Abstract
Background and objectives
The independent link between arterial stiffness and CKD remains unknown. We investigated the association of indicators of arterial stiffness with decline in kidney function.
Design, setting, participants, & measurements
We studied 3666 participants (mean age =65 years old; 58% women) from the Rotterdam Study. Pulse pressure (PP), carotid stiffness, and pulse wave velocity (PWV) were measured. We created genetic risk scores for PP and PWV. Annual declines in kidney function and incident CKD were assessed using eGFR. To put our findings in context of the literature, we performed a meta-analysis of the available population–based studies.
Results
After a median (interquartile range) follow–up time of 11 (10.7–11.3) years, 601 participants with incident CKD were recognized. In the model adjusted for age, sex, mean arterial pressure, heart rate, and baseline GFR, each SD higher PP was associated with 0.15-ml/min per 1.73 m2 steeper annual eGFR decline (95% confidence interval [95% CI], 0.10 to 0.20) and 11% higher risk of incident CKD (95% CI, 1.05 to 1.18). Each SD greater carotid stiffness was associated with 0.08-ml/min per 1.73 m2 steeper annual eGFR decline (95% CI, 0.04 to 0.13) and 13% higher risk of incident CKD (95% CI, 1.05 to 1.22). Each SD higher PWV was associated with 7% higher risk of incident CKD (95% CI, 1.00 to 1.14). Incorporating our findings in a meta-analysis, each SD higher PP and PWV were associated with 16% (95% CI, 1.12 to 1.21) and 8% (95% CI, 1.03 to 1.14) higher risks of incident CKD. Each SD higher PP genetic risk score was associated with 0.06-ml/min per 1.73 m2 steeper annual eGFR decline (95% CI, 0.01 to 0.10) and 8% higher risk of incident CKD (95% CI, 1.03 to 1.14). There was no association between PWV genetic risk score and kidney function decline.
Conclusions
Higher indices of arterial stiffness are associated with steeper decline in kidney function. This suggests that vascular stiffness could be considered as a target for delaying decline in kidney function.
Keywords: meta-analysis, chronic kidney disease, genetic risk score, arterial stiffness, pulse pressure, carotid stiffness, blood pressure, follow-up studies, pulse wave analysis, vascular stiffness
Introduction
Considerable proportions of patients with CKD carry multiple cardiovascular risk factors and die from cardiovascular causes (1). Accumulating evidence suggests a strong association between cardiovascular pathology and CKD. Nevertheless, exact mechanisms linking cardiovascular diseases with kidney impairment remain to be elucidated (2).
The role of vascular risk factors has been implicated in the association between cardiovascular disease and CKD (3). One of the novel risk factors proposed for cardiovascular disease is arterial stiffness (4). Arterial stiffness, independent of mean arterial pressure, results in end organ damage by imposing hemodynamic stress on vascular beds (5). Aortic stiffening, especially in older people, facilitates transmission of excessive pressure and flow pulsatility into the microvascular beds of the kidneys, a high-flow organ, which will potentially lead to microvascular ischemia and tissue damage (6).
Several studies have investigated an independent association between arterial stiffness and decline in kidney function, but the results have been inconsistent (7–14). Heterogeneity in the study populations and the limited power of the individual studies could underlie the inconsistent findings. In addition, all observational studies are subject to confounding and reverse causation. Relevant genetic variants could potentially be used to overcome these flaws (15).
We aimed to investigate the association between arterial stiffness as well as genetic variations related to arterial stiffness with the risk of decline in kidney function in the Rotterdam Study (RS), a population-based study of individuals 55 years old and older. Moreover, to put our findings in the context of the literature, we performed a meta-analysis of population-based studies on the association of arterial stiffness markers and risk of kidney disease.
Materials and Methods
Population for Analysis
This study was performed within the framework of the population–based RS. The cohort originated in 1990 and included 7983 participants from Ommoord, a district of Rotterdam in The Netherlands, age 55 years old or older (RS-I). In 2000, the first extension of the RS (RS-II) started, adding 3011 new participants. Arterial stiffness was evaluated at the third visit of the RS-I and the first visit of the RS-II. All individuals with available data on arterial stiffness markers at baseline and repeated creatinine measurements (at baseline and the next visit) were included in the analyses. The median (interquartile range) follow–up time elapsed between two creatinine measurements was 11 (10.7–11.3) years. This resulted in 2950 participants with available data on brachial pulse pressure, 2665 participants with pulse wave velocity (PWV) data, and 2344 participants with carotid stiffness data.
