Abstract
The ability of immune-based cancer therapies to elicit beneficial CD8+ CTL is limited by tolerance pathways that inactivate tumor-specific CD4 helper T cells. A strategy to bypass this problem is to engage tumor-unrelated CD4 helper T cells. Thus, CD4 T cells, regardless of their specificity per se, can boost CD8+ CTL priming so long as the cognate epitopes are linked via presentation on the same dendritic cell. Here, we assessed the therapeutic impact of engaging tumor-unrelated CD4 T cells during dual costimulation with CD134 plus CD137 that not only provide help via the above-mentioned classical linked pathway, but also provide non-linked help that facilitates CTL function in T cells not directly responding to cognate antigen. We found that engagement of tumor-unrelated CD4 helper T cells dramatically boosted the ability of dual costimulation to control the growth of established B16 melanomas. Surprisingly, this effect depended upon a CD134-dependent component that was extrinsic to the tumor-unrelated CD4 T cells, suggesting that the dual-costimulated helper cells are themselves helped by a CD134+ cell(s). Nevertheless, the delivery of therapeutic help tracked with an increased frequency of tumor-infiltrating granzyme B+ effector CD8 T cells and a reciprocal decrease in Foxp3+CD4+ cell frequency. Notably, the tumor-unrelated CD4 helper T cells also infiltrated the tumors, and their deletion several days following initial T cell priming negated their therapeutic impact. Taken together, dual costimulation programs tumor-unrelated CD4 T cells to deliver therapeutic help during both the priming and effector stages of the anti-tumor response.
Introduction
CD8+ CTL have been the major focus of immune-based cancer therapies. Nevertheless, CD4+ T cells can also play an integral role in tumor immunity (1) by engaging tumoricidal innate immune cells (2), targeting tumor-supporting stroma (3, 4), activating dendritic cells (5), and acquiring cytolytic capacity that enables direct killing of MHC class II+ tumors (6, 7). Additionally, similar to their ability to optimize CD8 T cell responses during infection (8), CD4 T cells can also help tumor-specific CD8+ CTL (9–13). Further, although CD4 help can be dispensable for CD8+ CTL response during certain viral infections (8), it is more likely to be critical for tumor immunity given that CD8 T cells with high tumor avidities tend to be susceptible to tolerization (14, 15). Tumor-specific CD4 T cells are, however, also susceptible to tolerization (16, 17).
A strategy to bypass tumor-specific CD4 T cell tolerance to support tumor-specific CD8+ CTL during adoptive cell therapy (ACT) involves treating patients with in vitro expanded autologous tumor-infiltrating lymphocytes (TIL) that contain tumor-reactive CD4 T cells, although this does not, however, consistently improve therapeutic outcome (18). Alternatively, vaccines can be engineered to incorporate a tumor-unrelated MHC class II-restricted helper epitope along with tumor-specific MHC class I-restricted peptides (19). Thus, in secondary lymphoid organs CD4 T cells, regardless of their specificity per se, can boost CD8+ CTL priming so long as their cognate epitopes are presented on the same dendritic cell (20–22). This approach of providing tumor-unrelated—but dendritic cell-linked—CD4 T cell help can indeed augment tumor-specific CD8 T cell responsiveness in human cancer patients, although therapeutic benefit has not yet been observed (19).
There are several reasons why vaccines can elicit tumor-specific CTL without producing durable therapeutic benefit. One issue is the size of tumor mass that must be targeted in advanced disease. This point is highlighted in human ACT studies where therapeutic response requires multiple infusions of large numbers of tumor-specific CTL (23, 24). Another problem is the outgrowth of antigen-loss (escape) variants that lack targeted MHC class I-restricted epitopes (23, 25, 26). Engaging the fullest possible repertoire of tumor-specific CTL would likely be helpful in dealing with both issues. One approach towards this involves next-generation sequencing technologies that can identify tumor neoepitopes to enable construction of patient-specific multivalent vaccines (27, 28). For simplicity, however, it would ultimately be desirable to develop precision, off-the-shelf immunotherapies that elicit multivalent anti-tumor T cell responses.
The current study analyzed the impact of engaging tumor-unrelated CD4 T cell help on anti-tumor immunity elicited by agonists to the TNF receptor costimulatory members CD134 (OX40) and CD137 (4-1BB). Similar to other costimulatory agonist combinations (29), costimulation with CD134 plus CD137 (dual costimulation) acts synergistically to elicit robust CD8 T cell effector and tumoricidal activity (30–33). Importantly, dual costimulation also induces cytotoxic CD4 Th1 cells that can directly kill MHC class II+ tumors (34) and provide linked-help to CD8 T cells (35, 36). We thus reasoned that a tumor-unrelated helper epitope might augment dual costimulation therapeutic efficacy by helping CD8 T cells responding to cross-presented tumor epitopes (37). Because cross-presentation is biased towards highly abundant antigens (38, 39), however, this linked helper pathway may not boost CTL specific to less abundant tumor epitopes. Nevertheless, this potential therapeutic gap might be bridged by an additional dual costimulation-elicited non-linked helper activity. Thus, dual-costimulated antigen-responding CD4 T cells can also help cognate antigen-non-responding T cells to develop CTL function (34). This non-linked help might influence tumor-specific CTL in draining lymph nodes that would otherwise not be cross-primed, or alternatively boost CTL encountering cognate tumor epitopes intratumorally (13).
To assess the potential of tumor-unrelated CD4 helper T cells to augment dual costimulation therapy, mice were challenged with tumors lacking an MHC class II-restricted peptide that was used as an immunogen with dual costimulation. Thus, although dual-costimulated CD4 T cells become cytolytic, they could only enhance tumor immunity by providing help. Notably, engagement of tumor-unrelated CD4 help dramatically augmented dual costimulation-mediated control of established B16 melanomas. Although these tumor-unrelated CD4 T cells were directly costimulated, their ability to provide therapeutic help depended, surprisingly, on an indirect CD134-dependent component. This suggested that the dual-costimulated helpers are themselves helped by a CD134+ cell(s). Nevertheless, therapeutic help was associated with an increased frequency of tumor-infiltrating CD8+ CTL and a reciprocal decrease in Foxp3+CD4+ cell frequency. The tumor-unrelated CD4 helper T cells also infiltrated the tumors, and their deletion several days following initial T cell priming negated their therapeutic impact. Taken together, dual costimulation triggers tumor-unrelated CD4 helper T cells to facilitate therapeutic efficacy, which relies on feedback from other CD134+ cells.
