Abstract
Background and objectives
ESRD is associated with an increased risk of malignancies. We analyzed the incidence of cancer in patients with pediatric ESRD after long–term follow-up.
Design, setting, participants, & measurements
All Dutch patients born before 1979 who were transplanted at age <15 years old in 1972–1992 were followed until 2010. We explored type and incidence of malignancies in patients compared with the general population using the National Cancer Registry.
Results
After a median of 25.3 years (1.3–37.8) of transplantation and at a median age of 33.5 years old (11.0–49.0), 105 primary malignancies had occurred in 54 of 249 patients. Among them, cutaneous squamous cell carcinoma was most frequent. Patients ages 25–30 years old had developed 16.5 times (95% confidence interval, 7.9 to 34.6) as many de novo tumors and 991 times (95% confidence interval, 313 to 3137) as many de novo cutaneous squamous cell carcinomas as their general population counterparts; in survivors ages 45–50 years old, these numbers were 81.5 (95% confidence interval, 50.7 to 131.1) and 2610 (95% confidence interval, 1596 to 4267), respectively. Cumulative incidence competing risk analysis showed that, after 30 years of transplantation, 41% of the survivors had developed cancer; 31% had developed a second de novo cancer <1 year after initial cancer diagnosis.
Conclusions
Cancer is highly prevalent among patients with pediatric ESRD after 25.3 years of transplantation, with a high rate of recurrence.
Keywords: renal transplantation; end-stage renal disease; cancer; carcinoma; squamous cell; child; follow-up studies; kidney failure, chronic; neoplasms; renal insufficiency, chronic; survivors
Introduction
Currently, cancer is the major cause of death in upper–middle and high–income countries (1). This makes it important to detect populations that are at increased risk of developing a malignancy. Previous studies have shown that patients suffering from ESRD belong to such populations. The increased risk of cancer applies to both patients on dialysis and recipients of renal grafts (2–5). In recipients of organ transplants, the occurrence of malignancies has been strongly linked to the use of immunosuppressive agents, because such agents impair the body’s ability to locate and destroy cancer cells and control viral infections related to cancer (6).
Malignancies and frequent recurrences of malignancies contribute significantly to mortality and morbidity rates. However, because nearly all studies have been performed in patients receiving kidney transplants in adulthood, few data exist on the occurrence of malignancies in adult patients who have received a renal graft in childhood (3,7,8). In a previous report in 2000, we showed that, in the latter group, the probability of developing a malignancy was 17% (95% confidence interval [95% CI], 9% to 24%) after these children reached an age between 20 and 40 years old (9). This was consistent with a 10 times increase in the incidence of de novo malignancy compared with the general population (GP) (9) that was also found in studies in patients with adult onset of ESRD (3–5,10–12).
Because in our previous analyses, most malignancies occurred toward the end of follow-up, we hypothesized that their occurrence might increase dramatically with further ageing of our cohort. We, therefore, extended follow-up until 2010. The aim of this paper is to describe the occurrence and the type of cancer after transplantation at very long–term follow-up in patients who started RRT before the age of 15 years old.
Materials and Methods
Study Design
This study is part of a 10-year extension of a comprehensive long–term follow-up study into the Late Effects of Renal Insufficiency (LERIC) Study in patients on RRT since childhood in The Netherlands. The LERIC Study cohort comprised all Dutch patients born before 1979 who had started chronic RRT (dialysis or a preemptive transplant) at <15 years of age between 1972 and 1992. Patients who needed >3 consecutive months of RRT were considered to receive chronic RRT. In 2000, we conducted the first study on these 249 patients (9). In 2010, the 10-year extension study was performed, covering the period from the last chart review in 2000 to the last chart review in 2010 or the patient’s death. Incidence rates (IRs) of cancer were compared with those in the GP.
We obtained permission from the Medical Ethical Committee and informed consent from all patients who were alive in 2000.
Data Collection
Data were collected in 2000 and 2010. To this end, LERIC Study coworkers visited all 37 hospitals that had been involved in the medical care of study patients. Data collection of the first period up to 2000 has been described in detail (9). For the second follow-up period, we reviewed the medical charts of all patients between June 1, 2010 and February 1, 2011. Although the LERIC Study cohort includes patients on dialysis, this paper is confined to recipients of transplants. Patient-years are defined as the number of years between first transplantation and death or the day of chart review.
Among others, we collected data on the total period that the patient lived on a functioning graft, the use of immunosuppressive agents, the diagnosis of noncutaneous cancer, and the diagnosis of nonmelanoma skin cancer (NMSC). Only patients with histologically proven basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) were considered to have NMSC. A second de novo cancer was defined as a cancer that was located at a different site and occurred at least 3 months after treatment of the previous malignancy.