DNA was extracted from samples taken at the first visit of the RS-I and at the first visit of the RS-II (n=8131). Among them, 3666 had repeated measurements of creatinine for longitudinal assessment of kidney function.
The RS has been approved by the medical ethics committee according to the Population Study Act RS executed by the Ministry of Health, Welfare and Sports of The Netherlands. Written informed consent was obtained from all participants (16).
Measurement of Arterial Stiffness
Carotid femoral PWV was measured with participants in supine position with an automatic device (Complior Artech Medical, Pantin, France) that measures the time delay between the rapid upstroke of the feet of simultaneously recorded pulse waves in the carotid artery and the femoral artery (17). The distance between the recording sites in the carotid and the femoral artery was measured with a tape over the surface of the body. PWV was calculated as the ratio between distance and the foot-to-foot time delay, and it was expressed in meters per second.
Common carotid stiffness was assessed with the participants in supine position with heads tilted slightly to the contralateral side for the measurement in the common carotid artery (18). The vessel wall motion of the right common carotid artery was measured by means of a duplex scanner (ATL Ultramark IV; operating frequency =7.5 MHz) connected to a vessel wall movement detector system (18,19). After 5 minutes of rest, a region at 1.5 cm proximal to the origin of the bulb of the carotid artery was identified with the use of B-mode ultrasound. The displacement of the arterial walls was obtained by processing the radiofrequency signals originating from two selected sample volumes positioned over the anterior and posterior walls. The end diastolic diameter (D), the absolute stroke change in diameter during systole (ΔD), and the relative stroke change in diameter (ΔD/D) were computed as the mean of four cardiac cycles of three successive recordings. The cross–sectional arterial wall distensibility coefficient was calculated according to the following equation: distensibility coefficient =2ΔD/(D× pulse pressure) (10−3 per kilopascal) (18,20). Lower carotid distensibility represents greater carotid stiffness.
Heart rate was measured simultaneously with arterial stiffness measurements. Three observers performed all measurements. In a reproducibility study performed among 47 individuals who were invited two times exactly 1 week apart, the intraclass correlation coefficient was 0.80 for both the PWV and the carotid distensibility coefficient (19,21). After 5 minutes of rest, systolic and diastolic BPs were measured two times on the right arm with a random zero sphygmomanometer, and the mean was taken as the individuals’ reading. Pulse pressure was estimated as the difference between systolic and diastolic BPs.
Genetic Risk Score
Genotyping was conducted using the Illumina 550K Array among self–reported white individuals. Imputation was done with reference to HapMap release 22 Utah residents of Northern and Western European ancestry using the maximum likelihood method implemented in MaCH (version 1.0.15).
We selected single-nucelotide polymorphisms (SNPs) reported in genome–wide association studies (GWAS) to be associated with pulse pressure and PWV (22,23). There is no GWAS available on carotid stiffness. Genetic risk score was formed using 10 SNPs associated with pulse pressure and 9 SNPs associated with PWV (Supplemental Table 1). For variants in the same locus, the variant with the smallest P value was selected. We calculated a weighted genetic risk score by multiplying the number of risk alleles at each locus by the corresponding reported coefficient from the previous GWASs and summing the products. The total score was then divided by the average effect size and multiplied by 100 to rescale the scores to a range between 0 and 100.
Measurement of eGFR
Serum creatinine was determined using an enzymatic assay method. Interassay and intra–assay coefficient variations were <0.92% and <1.37%, respectively. We calibrate creatinine measurements by aligning the mean values of creatinine with creatinine values of the participants of the National Health and Nutrition Examination Survey III in different sex and age groups (<60, 60–69, and ≥70 years old) (24). eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration Equation (25). To calculate the annual eGFR decline, we first subtracted the eGFR estimates of the follow-up examination from the eGFR estimates at baseline and then divided by the time between the two visits. CKD was defined as eGFR<60 ml/min per 1.73 m2. Patients with incident CKD were defined as individuals free of CKD at baseline (eGFR>60 ml/min per 1.73 m2) who had a decline in eGFR to <60 ml/min per 1.73 m2 between the two periodic examinations (26).
