Abstract
To prevent potentially damaging inflammatory responses, the eye actively promotes local immune tolerance via a variety of mechanisms. Due to trauma, infection, or other ongoing autoimmunity, these mechanisms sometimes fail, and an autoimmune disorder may develop in the eye. In mice of the C57BL/10 (B10) background, autoimmune keratitis often develops spontaneously, particularly in the females. Its incidence is greatly elevated in the absence of γδ T cells, such that about 80% of female B10.TCRδ−/− mice develop keratitis by 18 weeks of age. Here, we show that CD8+ αβ T cells are the drivers of this disease, because adoptive transfer of CD8+ but not CD4+ T cells to keratitis-resistant B10.TCRβ/δ−/− hosts induced a high incidence of keratitis. This was unexpected because in other autoimmune diseases, more often CD4+ αβ T cells, or both CD4+ and CD8+ αβ T cells, mediate the disease. Compared to wildtype B10 mice, B10.TCRδ−/− mice also show increased percentages of peripheral memory phenotype CD8+ αβ T cells, along with an elevated frequency of CD8+ αβ T cells biased to produce inflammatory cytokines. B10.TCRδ−/− mice in addition have fewer peripheral CD4+ CD25+ FoxP3+ regulatory αβ T cells (Tregs), which express lower levels of receptors needed for Treg development and function. Together, these observations suggest that in B10 background mice, γδ T cells are required to generate adequate numbers of CD4+ CD25+ FoxP3+ Tregs, and that in B10.TCRδ−/− mice a Treg deficiency allows dysregulated effector or memory CD8+ αβ T cells to infiltrate the cornea and provoke an autoimmune attack.
Keywords: Gamma delta T cells, keratitis, Tregs, CD8 memory cells, CD4+ CD25+ FoxP3+ T cells, ocular immune tolerance, keratitis
Introduction
Although immune responses are beneficial in clearing the host of infectious microbes, they also can cause collateral tissue damage that threatens the function of organs, and in the eye, a vigorous immune response can jeopardize the vision of the host. For this reason, immune responses in the eye are dampened by a variety of different mechanisms that collectively are referred to as ocular immune tolerance. γδ T cells have been shown in several experimental systems to be important for the development of ocular immune tolerance. They are necessary for the phenomenon of ACAID (Anterior Chamber-Associated Immune Deviation), in which antigen initially introduced into the anterior chamber of the eye induces systemic immune tolerance, since both TCRδ−/− mice and mice treated with an antibody that blocks the γδ TCR failed to develop ACAID (1, 2). As well, corneal transplant allografts, which due to ocular immune privilege are generally accepted even when they originate from histo-incompatible donors, were rejected in mice pre-treated with anti-γδ TCR antibody (1). However, γδ T cells conversely can play a role in immune protection in the eye; in mice infected with herpes simplex virus-1 by an ocular route, γδ T cells were able to reduce epithelial lesions and prevent the subsequent development of lethal viral encephalitis (3). A series of recent reports indicated that γδ T cells that reside in the eye in fact walk a fine line between promoting inflammation and enhancing healing (4–6). These γδ T cells, which make up >90% of the T cells normally present in the limbal epithelium of the eye, clearly mediate wound healing but also promote neutrophil infiltration and subsequent platelet deposition (4). Despite the “proinflammatory” potential of the γδ T cells infiltrating the wounded cornea, which are CCR6+ and IL-17+ (5), inflammatory damage appears to be mitigated by their co-production of IL-22 (6). This cytokine, which like IL-17 mobilizes neutrophils, also protects against inflammatory tissue destruction by promoting regeneration of damaged epithelia and stimulating epithelial cells to secrete anti-microbial peptides (7). Importantly, topical treatment of the cornea with recombinant IL-22 functionally replaced the γδ T cells in their ability to promote corneal wound healing (6). Thus, a variety of experimental models revealed that γδ T cell are critical players during immune responses in the eye.
Likewise, many papers have been published regarding regulatory T cells that are important in maintaining ocular immune tolerance and are needed for the development of ACAID. In additional to γδ T cells (1, 2), at least two distinct αβ TCR+ regulatory subsets, one CD4+ and one CD8+, have long been recognized (8, 9). Whether or not the CD4+ regulatory cells in ACAID represent classical adaptive CD4+ CD25+ Foxp3+ Tregs is somewhat controversial, because although they were found to be CD25+ IL-10-producers in one study (10), in another, the CD4+ regulatory cells appeared to be able to develop from CD25− precursors (11). However, CD4+ CD25+ FoxP3+ IL-10-producing Tregs with suppressive capacity clearly increase in the spleens of mice with ACAID (12), and appear to be part of the process that brings about ocular immune tolerance. In one study, γδ T cells appeared to be needed to produce the IL-10 needed for generation of CD8+ regulatory cells that help to bring about ACAID (13). γδ T cells have also been found to be promote the development of autoimmune uveitis by enhancing the activation of uveitogenic αβ T cells (14, 15).
We noted in previous studies that spontaneous keratitis develops at a high rate in female C57BL/10 (B10) mice that lack γδ T cells (B10.TCRδ−/− mice), implying that γδ T cells normally promote immune regulation in the cornea of B10 mice (16, 17). Approximately ~80% of female B10.TCRδ−/− mice show overt keratitis by 18 weeks of age, whereas the rate among males of this age is only ~15%. Despite the large difference between genders, we found no evidence that female hormones promote keratitis (17), or that male hormones protect against it (16). αβ T cells in contrast promoted the development of the disease, suggesting that it is autoimmune-mediated, whereas adoptive transfer of wildtype γδ T cells into B10.TCRδ−/− hosts decreased its incidence (16). We failed to detect any infectious component contributing to the disease, and rederived B10.TCRδ−/− mice developed keratitis at a rate similar to that of the original strain, supporting the interpretation that the disease arises from an autoimmune attack (16). B cells do not appear to contribute to the development of keratitis (17). However, the disease can be adoptively transferred to keratitis-resistant B10.TCRβ/δ−/− hosts by αβ T cells from keratitic B10.TCRδ−/− donors (16, 17). Overall, our previous work suggested that B10 wildtype mice rarely develop spontaneous keratitis because autoaggressive αβ T cells specific for corneal antigens are either suppressed or fail to develop in γδ T cell-sufficient animals.
In the current study, we therefore investigated whether the absence of γδ T cells leads to disruptions in peripheral immune cells that might explain the tendency of B10.TCRδ−/− mice to develop keratitis. Our findings indicate that CD4+ CD25+ FoxP3+ regulatory αβ T cells in B10.TCRδ−/− mice are deficient, due to either their decreased numbers and/or function, and suggest that this deficiency leads to dysregulated responses by CD8+ effector αβ T cells, which then infiltrate and attack the cornea. Thus, γδ T cells are required for fully functional T cell regulation in this system.