Statistical Analyses
We used cumulative incidence competing risk for calculation of the probability of first and second de novo malignancies after the first transplant. This analysis corrects for death from other causes.
In patients who were still alive, we calculated IRs, which were defined as the number of malignancies per 100 patient-years after the first transplant. We also calculated incidence rate ratios (IRRs), which were defined as the IR of a certain malignancy in a given age category in patients on RRT divided by the IR in the Dutch GP for the same malignancy in that age category as obtained from the National Cancer Registry (13). This national database maintains records of cancer incidence in the GP, with a completeness of >95%. All malignancies are registered, except for BCC and in situ cSCC. Because both of these types of malignancies are often removed without confirmation of the diagnosis by pathologist laboratories, registration of their incidence would not be reliable. IRs and IRRs of these types of cancer could not, therefore, be calculated.
All comparisons of the LERIC Study cohort used an age-matched group of the GP. Unfortunately, no statistics exist on the incidence of malignancies in the Dutch GP before 1989. To compare the incidence of malignancies among the LERIC Study patients and the Dutch GP, we, therefore, calculated the IRR of malignancies from 1990 to 2010.
IRs of de novo malignancies of all types, cSCC, and noncutaneous malignancies were calculated. The latter comprises all malignancies except for BCC, cSCC, and malignant melanoma (MM).
SPSS 21.0 was used for statistical analysis.
Results
Cohort Description
Information on the total LERIC Study cohort of 249 patients is displayed in Table 1. From first RRT until the end of follow-up in 2010, in total, 5709 patient-years were recorded. For 72 (29%) patients, follow-up was >30 years. Three patients were lost to follow-up; 91 patients had been transplanted one time, 85 patients had been transplanted two times, 43 patients had been transplanted three times, and 12 patients had been transplanted more than three times, whereas 18 patients never underwent transplantation. Those 18 patients died relatively soon after starting RRT after a median time on dialysis of 1.2 years.
Table 1.
Cohort characteristics
| Characteristics | LERIC Study Cohort | Range | Percentage |
|---|---|---|---|
| All patients | |||
| No. of patients starting RRT | 249 | ||
| Median age (yr) of survivors in 2010 | 39.9 | 20.9–52.4 | |
| Median age (yr) at start of RRT | 11.2 | 1.9–15.0 | |
| Median time (yr) on RRT | 25.3 | 0.3–39.3 | |
| Median time (yr) with transplant | 19.7 | 0.0–39.3 | |
| Median time (yr) on dialysis | 5.6 | 0.0–36.5 | |
| Deaths | |||
| No. of deaths | 95 | 38.2 | |
| Median age (yr) at death | 22.7 | 4.2–46.3 | |
| Median time (yr) on RRT at death | 11.0 | 0.3–32.3 | |
| Median time (yr) after first transplant at death | 14.4 | 0.0–29.7 | |
| Cause of death | |||
| Cardiovascular | 29 | 30.5 | |
| Infection | 29 | 30.5 | |
| Malignancy | 12 | 12.6 | |
| Other | 23 | 24.5 | |
| Unknown | 2 | 2.1 | |
| Recipients of transplants | |||
| No. of recipients of transplants | 231 | ||
| Median age (yr) at first transplant | 12.7 | 3.9–23.1 | |
| Median time (yr) on RRT | 26.1 | 1.5–39.9 | |
| Median time (yr) with transplant | 19.7 | 0.0–39.3 | |
| Median time (yr) on dialysis | 3.1 | 0.0–36.5 | |
| Recipients of transplants developing malignancy | |||
| No. of recipients of transplants developing malignancy | 53 | ||
| Median age (yr) at first malignancy | 33.5 | 11.0–49.0 | |
| Median time (yr) on RRT at development of first malignancy | 25.0 | 1.3–37.8 | |
| Median time (yr) with transplant at development of first malignancy | 18.4 | 0.0–37.2 | |
| Median time (yr) on dialysis at development of first malignancy | 3.3 | 0.0–36.5 | |
| Malignancies | |||
| No. of de novo malignancies | 105 |
LERIC Study, Late Effects of Renal Insufficiency Study.
