Abstract
Background and objectives
Patients of all ages undergoing hemodialysis (HD) have a high prevalence of cognitive impairment and worse cognitive function than healthy controls, and those with dementia are at high risk of death. Frailty has been associated with poor cognitive function in older adults without kidney disease. We hypothesized that frailty might also be associated with poor cognitive function in adults of all ages undergoing HD.
Design, setting, participants, & measurements
At HD initiation, 324 adults enrolled (November 2008 to July 2012) in a longitudinal cohort study (Predictors of Arrhythmic and Cardiovascular Risk in ESRD) were classified into three groups (frail, intermediately frail, and nonfrail) based on the Fried frailty phenotype. Global cognitive function (3MS) and speed/attention (Trail Making Tests A and B [TMTA and TMTB, respectively]) were assessed at cohort entry and 1-year follow-up. Associations between frailty and cognitive function (at cohort entry and 1-year follow-up) were evaluated in adjusted (for sex, age, race, body mass index, education, depression and comorbidity at baseline) linear (3MS, TMTA) and Tobit (TMTB) regression models.
Results
At cohort entry, the mean age was 54.8 years (SD 13.3), 56.5% were men, and 72.8% were black. The prevalence of frailty and intermediate frailty were 34.0% and 37.7%, respectively. The mean 3MS was 89.8 (SD 7.6), TMTA was 55.4 (SD 29), and TMTB was 161 (SD 83). Frailty was independently associated with lower cognitive function at cohort entry for all three measures (3MS: −2.4 points; 95% confidence interval [95% CI], −4.2 to −0.5; P=0.01; TMTA: 12.1 seconds; 95% CI, 4.7 to 19.4; P<0.001; and TMTB: 33.2 seconds; 95% CI, 9.9 to 56.4; P=0.01; all tests for trend, P<0.001) and with worse 3MS at 1-year follow-up (−2.8 points; 95% CI, −5.4 to −0.2; P=0.03).
Conclusions
In adult incident HD patients, frailty is associated with worse cognitive function, particularly global cognitive function (3MS).
Keywords: african american; black; hemodialysis; ESRD; epidemiology and outcomes; frailty; cognition; dementia; depression; kidney failure, chronic; renal dialysis
Introduction
Cognitive impairment is common in adults of all ages undergoing hemodialysis: 22% have mild cognitive impairment and 8% have moderate to severe impairment (1). Worse, there is a high burden of unrecognized cognitive impairment in this population, even among younger adults. Although there is growing awareness of the burden of cognitive impairment in patients undergoing hemodialysis, it remains an important issue in this highly vulnerable population. Adults undergoing hemodialysis who have cognitive dementia are at a high risk of mortality (2). Better understanding of cognitive function and correlates of poor cognitive function in adults of all ages initiating hemodialysis is the first step in identifying early interventions to delay the onset of impairment.
Frailty, a measure of physiologic reserve (or the body’s ability to respond to external stressors), was initially described and validated by Fried et al. in a geriatric population (3) and is characterized as a unique domain of risk that is related to, yet distinct from, comorbidity and disability (3,4). In older adults, there is evidence that frailty and cognitive function interact with age-related cycles of decline (4). Frailty (3), as defined by Fried et al., is emerging as an important risk factor in patients with ESRD of all ages. Among adults undergoing hemodialysis, this validated measure of frailty is associated with higher risk of mortality (5), hospitalization (5), and falls (6). These findings also suggest that frailty is associated with poor hemodialysis outcomes regardless of age (5,6). Poor cognitive function may be one pathway linking frailty to adverse outcomes on hemodialysis. Although there is some evidence that frailty is associated with poor cognitive function (3,7–10), cognitive decline (11,12), and dementia (13,14) in middle-aged and older adults, the association between frailty and cognitive function in adults of all ages undergoing hemodialysis is unclear. We hypothesize that frail adults initiating hemodialysis are more likely to have worse cognitive function.
To better understand the relationship between frailty and cognitive function, we estimated the association between these two constructs among 324 adults of all ages initiating hemodialysis.
Materials and Methods
Study Design
This study (ancillary to the Predictors of Arrhythmic and Cardiovascular Risk in ESRD [PACE] trial; R01DK072367) (15,16) was an in-person assessment of frailty and other factors in patients who had initiated hemodialysis at 27 free-standing dialysis centers in Baltimore, Maryland, and six surrounding counties (17). Although all participants were hemodialysis initiates (within 6 months), participants were not necessarily enrolled on their first day of hemodialysis; 95% were enrolled within the first month of dialysis. The parent study was designed to investigate sudden unexpected cardiac death in an incident dialysis population. Eligibility criteria included age ≥18 years at enrollment and the ability to speak English. Parent study exclusion criteria included living in a hospice, nursing facility, or prison; having a pacemaker or automatic implantable cardioverter defibrillator; having cancer other than nonmelanoma skin cancer within the prior year; or being pregnant or breastfeeding. Those with a diagnosis of dementia, Alzheimer’s disease, or schizophrenia were excluded, as were those who were unable to complete consent or follow through on a study visit. In addition, we excluded participants who were illiterate or had a disability that limited their ability to complete the cognitive function assessments. All participants in the parent study with a complete measure of frailty (n=62 excluded) and at least one measure of cognitive function (n=8 excluded) (both described below) were included in this analysis and resulted in an analytic sample of 324 participants (enrolled between November 2008 and July 2012). All study procedures were approved by the Johns Hopkins University Institutional Review Board; all participants provided informed consent.
