Sachs DH, Salomon DR, Sanfilippo F, Verani R, von Willebrand E, Yamaguchi Y: International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int 44: 411–422, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al. : The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E: The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg 252: 662–674, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Kaplan B: Immunosuppression in elderly renal transplant recipients: Are current regimens too aggressive? Drugs Aging 18: 751–759, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Silva HT, Jr., Yang HC, Meier-Kriesche HU, Croy R, Holman J, Fitzsimmons WE, First MR: Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 97: 636–641, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koller MT, van Delden C, Müller NJ, Baumann P, Lovis C, Marti HP, Fehr T, Binet I, De Geest S, Bucher HC, Meylan P, Pascual M, Steiger J: Design and methodology of the Swiss Transplant Cohort Study (STCS): A comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol 28: 347–355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehr T, Sykes M: Tolerance induction in clinical transplantation. Transpl Immunol 13: 117–130, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Danovitch GM, Gill J, Bunnapradist S: Immunosuppression of the elderly kidney transplant recipient. Transplantation 84: 285–291, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Fehr T, Cohen CD: Predicting an allograft’s fate. Kidney Int 80: 1254–1255, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Giese T, Sommerer C, Zeier M, Meuer S: Approaches towards individualized immune intervention. Dig Dis 28: 45–50, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R: Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol 22: 2107–2118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawitzki B, Schlickeiser S, Reinke P, Volk HD: Pretransplant immune risk assessment. Curr Opin Organ Transplant 14: 650–655, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Naesens M, Sarwal MM: Molecular diagnostics in transplantation. Nat Rev Nephrol 6: 614–628, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Budde K, Matz M, Dürr M, Glander P: Biomarkers of over-immunosuppression. Clin Pharmacol Ther 90: 316–322, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huskey J, Gralla J, Wiseman AC: Single time point immune function assay (ImmuKnow) testing does not aid in the prediction of future opportunistic infections or acute rejection. Clin J Am Soc Nephrol 6: 423–429, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia GG, Harden P, Chapman J, World Kidney Day Steering Committee 2012 : The global role of kidney transplantation. Lancet 379: e36–e38, 2012. 22405254 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.