Probability of Developing a Malignancy after Transplantation
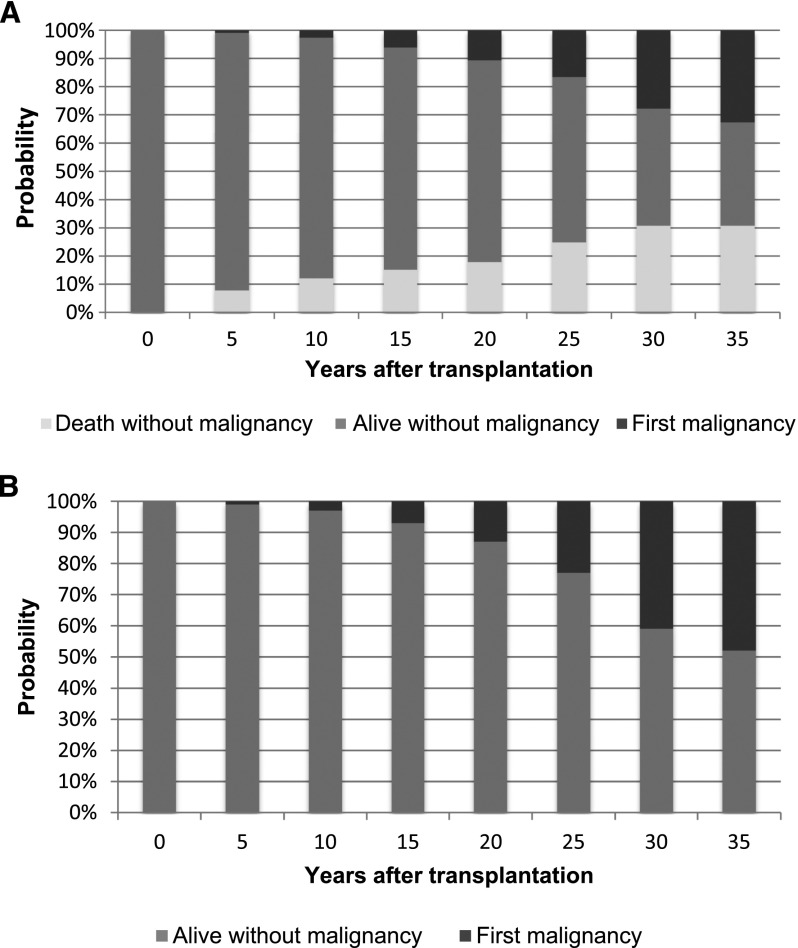
Of all 249 patients, 54 patients developed cancer. Of all 54 patients who developed cancer, only 1 patient did not receive a renal transplant. Figure 1A shows the probabilities of first malignancy, death without malignancy, and being alive without malignancy at several time points after transplantation.
Figure 1.
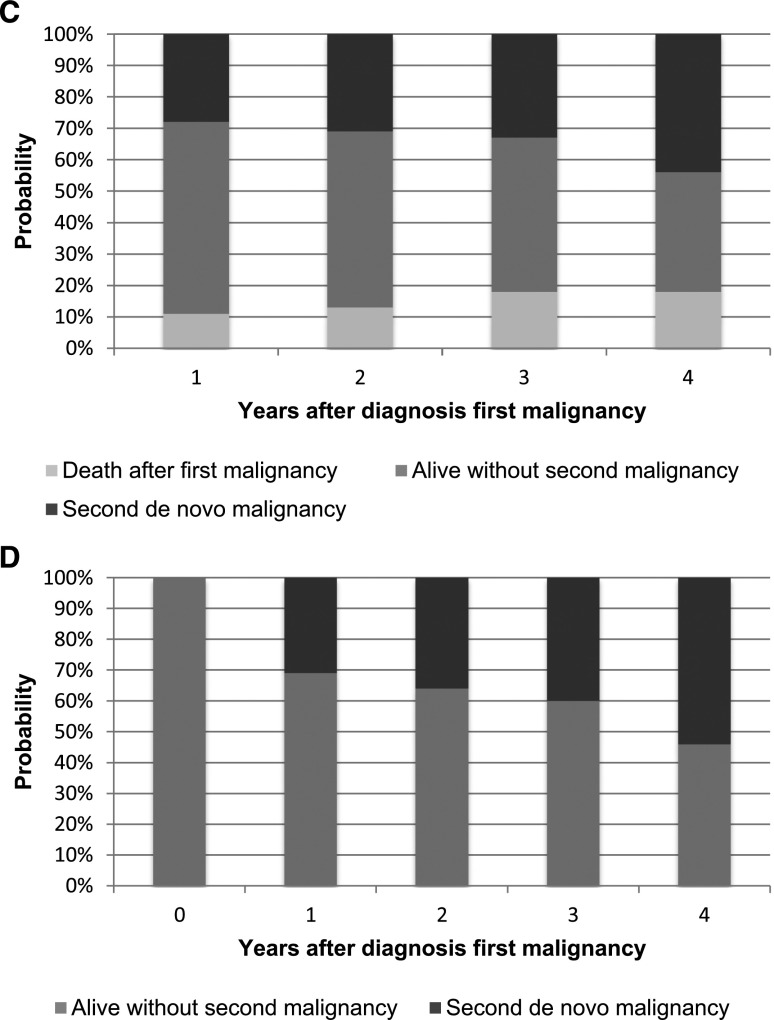
Cumulative incidence competing risk of de novo malignancy. (A) Cumulative incidence competing risk of de novo malignancy in patients with pediatric transplants. (B) Cumulative incidence competing risk of de novo malignancy in survivors of pediatric transplantation. (C) Cumulative incidence competing risk of developing a second de novo malignancy in patients with pediatric transplants. (D) Cumulative incidence competing risk of developing a second de novo malignancy in survivors of pediatric transplantation.