Materials and Methods
Mice, adoptive transfer, tumor challenge and cell depletion
One million 6.5 TCR transgenic (Tg) CD4 T cells specific to an I-Ed-restricted influenza hemagglutinin (HA) epitope (110SFERFEIFPKE120) on the Thy1.1+ BALB/c background (40) were prepared from CD8-depleted spleens plus lymph nodes of Tg donors and adoptively transferred into Thy1.2+ BALB/c recipients. TEa TCR Tg CD4 T cells specific to an I-Ab-restricted I-Ed epitope (Eα, 52ASFEAQGALANIAVDKA68) on the Thy1.1+ C57BL/6 background (41) that were either WT or lacking CD134 (Tnfrsf4 null mutation) (42) (Jackson Laboratory) were similarly prepared and transferred into WT or CD134−/− Thy1.2+ C57BL/6 recipients. As indicated, mice were treated i.p. with dual costimulation (50 μg OX86 monoclonal antibody (mAb) (anti-OX40/CD134) plus 25 μg 3H3 mAb (anti-4-1BB/CD137)) (BioXCell) or 75 μg control rat Ig (Sigma-Aldrich) one day following adoptive T cell transfer, recombinant vaccinia expressing HA (viral-HA, 106 plaque forming units) one day prior to T cell transfer, or 250 μg soluble Eα peptide the day of T cell transfer (35).
The A20 B cell lymphoma (A20WT, ATCC) and A20HA subline (16) (generously provided by Dr. Hyam Levitsky) were inoculated s.c. at 106 cells into BALB/c recipients. B16-F10 (B16) melanoma cells (ATCC) were inoculated i.d. at 5 x 105 cells into C57BL/6 recipients. Tumors were measured using calipers and multiplying perpendicular diameters to calculate surface areas in square millimeters. Area under the curve (AUC) analysis was performed as described (43).
CD8+, CD4+ and Thy1.1+ cells were depleted by i.p. injection of 200 μg each of the mAbs 2.43, GK1.5 and 19E12, respectively (BioXCell).
Flow cytometry
Transferred 6.5 and TEa TCR Tg CD4 T cells were identified as CD4+Thy1.1+, while host-derived T cells were identified as Thy1.1negCD4+ or CD8+Thy1.1neg. Ex vivo GzmB and CD25 staining was performed as described (34). Intracellular IFN-γ and TNF-α staining was performed following 5 h in vitro stimulation with either 100 μg/ml HA peptide for 6.5 CD4 T cells or 2.5 μg/ml soluble anti-CD3 mAb (eBioscience) for TEa CD4 T cells and host-derived T cells (34). Intratumoral T cells were extracted by mechanical crushing, straining and washing with PBS. The frequency of CD4 and CD8 T cells are expressed as their percent frequency within the lymphocyte gate (determined by forward and side scatter using splenocytes as a reference).
Statistical analysis
P values were calculated using an unpaired two-tailed t test, and p < 0.05, < 0.01, < 0.001 and < 0.0001 are indicated by *, **, *** and ****, respectively. Asterisks located above horizontal lines indicate comparisons between circumscribed groups, while all other asterisks indicate differences relative to the control group at the far left of each graph. Hashtags (#, ##, ### and ####) indicate significance between a CD134−/− group and its corresponding WT counterpart. Sample sizes and number of experimental trials are indicated in the figure legends.
Results
Tumor-unrelated CD4 T cell help augments dual costimulation therapy
The BALB/c-derived MHC class II+ B cell lymphoma A20 and the A20HA subline (16) were initially used to assess the tumoricidal potential of dual-costimulated CD4 T cells. A20HA presents an I-Ed-restricted HA epitope that can be recognized by adoptively transferred 6.5 TCR Tg CD4 T cells (16), raising the potential for direct CD4 T cell-mediated tumor killing (34). In contrast, the potential of dual-costimulated 6.5 CD4 T cells to control the parental A20WT lymphoma would presumably depend entirely on their helper capacity.
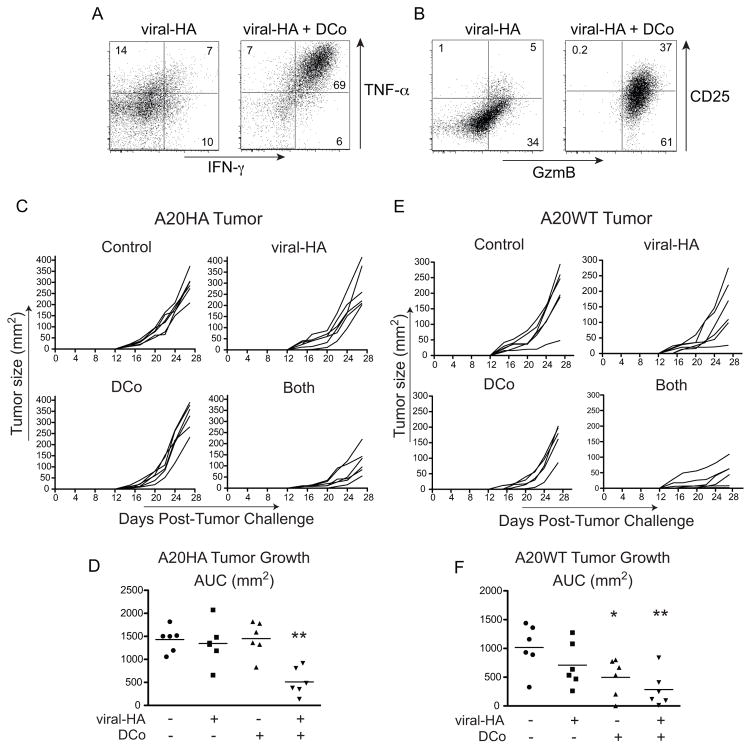
Because the potential of dual costimulation (DCo) to elicit cytotoxic CD4 Th1 cells was established previously in the Th1-predisposed C57BL/6 and B10.D2 mouse strains (34), it was first necessary to assess the CD4 T cell response to DCo in BALB/c mice that are Th2-predisposed (44). Thy1.1+ BALB/c 6.5 CD4 T cells were transferred into Thy1.2+ BALB/c recipients infected with a recombinant vaccinia virus expressing HA (viral-HA) and treated with DCo or control IgG (Fig. 1). In B10.D2 mice viral-HA programs 6.5 CD4 T cells to become standard Th1 effectors that express IFN-γ and TNF-α, while the addition of DCo pushes these CD4+ effectors to also express granzyme B (GzmB) (34). In BALB/c mice viral-HA without DCo programmed the 6.5 CD4 T cells to express only modest amounts of IFN-γ and TNF-α (Fig. 1A), but importantly the addition of DCo pushed the viral-HA-specific CD4 T cells to express high levels of IFN-γ, TNF-α (Fig. 1A) and GzmB (Fig. 1B). DCo also increased CD25 expression (Fig. 1B), consistent with the essential role of IL-2 in programming GzmB expression (34). Taken together, DCo can program cytotoxic Th1 differentiation in Th2-predisposed BALB/c mice.