Participant Characteristics
Demographics (age, sex, and race) and smoking status were obtained by participant self-report. Body mass index (BMI) was calculated from self-reported height and dry weight. Comorbidities were assessed based on participant-reported medical history, which was augmented with data from US Centers for Medicare and Medicaid Services Form-2728 and from chart abstraction. Comorbidities included cardiovascular disease (coronary artery disease, myocardial infarction, angina), congestive heart failure, rheumatoid arthritis (only by self-report), cancer (only by chart abstraction), diabetes, hypertension, or chronic obstructive pulmonary disease. Comorbidity was defined as ≥4 of these comorbidities, to be consistent with the hallmark article on frailty (3) and the frailty and ESRD literature (5,6) as well as by the Charlson Comorbidity Index (modified for patients with ESRD). Diagnosed depression and primary cause of ESRD were also ascertained from chart abstraction. Participants self-reported the highest level of education and were classified as having a high school degree or a high school equivalent degree or higher. As an alternative to adjusting for education, which is highly associated with socioeconomic status, the Wide Range Achievement Test (WRAT-4), a test of reading achievement, was administered at cohort entry. The WRAT provides a measure of premorbid intelligence that is minimally affected by cognitive decline during the life course.
Frailty
At enrollment, frailty was measured as defined and validated by Fried in older adults (3,18–27) and by our group in ESRD and kidney transplantation (5,6,28–31). Frailty was based on five components: shrinking (self-report of unintentional weight loss of >10 lb in the past year based on dry weight), weakness (grip strength below an established cutoff based on sex and BMI), exhaustion (self-report), low activity (kilocalories per week below an established cutoff), and slowed walking speed (walking time of 15 ft below an established cutoff by sex and height) (3). The previously published cutpoints for weakness, low physical activity, and slowed walking speed were used (3). Each of the five components was scored as 0 or 1 representing the absence or presence of that component. The aggregate frailty score was calculated as the sum of the component scores (range, 0–5); nonfrail was defined as a score of 0 or 1, intermediate frailty was defined as a score of 2, and frailty was defined as a score of ≥3, as we previously published (5).
Cognitive Function
Trained research coordinators measured cognitive function using the Modified Mini-Mental State (3MS) test and Trail Making Tests A and B (TMTA and TMTB, respectively) at enrollment and 1 year follow-up on nondialysis days. Participants were missing 1-year follow-up assessments because they did not attend the 1-year follow-up visit (n=77), died before the visit (n=32), were no longer eligible because they were not on hemodialysis (n=36), or withdrew consent or were withdrawn due to noncompliance (n=5). The 3MS is a measure of global cognitive function (range, 0–100), with higher scores representing better cognitive function (32). The TMTA and TMTB are time tests that measure executive function (33). Both of these tests assess scanning, speed of processing, attention and concentration, and psychomotor speed, and the TMTB further assesses cognitive shifting and complex sequencing function. The tests measure the time required to connect a series of sequentially numbered (TMTA) and numbered/lettered (TMTB) circles. Scores are based on the total time of test completion without error. Greater time needed to complete the tests indicates worse cognitive function; times were capped at 3 minutes for TMTA and 5 minutes for TMTB (33). Cognitive impairment was defined as a score <80 for the 3MS, a time 1.5 SD above the mean (from this cohort) for the TMTA/TMTB.
Statistical Analyses
Differences in participant characteristics by frailty status were tested using ANOVA and Fisher’s exact tests where appropriate.
We tested whether frailty was independently associated with cognitive function scores at cohort enrollment using linear regression for the 3MS and the TMTA. For the TMTB, we observed a ceiling effect, with 13% of participants stopped at 5 minutes during the baseline assessment. To account for this ceiling effect in TMTB, we used Tobit regression (34), which is an analytic model that assumes a linear distribution up to a dichotomous threshold (5 minutes for TMTB). In all models, we included frailty at hemodialysis initiation and adjusted for participant and dialysis factors (sex, age, race, BMI, education, and comorbidity at baseline) that were associated with frailty in this cohort or factors that were associated with frailty from the geriatrics and gerontology literature. For participants with baseline measures of cognitive function, we used the same models to test whether frailty at hemodialysis initiation was associated with these three measures of cognitive function scores ascertained at 1 year. In addition, we used linear regression to test whether frailty was associated with a change in cognitive function between initiation and 1-year follow-up, adjusting for participant and dialysis factors as well as baseline cognitive function scores.
We tested for an interaction of the association between frailty and cognitive function score at hemodialysis initiation by age, sex, and race using a Wald test. For all analyses, a P value <0.05 was considered significant. All analyses were performed using STATA 13.0 software (StataCorp, College Station, TX).