As outlined in Figure 1B, 20, 25, and 30 years after renal transplantation, 13%, 23%, and 41% of survivors had suffered from a malignancy, respectively.
Finally, Figure 1C shows the probabilities of being alive with a second de novo malignancy, being alive without a second de novo malignancy, and death after first malignancy 1, 2, 3, and 4 years after the first malignancy. In Figure 1D, patients who died after their first malignancy were excluded (see also Supplemental Material).
IRs of De Novo Malignancies
IRs and IRRs for all tumors (except BCC), all noncutaneous tumors, cSCC, and BCC are displayed in Table 2. Table 2 shows that, overall, the risk to develop a malignancy in this cohort was 21.7 (95% CI, 17.3 to 27.2) times higher than that in the Dutch GP. Patients in the age category 25–30 years old are 16.5 (95% CI, 7.9 to 34.6) times as likely to suffer from malignancies than their peers in the GP. In the age category 45–50 years old, the IRR has increased to 81.5 (95% CI, 50.7 to 131.1) for all tumors. Patients in that age category are 2610 (95% CI, 1596 to 4267) times as likely to develop a cSCC than the age-matched GP.
Table 2.
Incidence rates and incidence rate ratios of different types of malignancies after first transplantation
| Malignancies | Incidence Rate | 95% Confidence Interval | Incidence Rate Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| All malignanciesa | ||||
| 20–≤25 yr old | 1.1 | 1.1 to 1.1 | 35.6 | 16.9 to 74.7 |
| 26–≤30 yr old | 0.8 | 0.8 to 0.8 | 16.5 | 7.9 to 34.6 |
| 31–≤35 yr old | 1.5 | 1.5 to 1.5 | 19.4 | 11.2 to 33.4 |
| 36–≤40 yr old | 3.4 | 3.3 to 3.4 | 28.5 | 18.2 to 44.7 |
| 41–≤45 yr old | 4.1 | 4.0 to 4.1 | 21.2 | 11.7 to 38.2 |
| 46–50 yr old | 26.0 | 25.6 to 26.3 | 81.5 | 50.7 to 131.1 |
| All | 2.0 | 1.9 to 2.0 | 21.7 | 17.3 to 27.2 |
| All noncutaneous cancer | ||||
| 20–≤25 yr old | 0.5 | 0.1 to 1.3 | 19.7 | 7.4 to 52.4 |
| 26–≤30 yr old | 0.4 | 0.1 to 1.0 | 9.8 | 3.7 to 26.1 |
| 31–≤35 yr old | 0.4 | 0.1 to 1.0 | 6.3 | 2.4 to 16.8 |
| 36–≤40 yr old | 0.5 | 0.1 to 1.3 | 4.6 | 1.5 to 14.3 |
| 41–≤45 yr old | 0.3 | 0.3 to 0.3 | 1.7 | 0.2 to 12.3 |
| 46–50 yr old | 1.2 | 1.1 to 1.3 | 4.2 | 0.6 to 29.7 |
| All | 0.4 | 0.4 to 0.4 | 4.7 | 2.9 to 7.6 |
| Squamous cell carcinoma | ||||
| 20–≤25 yr old | 0.3 | 0.0 to 1.1 | 1915 | 416 to 7954 |
| 26–≤30 yr old | 0.3 | 0.1 to 1.0 | 991 | 313 to 3137 |
| 31–≤35 yr old | 0.9 | 0.4 to 1.8 | 927 | 459 to 1875 |
| 36–≤40 yr old | 2.8 | 1.6 to 4.6 | 1235 | 752 to 2029 |
| 41–≤45 yr old | 3.7 | 1.8 to 6.8 | 756 | 406 to 1409 |
| 46–50 yr old | 24.4 | 13.9 to 39.6 | 2610 | 1596 to 4268 |
| All | 1.5 | 1.0 to 1.9 | 744 | 570 to 970 |
| Basal cell carcinomab | ||||
| 20–≤25 yr old | 0 | |||
| 26–≤30 yr old | 0.1 | 0.1 to 0.1 | ||
| 31–≤35 yr old | 0.6 | 0.6 to 0.6 | ||
| 36–≤40 yr old | 1.4 | 1.2 to 1.2 | ||
| 41–45 yr old | 2.9 | 2.3 to 2.4 | ||
| All | 0.7 | 5.8 to 6.1 |
Incidence rate is defined as malignancy per 100 patient-years. Incidence rate ratio is defined as the incidence rate of our cohort divided by the incidence rate of the general population.