Statistical Analyses
Association of measures of arterial stiffness with annual decline in eGFR and incidence of CKD was evaluated using linear regression models and log binomial regressions, respectively. Coefficients were estimated per SD higher PWV and pulse pressure. Coefficients were estimated per negative SD higher measures of carotid distensibility, which represents greater carotid stiffness. In the first model, analyses were adjusted for age, sex, mean arterial pressure, heart rate, baseline eGFR, and follow-up time (for analyses on incidence of CKD). In the second model, we further adjusted for body mass index, alcohol consumption, smoking, HDL cholesterol, total cholesterol, history of diabetes mellitus and coronary heart disease, and different types of antihypertensive medications (diuretics, β-blockers, angiotensin-converting-enzyme inhibitors, and calcium channel blockers). Missing values on covariates were imputed using the expectation maximization method (single imputation). The percentage of missing values on covariates was not substantial and ranged from 0.2% to 13.3%. In addition, because an interaction between BP and PWV as biomarkers and indicators of hemodynamic status has been suggested previously (27), we assessed the interaction of PWV and systolic and diastolic BPs by adding an interaction term in the regression model. The interaction term was the product of the PWV and systolic or diastolic BP. In an extra analysis, we adjusted the associations of arterial stiffness genetic risk scores with decline in kidney function for measures of pulse pressure and PWV. All analyses were adjusted for the effect of the two RS cohorts and carried out using STATA 13.1 or R, version 2.15.0.
Meta-Analysis
We searched for studies published in MEDLINE, EMBASE, Web of Science, and Google Scholar using the common key words related to arterial stiffness and incident CKD (Supplemental Appendix). Population-based studies evaluating the association between indicators of arterial stiffness and incidence of CKD were included (Supplemental Table 2) (10–14). Supplemental Figure 1 shows the flow diagram for inclusion of the relevant studies in our meta-analyses. Incident CKD was defined as eGFR<60 ml/min per 1.73 m2 in all included studies except one study, in which eGFR loss of >3 ml/min per 1.73 m2 was used (13). We excluded one study from the meta-analysis of pulse pressure, because the outcome was reported continuously for each milliliter per minute per 1.73 meter2 decline in eGFR (14). We performed random and fixed effect meta–analyses including the current RS. The heterogeneity assumption was investigated using a commonly used statistical method, namely the I2 statistic. There was no evidence of publication bias using Egger’s test.
Results
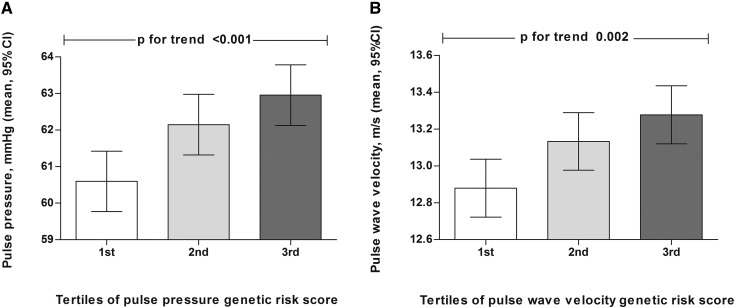
Baseline characteristics of participants are presented in Table 1. Mean age of the participants was 65±6.7 years old, and 58.3% were women. Figure 1 shows the means and SEMs of pulse pressure and PWV in tertiles of pulse pressure and the PWV genetic risk scores.
Table 1.
Baseline characteristics of participants
| Characteristics (n=3666) | Value |
|---|---|
| Age (yr), mean (SD) | 65.0 (6.7) |
| Women, n (%) | 2139 (58.3) |
| Body mass index (kg/m2), mean (SD) | 26.6 (3.6) |
| Total cholesterol (mg/dl), mean (SD) | 246.9 (46.1) |
| HDL cholesterol (mg/dl), mean (SD) | 52.5 (14.5) |
| Alcohol intake (g/d), median (interquartile range) | 4.8 (0.33–16.6) |
| Smoking, n (%) | |
| Current | 755 (20.6) |
| Former | 1649 (45.0) |
| Systolic BP (mmHg), mean (SD) | 137.3 (19.9) |
| Diastolic BP (mmHg), mean (SD) | 75.4 (10.9) |
| Pulse rate (beats per minute), mean (SD) | 72.2 (11.4) |
| Mean arterial pressure (mmHg), mean (SD) | 96.0 (12.6) |
| Pulse pressure (mmHg), mean (SD)a | 62.5 (15.5) |
| Pulse wave velocity (m/s), mean (SD)a | 12.2 (2.5) |
| Carotid distensibility coefficient (10−3/kPa), mean (SD)a | 12.9 (4.6) |
| GFR (ml/min per 1.73 m2), mean (SD) | 79.3 (13.7) |
| Diabetes mellitus, n (%) | 279 (7.6) |
| History of coronary heart disease, n (%) | 327 (8.9) |
| Antihypertensive medication, n (%) | |
| Diuretics | 355 (9.7) |
| ACE inhibitors | 204 (5.6) |
| Calcium channel blocker | 177 (4.8) |
| β-Blocker | 503 (13.7) |
ACE, angiotensin-converting-enzyme.