Materials and Methods
Mice
C57Bl/10 J (B10) mice were originally obtained from the Jackson Laboratory and thereafter maintained in our facility. All mice were housed under SPF conditions. B10.TCRδ−/− and B10.TCRβ/δ−/− mice were established in our facility as previously described (16). All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Keratitis Scoring
Briefly, mice were examined once per week and each eye scored for disease severity, on a scale of 0 (no keratitis) to 5 (very severe keratitis), as has been previously described (16). Scores for each eye were added together for a total score; the maximum possible disease score for a given mouse was therefore 10.
Corneal cell preparation
After cutting eyes in half and removing the lens, corneas were placed in a small amount of BSS (18) under a dissecting microscope, and dissected away from other eye tissue using a scalpel. Corneas were then placed in BSS containing 0.25 mg/ml Liberase DH (Roche Diagnostics Corp., Indianapolis, IN), and incubated on a rotator at 37°C for 1–2 hours. When the corneal structure appeared to have largely disintegrated, cells were diluted by adding 5–10 volumes of BSS + 5% FCS, and then passed through a 70 μm cell strainer (Becton Dickinson, Franklin Lakes, NJ) to remove any debris or large aggregates remaining. The cells were then pelleted by centrifugation at 1200 rpm for 8 minutes, and used in staining experiments.
Flow cytometry
See Supplemental Table 1 for a list of monoclonal antibodies used in this study. To block non-specific binding via Fc receptors, cells were pre-treated with 10 μg/ml of monoclonal antibody 2.4G2 (19) for 15 minutes at 4°C before staining. Cell surface staining was carried out as previously described (20). For intracellular cytokine staining, cells were first activated for 4–6 hours with PMA/ionomycin in the presence of Brefeldin A, as previously described (21); after activation, cells were washed and surface stained, and then fixed, permeabilized, and stained for intracellular cytokines. Lymphocytes were gated as previously described (22). Flow cytometry data were analyzed using FlowJo 9.3.1 for Mac software (Tree Star, Inc., Ashland, OR).
Adoptive transfer
For CD8+ adoptive transfers, cells were purified from the spleens of B10.TCRδ−/− or B10 donor females; spleens were dissociated in BSS + 5% FCS using a stainless steel screen mesh, the cells then placed on ice and the debris allowed to out settle out for a few minutes, and the cells then pelleted by centrifugation. After lysis of red blood cells using Gey’s solution, the cells were washed and resuspended in BSS + 5% FCS, then passed over nylon wool columns as previously described (23). The nonadherent cells were then blocked by incubation with an antibody against FcR II/III [clone 2.4G2 (19)] at 10 μg/ml for 15 minutes at 4°C, then washed one time, followed by treatment with biotinylated anti-CD8α or biotinylated anti-CD8β mAb, as indicated in the figures, for 15 minutes at 4°C. After one wash, the cells were next incubated with MACS streptavidin microbeads (Miltenyi Biotec Inc., San Diego, CA), and passed over a MACS MS minicolumn mounted on a magnet, according to the manufacturer’s instructions, to purify the positive cells. In some experiments, after removing the column from the magnetic field to elute the bound cells, they were passed again over a second MS column mounted on a magnet, then removed from the magnetic field and the bound cells again eluted, to improve the purity of the cells. The purity of the final preparations was verified by flow cytometric analysis using streptavidin-APC and anti-TCRβ-PE mAbs; in all experiments shown, 90–95% of the purified lymphocytes were CD8+ T cells. In some experiments, CD8β+ spleen cells alternatively were stained with fluorescently labeled anti-CD8β μAb and purified using a MoFlo flow cytometer. Cells to be transferred were resuspended in BSS + 5% FCS, or BSS only, and kept on ice until ready to be injected. CD8+ cells, 1–10 × 10^5/mouse, were transferred into 7–10 weeks old B10.TCRβ/δ−/− females by i.v. injection via the tail vein, in a volume of 0.1 cc; sham-treated controls were injected at the same time with an equal volume of BSS + 5% FCS, or BSS only. Mice were afterwards scored weekly for keratitis for the period of time indicated in individual figures.
CD4+ T cell adoptive transfers were carried out in a similar fashion as the CD8+ T cell adoptive transfers, but using instead a biotinylated anti-CD4 mAb plus MACS streptavidin beads, passing the cells twice over MACS MS minicolumns to improve purity. The purity of the final preparations was verified by flow cytometric analysis using streptavidin-APC and anti-TCRβ-PE mAbs; in all experiments shown, at least 90% of the cells were CD4+ T cells. Cells to be transferred were resuspended in BSS + 5% FCS and kept on ice until ready to be injected. CD4+ cells, 1.9–3.8 × 10^5/mouse, were transferred into 6–11 week old B10.TCRβ/δ−/− females by i.v. injection; sham-treated controls received the same volume of BSS + 5% FCS.
For Treg adoptive transfers, CD4+ cells were purified from the spleens of B10 female donors by staining with anti-CD4-FITC or -eFluor450, together with biotinylated anti-CD25 plus streptavidin-APC, using a MoFlo flow cytometer. The degree of purity of the sorted cells was ascertained by post-sort analysis of the cells; preparations were 95% pure or better in all experiments. 0.5 – 0.9 × 10^6 cells per mouse, or an equal volume of BSS only for sham-treated controls, was injected into each host by i.v. injection as described above, 6 days prior to CD8+ T cell adoptive transfer. B10.TCRβ/δ−/− females at 6–9 weeks of age were used as recipients. Treg adoptively transferred mice, or sham-treated controls, received purified CD8+ cells from B10.TCRδ−/− female donors 6 days later. The CD8+ cells were prepared and administered as described above using a flow cytometer; approximately 1 × 10^6 cells per mouse were transferred. Mice were scored weekly for keratitis for the time periods shown in Fig. 4.
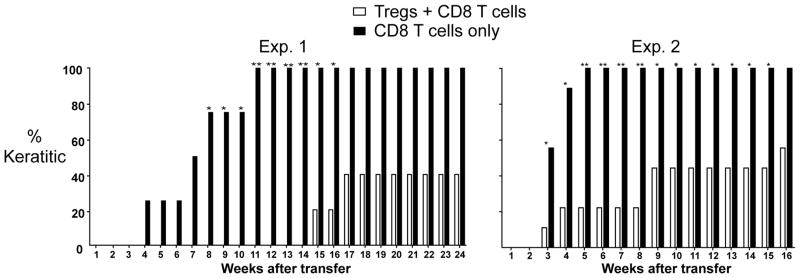
Fig. 4. CD4+ Tregs reduce adoptive transfer keratitis.
B10.TCRβ/δ−/− recipients females were pre-treated with Tregs from B10 female donors, or with cell diluent only, and received 6 days later an adoptive transfer of CD8+ cells from B10.TCRδ−/− donors. Results from two independent experiments are shown. All mice received 1 × 10^6 CD8+ cells in both experiments, but the Treg treated mice received 0.9 × 10 ^6 Tregs in exp. 1 vs. 0.5 × 10^6 Tregs in exp. 2. Disease incidence is shown as the percent of mice in each group with keratitis each week following adoptive transfer of CD8+ cells. Experiment 1 had 5 recipient mice in the Tregs + CD8 T cells-treated group and 4 recipient mice in the CD8 T cells only-treated group; experiment 2 had 9 recipient mice in each group. Errors bars indicate SEM.
