Abstract
Multiple mechanisms are likely to account for the link between obesity and increased risk of post-menopausal breast cancer. Two adipokines, leptin and adiponectin, are of particular interest due to their opposing biological functions and associations with breast cancer risk. In the current study, we investigated the effects of leptin and adiponectin on normal breast epithelial stem cells. Levels of leptin in human adipose explant-derived conditioned media positively correlated with the size of the normal breast stem cell pool. By contrast, an inverse relationship was found for adiponectin. Moreover, a strong linear relationship was observed between the leptin/adiponectin ratio in adipose conditioned media and breast stem cell self-renewal. Consistent with these findings, exogenous leptin stimulated whereas adiponectin suppressed breast stem cell self-renewal. In addition to local in-breast effects, circulating factors including leptin and adiponectin may contribute to the link between obesity and breast cancer. Increased levels of leptin and reduced amounts of adiponectin were found in serum from obese compared to age-matched lean post-menopausal women. Interestingly, serum from obese women increased stem cell self-renewal by 30% compared to only 7% for lean control serum. Taken together, these data suggest a plausible explanation for the obesity-driven increase in post-menopausal breast cancer risk. Leptin and adiponectin may function as both endocrine and paracrine/juxtacrine factors to modulate the size of the normal stem cell pool. Interventions that disrupt this axis and thereby normalize breast stem cell self-renewal could reduce the risk of breast cancer.
Keywords: adipose, leptin, adiponectin, obesity, breast cancer
INTRODUCTION
Obesity is associated with an increased risk of post-menopausal breast cancer (1, 2). Several mechanisms have been suggested to account for this increased risk including changes in breast adipose tissue (3). Adipose tissue is a complex energy storage and endocrine organ that secretes a wide range of biologically active factors including adipokines. The adipose-rich stroma that makes up the stem cell niche in the human breast is thought to influence ductal and alveolar morphogenesis during pubertal development, and again on a regular basis throughout a woman’s reproductive years, possibly by altering stem cell activity (4). Although obesity is associated with altered adipokine production (5), little is known about whether either local or systemic changes in adipokine levels can affect stem cell self-renewal in the normal breast.
Leptin and adiponectin, two of the most abundant adipokines, have been suggested to play a role in breast cancer. In post-menopausal women, plasma levels of leptin increase in association with BMI whereas levels of adiponectin decrease in obese subjects (6). Concentrations of leptin and adiponectin are generally higher in normal breast tissue than in blood, and may be part of an important paracrine or juxtacrine signaling system between the adipose-rich stroma and breast epithelial stem cells (7). Leptin is a 16 kDa protein encoded by the ob gene (8) that is primarily secreted from fat cells for the regulation of body weight. At normal physiologic concentrations, leptin crosses the blood-brain barrier to activate the ObR receptor in hypothalamic neurons which inhibit the drive to feed (9–11). In addition to the hypothalamus, the leptin receptor is expressed in the human breast (12, 13) and is often up-regulated in breast cancer cells. Notably, some studies have suggested that high levels of leptin in the circulation are associated with increased breast cancer risk (14). In vitro studies have demonstrated that leptin stimulates the proliferation and survival of tumor cells. Zheng et al transplanted MMTV-Wnt1 mammary tumor xenografts into obese ob/ob mice, and showed that leptin-deficiency suppressed tumor growth, while it was enhanced in obese hyper-leptinemic db/db mice (15–17). In addition, silencing the leptin receptor in triple-negative breast cancer cells leads to the loss of cancer cell stemness, as evidenced by decreased expression of the stem cell self-renewal transcription factors NANOG, SOX2, and OCT4 and reduced stem cell self-renewal (17). While these results in breast cancer are intriguing, leptin’s role in the maintenance of the non-transformed stem cell population in the healthy human breast is unknown.
Adiponectin is produced almost exclusively by adipocytes. In contrast to leptin, circulating levels of adiponectin are inversely correlated with BMI. Multiple human studies have demonstrated an inverse association between levels of circulating adiponectin and risk of post-menopausal breast cancer (18–23). Adiponectin activates two different receptors, AdipoR1 and AdipoR2; these receptors are expressed by most cells including normal mammary epithelial cells and breast cancer cells (24). Binding of adiponectin to its receptors activates AMPK, a nutrient-sensing enzyme, which regulates several key pathways involved in protein synthesis and cellular energy metabolism. Adiponectin can induce apoptosis and inhibit the growth of tumor cells (25). Adiponectin haploinsufficiency promotes mammary tumor formation by down-regulation of PTEN activity and activation of PI3K/Akt signaling (26). Whether adiponectin modulates normal mammary stem cell self-renewal is uncertain and could help to explain its anti-tumor activity.