Figure 1.
Dual costimulation (DCo) induces cytotoxic CD4 Th1 T cells and controls lymphoma growth control in BALB/c mice. HA-specific Thy1.1+ 6.5 TCR Tg CD4 T cells were transferred into Thy1.2+ BALB/c recipients followed by immunization with viral-HA or viral-HA plus DCo. Spleens were analyzed 5 days later. The transferred CD4 T cells were analyzed for intracellular IFN-γ versus TNF-α expression (A) and GzmB versus CD25 expression (B). The FACS plots shown are representative of an experiment containing 3 mice per group, and also of two other experiments. (C–F), BALB/c mice were inoculated with A20HA (C and D) or parental A20WT (E and F) lymphoma 4 days prior to receiving 6.5 CD4 T cells and subsequent immunization with rat Ig (Control), viral-HA, DCo, or viral-HA plus DCo (Both). (C and E), Tumor growth curves for individual mice. (D and F), Scatter plots of area under the curve (AUC) values with horizontal bars indicating the means corresponding to the data in Panels C and E, respectively. The experiment shown in C–F contained 6 mice per group, and similar results were observed in another trial.
BALB/c mice were inoculated with A20HA tumor and 4 days later received transferred 6.5 CD4 T cells and were immunized with viral-HA, DCo or both. Viral-HA alone had no impact on A20HA tumor growth compared to control IgG immunized mice (Fig. 1C, 1D). DCo alone also had no impact on A20HA tumor growth, but notably DCo given with viral-HA (Both) reduced tumor growth 3-fold (p≤0.009) (Fig. 1C, 1D).
The ability of DCo plus viral-HA to control A20HA tumor growth (Fig. 1C, 1D) is consistent with the potential of HA-specific 6.5 cytotoxic CD4 Th1 cells to directly kill A20 cells presenting the cognate MHC class II-restricted HA epitope (34). We next used a similar experimental setup but with A20WT tumor to assess whether the dual-costimulated CD4 T cells can control tumor growth through their helper activity. Similar to the results with A20HA (Fig. 1C, 1D), immunization with viral-HA alone did not significantly impact A20WT tumor growth compared to non-immunized controls (Fig. 1E, 1F). DCo alone significantly slowed tumor growth in some mice (2-fold, p=0.03), although a more substantial effect was observed in mice treated with both DCo plus viral-HA (4-fold, p=0.005).
That the priming of HA-specific CD4 T cells by viral-HA was associated with an enhanced ability of DCo to control A20 lymphoma growth regardless of whether this tumor expressed HA (Fig. 1C-F) suggested that DCo tumor-unrelated CD4 T cells can augment tumor immunity by providing help. To rigorously test this possibility, we devised a second system in which C57BL/6 mice were challenged with the highly aggressive B16-F10 melanoma and received transferred TEa CD4 T cells along with cognate I-Ab-restricted Eα peptide (41). Importantly, B16 melanoma does not express I-Ed from which the Eα epitope derives and thus cannot be directly targeted by the TEa CD4 T cells.
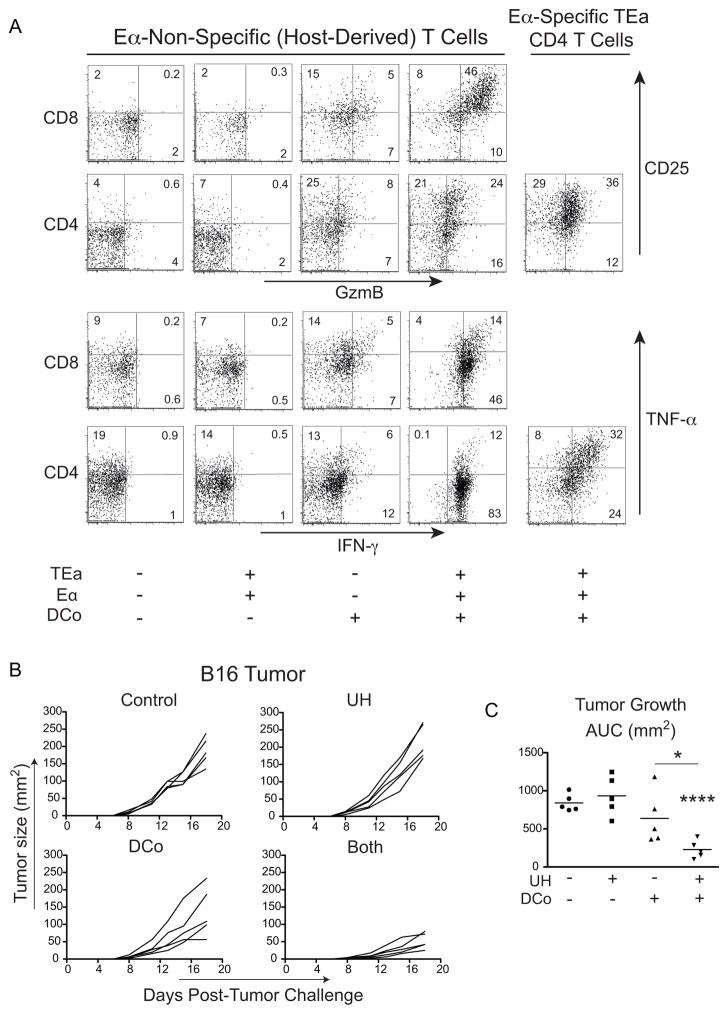
Because our previous studies demonstrating the potential of dual-costimulated CD4 T cells to acquire the cytotoxic Th1 phenotype and deliver non-linked help were performed using different antigens and cognate CD4 T cells (34), it was first necessary to characterize the response of transferred TEa CD4 T cells to DCo in non-tumor-bearing mice. TEa CD4 T cells induced by Eα peptide plus DCo expressed GzmB, CD25 (Fig. 2A, end of second row), and IFN-γ and TNF-α (Fig. 2A, end of fourth row). Further, host-derived (antigen-non-responding) T cells were also programmed to express GzmB, CD25 and IFN-γ, albeit GzmB expression was greater in CD8 T cells compared to CD4 counterparts (Fig. 2A, fourth column). Importantly, DCo given without transferred TEa CD4 T cells and Eα peptide induced lower amounts of these effector molecules in host-derived T cells (Fig. 2A, third column). TEa CD4 T cells plus Eα peptide but without DCo had no effect (Fig. 2A, second column). Thus, dual-costimulated TEa CD4 T cells become cytotoxic and provide non-linked unrelated help.
Figure 2.