Statistical Analysis
GraphPad Prism 6 software (San Diego, CA) was used to calculate p-values for comparison of two groups. To compare percentages of cell types or total cell numbers, 2-tailed unpaired t-tests were carried out, assuming parametric data. For comparing the incidence of keratitis between two groups, results of a 2 × 2 contingency table were analyzed using a two-tailed Fisher’s exact test. For comparing average keratitis scores (which are assigned ranks rather than quantitative values), differences were analyzed with a nonparametric one-tailed Wilcoxon rank sum or Mann-Whitney U test. The following symbols used in the figures correspond to p-values as shown: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p <0.0001.
Results
CD8+ but not CD4+ αβ T cells are sufficient to transfer the disease
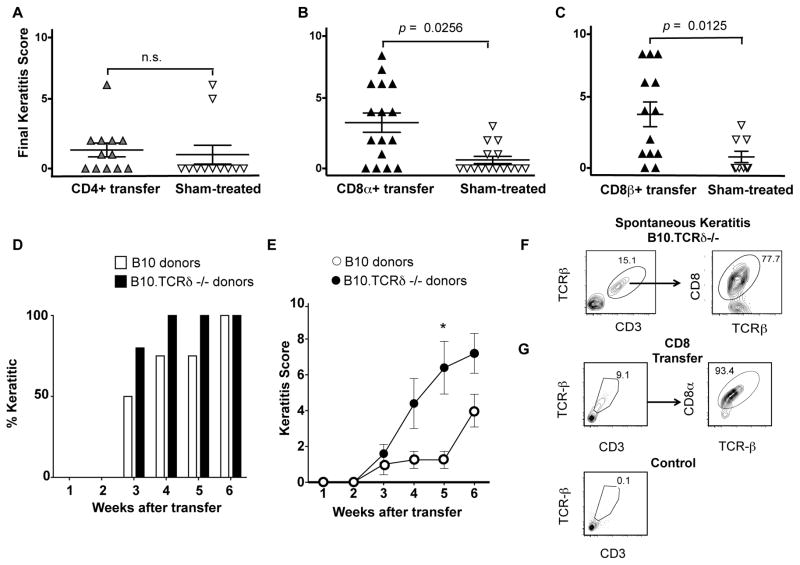
In a previous study, we showed that the incidence of keratitis in B10.TCRβ/δ−/− females, which are able to produce neither αβ nor γδ T cells and normally have a low incidence of keratitis [~20% develop keratitis by 18 weeks of age (16)], is increased following adoptive transfer of the mice with αβ T cells from the spleens of keratitic B10/TCRδ−/− females (17). We therefore went on to compare CD4+ αβ T cells and CD8+ αβ T cells for their ability to transfer the disease. We found that whereas the transfer of CD4+ cells from keratitic B10.TCRδ−/− donors had little or no effect (Fig. 1A), both the incidence and severity of keratitis were markedly increased following adoptive transfer of CD8+ cells (Fig. 1B). We used a CD8α-specific mAb to purify the donor cells from the spleen, and although our cell preparations contained at least 95% CD3+ TCRβ+ CD8α+ T cells, other CD8α+ cells, including a subset of dendritic cells (24), were likely present at low levels. Because such contaminating cells might instead or as well be needed to transfer the disease, we repeated this experiment using an anti-CD8β mAb to purify CD8+ TCR-αβ+ T cells, which express CD8αβ heterodimers (24), from the B10.TCRδ−/− donors, and obtained essentially the same results (Fig. 1C). Thus, CD8+ αβ T cells from B10.TCRδ−/− donors are sufficient to induce keratitis in B10.TCRβ/δ−/− female hosts.
Fig. 1. CD8+ T cells transfer keratitis.
For A, B, and C, each symbol represents the final score obtained for an individual recipient mouse; error bars show SEM. CD4+ or CD8+ cells were enriched to 90–95% purity by passing them twice over MACS columns (see Methods), or for some mice in C, by flow cytometry. A. B10.TCRβ/δ−/− female mice, 6–10 weeks old, were adoptively transferred with CD4+ cells from keratitic B10.TCRδ−/− female donors, then scored weekly for keratitis until 18–19 week old. Sham-treated controls received the same volume of cell diluent only. B. As for A, except that the mice received CD8α+ cells from keratitic B10.TCRδ−/− female donors, or were sham-treated with cell diluent. C. As for B, except that the mice received CD8β+ cells from keratitic B10.TCRδ−/− female donors, or were sham-treated with cell diluent. D. B10.TCRβ/δ−/− female mice were adoptively transferred with CD8α+ cells from either keratitic B10.TCRδ−/− female donors (4 recipients) or wildtype B10 females (5 recipients). Note that the number of CD8+ T cells adoptively transferred per mouse in this experiment (~1 × 10^6 cells/recipient) was about ten times higher than in experiments shown in B and C; this results in more rapid disease onset than using lower numbers (data not shown). The percent of mice that developed keratitis at each timepoint shown is indicated for both groups. E. The mean values of keratitis scores obtained for the same mice tested in D; error bars show SEM. F. Example flow cytometric profiles of cells prepared from pooled corneas following digestion with Liberase, obtained from B10.TCRδ−/− keratitic females. G. Example flow cytometry profiles of cells prepared from pooled corneal digests from either B10.TCRβ/δ−/− keratitic females that had been adoptively transferred with CD8α+ cells from B10.TCRδ−/− female donors 3 weeks previously, or from healthy untreated B10.TCRβ/δ−/− females.
Although most are disease-resistant, a low percentage (~10%) of wildtype B10 female mice develop keratitis as well, but the disease usually remains very mild. Unlike in B10.TCRδ−/− mice, the disease in B10 mice appears at a relatively young age (by 7 weeks) and does not become more common with increasing age (16). We therefore wondered whether pathogenic CD8+ αβ T cells can only develop in mice lacking γδ T cells, or instead are also present in mature wildtype mice but are less likely to cause disease in that setting. To test this, we adoptively transferred into B10.TCRβ/δ̃ female hosts purified CD8+ T cells from B10 wildtype female donors, and compared them to hosts that instead received an equivalent dose of CD8+ cells from B10.TCRδ−/− female donors. Although the disease developed somewhat slower in mice that received B10-derived cells, all mice in both groups developed keratitis within 6 weeks after transfer (Fig. 1D). The severity of the disease was diminished in recipients that were given B10 CD8+ cells compared to those given B10.TCRδ−/− CD8 cells (Fig. 1E). This result suggests that pathogenic CD8+ T cells do not require the absence of γδ T cells in order to develop, although a consequence of the absence of γδ T cells can be that pathogenic CD8+ αβ T cells are able to expand and/or become more active, increasing the chance of an autoimmune attack on the cornea.