Results
Study Population
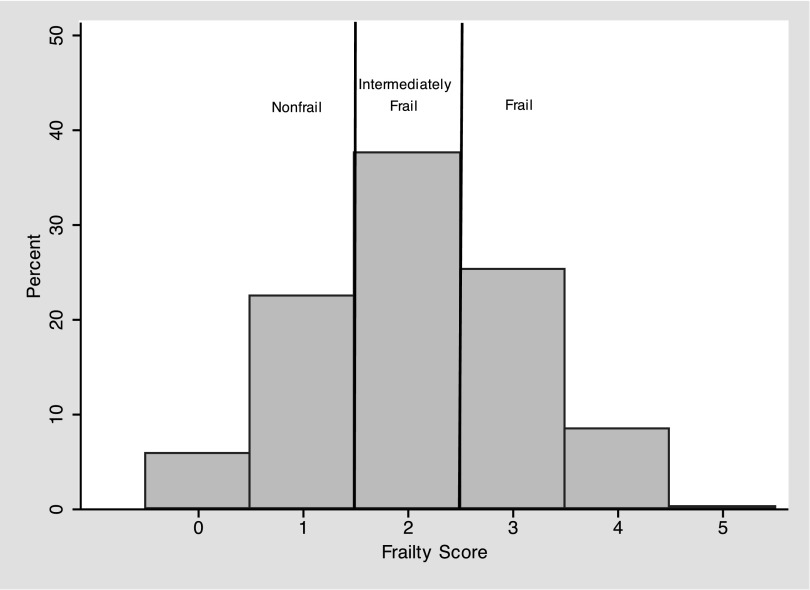
Among 324 adults initiating hemodialysis, the mean age was 54.8 years (SD 13.3), 56.5% were men, and 72.8% were self-reported black; 34.0% of participants were frail and 37.7% were intermediately frail at the time of hemodialysis initiation (Figure 1). Consistent with previous findings of frailty as an independent domain (5,6), few dialysis factors were statistically significantly associated with frailty. The mean age (frail, 57.0 years; intermediately frail, 54.8 years; and nonfrail, 52.0 years; P=0.03) and mean dry weight BMI (frail, 31.5 kg/m2; intermediately frail, 29.7 kg/m2; and nonfrail, 27.6 kg/m2; P=0.004) were highest in those who were frail (Table 1). In addition, frail adults initiating hemodialysis were more likely to be obese (frail, 51.8%; intermediately frail, 41.8%; and nonfrail, 23.9%; P<0.001) and have cerebrovascular disease (frail, 26.4%; intermediately frail, 22.1%; and nonfrail, 12.0%; P=0.03) but not other comorbidities (Table 1). As expected, frailty was not associated with the WRAT score, a measure of reading achievement that reflects premorbid intelligence.
Figure 1.
Distribution of the Fried Frailty Score in adults initiating hemodialysis. The distribution of the Fried Frailty Score is presented as a histogram of the percentage of participants with a given score. The Fried Frailty Score ranges from 0 to 5, with a higher score representing worse frailty.
Table 1.
Characteristics of hemodialysis initiates (N=324), by frailty status
| Characteristic | Nonfrail (n=92) | Intermediately Frail (n=122) | Frail (n=110) | P Value |
|---|---|---|---|---|
| Women | 45.7 | 38.5 | 47.3 | 0.37 |
| Age (yr) | 52.0 (11.7) | 54.8 (14.1) | 57.0 (13.3) | 0.03 |
| Black race | 80.4 | 68.9 | 70.9 | 0.14 |
| Dry weight body mass index (kg/m2) | 27.6 (7.3) | 29.7 (8.1) | 31.5 (8.9) | 0.004 |
| Obese | 23.9 | 41.8 | 51.8 | <0.001 |
| Current smoker | 21.7 | 24.6 | 22.7 | 0.89 |
| High school or higher education | 63.0 | 65.6 | 61.8 | 0.85 |
| Primary cause of ESRD | 0.47 | |||
| Diabetes | 28.3 | 33.6 | 39.1 | |
| Hypertension | 27.2 | 27.9 | 27.3 | |
| GN | 20.7 | 13.9 | 10.0 | |
| Other | 23.9 | 24.6 | 23.6 | |
| Comorbidities | ||||
| Cardiovascular disease | 40.2 | 43.4 | 52.7 | 0.17 |
| Peripheral vascular disease | 14.1 | 20.5 | 19.9 | 0.49 |
| Rheumatoid arthritis | 7.6 | 15.6 | 15.5 | 0.16 |
| History of cancer | 7.6 | 5.7 | 11.8 | 0.25 |
| Hypertension | 100 | 100 | 100 | >0.99 |
| Chronic pulmonary disease | 27.2 | 27.9 | 32.7 | 0.64 |
| Diabetes | 51.1 | 53.3 | 63.6 | 0.14 |
| Congestive heart failure | 30.4 | 30.3 | 31.8 | 0.97 |
| Cerebrovascular disease | 12.0 | 22.1 | 26.4 | 0.03 |
| Comorbiditya | 39.1 | 40.2 | 48.2 | 0.35 |
| Diagnosed depression | 17.1 | 25.2 | 24.5 | 0.32 |
| Wide Range Achievement Test score | 52.5 (11) | 53.3 (11) | 52.0 (12) | 0.68 |
Data are presented as percentages or means (SD).
Comorbidity is ≥4 of the following comorbidities: cardiovascular disease (coronary artery disease, myocardial infarction, angina), congestive heart failure, arthritis, cancer, diabetes, hypertension, or chronic obstructive pulmonary disease.