DCo tumor-unrelated CD4 T cells provide non-linked help and control melanoma growth. Thy1.2+ C57BL/6 mice received Thy1.1+ TEa TCR Tg CD4 T cells and were immunized with or without 250 μg cognate Eα peptide and with or without DCo. (A) Eα-specific TEa CD4 T cells and host-derived CD4 and CD8 T cells from spleens were analyzed 5 days post-transfer. GzmB versus CD25 expression (top two rows) and IFN-γ versus TNF-α expression (bottom two rows). FACS plots are representative of 3-7 replicates per group that were pooled from two separate experiments. (B and C) C57BL/6 mice were challenged with B16-F10 melanoma cells the same day they received 106 TEa CD4 T cells and were subsequently treated with Eα peptide (UH, Unrelated Help), DCo, or DCo plus UH (Both). Tumor growth curves for individual mice (B) and AUC analysis (C). N=5 mice per group, and similar results were observed in two other trials.
C57BL/6 mice were challenged with B16 melanoma and received TEa CD4 T cells. Tumor growth in mice with TEa CD4 T cells and Eα peptide (Unrelated Help (UH)) but without DCo was comparable to IgG-treated controls (Fig. 2B, 2C). DCo given alone reduced tumor growth in some mice (Fig. 2B, 2C), but this trend did not yet reach statistical significance (p=0.24, compared to no treatment) (Fig. 2C). Nevertheless, DCo with Eα peptide (Both) markedly reduced tumor growth (3.5-fold compared to controls, p<0.0001) (Fig. 2B, 2C).
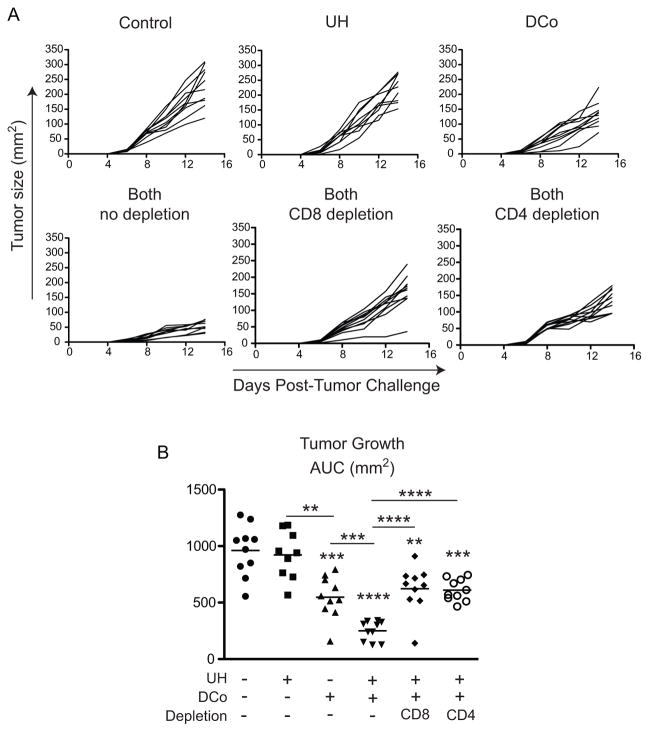
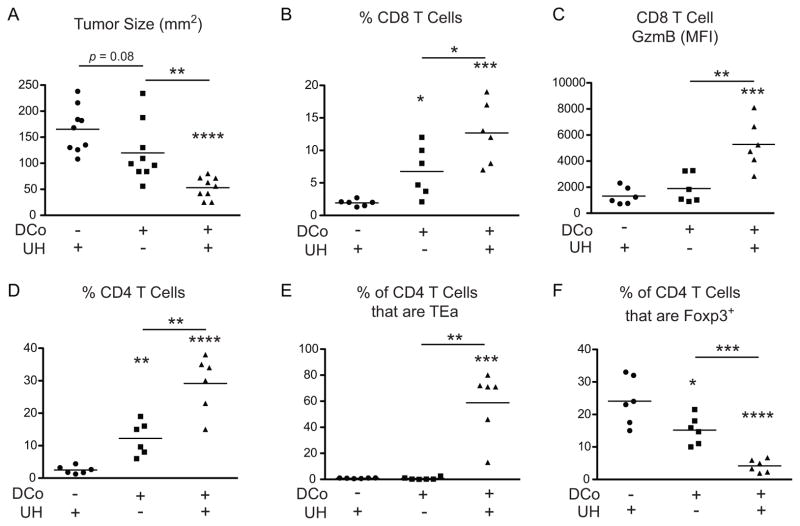
To delineate the tumoricidal T cell subsets helped by the DCo tumor-unrelated TEa CD4 T cells, B16-challenged mice were given TEa CD4 T cells and immunized with Eα peptide and DCo (Both, as in Fig. 2). Three days later the mice were treated with anti-CD4 or -CD8 depleting mAbs (Fig. 3). Depletion of either CD4+ or CD8+ cells accelerated tumor growth (p<0.0001) similar to the rate observed in mice treated with DCo only (Fig. 3A, 3B), suggesting that both CD4 and CD8 T cells provide complementary tumoricidal functions. Consistent with this possibility, in a repeat experiment where UH augmented DCo-mediated tumor growth control (Fig. 4A), the tumors controlled in mice treated with both DCo plus UH exhibited an increased frequency of infiltrating CD8 T cells (Fig. 4B) that expressed greater amounts of GzmB (Fig. 4C) compared to mice treated with DCo only or not given DCo. DCo plus UH also boosted the frequency of intratumoral CD4 T cells (Fig. 4D), which were mostly comprised of the DCo tumor-unrelated TEa CD4 T cells (Fig. 4E), and reciprocally diminished the frequency of CD4+Foxp3+ Tregs (Fig. 4F).
Figure 3.
CD8+ and CD4+ cells are both critical for the DCo therapeutic effect with tumor-unrelated help. B16-challenged mice received TEa CD4 T cells and were treated with Eα peptide (UH) and/or DCo. Mice treated with DCo plus UH (Both) were treated 3 days post-tumor challenge with CD8, CD4 or no depleting antibodies. Tumor growth curves for individual mice (A) and AUC analysis (B). N=9-10 mice per group.
Figure 4.
Intratumoral T cell subsets following treatment with DCo plus tumor-unrelated help (UH). Tumor challenge and treatments were performed as in Figure 2B, and on day 16 tumor sizes were measured and lymphocytes extracted. (A) Tumor size, n=9 mice per group. CD8 T cell frequency (B) and GzmB expression levels (C). Total CD4 T cell frequency (D), the percentage of CD4+ cells that are TEa (E) and the percentage of CD4+ cells that express Foxp3 (F). N=6 mice per group. Similar results were observed in another trial.