CD8+ T cells infiltrate the corneas of keratitic mice
The eye is an immune-privileged site, due in part to a lack of blood vessels in the cornea (a component of the blood/ocular barrier) which makes it unlikely that lymphocytes come into contact with corneal antigens (25). A lack of the γδ T cells that normally reside in the limbus surrounding the cornea could make this site more penetrable to blood-borne lymphocytes in B10.TCRδ−/− mice, including auto-aggressive CD8+ αβ T cells which, if specific for corneal antigens, might then become locally activated and able to induce inflammation and/or corneal cell damage. To test whether CD8+ αβ T cells are present in keratitic lesions, we dissected out corneas, digested them with Liberase to release the infiltrating cells, and stained the released cells with antibodies against the αβ TCR and CD8. We found that most of the T cells in the corneas of B10.TCRδ−/− mice with spontaneous keratitis are CD8+ (Fig. 1F), although CD8+ T cells are normally less abundant than CD4+ T cells in the peripheral lymphoid organs. We were also readily able to detect CD8+ T cells in the corneas of keratitic B10.TCRβ/δ−/− hosts following adoptive transfer with CD8+ T cells; these were not present in the corneas of healthy untreated control B10.TCRβ/δ females (Fig. 1G). Therefore, in both systems, the CD8+ T cells could be inducing keratitis by a direct cytolytic attack on corneal stromal cells, and/or by local cytokine release, eliciting corneal inflammatory damage by activating innate immune cells.
The frequency of CD8 memory T cells is elevated in B10.TCRδ−/− mice
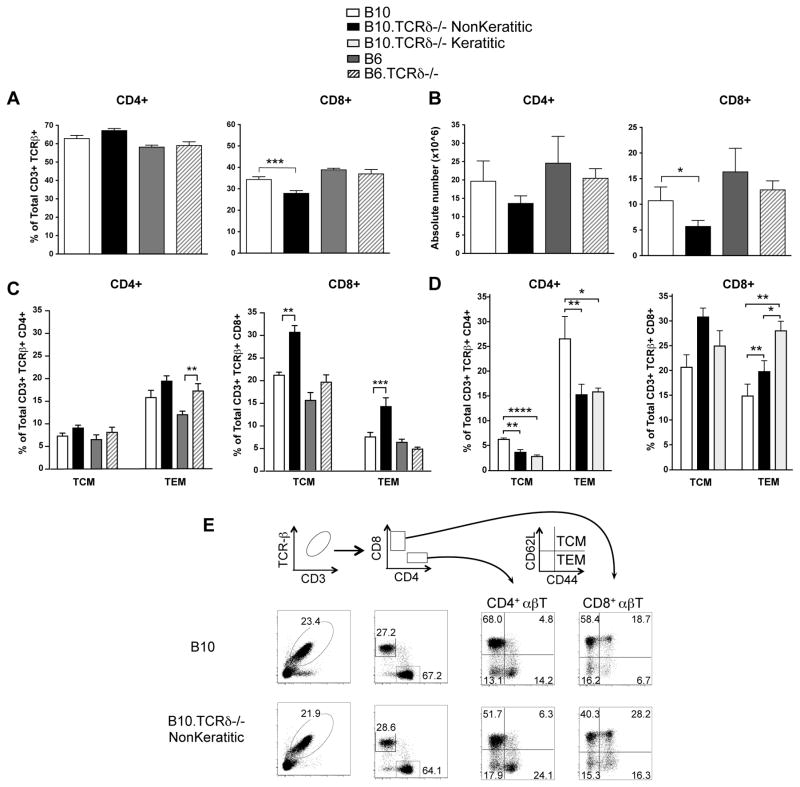
Because splenic CD8+ T cells from B10.TCRδ−/− mice were able to transfer disease more effectively, we went on to examine by flow cytometry whether splenic T cells in B10.TCRδ−/− females differed from those in wildtype B10 females. Although the percent and number of CD4+ αβ T cells was similar in both, we found to our surprise a small but significantly lower percentage (Fig. 2A) and absolute number (Fig. 2B) of CD8+ αβ T cells in B10.TCRδ−/− mice, even at a very young age (5 weeks old), before keratitis generally develops. This was not the case in B6.TCRδ−/− mice (which do not develop keratitis) when compared to B6 wildtype controls. We also stained the same T cells with anti-CD44 and anti-CD62L mAbs to compare T cells having a central memory (TCM, CD44-high CD62L-high) or effector memory phenotype (TEM, CD44-high CD62L-low) in these mice (26). A small difference between CD4+ TEM cells in female B10.TCRδ−/− mice vs. female B10 controls was seen but this was not statistically significant (Fig. 2C, left), and because a similar difference was apparent in keratitis-resistant B6.TCRδ−/− females compared to B6 female controls, this finding did not suggest a potential pathologic effect. However, despite the decrease in total CD8+ αβ T cells, a substantial increase in the relative frequency of both CD8+ TCM and CD8+ TEM was evident in female B10.TCRδ−/− mice compared to B10 age- and sex-matched controls (Fig. 2C, right). Interestingly, these differences were not evident in B6.TCRδ−/− females compared to matched B6 females, although similar differences in CD8 memory phenotype cells were found in B10.TCRδ−/− male mice of the same age compared to matched B10 male controls (see Supp. Fig. 1A). The higher frequency of memory phenotype CD8+ T cells in keratitis-susceptible B10.TCRδ−/− mice, despite an overall decrease in CD8+ T cell numbers in this strain compared to B10 controls, is even more striking in light of the fact that such differences were already clear when the mice were only 5 weeks old, at which time almost none have developed keratitis. We therefore went on to test how the TCM and TEM αβ T cells in B10 vs. B10.TCRδ−/− mice compared in older animals in which keratitis either had or had not developed. As can be seen (Fig. 2D), the frequency of CD8+ TEM cells was higher in older keratitic B10.TCRδ−/− females compared to that in matched nonkeratitic females of the same strain. Although in the older B10 mice, the frequency of CD8+ TEM cells had increased substantially compared to that of 5 week old B10 females, the difference in CD8+ TEM cells between B10 and B10.TCRδ−/− females, particularly those with keratitis, was still nearly 2-fold. Moreover, a substantial decrease in both CD4+ TCM and TEM cells was also now clear in the older animals. These findings suggest that the absence of γδ T cells favors the development or survival of CD8 memory phenotype T cells, a condition that might predispose the mice to autoimmune attack of the cornea.
Fig. 2. CD8 memory phenotype cells are increased in B10.TCRδ−/− mice.
Spleen cells from five week-old female mice were analyzed by flow cytometry. Each column shows the mean value obtained from multiple mice; errors bars indicate SEM. A. After gating on CD3+ TCRβ+ cells, the proportion expressing CD4 or CD8 was determined, using 4 mice per group. B. Using the proportions obtained in A, the number of CD4+ and CD8+ spleen cells in each mouse was calculated; the average obtained from 4 individual mice is shown. C. After gating on CD3+ TCRβ+ CD4+ spleen cells (left), or CD3+ TCRβ+ CD8+ spleen cells (right), the proportion having the T effector memory phenotype (TEM) was determined as those cells staining brightly with anti-CD44 and dimly with anti-CD62L. The proportion having the T central memory cell phenotype was determined as those cells staining brightly for both CD44 and CD62L. For each group, data were averaged from 3–6 five week-old mice. D. As for C, except that 6–10 females aged 18–30 weeks were used to calculate averages. E. Representative flow cytometry plots of samples analyzed in A and B, including the gating strategy used to test for memory phenotype αβ T cells.