Data are on the basis of the correspondence sample size (pulse pressure, n=2950; pulse wave velocity, n=2665; and carotid distensibility, n=2344).
Figure 1.
Linear association between arterial stiffness measures and their corresponding genetic risk scores. (A) Mean and SEM of pulse pressure in tertiles of pulse pressure genetic risk score. (B) Mean and SEM of pulse wave velocity in tertiles of pulse wave velocity genetic risk score. Analyses are adjusted for age and sex. 95% CI, 95% confidence interval.
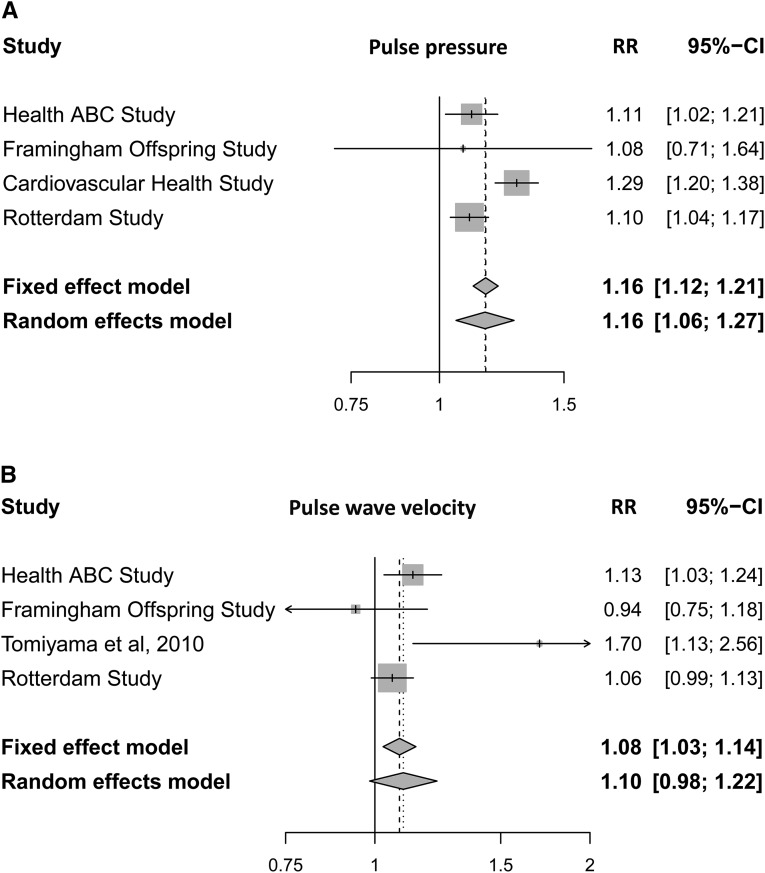
Table 2 shows the association between indicators of arterial stiffness and kidney function. In the first model, we observed that higher pulse pressure and greater carotid stiffness were associated with steeper annual decline in eGFR and higher risk of incident CKD. Adjusting for additional potential confounders, in the second model, did not substantially change the association. There was no association between PWV and annual decline in eGFR. Higher PWV was associated with higher risk of incident CKD. Furthermore, we did not observe any statistically significant interaction between PWV and systolic or diastolic BP (Supplemental Table 3). However, the association was not present after adjustment for potential confounders in the second model (Table 2). To provide more reliable estimates, we performed a meta-analysis of the available studies (including this study) to report the association of pulse pressure and PWV with incident CKD (Figure 2). Combining the effect estimates of our study with three previous population–based studies, we observed the overall relative risk of 1.16 (95% confidence interval [95% CI], 1.12 to 1.21) for each SD higher pulse pressure in respect to incident CKD. Test for heterogeneity resulted in moderate estimates (I2=75%; 30.6%–91%). Excluding the study with outcome defined as eGFR loss of >3 ml/min per 1.73 m2 resulted in no heterogeneity (Supplemental Figure 2). Regarding PWV, we observed the overall relative risk of 1.08 (95% CI, 1.03 to 1.14) for incident CKD per each SD higher PWV. The test for heterogeneity resulted in moderate estimates (I2=59.5%; 0%–86.5%). Excluding the study with carotid brachial PWV measures reduced the heterogeneity (I2=24%; 0%–92.1%) (Supplemental Figure 2).