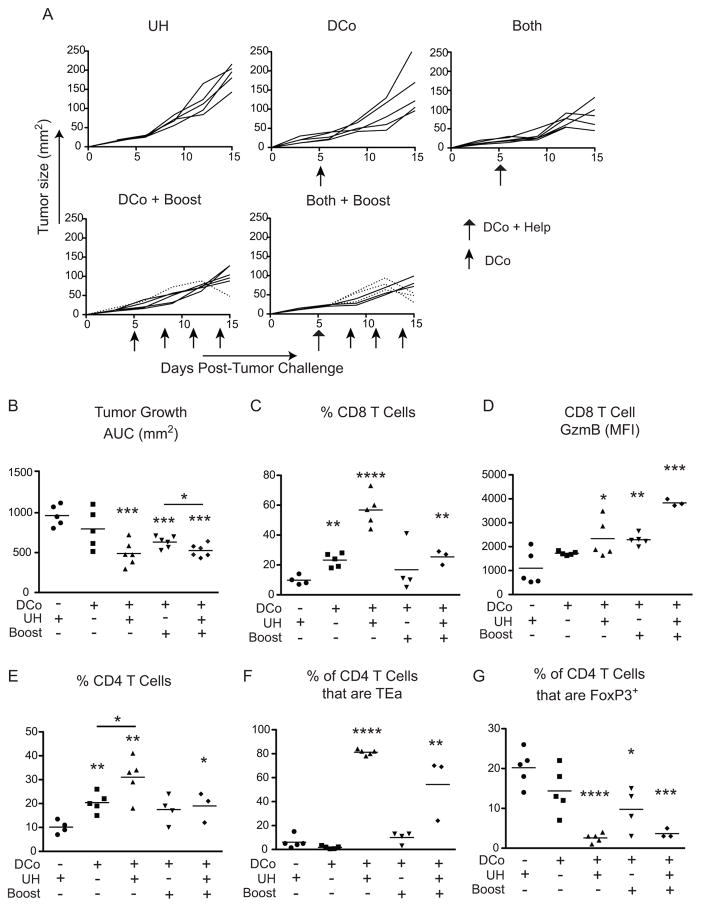
To test the potential of tumor-unrelated CD4 T cell help to augment DCo in a therapeutic scenario, pre-established B16 tumors were allowed to grow for 5 days (reaching an average size of 25-50 mm2) prior to initiating treatment (Fig. 5). Under these stringent conditions a single DCo dose failed to slow tumor growth compared to controls that did not receive DCo, although booster treatments given on days 8, 11 and 14 did reduce tumor growth (DCo + Boost) (p<0.001) (Fig. 5A, 5B). In contrast, engagement of UH enabled a single dose of DCo to slow tumor growth (Both) (p<0.001), and provision of additional DCo boosters (Both + Boost) resulted in tumor regression between days 12 and 15 in 3 out of 6 mice (dotted lines in Fig. 5A). These 3 mice were eventually euthanized on day 30 following resumption of tumor growth (Supplemental Fig. 1). All the other mice were euthanized on day 15 (Fig. 5A). Taken together, engagement of unrelated help substantially augments DCo therapeutic efficacy. Similar to the previous challenge study (Fig. 4), in this therapeutic model DCo with engagement of unrelated help increased the frequency of intratumoral CD8 T cells (Fig. 5C) and their GzmB expression (Fig. 5D). The increase in total (Fig. 5E) and TEa (Fig. 5F) CD4 T cell frequency was also associated with a reciprocally decreased Foxp3+ Treg frequency (Fig. 5G). Although DCo booster treatments did not further increase the frequency of intratumoral Foxp3neg T cells or further decrease CD4+Foxp3+ cells (Fig. 5C, 5F and 5G), they did increase GzmB expression in the CD8 T cells (Fig. 5D). Thus, therapeutic efficacy tracks with increased intratumoral effector T cell function.
Figure 5.
Tumor-unrelated help facilitates DCo-mediated control of established B16 melanomas. Mice harboring established B16 melanomas that received TEa CD4 T cells were treated with DCo, Eα peptide (UH) or DCo plus UH (Both) on day 5, and as indicated (with arrows) given booster DCo treatments (Boost) on days 8, 11 and 14. Intratumoral T cells were analyzed on day 15. Tumor growth curves for individual mice (A) and AUC analysis (B) N=5–6 mice per group. CD8 T cell frequency (C) and GzmB expression (D). Total CD4 T cell frequency (E), the percentage of CD4+ cells that are TEa (F) and the percentage of CD4+ cells that express Foxp3 (G). N=3–5 mice per group.
Delivery of therapeutic help requires CD134-dependent feedback that is extrinsic to the dual-costimulated tumor-unrelated CD4 T cells
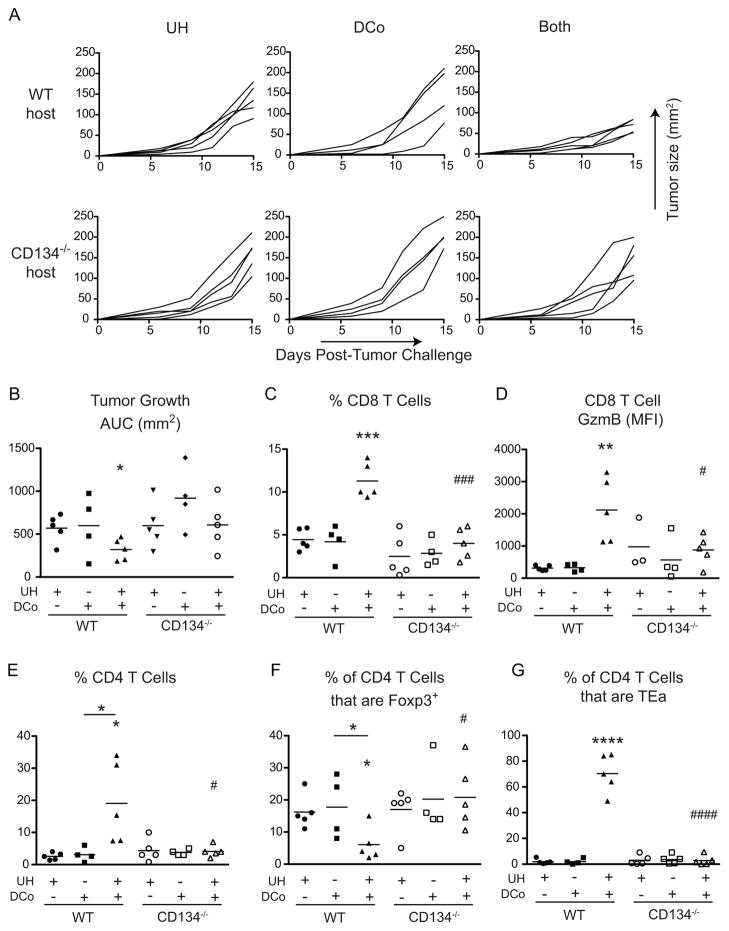
To study the mechanistic basis of how tumor-unrelated CD4+ helpers augment the therapeutic effect of DCo, we first hypothesized that tumor-specific T cells receiving help require direct costimulation. Hence, the potential of DCo plus UH to control established B16 tumors was compared in WT versus CD134−/− mice. As before, DCo plus engagement of WT tumor-unrelated CD4 helper T cells slowed tumor growth in WT recipients (p<0.05) but, importantly, not in CD134−/− counterparts (Fig. 6A, 6B). The reduced intratumoral CD8+ cell frequency (p<0.001, Fig. 6C) and GzmB expression (p<0.05, Fig. 6D) in CD134−/− compared to WT DCo + UH mice indeed suggested that the therapeutic effect might require that the helped-CD8 T cells receive direct CD134 costimulation. DCo + UH also failed to reduce the frequency of intratumoral CD4+Foxp3+ cells in the CD134−/− mice (Fig. 6F). This result was consistent with the known ability of CD134 agonist to deplete intratumoral Tregs (45), and could potentially contribute to the reduction in effector CD8 T cell accumulation (Fig. 6C, 6D). Nevertheless, DCo + UH CD134−/− mice also contained fewer intratumoral TEa helper CD4 T cells (Fig. 6G). This was unexpected given that these tumor-unrelated helper CD4 T cells were WT and thus presumed to express CD134 in response to specific antigen (46). Hence, it was also possible that the responding DCo CD4 T cells, with the CD8 T cells they help, depend on CD134-dependent host cell feedback.