Excluding basal cell carcinoma.
We were not able to calculate the incidence rate ratio of basal cell carcinoma, because national cancer statistics do not register basal cell carcinoma.
Tumor Characteristics
In total, 105 de novo malignancies were registered in 54 patients. Table 3 shows the characteristics of the malignancies. We found 82 NMSCs (78% of all de novo tumors) in 39 patients. NMSC mortality rates were low. No one died of BCC; two patients died because of metastases of a cSCC. Of all malignancies, NMSC was most common to recur. There were almost two times as many cSCCs as BCCs.
Table 3.
Tumor characteristics
| Malignancies | Patients | N De Novo Tumorsa | Median Age (yr) at Diagnosis (Range) | Median Interval (yr) between Transplant and Diagnosis (Range) | Deaths Related to Malignancy |
|---|---|---|---|---|---|
| Total | 54 | 105 | 37.6 (11.1–50.4) | 25.0 (0.0–37.8) | 12 |
| Skin cancer | |||||
| Nonmelanoma skin cancer | 40 | 82 | 39.1 (21.8–50.4) | 26.2 (5.5–36.2) | 2 |
| Basal cell carcinoma | 21 | 29 | 38.8 (21.8–49.3) | 26.7 (5.5–36.1) | 0 |
| Squamous cell carcinoma | 29 | 53 | 39.9 (22.5–50.4) | 26.2 (10.2–36.2) | 2 |
| Malignant melanoma | 5 | 5 | 31.1 (14.3–37.6) | 22.5 (15.7–25.8) | 1 |
| Noncutaneous cancer | |||||
| Leiomyosarcoma | 1 | 1 | 32.9 | 21.1 | 1 |
| Lymphoproliferative disorders | 6 | 7 | 25.5 (11.1–32.0) | 15.1 (0.6–20.8) | 3 |
| Leukemia | 2 | 2 | 29.2 (27.3–32.2) | 16.7 (11.1–22.2) | 1 |
| Fibrosarcoma | 1 | 1 | 12.8 | 0.1 | 1 |
| Adenocarcinoma | 1 | 1 | 45.8 | 27.5 | 1 |
| Brown’s tumor | 1 | 1 | 35.6 | 25.0 | 0 |
| Grawitz tumor | 1 | 1 | 23.3 | 8.4 | 0 |
| Parotis carcinoma | 1 | 1 | 42.1 | 26.0 | 0 |
| Thyroid carcinoma | 1 | 1 | 28.3 | 18.6 | 1 |
| Lymphoreticular malignancy | 1 | 1 | 35.1 | 23.4 | 1 |
| Mammacarcinoma | 1 | 1 | 44.5 | 26.7 | 0 |
Some patients had more than one type of malignancy and were, therefore, included in both categories.
Other than NMSC, there were 11 other types of tumors. MM accounted for five de novo tumors in five patients. The median age at diagnosis was 29.0 years old (range =14.3–37.6; median =31.1). All but one patient with MM had been transplanted at least one time. One patient died because of metastases of an MM.
Lymphoproliferative disorders accounted for seven de novo tumors in six patients. Three of those six patients died, whereas one of two patients with leukemia died. The mortality rates for those diseases were, therefore, considerably higher than those for NMSC and MM.
Discussion
This study provides the longest complete follow-up of a cohort of patients with pediatric ESRD to date. We found an incidence of at least one malignancy of 41% in patients who survived and had 30 years of follow-up starting from first transplantation, the majority being NMSC and lymphoproliferative disorders. Malignancies recurred frequently; 31% of patients with cancer developed a second de novo malignancy within 1 year. Compared with the Dutch GP, the risk of developing a malignancy in this cohort was >20-fold higher. Malignancies contributed to a significant proportion of overall mortality (12.6%).