Table 2.
Association of measures of arterial stiffness with decline in eGFR and incidence of CKD
| Regression Models | eGFR Decline | Incident CKD | ||||
|---|---|---|---|---|---|---|
| Difference | 95% Confidence Interval | P Value | Relative Risk | 95% Confidence Interval | P Value | |
| Pulse pressure (n=2950) | ||||||
| Model 1 | 0.15 | 0.10 to 0.20 | <0.001 | 1.11 | 1.05 to 1.18 | <0.001 |
| Model 2 | 0.13 | 0.09 to 0.18 | <0.001 | 1.10 | 1.03 to 1.17 | 0.002 |
| Carotid stiffness (n=2342) | ||||||
| Model 1 | 0.08 | 0.04 to 0.13 | <0.001 | 1.13 | 1.05 to 1.22 | 0.001 |
| Model 2 | 0.07 | 0.02 to 0.11 | 0.002 | 1.13 | 1.05 to 1.22 | 0.001 |
| Pulse wave velocity (n=2665) | ||||||
| Model 1 | 0.04 | −0.00 to 0.09 | 0.07 | 1.07 | 1.01 to 1.14 | 0.04 |
| Model 2 | 0.02 | −0.02 to 0.07 | 0.33 | 1.05 | 0.99 to 1.31 | 0.10 |
Differences (coefficients) and relative risks are calculated per each SD of arterial stiffness measures. Model 1 is adjusted for age, sex, mean arterial pressure, heart rate, baseline eGFR, and follow-up time (for analyses on incidence of CKD). Model 2 is additionally adjusted for body mass index, alcohol consumption, smoking, HDL cholesterol, total cholesterol, diuretics, angiotensin-converting-enzyme (ACE) inhibitors, β-blockers, calcium channel blockers, and history of diabetes and coronary heart disease.
Figure 2.
Higher pulse pressure and pulse wave velocity are associated with higher risk of incident CKD. Forest plots of multivariate–adjusted relative risks (RRs) for the association of each SD of (A) pulse pressure and (B) pulse wave velocity with incident CKD (11). Health ABC, health, aging, and body composition; 95% CI, 95% confidence interval.
Pulse pressure genetic risk score was associated with steeper annual decline in eGFR and higher risk of incident CKD (relative risk, 1.08; 95% CI, 1.03 to 1.14) (Table 3). There was no association between PWV genetic risk score and kidney function. Adjusting the associations for pulse pressure and PWV measurements changed the associations minimally (Supplemental Table 4).
Table 3.
Association of genetic risk scores for measures of arterial stiffness with annual decline in eGFR and incidence of CKD
| Regression Models | eGFR Decline | Incident CKD | ||||
|---|---|---|---|---|---|---|
| Difference | 95% Confidence Interval | P Value | Relative Risk | 95% Confidence Interval | P Value | |
| Pulse pressure GRS (n=3666) | ||||||
| Model 1 | 0.06 | 0.01 to 0.10 | 0.01 | 1.08 | 1.03 to 1.14 | 0.003 |
| Model 2 | 0.05 | 0.01 to 0.11 | 0.02 | 1.07 | 1.02 to 1.13 | <0.01 |
| Pulse wave velocity GRS (n=3666) | ||||||
| Model 1 | −7.4×10−4 | −0.04 to 0.04 | 0.99 | 1.03 | 0.98 to 1.08 | 0.18 |
| Model 2 | 3.6×10−3 | −0.04 to 0.05 | 0.87 | 1.03 | 0.98 to 1.08 | 0.17 |
Differences (coefficients) are per SD of pulse pressure genetic risk score (GRS) and pulse wave velocity GRS. Model 1 is adjusted for age, sex, mean arterial pressure, heart rate, baseline eGFR, and follow-up time (for analyses on incidence of CKD). Model 2 is additionally adjusted for body mass index, alcohol consumption, smoking, HDL cholesterol, total cholesterol, diuretics, angiotensin-converting-enzyme (ACE) inhibitors, β-blockers, calcium channel blockers, and history of diabetes and coronary heart disease.