Figure 6.
Tumor-unrelated help does not facilitate DCo-mediated tumor control in CD134−/− mice. WT and CD134−/− mice harboring established B16 melanomas that received WT TEa CD4 T cells were treated on day 5with DCo, UH or DCo plus UH (Both). Analysis of intratumoral T cells was performed on day 15. Tumor growth curves for individual mice (A) and AUC analysis (B). CD8 T cell frequency (C) and GzmB expression (D). Total CD4 T cell frequency (E), the percentage of CD4+ cells that express Foxp3 (F), and the percentage of CD4+ cells that are TEa (G). N=4–5 mice per group.
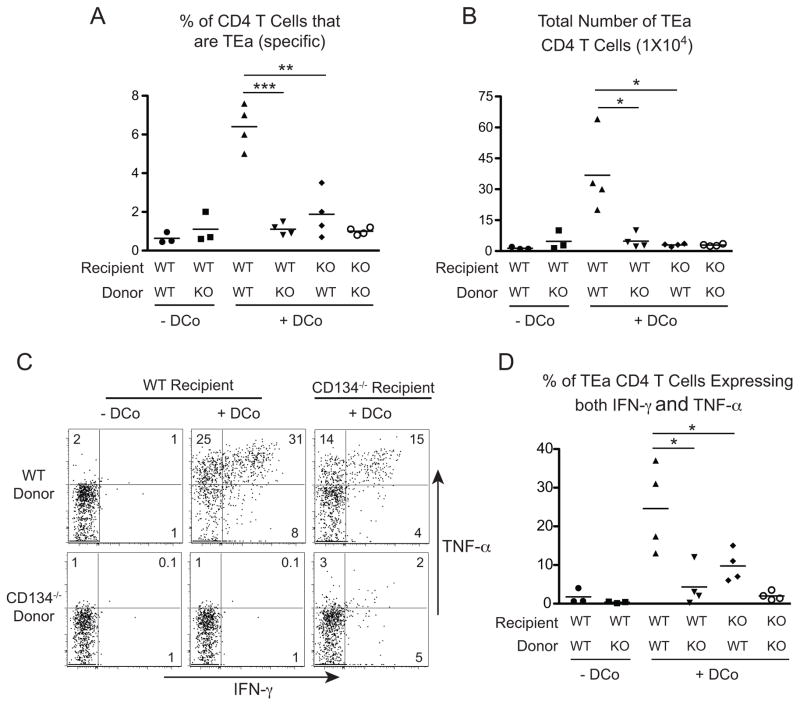
Thus, to directly assess how CD134 programs CD4 helper activity during DCo, the response of WT versus CD134−/− TEa CD4 T cells was compared in WT versus CD134−/− non-tumor-bearing recipients (Fig. 7). Consistent with our previous observation that CD134 plays a more dominant role than CD137 in driving CD4 T cell expansion and effector differentiation (34), CD134−/− TEa CD4 T cells transferred into WT recipients and recovered from spleens exhibited greatly diminished expansion (Fig. 7A, 7B) and capacity to express IFN-γ and TNF-α (Fig. 7C, 7D) compared to WT. Importantly, WT TEa CD4 T cells transferred into CD134−/− recipients also underwent substantially reduced expansion (p<0.05) (Fig. 7A, 7B) and expressed less IFN-γ and TNF-α (p<0.05) (Fig. 7C, 7D) compared to WT → WT controls. Thus, although the transferred CD4 T cells responding to specific antigen and DCo were directly costimulated by CD134 agonist, their expansion and differentiation also required CD134-dependent feedback from the endogenous host cells. This result was similar to the previous observation that CD137 can impact CD8 T cell responses both directly and indirectly (31).
Figure 7.
The response of DCo CD4 T cells requires both direct CD134 costimulation plus an indirect CD134-dependent component. WT and CD134−/− TEa CD4 T cells were transferred into WT or CD134−/− recipients and treated with Eα peptide with or without DCo. Spleens were analyzed on day 5 for TEa cell expansion expressed as both their percentage of the overall CD4 T cell population (A) and total number (B). Representative FACS plots (C) and scatter plot analysis (D) of intracellular IFN-γ versus TNF-α expression in the TEa cells. N=3–4 mice per group, and similar results were observed in two other trials.