Cognitive Function at Hemodialysis Initiation
3MS scores were available for 323 participants at hemodialysis initiation. TMTA and TMTB scores were available for 320 and 314 participants at hemodialysis initiation, respectively. The mean score was 89.8 (SD 7.6) for 3MS, 55.4 (SD 29) for TMTA, and 161 (SD 83) for TMTB at hemodialysis initiation and 10.2% had cognitive impairment by 3MS, 7.5% by TMTA, and 15.0% by TMTB scores (Table 2).
Table 2.
Distribution of cognitive function scores and cognitive impairment at hemodialysis initiation and 1-year follow-up, by frailty status at hemodialysis initiation
| Frailty Status Measured at Hemodialysis Initiation | 3MS (n=323) | Trail Making Test A (n=320) | Trail Making Test B (n=314) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | % Impairment | Mean (SD) | % Impairment | Mean (SD) | % Impairment | |
| Initiation | ||||||
| Full cohort | 89.8 (7.6) | 10.2 | 55.4 (29) | 7.5 | 161 (83) | 15.0 |
| Nonfrail | 91.1 (7.3) | 6.6 | 48.5 (27) | 4.4 | 143 (80) | 8.7 |
| Intermediately frail | 90.0 (7.6) | 9.8 | 54.7 (25) | 6.7 | 161 (81) | 15.4 |
| Frail | 88.5 (7.6)a | 13.6 | 62.1 (33)a | 11.1 | 176 (84)a | 20.0a |
| 1-yr follow-up | ||||||
| Cohort at follow-up | 88.9 (7.3) | 9.9 | 56.8 (32) | 10.7 | 157 (82) | 16.8 |
| Nonfrail | 90.3 (5.8) | 4.0 | 51.1 (32) | 10.2 | 151 (88) | 12.7 |
| Intermediately frail | 88.9 (8.3) | 11.9 | 57.7 (34) | 12.3 | 151 (83) | 9.3 |
| Frail | 87.7 (7.0)a | 12.7 | 60.7 (28) | 14.8 | 169 (76) | 17.3 |
P values test the difference in mean cognitive function score for those who are intermediately frail and frail compared with those who are nonfrail. 3MS, Modified Mini-Mental State test.
P<0.05.
Frailty and Cognitive Function at Hemodialysis Initiation
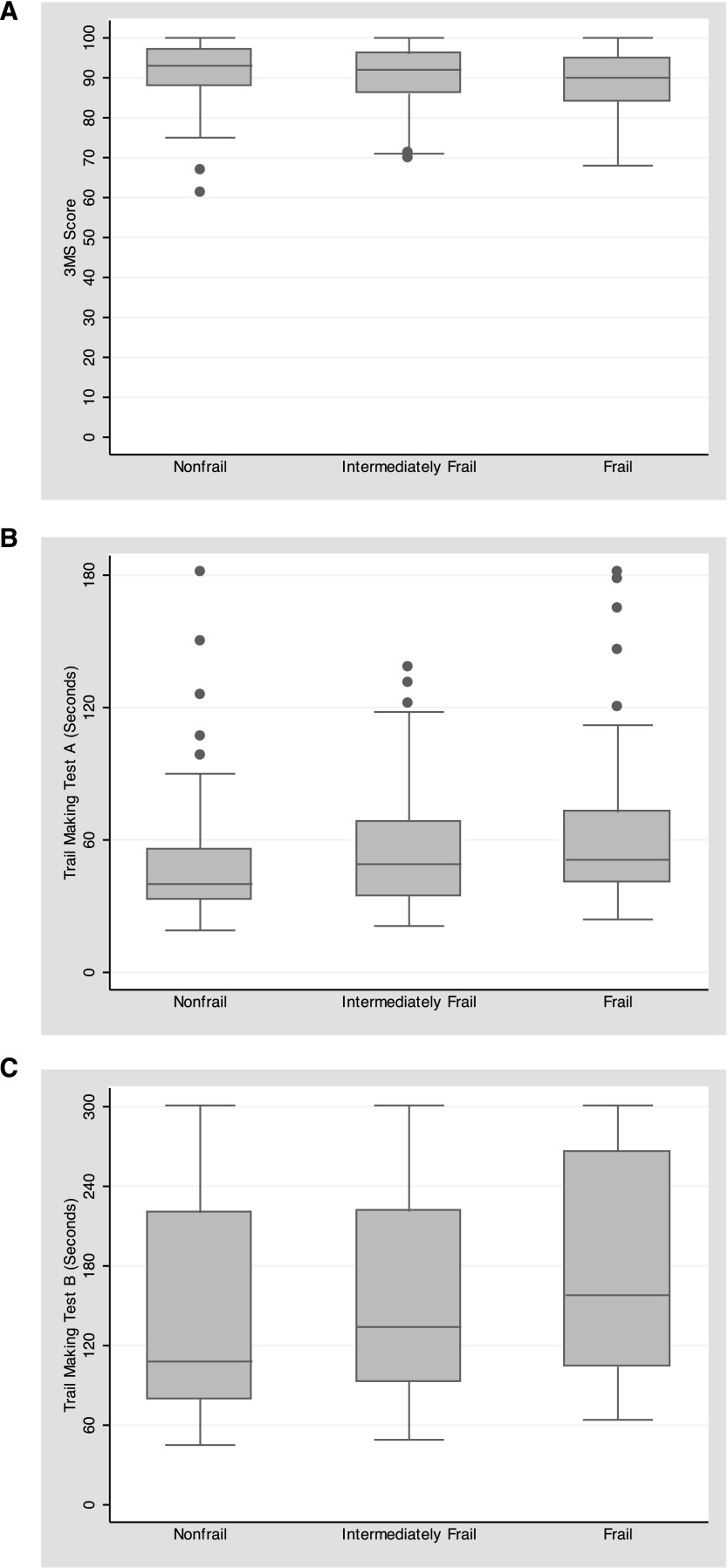
The unadjusted mean scores of the 3MS, TMTA, and TMTB were worse (lower for 3MS and higher for both Trail Making Tests) in those who were frail compared with those who were nonfrail (3MS, P=0.02; TMTA, P=0.002; and TMTB, P=0.01) (Figure 2, Table 2). When adjusted for potential confounders, frailty was associated with lower 3MS cognitive function scores at hemodialysis initiation (−2.4 points; 95% confidence interval [95% CI], −4.2 to −0.5; P=0.01), with higher TMTA scores (12.1 seconds; 95% CI, 4.7 to 19.4; P<0.001) and higher TMTB scores (33.2 seconds; 95% CI, 9.9 to 56.4; P=0.01) (Table 3). There was a dose response for each increasing level of frailty (3MS, P=0.01; TMTA, P=0.001; and TMTB, P=0.01).