While we recognize that the mammary tumor cell of origin has not been clearly determined, increasing evidence including lineage tracing experiments support the concept that clonal neoplastic epithelial transformation arises from a single stem cell or early progenitor, resulting in a hierarchically organized tumor (27, 28). In the human breast, normal mammary epithelial stem cells maintain the mammary gland throughout a woman’s reproductive years. The stem cell theory argues that these long-lived and slowly self-renewing cells may be exposed to genetic insults over their extremely long lifespans, thus accumulating and harboring tumorigenic mutations, ultimately giving rise to cancer (29–31). Despite this uncertainty in the literature, we have been able to use this model as a clinically relevant tool to interrogate the underlying mechanisms of carcinogenesis and to establish therapeutic efficacy. In this communication, we now address whether obesity-related factors related to adipokine biology may lead to expansion of the normal mammary stem cell population and increase the risk of cancer later in life by expanding the number of potential targets for tumorigenesis.
Here, we tested the hypothesis that the increase in the leptin/adiponectin ratio that commonly occurs in obese women promotes increased breast stem cell self-renewal leading to a larger population of stem cells in vivo. We infer that a larger population of breast stem cells provides more potential targets for transformation and carcinogenesis, increasing the risk of breast cancer.
MATERIALS and METHODS
Normal human mammary tissue
Human mammary tissue was obtained from women undergoing elective reduction mammoplasty surgery at the University of Michigan after giving informed consent (University of Michigan IRBMED approved tissue collection protocol). Reduction mammoplasty, tissue was obtained through a program collecting de-identified mammoplasty tissue from all reduction mammoplasty procedures at the University of Michigan Medical Center, was provided by 12 women, ranging in age from 18 to 48 years (X̄ = 32.8 ± 9.5 years). The specimens were carefully reviewed by a pathologist through the Tissue Procurement Service and the Department of Pathology at the University of Michigan Medical School to ensure the absence of malignancy.
Epithelial cell separation and the mammosphere formation assay
Resected mammary tissue was minced with scalpels and digested with collagenase overnight. The digested tissue was centrifuged at 40 xg to pellet the “organoids” containing intact mammary glands, and the top fat layer and fibroblasts were discarded as previously described (32, 33). The separated organoids were then digested in trypsin and dispase to generate a single cell suspension. Cells were counted with a hemacytometer and viability was assessed by trypan blue exclusion. 2×105 viable cells were plated into each well of a 24-well ultra-low attachment culture plate (Corning) with 400 μL of Mammocult Media (Stem Cell Technologies). Media was refreshed every 2–3 days. Resulting primary spheres were counted with an inverted microscope at 10 days.
Primary cultures were treated with physiologic concentrations of recombinant leptin (100 ng/mL), adiponectin (25 μg/mL), adipose-conditioned media (standardized to 0.1 mg/mL total protein) or human serum (2%), or empty carrier control during the period of primary mammosphere formation. The baseline/negative control condition (basal Mammocult media without recombinant adipokines) allowed determination of the baseline rate of mammary stem cell self-renewal activity, and thereby calculation of the change in this activity due to various stimuli. After 10 days, the spheres were counted and collected. The primary mammospheres were dispersed with 0.25% trypsin and FACSmax (Genlantis) and all viable cells were re-plated in new ultra-low adherent culture plates at a concentration of 2×105 viable cells/well in fresh Mammocult media. Thus, all stem cells in the primary mammospheres were available to produce secondary mammospheres. Media was refreshed every 2 days, and secondary mammospheres were counted after 10 days.
While in vivo transplantation is considered the gold standard assay for stem cell self-renewal, the secondary mammosphere formation assay is a high-throughput, reliable, and cost-effective in vitro assay that allows the relatively quick and simple interrogation of changes in stem cell self-renewal activity in response to specific or novel agents. The percent difference between primary (1°) and secondary (2°) mammosphere number is a measure of changes in stem cell self-renewal activity: (34–36).