Given the correlation between pulse pressure and BP, we investigated if any of the pulse pressure genes are associated with systolic or diastolic BP in our sample (Supplemental Table 5). After Bonferroni correction (adjusted P value of 0.002), none of the variants were significantly associated with BP measures; however, an SNP in the PIK3CG gene and an SNP in the PLCE-1 gene were suggestively associated with systolic BP (P=0.003). Excluding these SNPs from the genetic risk score of pulse pressure did not essentially change the associations (Supplemental Table 6).
Discussion
We showed that markers of arterial stiffness are independently associated with future decline in kidney function. This study provides additional evidence for the association between pulse pressure and decline in kidney function using genetic variability in pulse pressure.
Previous studies on the association between arterial stiffness and decline in kidney function have been inconsistent (7,9–14,28). In a study including patients with CKD, Ford et al. (9) showed that higher PWV but not pulse pressure was associated with the rate of change in kidney function. Similarly, in a Japanese cohort, an association was observed between higher brachial PWV and steeper decline in eGFR (11). In contrast, results of the Framingham Offspring Cohort showed that PWV is associated with the incidence of albuminuria but not mild to moderate CKD (12). In this study, we observed an association between pulse pressure and decline in kidney function, but the association between PWV and CKD disappeared after adjustment for cardiovascular risk factors. To improve the power, increase generalizability, and decrease heterogeneity, we combined our results with the effect estimates of the population–based studies and provided additional support for an independent link between PWV and decline in kidney function.
Pulse pressure and PWV are two commonly used measures of arterial stiffness. Arterial stiffness is not uniform along the arterial tree; therefore, assessment of arterial stiffness at different sites in relation to clinical outcomes is important (29). We have previously shown that arterial stiffness measured as carotid femoral PWV is associated with cardiovascular morbidity and mortality; however, we did not observe such an association with stiffness in the carotid artery. Previous studies showed that local carotid arterial stiffness is associated with brain outcomes (direct organ supplies by carotid arteries). In this study, we showed an independent association between carotid stiffness and decline in kidney function. Both kidney and brain are low–resistance, high–flow end organs, which renders them vulnerable to pulsatile changes in the blood flow. This might suggest that systemic pulsatile pressure can cause vascular injury in both organs. Future studies are needed to investigate the mechanism behind the association between carotid stiffness and decline in kidney function.
We observed that pulse pressure genetic variants but not PWV genetic variants are associated with kidney function decline. Our findings can be explained by relatively more power for pulse pressure given the stronger association of pulse pressure with kidney function compared with PWV. It is also known that pulse pressure is not only the indicator of arterial stiffness but also, influenced by peak systolic BP (30). Some of the genes found for pulse pressure are known to be associated with BP variation (31–33). However, adjustment for BP and excluding SNPs with suggestive association with systolic BP did not change the associations.
There are different putative mechanisms suggesting a role for arterial stiffness in the deterioration of kidney function. A plausible mechanism is that arterial stiffness increases circumferential and shear stresses in the arterial lumen. This hemodynamic stress on the kidney vasculature may result in endothelial dysfunction and microvascular ischemia, leading to kidney injury (34). Other possible mechanisms include chronic inflammation, oxidative stress, and activation of the renin-angiotensin system (12).