An increased percentage of CD8+ T cells in B10.TCRδ−/− mice is biased to produce inflammatory cytokines
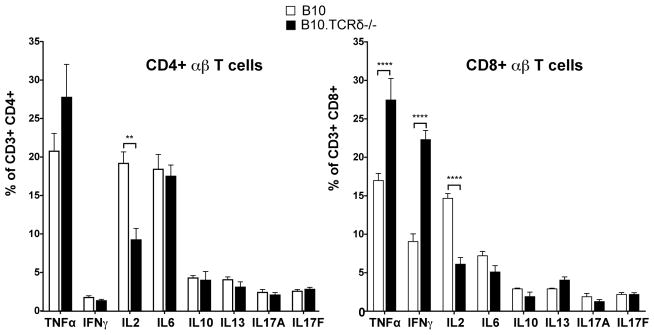
We went on to look at the cytokines produced by CD8+ T cells in B10.TCRδ−/− females, and found that an elevated percentage produce IFNγ and TNFα when non-specifically stimulated, compared to matched B10 controls (Fig. 3). In contrast, the percentage producing these two cytokines was not different in the CD4+ T cells from B10.TCRδ−/− mice vs. those from B10. This is consistent with the idea that it is the CD8+ T cells that promote keratitis in B10.TCRδ−/− mice. Interestingly, among both CD8+ and CD4+ cells from B10.TCRδ−/− mice, there was also a greater than two-fold reduction in the percentage biased to produce IL-2, compared to the same cells in B10 controls (Fig. 3, left). Although IL-2 can boost pro-inflammatory effector T cells and is required for their responses, IL-2 is also essential for the development of adequate numbers of regulatory T cells, including CD4+ CD25+ FoxP3+ T cells (Tregs; (27)). Thus, the low percentage of αβ T cells in B10.TCRδ−/− mice that are able to produce IL-2 could lead to a deficiency in Tregs in these mice.
Fig. 3. In B10.TCRδ−/− mice, the frequency of CD8+ T cells producing inflammatory cytokines is elevated.
Spleen cells were stimulated with PMA/ionomycin for 4 hours at 37°C in the presence of Brefeldin A. After surface staining with anti-CD3, anti-TCR-β, anti-CD8, and anti-CD4 mAbs, cells were fixed and permeabilized, and then stained with the indicated anti-cytokine mAb. The percent of CD3+ TCR-β+ CD8+ or CD3+ TCR-β+ CD4+ cells staining with each anti-cytokine mAb was determined by flow cytometric analysis. Means obtained from 4 five week-old female mice per group are shown; error bars indicate SEM.
Tregs can reduce CD8 T cell-transferred keratitis
To test whether inadequate Treg numbers could explain the susceptibility to keratitis in B10.TCRδ−/− females, we examined whether B10.TCRβ/δ−/− mice that had been adoptively transferred with CD8+ cells from B10.TCRδ−/− donors were less susceptible to keratitis if they were also given αβ T cells enriched in Tregs (CD4+ CD25+ cells) from wildtype B10 donors. Because CD4+ T cells compete poorly with CD8+ T cells in terms of homeostatic expansion following adoptive transfer (28), we transferred the CD4+ Tregs several days before the keratitogenic CD8+ T cells. As shown in Fig. 4, we found that mice that also received Tregs showed a delayed onset of the disease and reduced disease incidence. This was true for mice that initially received nearly as many CD4+ Tregs in the initial transfer as they did CD8+ T cells in the second transfer (Fig. 4, left), as well as for mice that received only half as many CD4+ Tregs as CD8+ T cells (Fig. 4, right), although the inhibitory effect with the lower number of CD4+ Tregs was weaker. These experiments show that a deficiency in CD4+ Tregs, as was seen in B10.TCRδ−/− females compared to B10 females, could well explain their higher susceptibility to spontaneous keratitis.
Tregs are reduced in B10.TCRδ−/− mice
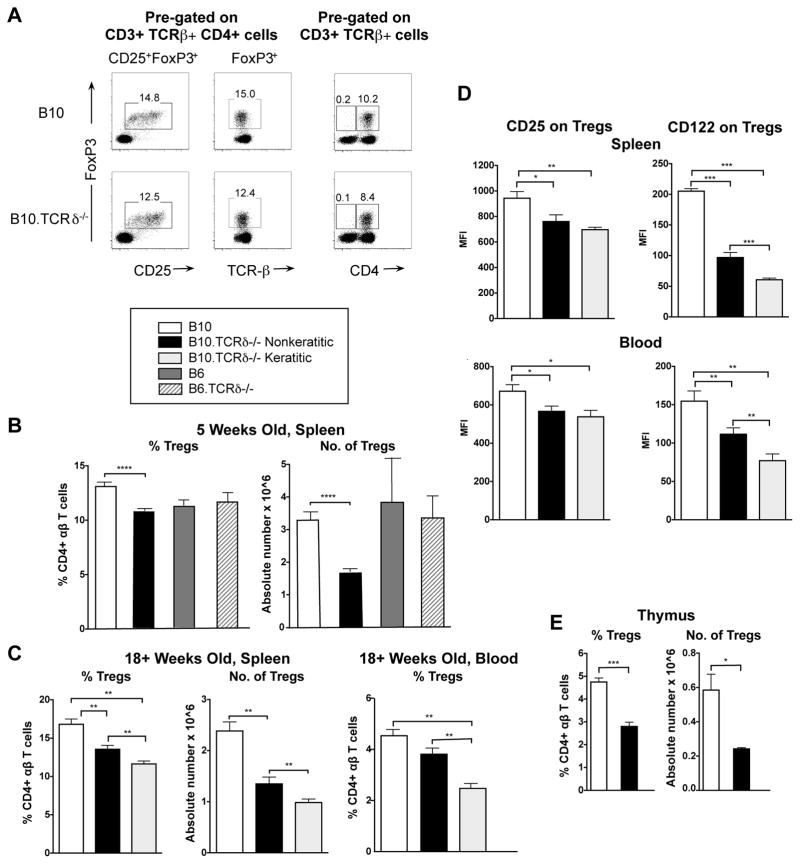
We went on to examine regulatory T cells present in B10 vs. B10.TCRδ−/− females by staining cells for intracellular expression of FoxP3. As shown in Fig. 5A, we found that virtually all FoxP3+ cells in both strains are also both CD4+ and CD25+, and therefore have the phenotype typical of classical natural Tregs (29). CD8+ FoxP3+ cells were very rare in both strains. However, even at 5 weeks of age, in the B10.TCRδ−/− females, these Tregs were indeed found to be significantly reduced, both in terms of percent and absolute numbers (Fig. 5B). CD4+ Tregs were similarly reduced in B10.TCRδ−/− males vs. B10 male controls (see Supp. Fig. 1B), but neither the frequency nor number of CD4+ Tregs was significantly different in B6.TCRδ−/− females vs. B6 females (Fig. 5B). Moreover, in older B10.TCRδ−/− females, the numbers and frequencies of CD4+ Tregs remained low, and were even more reduced in those with keratitis, when compared to either matched wildtype controls or to matched non-keratitic B10.TCRδ−/− females (Fig. 5C). We went on to examine the levels of two molecules present on Tregs that are important for their functionality: IL-2Rα (CD25) and IL-2Rβ (CD122) (29). As can be seen (Fig. 5D), in B10.TCRδ−/− females, CD25 was slightly but significantly reduced on CD4+ Tregs in both spleen and blood, whereas CD122 was substantially reduced, and significantly lower still on CD4+ Tregs taken from keratitic rather than nonkeratitic mice. Therefore, the CD4+ Tregs in B10.TCRδ−/− mice may also be functionally impaired. We went on to test whether or not B10.TCRδ−/− mice show a reduction in Tregs in the thymus as well as in the periphery. Compared to B10 wildtype controls, both the percent of Tregs (CD25+ FoxP3+) among thymic CD4+ CD8− T cells and the numbers of Tregs in the thymus were in fact substantially reduced in B10.TCRδ−/− mice (Fig. 5E). These data imply that the lack of γδ T cells in B10.TCRδ−/− mice in some way disrupts the development of regulatory αβ T cells. Because Treg deficiencies were found in mice that have a strong tendency to develop keratitis but not in those that do not, they may explain the increased survival and/or expansion of memory phenotype CD8+ T cells in these mice.