Figure 2.
Distribution of baseline cognitive function scores, by frailty status in adults initiating hemodialysis. The distribution of the cognitive function scores at baseline is presented as a boxplot. The median (center bar), 25th percentile (bottom of the box), and 75th percentile (top of the box) are plotted by frailty status. Outliers (data points that fall outside the lower quartile −1.5 × IQR, upper quartile +1.5 × IQR) are noted as dots. (A) Modified Mini-Mental State (3MS) test. (B) Trail Making Test A (TMTA). (C) Trail Making Test B (TMTB). IQR, interquartile range.
Table 3.
Frailty Status at Hemodialysis Initiation and Cognitive Function at Hemodialysis Initiation and 1-Year Follow-up
| Frailty Status Measured at Hemodialysis Initiation | 3MS | Trail Making Test A | Trail Making Test B |
|---|---|---|---|
| Initiation | |||
| Patients (n) | 323 | 320 | 314 |
| Nonfrail | Reference | Reference | Reference |
| Intermediately frail | −1.29 (−3.05 to 0.48) | 6.12 (−0.94 to 13.18) | 19.87 (−2.34 to 42.08) |
| Frail | −2.37 (−4.21 to −0.53)a | 12.08 (4.73 to 19.43)b | 33.15 (9.88 to 56.42)a |
| P for trend | 0.01 | 0.001 | 0.01 |
| 1-yr follow-up | |||
| Patients (n) | 171 | 168 | 161 |
| Nonfrail | Reference | Reference | Reference |
| Intermediately frail | −1.74 (−4.16 to 0.69) | 6.56 (−4.00 to 17.13) | 1.10 (−28.94 to 31.14) |
| Frail | −2.80 (−5.37 to −0.24)a | 8.48 (−2.62 to 19.58) | 17.42 (−14.29 to 49.14) |
| P for trend | 0.03 | 0.14 | 0.27 |
Data are presented with 95% confidence intervals and are adjusted for sex, age, race, BMI, education, and comorbidity at baseline. 3MS, Modified Mini-Mental State test.
P<0.05.
P<0.001.
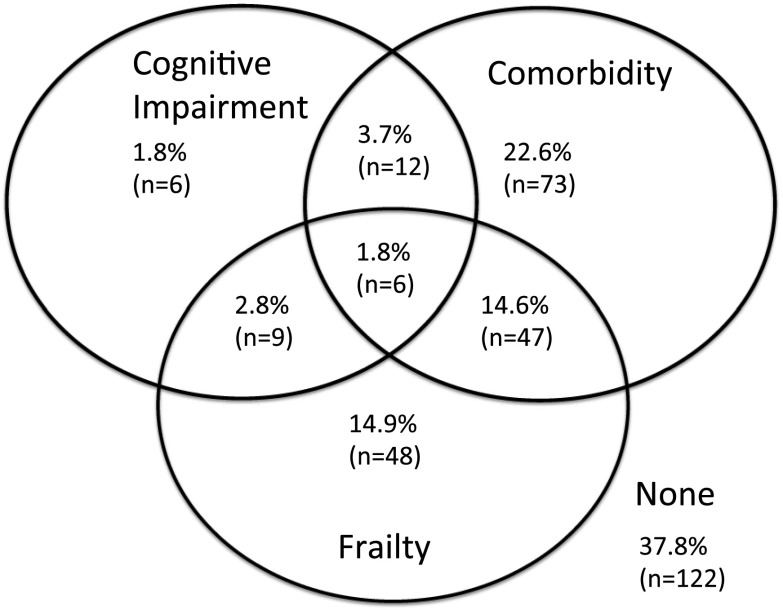
There was a higher prevalence of cognitive impairment by TMTB for frail participants (20.0% versus 8.7%; P=0.03) (Table 2). There was overlap between comorbidity, cognitive impairment (by 3MS), and frailty (Figure 3): 14.9% were frail without comorbidity or cognitive impairment, 14.6% were frail with comorbidity but no cognitive impairment, and 2.8% were frail with cognitive impairment but no comorbidity. Only 1.8% were frail with both cognitive impairment and comorbidity.