We performed these analyses in a large population–based study, which enables us to control for several potential confounders and see the small effects of the genes. In addition, we performed a meta-analysis to provide a more precise estimate of the association. We confirmed the association between pulse pressure and kidney function using genetic variants as the less-biased proxies for the arterial stiffness parameters. As a limitation, data on albuminuria were unavailable, and it is an important element in defining CKD. However, eGFR<60 ml/min per 1.73 m2 is a well accepted definition for CKD in population–based research settings (35). Furthermore, adjustments for pulse pressure changed the association between genetic variants of pulse pressure and kidney disease only minimally; this might indicate that our findings with pulse pressure genetic risk score could be partially explained by pleiotropic effects in the genetic risk score, such as BP genetic variants. In computing the carotid distensibility coefficient, we used the brachial pulse pressure rather than the carotid pulse pressure. Substantial differences have been reported between carotid and brachial pulse pressures, which can lead to an underestimation of the distensibility measurements and subsequently, an underestimation of the association with the disease (4).
Currently, major strategies to prevent CKD are focused on conventional cardiovascular risk factors, and in this study, we showed that vascular stiffness independent of cardiovascular risk factors is associated with decline in kidney function. This highlights that vascular stiffness can be considered as a target for delaying decline in kidney function.
Disclosures
None.
Supplementary Material
Acknowledgments
The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; The Netherlands Organization for Scientific Research; The Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission; and the Municipality of Rotterdam. Support for genotyping was provided by The Netherlands Organisation of Scientific Research (NWO) Investments grants 175.010.2005.011 and 911-03-012; Research Institute for Diseases in the Elderly grant 014-93-015, RIDE2; and The Netherlands Genomics Initiative/Netherlands Consortium for Healthy Aging Project 050-060-810. O.H.F. works at ErasmusAGE, a center for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd.), Metagenics Inc., and AXA. A.D. is supported by NWO grant veni, 916.12.154 and the Erasmus University Rotterdam Fellowship. E.J.H is supported by an NWO grant veni, 916.12.140.
Nestlé Nutrition (Nestec Ltd.), Metagenics Inc., and AXA had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Targeting Blood Vessel Stiffness to Protect Kidney Function,” on pages 2107–2109.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03000315/-/DCSupplemental.
References
- 1.Waheed S, Matsushita K, Sang Y, Hoogeveen R, Ballantyne C, Coresh J, Astor BC: Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 207–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Karbasi-Afshar R, Saburi A, Taheri S: Clinical associations between renal dysfunction and vascular events: A literature review. ARYA Atheroscler 9: 203–209, 2013 [PMC free article] [PubMed] [Google Scholar]
- 4.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC: Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation 113: 657–663, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ: Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 121: 505–511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ: Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility–Reykjavik study. Brain 134: 3398–3407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P, Nephrotest Study Group : Arterial remodeling associates with CKD progression. J Am Soc Nephrol 22: 967–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fesler P, Safar ME, du Cailar G, Ribstein J, Mimran A: Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 25: 1915–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG: Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 55: 1110–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, Shlipak M, Simonsick E, Lakatta E, Patel K, Rifkin D, Hawkins M, Newman A, Sarnak M, Health ABC Study : Association of arterial rigidity with incident kidney disease and kidney function decline: The Health ABC study. Clin J Am Soc Nephrol 8: 424–433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A: Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 212: 345–350, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, Meigs JB, Larson MG, Levy D, Benjamin EJ, Fox CS: Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 20: 2044–2053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rifkin DE, Katz R, Chonchol M, Shlipak MG, Sarnak MJ, Fried LF, Newman AB, Siscovick DS, Peralta CA: Blood pressure components and decline in kidney function in community-living older adults: The Cardiovascular Health Study. Am J Hypertens 26: 1037–1044, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peralta CA, Jacobs DR, Jr., Katz R, Ix JH, Madero M, Duprez DA, Sarnak MJ, Criqui MH, Kramer HJ, Palmas W, Herrington D, Shlipak MG: Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 59: 41–49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, Stijnen T, Hofman A, Schram MT, Witteman JC: Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 56: 872–878, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hofman A, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, Tiemeier