Fig. 5. Tregs are reduced in B10.TCRδ−/− mice.
Cells from the indicated tissues were analyzed by flow cytometry, after cell surface staining for CD3, TCR-β, CD4, and CD25, plus intracellular staining for FoxP3 expression. For B-E, columns represent samples from the mice indicated in the key above panel B, and shows the mean value obtained from multiple mice; errors bars indicate SEM. A. Representative flow cytometry plots from a B10 and a B10.TCRδ−/− mouse, both five weeks old. The two left-most panels illustrate that among CD4+ αβ T cells (CD3+ TCR-β+), virtually all FoxP3+ cells are also CD25+. The right panel shows that within αβ T cells (CD3+ TCR-β+), virtually all FoxP3+ cells are also CD4+. Thus, using a relatively generous gate for CD25, the FoxP3 regulatory cells present in these mice almost entirely are represented by CD4+ CD25+ Tregs. B. Results are from spleens of five week-old female mice (9 mice for B10, 12 for B10.TCRδ−/−, and 4 each B6 and B6.TCRδ−/−). C. Results are from spleens and blood of 18–30 week old females (6–10 mice per group). D. Mean fluorescence intensity (MFI) was determined for CD25 and CD122 by flow cytometry using mAb specific for these molecules, among CD4+ CD25+ FoxP3+ cells from spleen (top panels) or blood (bottom panels) of female mice of the indicated strains. E. Results are from thymi of 9 week-old female mice (4 mice per strain were analyzed).
Discussion
We discovered in this study three abnormalities in the αβ T cells of keratitis-prone B10.TCRδ−/− female mice when compared to wildtype female controls: the B10.TCRδ−/− mice had reduced numbers of CD8+ αβ T cells overall, an increased proportion of memory phenotype CD8+ cells, and a decreased number and proportion of CD4+ CD25+ FoxP3+ Tregs. We also found that CD8+ αβ T cells efficiently promote keratitis when adoptively transferred to the relatively keratitis-resistant B10.TCRβ/δ−/− strain, and that Tregs diminish the ability of CD8+ T cells to transfer disease. The fact that CD8+ T cells are sufficient to cause keratitis, and that Tregs can counteract this, suggests the hypothesis that B10.TCRδ−/− mice are highly prone to develop keratitis because their lack of γδ T cells leads to a deficiency in Treg development and/or function, which in turn leads to failure to suppress CD8+ memory phenotype T cells that then promote or cause keratitis. The CD62L-low CD44-high CD8+ T cells could in fact represent innate memory phenotype-like CD8+ T cells that can generate potent responses to primary antigen, as have been previously described (30), and could therefore be directly responsible for the pathology. The reason for the overall reduction in CD8+ αβ T cells in B10.TCRδ−/− mice is less clear, but it could be a direct consequence of the increase in memory phenotype CD8+ αβ T cells, which may outcompete the naïve CD8+ cells for cytokines needed for maintenance, because they easily outstrip naïve CD8 cells in homeostatic expansion following adoptive transfer (31).
In a previous study, we noted that B10.TCRβ/δ−/− mice that had been adoptively transferred with αβ T cells from B10.TCRδ−/− mice often underwent major weight loss (17). In the current study, we also noted this same sort of wasting in mice that received CD4+ T cells, but not CD8+ T cells, from B10.TCRδ−/− keratitic donors (Supp. Fig. 1C). This is additional evidence that the T cells of B10.TCRδ−/− mice are deficient in Tregs, because colitis resulting in severe weight loss is known to develop when T cell deficient-mice are given an adoptive transfer containing CD4+ T cells depleted of Tregs (32). We have still to determine whether the Treg numbers in B10.TCRδ−/− mice are simply too low, or whether their functionality is also impaired. However, staining of CD4+ Tregs cells in B10.TCRδ−/− mice for expression of CD25 (IL-2Rα) and CD122 (IL-2Rβ) revealed that they carry lower surface levels of these receptors than Tregs from wildtype B10 mice, particularly those taken from keratitic mice (Fig. 5D above). IL-2 receptors receptors are clearly important for the functional differentiation and maintenance of CD4+ Tregs (33), and may be necessary for their activation or suppressive activity as well (29). Moreover, we also found that both CD4+ and CD8+ T cells in B10.TCRδ−/− mice produce substantially less IL-2 than those in B10 controls. These three findings together – reduced CD4+ Treg cell numbers, reduced levels of IL-2Rα and IL-2Rβ on the Tregs, and a lower frequency of CD4 and CD8 T cells biased to produce IL-2 – suggest that Treg influence could be substantially impaired in B10.TCRδ−/− mice.