Figure 3.
Overlap of frailty, comorbidity, and cognitive impairment in adults initiating hemodialysis. The overlap of frailty, comorbidity, and cognitive function is displayed through a Venn diagram. The percentages represent the proportion of the total population with only cognitive impairment, comorbidity, and frailty as well as the overlap of these three factors. Frailty is defined as ≥3 components as defined by Fried. Comorbidity is defined as ≥4 conditions as specified in the Materials and Methods. Cognitive impairment was defined as a Modified Mini-Mental State (3MS) score <80. The total represents 323 study participants on hemodialysis. The size of each subgroup is indicated in parentheses.
Frailty and Cognitive Function at 1-Year Follow-Up
3MS scores were available for 171 patients at 1-year follow-up. TMTA and TMTB scores were available for 168 and 161 at 1-year follow-up, respectively. Mortality at 1 year did not differ by frailty status (nonfrail, 8.7%; intermediately frail, 10.7%; and frail, 10.0%; P=0.91), nor did dropout among those remaining alive at 1 year (nonfrail, 41.7%; intermediately frail, 38.5%; and frail, 44.4%; P=0.68). Finally, among those who survived to 1 year after enrollment, there were no differences in participants with and without follow-up measures of cognitive function by their baseline cognitive function scores (3MS, P=0.61; TMTA, P=0.72; and TMTB, P=0.46), their baseline frailty status (P=0.67), sex (P=0.57), or comorbidity status (P=0.97). However, black (P=0.01) and older participants (P=0.02) were less likely to have a follow-up measure of cognitive function.
At 1-year follow-up, the mean cognitive function scores were 88.9 points (SD 7.3) for 3MS, 56.8 seconds (SD 31.7) for TMTA, and 157 seconds (SD 82) for TMTB, and frail participants had worse 3MS scores (P=0.04) at 1-year follow-up (Table 2). In addition, frailty was independently associated with a lower 3MS cognitive function score at 1-year follow-up (−2.8 points; 95% CI, −5.4 to −0.2; P=0.03), with a significant “dose response” by levels of frailty (P=0.03) (Table 3). There was no statistical association between frailty and either TMTA or TMTB scores at 1-year follow-up (Table 3).
The prevalence of cognitive impairment at 1-year follow-up was 9.9% based on 3MS, 10.7% based on TMTA, and 16.8% for TMTB; there was no difference in the prevalence of cognitive impairment at 1-year follow-up by frailty status (Table 2). In addition, there was no difference in the change in 3MS (P=0.80), change in TMTA (P=0.46), or change in TMTB (P=0.07) in adjusted analyses, regardless of whether cognitive function at initiation was included in the model.
Effect Heterogeneity
The association of intermediate frailty as well as frailty and all three cognitive function scores at hemodialysis initiation did not differ between men and women, older and younger adults, or black and nonblack participants (all P>0.05). Similar results were observed for effect modification of frailty and 3MS score measured at 1 year (P>0.05).
Sensitivity Analyses
The associations between frailty and cognitive function scores at hemodialysis initiation were similar when (1) adjusted for WRAT scores (a measure of premorbid reading achievement) instead of education, (2) adjusted for the Charlson Comorbidity Index scores (modified for patients with ESRD) instead of the previously published measure of comorbidity, (3) additionally adjusted for diagnosed depression, (4) adjusted for cardiovascular disease in addition to comorbidity, and (5) restricted to those without a previous history of cerebrovascular disease. Finally, frailty was associated with all three measures of cognitive function at dialysis initiation when the study population was limited to those who also had a 1-year measure of cognitive function.
Discussion
In this cohort of adults of all ages initiating hemodialysis, 34% were frail, which is >4 times the prevalence of frailty in community-dwelling older adults. We found an overlap between frailty, cognitive impairment, and comorbidity; frailty appeared to be distinct from yet related to cognitive impairment and comorbidity. Those who were frail were more likely to also have worse cognitive function scores at the time of hemodialysis initiation as measured by the 3MS and TMTA/TMTB and worse global cognitive function (measured by 3MS) at 1 year after initiation. In addition, frail patients initiating hemodialysis were more likely to have cognitive impairment in cognitive shifting and complex sequencing. Of note, the prevalence of cognitive dysfunction is quite small relative to the prevalence of frailty.
It is well recognized that, as kidney function declines, there is a decline in cognitive function and an increase in cognitive impairment (35–37). Although previous studies of adults with CKD have found that lower kidney function is associated with worse cognitive function (36,37), we have identified a high-risk subgroup of patients with ESRD: namely, those who are frail who have more profound cognitive loss. Our findings add some support for the association between frailty and cognitive function, cognitive decline, and dementia observed in frail middle-aged and older adults (3,7–14) and extend these findings to adults of all ages undergoing hemodialysis. Similar to a cross-sectional study of older adults, we found that frailty was associated with multiple domains of cognition, not just global cognitive function (10). However, global cognitive function at 1 year after hemodialysis initiation may be worse for frail adults undergoing hemodialysis because it represents overall function rather than domain-specific function, as is measured by TMTA/TMTB. The lack of association between frailty and TMTA/TMTB at 1 year of follow-up may also be attributable to a lack of statistical power.