Imaging of Mammosphere Stem Cells
Whereas the secondary mammosphere formation assay is a quantifiable high-throughput measure of stem cell self-renewal activity, we also used immunostaining and direct visualization of mammosphere stem cells to demonstrate ALDH+ stem cell self-renewal within the mammospheres, as previously described (37, 38). Briefly, primary mammary epithelial cells were separated from reduction mammoplasty tissue and plated in non-adherent culture dishes, as described above. Cultures were treated with physiologic concentrations of recombinant leptin (100 ng/mL), adiponectin (25 μg/mL), or control basal media during the period of primary mammosphere formation. After 10 days, the spheres were collected, fixed in 5% formalin for 30 minutes at room temperature, and washed in glycine buffer to quench background autofluorescence. The fixed mammospheres were stained with a monoclonal antibody to ALDH1a1 (BD 611195) at 1:1000 dilution in goat serum blocking solution overnight at 4°C. After washing in PBS, the mammospheres were then stained with secondary antibody AlexaFluor488 goat anti-mouse IgG highly cross-adsorbed (Molecular Probes A11029) for 45 minutes at room temperature. After washing with PBS, the stained mammospheres were placed on Superfrost Excell Adhesion slides (Fisher 22-037-247) with ProLong Gold containing DAPI to stain nuclei (Molecular Probes P36935). The stained mammospheres were then visualized with a Leica Inverted SP5X Confocal Microscope System (University of Michigan Medical School Biomedical Research Core Facility).
Human mammary adipose tissue explant cultures
Up to 20 grams of human breast adipose tissue was visually inspected and stripped of any grossly visible blood vessels or connective tissue. The tissue was finely minced into pieces less than 1 mm3 using scalpels. Minced adipose was partially digested with trypsin/EDTA and dispase for 10 minutes in order to partially disrupt the extracellular matrix and allow better nutrient and oxygen diffusion without completely disturbing tissue architecture as demonstrated by confocal imaging. 500 mg of processed adipose tissue was placed in each well of a 6-well tissue culture dish (Corning) containing a 40 micron cell strainer (BD Falcon). The buoyant adipose tissue explants were suspended in 4.0 mL of culture media (M199 base media supplemented with 1 μg/mL insulin, 400 ng/ml dexamethasone, and 1x PCN/Strep) and incubated at 37°C with 5% CO2. Adipocyte viability was determined by trypan blue exclusion at high magnification (39). Conditioned media was collected after 48 hours from outside the cell strainer to avoid disrupting the suspended tissue. Media was centrifuged at 1000 xg to pellet any cells or debris. To specifically examine adipokine activity without a confounding influence from smaller molecules such as free amino acids, glucose, fatty acids, or insulin the conditioned media from the human mammary adipose explants was filtered through a 10 kDa centrifugal filter unit (Amicon Ultra-10, Millipore). Total protein concentration was measured with the Bradford assay and samples were standardized to 1 mg/ml total protein.
Adipokine measurements
Leptin concentration in adipose conditioned media and human serum was quantified with the Leptin Quantikine ELISA kit from R&D Systems. Total adiponectin concentrations were similarly measured with the Total Adiponectin Quantikine ELISA kit from R&D Systems. Colorimetric determinations were made with a Powerwave XS plate reader (Bio-Tek).
Adipose explant conditioned media was assayed with the Proteome Profiler Human XL Cytokine Array Kit from R&D Systems according the manufacturer’s protocol. Resulting blots were exposed to film, which were scanned with a flatbed scanner and pixel intensity was measured with ImageJ. The leptin concentration quantified by ELISA was used to normalize pixel intensity to molar concentration in order to allow semi-quantitative determination of other adipokine concentrations.
Confocal Imaging of Adipose Tissue
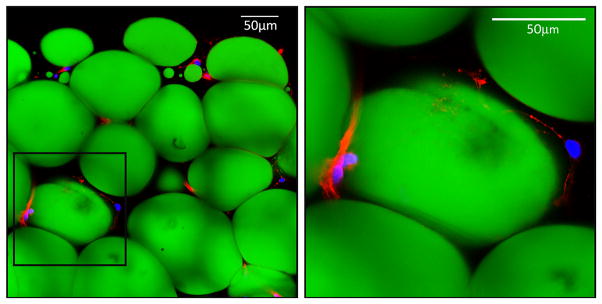
Minced breast adipose tissue was fixed overnight in 10% formalin. Tissue was then washed three times in PBS/0.1 mol/L glycine and permeabilized with 0.2% Triton-X for 15 minutes before blocking in PBS/1% BSA. With its nonpolar structure and long-wavelength absorption and bright green fluorescence, BODIPY493/503 is commonly used as an adipocyte stain for neutral fatty acids and other nonpolar lipids. Here, adipocytes were stained with BODIPY493/503 (2 μg/mL, Life Technology), and actin filaments were stained with phalloidin-AlexaFluor546, and DAPI to identify nuclei. Stained tissue was rinsed in PBS and mounted onto a coverslip using a Nunc Labtek II chamber system (Thermo Scientific) with Prolong Gold. The stained tissue was then visualized with a Leica Inverted SP5X Confocal Microscope System (University of Michigan Medical School Biomedical Research Core Facility) (Figure 1).