Dual-costimulated tumor-unrelated CD4 T cells provide therapeutic benefit beyond the T cell priming phase
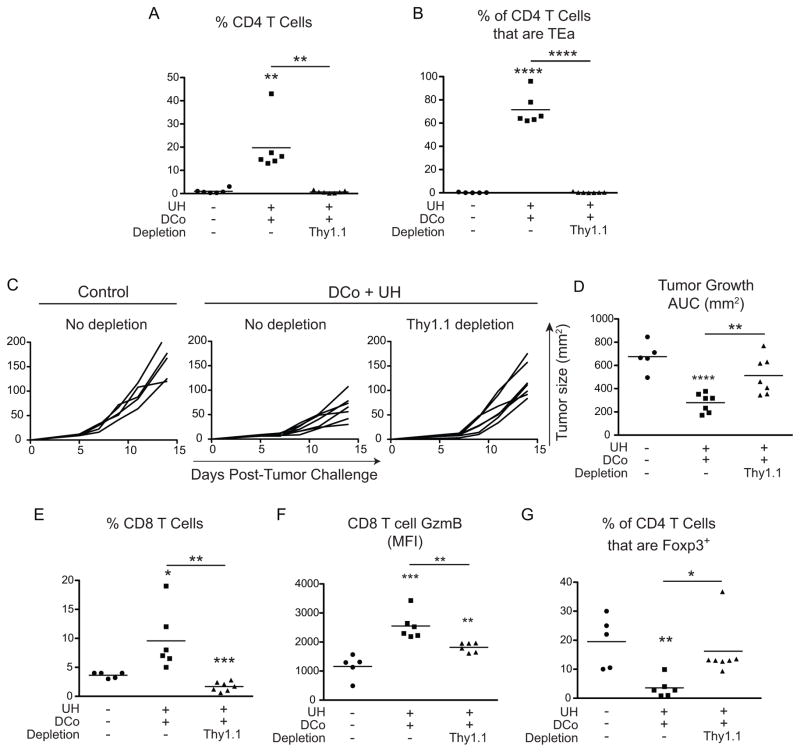
CD8 T cells are essential for controlling tumor growth when tumor-unrelated CD4+ helpers are engaged during DCo. Thus, depletion of CD8+ cells accelerates tumor growth (Fig. 3), and increased frequency of intratumoral GzmB+ CD8 T cells tracks with therapeutic efficacy (Figs. 4–6). CD4 help-mediated augmentation of CD8 T cell responses likely involves initial T cell priming in lymph nodes where both linked-help (35, 36) and non-linked help (34) (Fig. 2A) can be delivered. Nevertheless, DCo tumor-unrelated CD4 helper T cells also accumulated at high frequency within tumors, suggesting that they might also deliver tumoricidal help during the effector phase of the anti-tumor response. To test this possibility, mice with pre-established B16 tumors were treated with DCo plus UH, then 3 days later treated with or without an anti-Thy1.1 mAb to deplete the unrelated Thy1.1+ TEa cells (Fig. 8). At this time point the DCo TEa CD4 T cells have already delivered help to T cells undergoing initial priming in lymphoid organs (34), and thus their depletion should mostly impact the effector phase of the anti-tumor response. As expected, anti-Thy1.1 mAb treatment prevented total (Fig. 8A) and TEa (Fig. 8B) CD4 T cell accumulation within tumors. Importantly, Thy1.1 depletion accelerated tumor growth (Fig. 8C, 8D), diminished accumulation of intratumoral CD8 T cell (Fig. 8E) and their expression of GzmB (Fig. 8F), and prevented the reduction in intratumoral Foxp3+CD4+ cell frequency (Fig. 8G). Taken together, these data are consistent with a role for the DCo tumor-unrelated CD4 T cells in helping CD8+ CTL within the tumor microenvironment.
Figure 8.
DCo tumor-unrelated CD4 T cells provide therapeutic help beyond the initial T cell priming phase. Mice harboring established B16 melanomas that received TEa CD4 T cells were treated with DCo plus UH or rat Ig (Control) and 3 days later treated with or without a Thy1.1 depleting mAb. Intratumoral T cells were analyzed on day 14. Total CD4 T cell frequency (A) and the percentage of CD4+ cells that are TEa (B). Tumor growth curves for individual mice (C) and AUC analysis (D). CD8 T cell frequency (E) and GzmB expression (F). The percentage of CD4+ cells that express Foxp3 (G). N=5–7 mice per group.
Discussion
T cell-based cancer therapies should elicit multi-pronged responses in order to prevent outgrowth of tumor escape variants. An advantage of dual costimulation is that it not only generates potent tumor-reactive CD8+ CTL (30, 31) but also drives cytotoxic CD4 T cell-mediated tumor killing (34). This may be useful in treating tumors such as melanoma that can be induced to express MHC class II (6, 7).
A general issue limiting tumor vaccine efficacy is T cell-intrinsic tolerance mechanisms that limit the pool of tumor-reactive specificities (14, 15). A strategy to bypass tolerant tumor-specific CD4 helper T cells is to add a class II-restricted tumor-unrelated antigen to vaccines comprised of a cocktail of class I-restricted tumor peptides (19). This is based on the ability of CD4 T cells to help CD8 T cells responding to an unrelated antigen so long as both T cells are linked through priming by the same dendritic cell (20–22). Nevertheless, the repertoire of CTL generated is limited by the choice of class I peptides included in the vaccine, and may therefore exclude many therapeutically useful specificities. Dual costimulation, in contrast, does not target pre-determined CD8 T cell specificities, but rather can potentially augment the response of any tumor-reactive T cell. We reasoned that engaging tumor-unrelated CD4 T cells might augment dual costimulation therapy through the combined effects of their ability to provide help in both linked (35, 36) and non-linked (34) (Fig. 2A) manners.
Engagement of tumor-unrelated CD4 T cell help substantially enhanced dual costimulation efficacy in treating established B16 melanomas (Fig. 5). This effect could be mediated through two distinct mechanisms. First, during initial T cell priming in lymph nodes, linked help (35, 36) could boost the response of CD8 T cells responding to dendritic cells cross-presenting abundant tumor epitopes (37), while non-linked help (34) (Fig. 2A) might facilitate CTL function in T cells specific to tumor epitopes that are inefficiently cross-presented due to low abundance (38, 39). Second, dual-costimulated tumor-unrelated CD4 T cells also appear to support CD8+ CTL within tumors (Fig. 8). In a different immunization model CD4 T cells help CD8+ CTL within the tumor microenvironment provided that these CD4+ helpers are tumor-specific (13). This raises the intriguing question of how dual-costimulated tumor-unrelated CD4 T cells are triggered to help CD8+ CTL within tumors. One possibility, based on our recent observation that dual-costimulated CD8 T cells can be triggered in the absence of TCR ligation by IL-12 or IL-2 plus IL-33 to secrete IFN-γ (47), is that cytokines in the tumor microenvironment trigger dual-costimulated CD4 T cells to elaborate helper functions. This help may be delivered to the CD8 T cells through a variety of mechanisms such as DC licensing (5) or secretion of cytokines such as IL-2 (11) that can facilitate TCR-independent triggering of fully differentiated effector CD8 T cells (47). Another question relates to the specificity of the helped CD8 T cells. Thus, some might exert tumoricidal function upon recognizing cognate antigen on the surface of tumor cells, although TCR-independent triggering of intratumoral effector CD8 T cells whose TCR do not recognize tumor antigens may also occur.