Because we backcrossed the Cδ-null mutation (34) onto the B10 background, the B10.TCRδ−/− mice likely carry all or nearly all of the 129 strain TCR α/δ locus, which differs from that of B6 and B10 by a deletion comprising 27 functional Vα genes (35). The αβ T cells of B10.TCRδ−/− mice therefore intrinsically differ from those of B10 in this regard, and this could explain at least in part why the B10.TCRδ−/− strain is prone to develop keratitis. However, when we compared CD8+ cells from B10 vs. B10.TCRδ−/− females in terms of their ability to adoptively transfer keratitis, both were effective, although the B10 CD8+ cells were somewhat less efficient. This suggests that peripheral CD8+ T cells from both strains contain autoaggressive precursors that under the “correct” conditions can drive an autoimmune attack on the cornea, and would seem to rule out the possibility that abnormal CD8+ αβ T cells which only develop in B10.TCRδ−/− mice are needed to induce the disease. Alternatively, another potential difference between the CD8+ cells derived from B10.TCRδ−/− vs. B10 mice is that the latter also contain a few contaminating γδ T cells, because about 15% normally express CD8 (36), and this could instead explain why CD8+ T cells from B10 mice were comparatively less efficient in transferring disease. However, γδ T cells compete poorly with CD8+ αβ T cells in homeostatic proliferation following adoptive transfer (37, 38), and when we examined the composition of the transferred cells 3 weeks later (Supp. Fig. 2A), essentially all CD8+ T cells in the spleen were αβ TCR+ cells, whereas γδ T cells (CD3+ TCRβ− cells) could not be detected. Therefore, it seems unlikely that contaminating γδ T cells had much if any effect in this experiment. We found that the adoptively transferred CD8α+ cells, after homeostatic expansion, also virtually all displayed a memory phenotype in spleen, lymph node, and blood, representing either TCM (CD44 high, CD62L high) or TEM (CD44-high CD62L low) cells (Supp. Fig. 2B). The conversion of naïve CD8+ T cells into CD44-high memory-type CD8+ cells following adoptive transfer into lymphopenic hosts has been noted previously (39), and may have promoted the emergence of keratitogenic CD8+ T cells from normal B10 donors in this experiment.
Our results in this study suggest that γδ T cells are in some way needed to promote the development of CD4+ CD25+ FoxP3+ Tregs, and that the reduction of Tregs in B10.TCRδ−/− mice is sufficient to bring about the development of autoimmune keratitis. However, B6.TCRδ−/− mice, although they fail to develop ACAID in response to antigens experimentally introduced into the anterior chamber (1, 2), did not show a reduction in CD4+ Tregs. The B6.TCRδ−/− mice also did not have decreased CD8+ T cell numbers or an increased frequency of memory CD8+ T cells as was seen in B10.TCRδ−/− mice. Therefore, the lack of γδ T cells cannot by itself explain why the B10.TCRδ−/− mice develop these αβ T cell abnormalities. Instead, some other factor peculiar to the B10 background is also needed that, together with the lack of γδ T cells, predisposes the mice to develop these defects, which together with a characteristic found in the females, further predisposes the majority of them to spontaneously develop autoimmune keratitis. The decrease in CD4+ Tregs could, however, directly give rise to increased survival of CD8 memory cells, which could in turn reduce the overall number of CD8+ cells - the other two αβ T cell abnormalities that were evident in B10.TCRδ−/− mice. B10 mice differ genetically from B6 mice at only a few loci, one of which, the Lv locus, was previously implicated in the greater resistance of B10 mice to infection with the pathogenic fungus Coccidioides immitis, compared to B6 (40), so it is possible that the Lv locus is also key in keratitis susceptibility.
Our finding in this study that CD8+ T cells are sufficient to induce spontaneous autoimmune keratitis represents a relatively rare example in which it has been shown that CD8+ T cells alone, but not CD4+ cells, are responsible for the autoimmunity [reviewed in (41)]. Although CD8+ T cells are able to transfer autoimmunity in a mouse model of type 1 diabetes (NOD mice), and of multiple sclerosis (EAE), in both cases, CD4+ T cells were also able to transfer the disease. However, there is clear evidence that CD8+ T cells mediate disease in patients with vitiligo (42, 43), and CD8+ T cells may also be critical in Hashimoto’s thyroiditis (44). The CD8+ T cell requirement we found in our model could be a consequence of immune mechanisms unique to the eye, although in keratitis induced by Candida albicans infection, the disease was dependent upon IL-17-producing CD4+ T cells, and in herpes stromal keratitis (HSK), CD4+ T cells were also found to play a necessary role as a autoimmune component (45).
The ability to produce IL-10 is required for γδ T cells that promote the development of ACAID (13). Whether IL-10 produced in part by γδ T cells is likewise critical for producing CD4+ Tregs that prevent autoimmune keratitis in our system has yet to be determined, but seems unlikely because we did not find a significant reduction in the frequency of IL-10+ cells among either PMA/ionomycin-stimulated CD8+ or CD4+ cells from B10.TCRδ−/− mice, as compared to B10 controls (see Fig. 3 above). We did, however, find a more than two-fold decrease in both CD4+ and CD8+ cells biased to produce IL-2 in B10.TCRδ−/− mice, a cytokine known to be critical for CD4+ Treg development and survival. γδ T cells in certain settings have been shown to produce IL-2 (46), so it is possible that a lack of IL-2-producing γδ T cells could directly impair CD4+ Treg development in the B10TCRδ−/− strain. To examine this possibility, we therefore compared purified αβ T cells from the spleens of B10 mice to γδ T cells for their ability to produce IL-2, following non-specific activation in vitro. Splenic γδ T cells in fact produced much less IL-2 than did splenic αβ T cells (see Supp. Fig. 3A). However, when we similarly examined IL-2 production by γδ T cells isolated from the thymus (Supp. Fig. 3B), they produced IL-2 in amounts comparable to those produced by γδ TCR-negative thymocytes. Moreover, when we non-specifically activated thymocytes and then compared thymic γδ TCR+ cells to αβ TCR+ cells present in the same cultures, a similar percentage in each group was positive for IL-2 by intracellular cytokine staining (Supp. Fig. 3C). Thus, a reduction in IL-2 in the thymus due to the lack of γδ T cells is a possible mechanism to explain why Tregs are reduced in B10.TCRδ−/− mice. If in B10 mice, γδ T cells normally provide a significant fraction of the limiting amount of IL-2 necessary for the thymic development of CD4+ CD25+ Tregs (33), the lack of this additional IL-2 source could diminish the levels of Tregs coming out of the thymus, and lead to reduced peripheral Tregs as well. As noted above, thymic Tregs were in fact also substantially reduced in B10.TCRδ−/− mice Fig. 5E).
Different γδ T cell subsets, which can be partially defined by the TCRs they express, are biased to produce certain cytokines, usually including either IFNγ or IL-17 (47, 48). γδ T cells that reside in the limbus of the eye are known to promote healing in a corneal wounding model have been shown to secrete both IL-17 and IL-22 (4, 6), both neutrophil-mobilizing cytokines. Whether these IL-17 producing γδ T cells are the same γδ T cells that in B10 mice promote the development of CD4+ Tregs and prevent autoimmune keratitis therefore seems unlikely. A previous experiment suggested that the protective γδ T cells in our model express Vγ1 (17). We have yet to determine whether Vγ1+ cells are capable of promoting the development of CD4+ CD25+ FoxP3+ Tregs, and if so, how they may accomplish this.
Supplementary Material
Acknowledgments
The authors thank Philippa Marrack (National Jewish Health, Denver, CO), Hongbo Chi (St. Jude Children’s Research Hospital, Memphis, TN), and Ellen Robey (Univ. of California, Berkeley, CA) for helpful discussions.