HW, Uitterlinden AG, Vernooij MW: The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol 28: 889–926, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI: Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 26: 485–490, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Hoeks AP, Brands PJ, Smeets FA, Reneman RS: Assessment of the distensibility of superficial arteries. Ultrasound Med Biol 16: 121–128, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Sedaghat S, Dawkins Arce FG, Verwoert GC, Hofman A, Ikram MA, Franco OH, Dehghan A, Witteman JC, Mattace-Raso F: Association of renal function with vascular stiffness in older adults: The Rotterdam study. Age Ageing 43: 827–833, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Reneman RS, van Merode T, Hick P, Muytjens AM, Hoeks AP: Age-related changes in carotid artery wall properties in men. Ultrasound Med Biol 12: 465–471, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Kool MJ, van Merode T, Reneman RS, Hoeks AP, Struyker Boudier HA, Van Bortel LM: Evaluation of reproducibility of a vessel wall movement detector system for assessment of large artery properties. Cardiovasc Res 28: 610–614, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Verwoert GC, Tarasov KV, Isaacs A, Smith AV, Yasmin, Rietzschel ER, Tanaka T, Liu Y, Parsa A, Najjar SS, O’Shaughnessy KM, Sigurdsson S, De Buyzere ML, Larson MG, Sie MP, Andrews JS, Post WS, Mattace-Raso FU, McEniery CM, Eiriksdottir G, Segers P, Vasan RS, van Rijn MJ, Howard TD, McArdle PF, Dehghan A, Jewell ES, Newhouse SJ, Bekaert S, Hamburg NM, Newman AB, Hofman A, Scuteri A, De Bacquer D, Ikram MA, Psaty BM, Fuchsberger C, Olden M, Wain LV, Elliott P, Smith NL, Felix JF, Erdmann J, Vita JA, Sutton-Tyrrell K, Sijbrands EJ, Sanna S, Launer LJ, De Meyer T, Johnson AD, Schut AF, Herrington DM, Rivadeneira F, Uda M, Wilkinson IB, Aspelund T, Gillebert TC, Van Bortel L, Benjamin EJ, Oostra BA, Ding J, Gibson Q, Uitterlinden AG, Abecasis GR, Cockcroft JR, Gudnason V, De Backer GG, Ferrucci L, Harris TB, Shuldiner AR, van Duijn CM, Levy D, Lakatta EG, Witteman JC: Common genetic variation in the 3′-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk: The AortaGen Consortium. Circ Cardiovasc Genet 5: 81–90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dörr M, Bis JC, Aspelund T, Esko T, Janssens AC, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimäki T, Kühnel B, Lopez LM, Polašek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace-Raso FU, Rivadeneira F, Sijbrands EJ, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Völker U, Völzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sõber S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O’Donnell CJ, Salomaa V, d’Adamo AP, Fabretto A, Faletra F, Ulivi S, Del Greco F, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJ, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, van der Klauw MM, Zhang W, Li X, Scott J, Chen YD, Burke GL, Kähönen M, Viikari J, Döring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, König IR, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, Mitchell GF, Smith NL, Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM, LifeLines Cohort Study. EchoGen consortium. AortaGen Consortium. CHARGE Consortium Heart Failure Working Group. KidneyGen consortium. CKDGen consortium. Cardiogenics consortium. CardioGram : Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet 43: 1005–1011, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrone RD, Madias NE, Levey AS: Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem 38: 1933–1953, 1992 [PubMed] [Google Scholar]
- 27.Chirinos JA, Townsend RR: Reducing arterial stiffness in CKD: Revising the paradigms. Clin J Am Soc Nephrol 10: 547–550, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, Hanai K, Tanaka N, Ishii A, Uchigata Y, Iwamoto Y: Arterial stiffness is associated with incident albuminuria and decreased glomerular filtration rate in type 2 diabetic patients. Diabetes Care 34: 2570–2575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD: Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: The Hoorn study. J Am Coll Cardiol 63: 1739–1747, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Dart AM, Kingwell BA: Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol 37: 975–984, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J: Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 43: 531–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoi N, Soma M, Nakayama T, Rahmutula D, Kosuge K, Izumi Y, Matsumoto K: Variable number of tandem repeat of the 5′-flanking region of type-C human natriuretic peptide receptor gene influences blood pressure levels in obesity-associated hypertension. Hypertens Res 27: 711–716, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Meyer TE, Shiffman D, Morrison AC, Rowland CM, Louie JZ, Bare LA, Ross DA, Arellano AR, Chasman DI, Ridker PM, Pankow JS, Coresh J, Malloy MJ, Kane JP, Ellis SG, Devlin JJ, Boerwinkle E: GOSR2 Lys67Arg is associated with hypertension in whites. Am J Hypertens 22: 163–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safar ME, London GM, Plante GE: Arterial stiffness and kidney function. Hypertension 43: 163–168, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Bash LD, Coresh J, Köttgen A, Parekh RS, Fulop T, Wang Y, Astor BC: Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol 170: 414–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.