The IRR of all malignancies in our cohort was almost 22 (IRR, 21.7; 95% CI, 17.3 to 27.2) times higher than that in the GP. This number is higher than that seen in earlier adult studies, which consistently report a 3- to 7-fold increased incidence in patients with ESRD (4,5,11,12,14). This could be explained by the fact that our study is on the basis of a nationwide cohort with an exceptionally long period of follow-up. Our cohort was exposed to RRT and immunosuppression from childhood onward, and this may have led to an exponential growth in malignancies. Our results show that, after 20 years of transplantation, the incidence abruptly increases, confirming our hypothesis. However, it is not known how generalizable these results are to other populations, because these patients are mostly older and have a shorter time of follow-up.
Cumulative incidence competing risk analysis showed that the probability of death after 30 years of follow-up from first transplantation is 31% (Figure 1A). It is also showed a cumulative incidence of 41% after 30 years after first transplantation (Figure 1B). Figure 1B can be used to explain the risk of developing a malignancy, because the deceased patients are excluded. Therefore, if a patient is still alive after 30 years of follow-up, the chance of developing a malignancy in those past 30 years is 41%. This increased incidence of malignancies is associated with the use of immunosuppression. The presumed pathophysiology is that such agents impair the ability to locate and destroy cancer cells as well as control viral infections that are potentially oncogenic (6). Consequently, types of cancer with the highest standardized IRRs after organ transplantation with immunosuppression are those that are related to infection. Immunosuppression may enable different viruses to become carcinogenic (i.e., post–transplant lymphoproliferative disorder, Kaposi sarcoma, and anogenital dysplasias) or prevent repair of affected cells in areas exposed to solar radiation (i.e., skin cancer and head and neck cancers).
In a previous study, we showed an early effect on the occurrence of malignancy of cyclophosphamide. This effect had disappeared in 2010. An explanation might be that cyclophosphamide was only given in the early years of starting RRT and most often before the start of RRT. After 1990, cyclophosphamide was no longer used for outpatients.
Another problem that we faced while trying to analyze the effect of specific immunosuppressive therapy was the change over time in immunosuppressive regiments. In the 1970s and early 1980s, patients only received azathioprine and prednisolone as standard surveillance immunosuppressive medication. In the late 1980s, this was switched to cyclosporin and prednisolone, and by the middle 1990s, it was changed to triple therapy of cyclosporin, azathioprine, and prednisolone. In the early 2000s, this regime was gradually replaced with the combination of tacrolimus, prednisolone, and mycophenolate mofetil. From 2004 on, this was combined with anti–IL-2 induction therapy. These switches of immunosuppressive medication over the last 10–15 years seemed to be a major hurdle in the analysis of the specific effect of the various immunosuppressive regimes.
Nevertheless, the fact that we found such a high effect of long–lasting, relatively mild immunosuppressive therapy to current standards implies that the risk of cancer will certainly be even greater in children who are currently transplanted.
There is discussion in the literature as to what extent being on dialysis may contribute to the increased risk of cancer compared with transplantation (15). There are several insuperable hurdles that make investigation of the effect of dialysis on cancer occurrence virtually impossible. An increase in risk of cancer will only occur after a certain exposure time of the risk factor. All physicians will try to shorten dialysis time in children and young adults as much as possible. As a consequence, the average overall time on dialysis in young patients on RRT is relatively short. Those children who are forced to stay on dialysis for a relatively long period are at extremely high risk for early death caused by cardiovascular disease long before they may develop cancer. This practice is illustrated by the course of the patients in our cohort. Of the total RRT time, our patients spent six times as much time with a functioning graft as on dialysis.
The most important cancer registered in this study is cSCC. Many of the other more deadly cancers, especially lymfoproliferative disorders, occurred earlier and have killed some of the recipients of transplants, and therefore, cutaneous cancer, which has low mortality, was what was left behind. The incidence of cSCC was 744 times as high as that in the age-matched population. This outnumbers previous reports, which confirm NMSC and virus-related cancers to be the predominant types of cancers found in patients with transplants (3–5,10–12). Studies that have included NMSC have found a 3- to 5-fold increased risk for all cancers and 13- to 222-fold increased risk for NMSC after transplantation (4,9–12,16). Because these reports concern adult recipients, the total exposure time is far less than the exposure time in the LERIC Study cohort. Studies done in the Dutch population of adult recipients of kidney transplants found a lower IR of squamous cell carcinoma (SCC) than that presented in this paper (17). As mentioned above, the discrepancy between the results is because of the fact that the LERIC Study cohort is an unique cohort with an exceptionally long exposure to immunosuppressive agents and RRT.