Cognitive impairment is often unrecognized and underestimated in adults undergoing hemodialysis (38). Although the causes of poor cognitive function are not fully understood in adults undergoing hemodialysis (38), a recent review of cognitive function and frailty in older adults presented a hypothetical mechanism model of the association, which includes inflammation, cardiovascular disease, nutrition, and neuropathology (4). ESRD potentially leads to poor cognitive function through the retention of uremic toxins, as illustrated by the findings that restoration of kidney function after kidney transplantation improves cognitive performance (39). In addition, there are many common risk factors between cognitive impairment and ESRD, including cardiovascular and cerebrovascular disease (40,41). Frailty is an inflammatory phenotype (19,22,42) and thus may worsen cardiovascular disease or the symptoms of uremia in adults with ESRD, leading to lower cognitive function.
Age is the most important risk factor for cognitive impairment. Yet, as was noted in a recent review of the causal link between frailty and cognitive impairment, age-associated processes that lead to frailty are also responsible for cognitive decline (4). It is likely that although cognitive function is not a component of frailty, they result from a shared pathway. It is important to understand the link between these two separate yet related concepts so that new interventions can be identified.
Strengths of this study were the prospective measurement of a validated, objective frailty instrument, multiple tests of cognitive function, and a multicenter study. In addition, we studied adults of all ages, not just older adults undergoing hemodialysis. Blacks were overrepresented in this cohort as compared with all US hemodialysis initiates. However, we did not observe that race modified the association of frailty and cognitive function; thus, the findings are not likely biased by this over-representation. The main limitation is that there was limited follow-up at 1 year, which was primarily due to lack of a 1-year follow-up visit (n=77), death before the visit (n=32), or subsequent ineligibility for the study because the participant was not longer on hemodialysis (n=36). However, the association between frailty and cognitive function at cohort enrollment was similar among those who did and did not attend the 1-year visit. Baseline scores for the 3MS, TMTA, and TMTB were not associated with loss to follow-up at the 1-year follow-up. In addition, the loss to follow-up was not differential based on frailty status, so it is likely that the association is unbiased because of loss to follow-up. The main concern is that our ability to test the associations of frailty and cognitive tests at 1 year may be underpowered. Finally, we were unable to assess whether inflammation defined by IL-6 level mediated the association between frailty and cognitive function because levels of this inflammatory marker were not available in the PACE study.
On the basis of our findings, frailty is common at the time of hemodialysis initiation and is associated with worse cognitive function scores and impairment in cognitive shifting and complex sequencing, even after accounting for three of the most important predictors of cognitive function: namely, age, race, and education. Frailty at the time of hemodialysis initiation is also associated with poor global cognitive function (3MS) after 1 year, suggesting that intervening on frailty early may affect longer-term global cognitive function. Interventions to improve cognitive function in frail adults initiating hemodialysis should be identified.
Disclosures
None.
Acknowledgments
We thank the participants, nephrologists, and staff of the DaVita and MedStar dialysis units in the Baltimore area who contributed to the PACE study.
This study was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Grants R01AG042504 and K24DK101828 to D.L.S.). M.A.M.-D. was supported by the American Society of Nephrology (Carl W. Gottschalk Research Scholar Grant), the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, and the National Institute on Aging (Grants P30-AG021334 and K01AG043501). J.T. was supported by the National Heart, Lung, and Blood Institute (Grant T32 HL007024). The PACE study and faculty were supported by the NIDDK (Grant R01DK072367 to L.A.M., W.-H.L.K., and R.S.P.), the National Center for Research Resources (KL2RR025006 to S.M.S.), and the National Kidney Foundation of Maryland (Professional Development Award to S.M.S.).
An early version of this research was presented at the American Society of Nephrology Annual Meeting, held November 11–16, 2014, in Philadelphia, Pennsylvania.
Footnotes
Deceased.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Frailty and Cognitive Impairment in ESRD: Brain-Body Connections,” on pages 2104–2106.