An unexpected observation in this study was that dual-costimulated tumor-unrelated CD4 helper T cells require an indirect CD134-dependent component to undergo maximal expansion and effector differentiation (Fig. 7) and deliver therapeutic help (Fig. 6). This suggests that the dual-costimulated CD4 helper T cells are themselves helped. A “help for helpers” mechanism has been described previously where CD4 T cells responding to a strong foreign antigen engage otherwise tolerant tumor-specific CD4 helper T cells (48). Our model, however, is distinct in that a CD4 helper T cell responding to a strong foreign antigen appears to receive help from a cell(s) that expresses CD134 yet is not responding to a strong cognate antigen. Although the identity of this cell(s) is currently unknown, Foxp3+ Tregs represent a plausible candidate in part because they have been shown in other systems to undergo phenotypic reprogramming (49) and acquire helper capacity (50). Further, they also express CD134 constitutively (51), can lose their suppressive capacity in response to CD134 agonist (52) are the only detectable lymphocyte subset in secondary lymphoid organs that express high levels of CD134 during steady state conditions (Supplemental Fig. 2A). Finally, in response to DCo and unrelated help Tregs gain the capacity to express the Tc1/cytotoxic Th1 lineage transcription factor Eomesodermin (34, 53) as well as the effector molecules IFN-γ and GzmB (Supplemental Fig. 2B–F). Regardless of the identity of this helper facilitatory cell, this observation suggests that the dual costimulation helper response is a complex process. Importantly, although the current study defines this feedback mechanism as being CD134-dependent, it might be possible that CD137 is also involved. Thus, apart from antigen-stimulated conventional T cells CD137 is expressed on a variety of other cell types (54), included Foxp3+ Tregs (55).
Dual costimulation possesses powerful anti-tumor activity. This is likely due in part to the individual abilities of CD137 agonist to activate NK cells (56) and CD134 agonist to deplete intratumoral Tregs (45). Additionally, combining the two agonists elicits both cytotoxic CD8 (30) and CD4 (34) tumoricidal T cells. We currently found that engaging tumor-unrelated CD4 T cell help substantially augments the ability of dual costimulation to control an inherently difficult to treat, highly aggressive tumor. This approach has strong potential for human translation. Thus, humanized agonists to both CD134 (57) and CD137 (58) are currently being tested as monotherapies in human cancer patients. Further, it has already been demonstrated that tumor-unrelated CD4 T cell help can be effectively engaged in human patients by adding tetanus toxoid to vaccines containing MHC class I-restricted tumor peptides (19). By taking advantage of the fact that most people have CD4+ memory pools against pathogens they have been vaccinated against (e.g., tetanus), it should be possible to call upon these responses to help fight cancer and perhaps provide a lower risk of autoimmunity since they are directed against foreign entities. In sum, triggering tumor-unrelated CD4 helper T cells with costimulation will likely be both safe and effective in patients suffering from cancer.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants RO1CA109339 and RO1AI094640 (to A.J. Adler and A.T. Vella).
References
- 1.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 2.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci USA. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, Conejo-Garcia JR. CD4+ T cells elicit host immune responses to MHC class II- ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol. 2010;184:5654–5662. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 9.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BWS, Scott B. Tumor-specific CD4+ T cells have a major "post-licensing" role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 11.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207:2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 15.Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, Pierce RA, Restifo NP, Engelhard VH. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J Exp Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stavely-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, White DE, Nathan D, Restifo NP, Steinberg SM, Wunderlich JR, Kammula US, Sherry RM, Yang JC, Phan GQ, Hughes MS, Laurencot CM, Rosenberg SA. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152–2159. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slingluff CL, Jr, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, Kittlesen D, Deacon D, Hibbitts S, Grosh WW, Petroni G, Cohen R, Wiernasz C, Patterson JW, Conway BP, Ross WG. Phase I trial of a melanoma vaccine with gp100(280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 20.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 21.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 22.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell response is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 23.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschalk S, Ng CY, Perez M, Smith CA, Sample C, Brenner MK, Heslop HE, Rooney CM. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 26.Schmollinger JC, Vonderheide RH, Hoar KM, Maecker B, Schultze JL, Hodi FS, Soiffer RJ, Jung K, Kuroda MJ, Letvin NL, Greenfield EA, Mihm M, Kutok JL, Dranoff G. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci USA. 2003;100:3398–3403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, Koslowski M, Kuhn AN, Britten CM, Huber C, Tureci O, Sahin U. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 28.Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, Baker BM, Mandoiu, Srivastava PK. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, Mittler RS, Vella AT. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, Vella AT. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 32.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 33.Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38:2499–2511. doi: 10.1002/eji.200838208. [DOI] [PubMed] [Google Scholar]
- 34.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, Adler AJ. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187:3555–3564. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandyopadhyay S, Long M, Qui HZ, Hagymasi AT, Slaiby AM, Mihalyo MA, Aguila HL, Mittler RS, Vella AT, Adler AJ. Self-Antigen Prevents CD8 T Cell Effector Differentiation by CD134 and CD137 Dual Costimulation. J Immunol. 2008;181:7728–7737. doi: 10.4049/jimmunol.181.11.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St Rose MC, Taylor RA, Bandyopadhyay S, Qui HZ, Hagymasi AT, Vella AT, Adler AJ. CD134/CD137 dual costimulation-elicited IFN-gamma maximizes effector T-cell function but limits Treg expansion. Immunol Cell Biol. 2013;91:173–183. doi: 10.1038/icb.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 38.Kurts C, Miller JFAP, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes "ignorance" to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 40.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 42.Pippig SD, Pena-Rossi C, Long J, Godfrey WR, Fowell DJ, Reiner SL, Birkeland ML, Locksley RM, Barclay AN, Killeen N. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–6529. [PubMed] [Google Scholar]
- 43.Duan F, Simeone S, Wu R, Grady J, Mandoiu I, Srivastava PK. Area under the curve as a tool to measure kinetics of tumor growth in experimental animals. J Immunol Meth. 2012;382:224–228. doi: 10.1016/j.jim.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 46.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 47.Ngoi SM, St Rose MC, Menoret AM, Smith DE, Tovey MG, Adler AJ, Vella AT. Presensitizing with a Toll-like receptor 3 ligand impairs CD8 T-cell effector differentiation and IL-33 responsiveness. Proc Natl Acad Sci USA. 2012;109:10486–10491. doi: 10.1073/pnas.1202607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanetti M. T for two: when helpers need help. Autoimmun Rev. 2005;4:571–578. doi: 10.1016/j.autrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, Blazar BR, Mellor AL, Munn DH. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 52.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Chang Li X. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 54.Kwon B. Regulation of Inflammation by Bidirectional Signaling through CD137 and Its Ligand. Immune Netw. 2012;12:176–180. doi: 10.4110/in.2012.12.5.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang P, Gao F, Wang Q, Wang X, Zhu F, Ma C, Sun W, Zhang L. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol. 2007;66:435–440. doi: 10.1111/j.1365-3083.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 56.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol Rev. 2011;244:218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.