In contrast to the GP (13), the incidence of cSCC in this patient population exceeded that of BCC. The cSCC incidence was nearly two times that of BCC. Earlier studies also described a reversed ratio of BCC to cSCC with the GP, in which BCC is the most common one. The predominance of SCC over BCC is more pronounced in pediatric than in adult recipients of transplants (SCC:BCC is 2.8:1 versus 1.7:1) (18). Ultraviolet (UV) exposure and human papilloma virus (HPV) combined with immunosuppression are thought to be the most important causative factors for the development of post-transplant cSCC. UV radiation is probably one of the most important factors (19). This would explain the extremely high incidence of cSCC in Australia, a country with a majority of genetically ill–protected white people and a very high sun exposure; there is 93% skin cancer among all post-transplant malignancies (20). Sun protection is, therefore, of utmost importance. cSCC may appear in the absence of any preexisting skin lesion but is often preceded by actinic keratosis, which could mean some involvement of HPV in its pathogenesis. There is, however, no conclusive evidence regarding the causal role of HPV in actinic keratosis at this time. Additional evidence for the role of HPV comes from a study on post-transplant SCC that occurred in a cohort of 500 recipients of allografts; HPV DNA could be detected in nearly 50% of all SCCs (21–23).
Malignancies recurred often; 31% of the patients had a second de novo malignancy within 1 year (Figure 1C). Figure 1D can be used to explain the risk of developing a second de novo cancer (i.e., if a patient developed cancer, the chance of a de novo malignancy within 4 years is 54%). This number emphasizes the need for regular follow-up.
Most of them were NMSC, one was a leiomyosarcoma, and two were MM. Although in most patients, it is not lethal at the time of investigation, NMSC (especially cSCC) tended to recur very often within a short interlude. Some patients seemed more susceptible for multiple reoccurrences or distant metastasis of NMSC than others. One patient was diagnosed 12 times with NMSC. Eventually, two patients died because of metastases of cSCCs. Unfortunately, we had no information on potential risk factors, such as HPV status and the extent of UV exposure (24). Previous studies in adult transplantation confirm that post-transplant cSCC tends to be far more aggressive than that in the GP.
As we continue to improve the life expectancy of our patients by better cardiovascular risk management and prevention of infection, we elongate the exposure on RRT. With longer exposure, the risk of malignancy will increase. The follow-up in our patient cohort may be too short to fully appreciate its final effect on mortality, and therefore, additional research must be conducted.
This long–term follow-up study of late effects of renal insufficiency in children shows a cumulative cancer incidence of 41% after 30 years post-transplantation. cSCC occurs, by far, most frequently, occurs relatively late after transplantation, and tends to recur frequently. Although in most patients, it is not lethal at short follow-up, the high recurrence rate may affect the outcome on the longer term. Moreover, the chronicity of the disease may have an important negative effect on quality of life because of frequent hospital visits, excisions, and cosmetic consequences. Additionally, the threat of becoming a lethal disease is always present.
We, therefore, advise that all risk factors concerning skin cancer must be reduced. The most important risk factor is sunburn at an early age. This reduction could take place through education, parental role modeling, and sunscreen vigilance.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to the Department of Medical Informatics and all participating centers in the Late Effects of Renal Insufficiency (LERIC) Study, with special thanks to the participating nephrologists: Dr. J.M.A. Ampting, Dr. A.J. Apperloo, Dr. C.H. Beerenhout, Dr. F.J. Bemelman, Dr. M. Boekhout, Dr. P.J.M. van der Boog, Dr. M.H.L. Christiaans, Dr. S.H.A. Diepeveen, M.A. van den Dorpel, Dr. A. van Es, Dr. W.J. Fagel, Dr. B.A.T.F. Gabreels, Dr. A.B.M. Geers, Dr. P.G.G. Gerlag, Dr. E.C. Hagen, Dr. M. den Hartog, Dr. R.J. Hene, Dr. J.J. Homan van der Heide, Prof. Dr. A.J. Hoitsma, Dr. M. Huisman, Dr. B.C. van Jaarsveld, Dr. K. Jie, Dr. G.M.T. de Jong, Dr. W.A.H. Koning-Mulder, Prof. Dr. M.P. Kooistra, Dr. W.H.M. van Kuijk, Dr. A.G. Lieverse, Dr. R.R.H. Nap, Dr. M.J. Nubé, Dr. K.J. Parlevliet, Dr. E.J. Roodnat, Dr. van Schie, Dr. M.A.J. Seelen, Dr. H.E. Sluiter, Dr. C.R. Susanto, and Dr. R.M. Valentijn.
This study was performed as part of the LERIC Follow-Up Study, which is mainly funded by the Dutch Kidney Foundation.