References
- 1.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ: Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 30: 41–49, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Robertson DA, Savva GM, Kenny RA: Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev 12: 840–851, 2013 [DOI] [PubMed] [Google Scholar]
- 5.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG, Walston JD, Segev DL: Frailty and falls among adult patients undergoing chronic hemodialysis: A prospective cohort study. BMC Nephrol 14: 224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yassuda MS, Lopes A, Cachioni M, Falcao DV, Batistoni SS, Guimaraes VV, Neri AL: Frailty criteria and cognitive performance are related: Data from the FIBRA study in Ermelino Matarazzo, São Paulo, Brazil. J Nutr Health Aging 16: 55–61, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Mitnitski A, Fallah N, Rockwood MR, Rockwood K: Transitions in cognitive status in relation to frailty in older adults: A comparison of three frailty measures. J Nutr Health Aging 15: 863–867, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Sanders JL, Boudreau RM, Fried LP, Walston JD, Harris TB, Newman AB: Measurement of organ structure and function enhances understanding of the physiological basis of frailty: The Cardiovascular Health Study. J Am Geriatr Soc 59: 1581–1588, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson DA, Savva GM, Coen RF, Kenny RA: Cognitive function in the prefrailty and frailty syndrome. J Am Geriatr Soc 62: 2118–2124, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA: Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc 58: 248–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA: Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med 69: 483–489, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gray SL, Anderson ML, Hubbard RA, LaCroix A, Crane PK, McCormick W, Bowen JD, McCurry SM, Larson EB: Frailty and incident dementia. J Gerontol A Biol Sci Med Sci 68: 1083–1090, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solfrizzi V, Scafato E, Frisardi V, Seripa D, Logroscino G, Maggi S, Imbimbo BP, Galluzzo L, Baldereschi M, Gandin C, Di Carlo A, Inzitari D, Crepaldi G, Pilotto A, Panza F, Italian Longitudinal Study on Aging Working Group : Frailty syndrome and the risk of vascular dementia: The Italian Longitudinal Study on Aging. Alzheimers Dement 9: 113–122, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Jaar BG, Zhang L, Chembrovich SV, Sozio SM, Shafi T, Scialla JJ, Tomaselli GF, Lima JA, Kao WH, Parekh RS, Meoni LA: Incidental findings on cardiac computed tomography in incident hemodialysis patients: The predictors of arrhythmic and cardiovascular events in end-stage renal disease (PACE) study. BMC Nephrol 15: 68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parekh RS, Meoni LA, Jaar BG, Sozio SM, Shafi T, Tomaselli GF, Lima JA, Tereshchenko LG, Estrella MM, Kao WH: Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. BMC Nephrol 16: 63, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter ML, Orandi B, McAdams-DeMarco MA, Law A, Meoni LA, Jaar BG, Sozio SM, Kao WH, Parekh RS, Segev DL: Patient- and provider-reported information about transplantation and subsequent waitlisting. J Am Soc Nephrol 25: 2871–2877, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP: Phenotype of frailty: Characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 61: 262–266, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP: Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med 167: 635–641, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Cappola AR, Xue QL, Fried LP: Multiple hormonal deficiencies in anabolic hormones are found in frail older women: The Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci 64: 243–248, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP: White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci 64: 499–502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leng SX, Xue QL, Tian J, Walston JD, Fried LP: Inflammation and frailty in older women. J Am Geriatr Soc 55: 864–871, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP, Cardiovascular Health Study Research Group : Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 56: M158–M166, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular Health Study : Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med 162: 2333–2341, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP: Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci 63: 984–990, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Chang SS, Weiss CO, Xue QL, Fried LP: Association between inflammatory-related disease burden and frailty: Results from the Women’s Health and Aging Studies (WHAS) I and II. Arch Gerontol Geriatr 54: 9–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang SS, Weiss CO, Xue QL, Fried LP: Patterns of comorbid inflammatory diseases in frail older women: The Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci 65: 407–413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, Jain V, Ros RL, James NT, Kucirka LM, Hall EC, Berger JC, Montgomery RA, Desai NM, Dagher NN, Sonnenday CJ, Englesbe MJ, Makary MA, Walston JD, Segev DL: Frailty and delayed graft function in kidney transplant recipients. Arch Surg 147: 190–193, 2012 [DOI] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL: Frailty and early hospital readmission after kidney transplantation. Am J Transplant 13: 2091–2095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B, Salter M, Alachkar N, Desai N, Grams M, Walston J, Segev DL: Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation 99: 805–810, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, Walston J, Segev DL: Frailty and mortality in kidney transplant recipients. Am J Transplant 15: 149–154, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48: 314–318, 1987 [PubMed] [Google Scholar]
- 33.Spreen O, Spreen E: A Compendium of Neuropsychological Tests: Administration, Norms and Commentary, New York, Oxford University Press [Google Scholar]
- 34.Tobin J: Estimation of relationships for limited dependent variables. Econometrica 26: 24–36, 1958 [Google Scholar]
- 35.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G, Go AS, Chronic Renal Insufficiency Cohort Investigators : Chronic kidney disease and cognitive function in older adults: Findings from the Chronic Renal Insufficiency Cohort cognitive study. J Am Geriatr Soc 58: 338–345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurella Tamura M, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, Messé SR, Sehgal AR, Kusek J, DeSalvo KB, Cornish-Zirker D, Cohan J, Seliger SL, Chertow GM, Go AS: Vascular risk factors and cognitive impairment in chronic kidney disease: The Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol 6: 248–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown EA, Johansson L: Old age and frailty in the dialysis population. J Nephrol 23: 502–507, 2010 [PubMed] [Google Scholar]
- 39.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP: Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrol Dial Transplant 21: 3275–3282, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Pereira AA, Weiner DE, Scott T, Sarnak MJ: Cognitive function in dialysis patients. Am J Kidney Dis 45: 448–462, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Weiner DE, Scott TM, Giang LM, Agganis BT, Sorensen EP, Tighiouart H, Sarnak MJ: Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 58: 773–781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walston JD, Matteini AM, Nievergelt C, Lange LA, Fallin DM, Barzilai N, Ziv E, Pawlikowska L, Kwok P, Cummings SR, Kooperberg C, LaCroix A, Tracy RP, Atzmon G, Lange EM, Reiner AP: Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol 44: 350–355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]