Footnotes
This work was supported by NIH grant R01EY021199 to R.L.O. In addition, Y.H. received salary support from NIH training grant T32AI007405, and Z.Y. received partial salary support from the Dept. of Breast and Thyroid Surgery, Tongyi Hospital.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Skelsey ME, Mellon J, Niederkorn JY. γδ T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–4333. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Kapp JA. γδ T cells are critical for the induction of anterior chamber-associated immune deviation. Immunol. 2001;104:142–148. doi: 10.1046/j.0019-2805.2001.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-γδ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Burns AR, Rumbaut RE, Smith CW. γδ T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epitheial repair after corneal abrasion. Am J Pathol. 2007;171:838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178:1106–1016. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Burns AR, Byeseda Miller S, Smith CW. CCL20, γδ T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurence A, O’Shea JJ, Watford WT. Interleukin-22: a sheep in wolf’s clothing. Nat Med. 2008;14:247–249. doi: 10.1038/nm0308-247. [DOI] [PubMed] [Google Scholar]
- 8.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunol. 1990;71:383–389. [PMC free article] [PubMed] [Google Scholar]
- 9.Kosiewicz MM, Streilein JW. Intraocular injection of class II-restricted peptide induces an unexpected population of CD8 regulatory cells. J Immunol. 1996;157:1905–1912. [PubMed] [Google Scholar]
- 10.Skelsey ME, Mayhew E, Niederkorn JY. CD25+, interleukin-10-producing CD4+ T cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology. 2003;110:18–29. doi: 10.1046/j.1365-2567.2003.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keino H, Takeuchi M, Kezuka T, Hattori T, Usui M, Taguchi O, Streilein JW, Stein-Streilein J. Induction of eye-derived tolerance does not depend on naturally occurring CD4+CD25+ T regulatory cells. Invest Ophthalmol Vis Sci. 2006;47:1047–1055. doi: 10.1167/iovs.05-0110. [DOI] [PubMed] [Google Scholar]
- 12.Ji SX, Yin XL, Yang PZ. Effect of CD4(+)CD25(+) regulatory T cells in the development of anterior chamber-associated immune deviation. Int J Ophthalmol. 2011;4:19–25. doi: 10.3980/j.issn.2222-3959.2011.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashour HM, Niederkorn JY. γδ T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J Immunol. 2006;177:8331–8337. doi: 10.4049/jimmunol.177.12.8331. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major role of γδ T cells in the generation of IL-17 uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nian H, Shao H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Activated γδ T cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest Ophthalmol Vis Sci. 2011;52:5920–5927. doi: 10.1167/iovs.10-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien RL, Taylor MA, Hartley J, Nuhsbaum T, Dugan S, Lahmers K, Aydintug MK, Roark C, Born WK. Protective role of γδ T cells in spontaneous ocular inflammation. Invest Ophthal Vis Sci. 2009;50:3266–3274. doi: 10.1167/iovs.08-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien RL, Chain JL, Aydintug MK, Bohrer-Kunter D, Huang Y, Hardy IR, Cambier JC, Lahmers K, Nuhsbaum T, Davidson R, Sun D, Born WK. αβ TCR(+) T cells, but not B cells, promote autoimmune keratitis in B10 mice lacking γδ T cells. Invest Ophthal Vis Sci. 2012;53:301–308. doi: 10.1167/iovs.11-8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishell RI, Dutton RW. Immunization of dissociated spleed cell cultures from normal mice. J Exp Med. 1969;67:423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–588. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O’Brien RL. Subset-specific, uniform activation of Vγ6/Vδ1+ γδ T cells elicited by inflammation. J Leuk Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 21.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Aydintug MK, Loomis J, Macleod MK, McKee AS, Kirchenbaum G, Jakubzick CV, Kedl RM, Sun D, Jacobelli J, O’Brien RL, Born WK. Antigen-specific regulation of IgE antibodies by non-antigen-specific γδ T cells. J Immunol. 2013;190:913–921. doi: 10.4049/jimmunol.1202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 24.Gibbings D, Befus AD. CD4 and CD8: an inside-out coreceptor model for innate immune cells. J Leukoc Biol. 2009;86:251–259. doi: 10.1189/jlb.0109040. [DOI] [PubMed] [Google Scholar]
- 25.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leuk Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkdahl O, Barber KA, Brett SJ, Daly MG, Plumpton C, Elshourbagy NA, Tite JP, Thomsen LL. Characterization of CC-chemokine receptor 7 expression on murine T cells in lymphoid tissues. Immunology. 2003;110:170–179. doi: 10.1046/j.1365-2567.2003.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. Differential survival of naive CD4 and CD8 T cells. J Immunol. 2000;165:3689–3694. doi: 10.4049/jimmunol.165.7.3689. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1011. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Hu J, August A. Cutting edge: innate memory CD8+ T cells are distinct from homeostatic expanded CD8+ T cells and rapidly respond to primary antigenic stimuli. J Immunol. 2013;190:2490–2494. doi: 10.4049/jimmunol.1202988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16:635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 35.Bosc N, Lefranc MP. The mouse (Mus musculus) T cell receptor α (TRA) and δ (TRD) variable genes. Dev Comp Immunol. 2003;27:465–497. doi: 10.1016/s0145-305x(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 36.French JD, Roark CL, Born WK, O’Brien RL. γδ T lymphocyte homeostasis is negatively regulated by β2-microglobulin. J Immunol. 2009;182:1892–1900. doi: 10.4049/jimmunol.0803165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French JD, Roark CL, Born WK, O’Brien RL. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proc Natl Acad Sci USA. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, Theofilopoulos AN. γδ T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 39.Schuler T, Hammerling GJ, Arnold B. Cutting edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol. 2004;172:15–19. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- 40.Fierer J, Walls L, Kirkland TN. Genetic evidence for the role of the Lv locus in early susceptibility but not IL-10 synthesis in experimental coccidioidomycosis in C57BL mice. J Inf Diseases. 2000;181:681–685. doi: 10.1086/315256. [DOI] [PubMed] [Google Scholar]
- 41.Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun. 2013;45:68–79. doi: 10.1016/j.jaut.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One. 2012;7:e37513. doi: 10.1371/journal.pone.0037513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, Li M. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta, Derm, Venereol. 2015;95:664–670. doi: 10.2340/00015555-2080. [DOI] [PubMed] [Google Scholar]
- 44.Fountoulakis S, Vartholomatos G, Kolaitis N, Frillingos S, Philippou G, Tsatsoulis A. Differential expression of Fas system apoptotic molecules in peripheral lymphocytes from patients with Graves’ disease and Hashimoto’s thyroiditis. Eur J Endocrinol. 2008;158:853–859. doi: 10.1530/EJE-08-0092. [DOI] [PubMed] [Google Scholar]
- 45.Lepisto AJ, Frank GM, Hendricks RL. How Herpes Simplex Virus type I rescinds corneal privilege. Chem Immunol Allergy. 2007;92:203–212. doi: 10.1159/000099271. [DOI] [PubMed] [Google Scholar]
- 46.Hayday AC. γδ T cells: A right time and a right place for a conserved third way of protection. Ann Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien RL, Born WK. γδ T cell subsets: A link between TCR and function? Sem Immunol. 2010;22:193–198. doi: 10.1016/j.smim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chien YH, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Ann Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.