The funder had no role in the design and conduct of the study, data gathering or interpretation, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03630415/-/DCSupplemental.
References
- 1.Centraal Bureau voor de Statistiek : Statistics Netherlands. Available at: http://www.cbs.nl. Accessed February 8, 2015
- 2.Holley JL: Screening, diagnosis, and treatment of cancer in long-term dialysis patients. Clin J Am Soc Nephrol 2: 604–610, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Port FK, Ragheb NE, Schwartz AG, Hawthorne VM: Neoplasms in dialysis patients: A population-based study. Am J Kidney Dis 14: 119–123, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Birkeland SA, Løkkegaard H, Storm HH: Cancer risk in patients on dialysis and after renal transplantation. Lancet 355: 1886–1887, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Nocera A, Ghio L, Dall'Amico R, Fontana I, Cardillo M, Berardinelli L, Zanon GF, Scalamogna M, Zacchello G, Valente U, Ginevri F: De novo cancers in paediatric renal transplant recipients: A multicentre analysis within the North Italy Transplant programme (NITp), Italy. Eur J Cancer 36: 80–86, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Debray D, Baudouin V, Lacaille F, Charbit M, Rivet C, Harambat J, Iserin F, Di Filippo S, Guyot C, Pediatric Transplantation Working Group of the French Speaking Society of Transplantation : De novo malignancy after solid organ transplantation in children. Transplant Proc 41: 674–675, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Coutinho HM, Groothoff JW, Offringa M, Gruppen MP, Heymans HS: De novo malignancy after paediatric renal replacement therapy. Arch Dis Child 85: 478–483, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F: Cancer risk following organ transplantation: A nationwide cohort study in Sweden. Br J Cancer 89: 1221–1227, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohmé I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frödin L, Glattre E, Halvorsen S, Holm NV, Jakobsen A, Jorgensen HE, Ladefoged J, Lindholm T, Lundgren G, Pukkala E: Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer 60: 183–189, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Gaya SB, Rees AJ, Lechler RI, Williams G, Mason PD: Malignant disease in patients with long-term renal transplants. Transplantation 59: 1705–1709, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Nederlandse Kanker Registratie: 2013. Available at: http://www.cijfersoverkanker.nl. Accessed January 1, 2015
- 14.Piselli P, Serraino D, Segoloni GP, Sandrini S, Piredda GB, Scolari MP, Rigotti P, Busnach G, Messa P, Donati D, Schena FP, Maresca MC, Tisone G, Veroux M, Sparacino V, Pisani F, Citterio F, Immunosuppression and Cancer Study Group : Risk of de novo cancers after transplantation: Results from a cohort of 7217 kidney transplant recipients, Italy 1997-2009. Eur J Cancer 49: 336–344, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Butler AM, Olshan AF, Kshirsagar AV, Edwards JK, Nielsen ME, Wheeler SB, Brookhart MA: Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996-2009. Am J Kidney Dis 65: 763–772, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM: Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 370: 59–67, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Wisgerhof HC, Wolterbeek R, de Fijter JW, Willemze R, Bouwes Bavinck JN: Kidney transplant recipients with cutaneous squamous cell carcinoma have an increased risk of internal malignancy. J Invest Dermatol 132: 2176–2183, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Penn I: De novo malignances in pediatric organ transplant recipients. Pediatr Transplant 2: 56–63, 1998 [PubMed] [Google Scholar]
- 19.Bouwes Bavinck JN: Epidemiological aspects of immunosuppression: Role of exposure to sunlight and human papillomavirus on the development of skin cancer. Hum Exp Toxicol 14: 98, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Hardie IR, Strong RW, Hartley LC, Woodruff PW, Clunie GJ: Skin cancer in Caucasian renal allograft recipients living in a subtropical climate. Surgery 87: 177–183, 1980 [PubMed] [Google Scholar]
- 21.Euvrard S, Chardonnet Y, Pouteil-Noble C, Kanitakis J, Chignol MC, Thivolet J, Touraine JL: Association of skin malignancies with various and multiple carcinogenic and noncarcinogenic human papillomaviruses in renal transplant recipients. Cancer 72: 2198–2206, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sørensen HT: Skin cancer risk among solid organ recipients: A nationwide cohort study in Denmark. Acta Derm Venereol 90: 474–479, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Muñoz N, Castellsagué X, de González AB, Gissmann L: Chapter 1: HPV in the etiology of human cancer. Vaccine 24[Suppl 3]: S3/1–S3/10, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Reichrath J: Dermatologic management, sun avoidance and vitamin D status in organ transplant recipients (OTR). J Photochem Photobiol B 101: 150–159, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.