Figure 1. Human breast adipose explant cultures.
Adipose was harvested from primary reduction mammoplasty tissue and grown in explant culture for 48 hours to collect conditioned media. Adipocyte lipid droplets were stained with BODIPY493/503 (green), actin was stained red with phalloidin-AlexaFluor546, and DAPI stained nuclei blue.
Human Serum
This study was approved by the Institutional Review Board of Rockefeller University. Pooled human serum was prepared from lean (n=9, BMI 18–25) and obese (n=10, BMI >35) post-menopausal women and lean (n=10) and obese (n=10) men. All subjects were paired by age (± 5 years).
STATISTICS and DATA ANALYSIS
Manual counts of primary and secondary mammosphere numbers and measured adipokine concentrations were entered into Microsoft EXCEL. The population of normal mammary stem cells in vivo was determined by counting the number of primary mammospheres and dividing by the number of total epithelial cells plated to generate a percentage to represent the stem cell population. The rate of stem cell self-renewal was similarly determined by counting the number of secondary mammospheres formed and dividing by the number of primary mammospheres to determine a percent change from baseline (negative control). Percent changes in mammosphere formation were plotted against measured adipokine concentrations, and correlation coefficients were calculated. An increased number of secondary mammospheres compared to primary spheres is an indicator of increased stem cell self-renewal, while a decreased percentage of secondary mammospheres suggests inhibition of self-renewal, possibly by promotion of stem cell quiescence, apoptosis, or terminal differentiation.
RESULTS
Primary breast adipose explants can be maintained in culture
As seen in Figure 1, the stripped and partially digested adipose tissue is enriched for adipocytes (stained green) with some remaining support cells (e.g. macrophages, endothelial cells) and maintains its three-dimensional architecture. The inset image (Figure 1, right hand panel) shows a representative breast adipocyte with a single large lipid droplet and a small area of peripheral cytoplasm outlined by actin filaments and the nucleus pushed to the periphery. After 48 hours in culture, >97% of the mature adipocytes remained viable, as assessed by trypan blue exclusion (data not shown). Subsequent follow-up determined that these explants can be maintained in culture with periodic media changes for at least 10 days with >90% viability.
Locally-produced breast adipokine concentrations predict the stem cell population in the breast
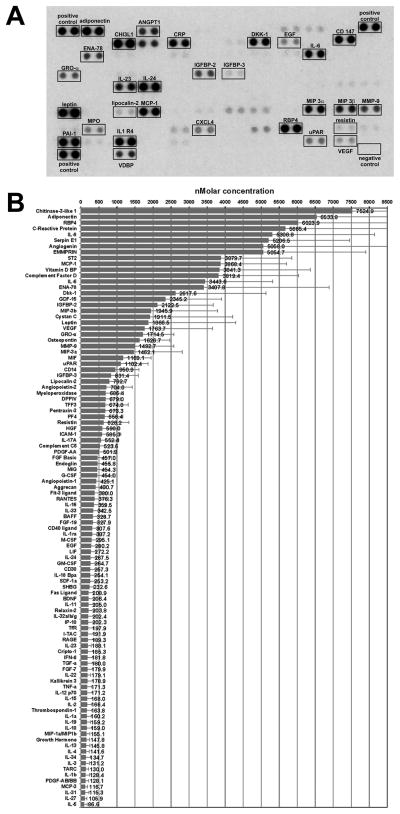
Adipose conditioned media contained a wide range of known adipokines and cytokines as demonstrated by protein array, with adiponectin being among the most abundant (Figure 2A). We used an ELISA to quantify both the leptin and adiponectin concentrations in the media; these data were used to normalize the blot intensity to generate semi-quantitative measurements of the other factors (Figure 2B). Some of these factors would be expected to come from macrophages and endothelial cells within the adipose tissue, while others are made exclusively by adipocytes. Given the potential importance of leptin and adiponectin in both stem cell biology and obesity-related breast cancer, subsequent experiments focused on these two adipokines.
Figure 2. Measurement of adipocytokines in breast adipose conditioned media.
A. The Proteome Profiler Human Cytokine XL Array was exposed to adipose conditioned media to measure relative molar concentrations of secreted proteins. B. Leptin was quantified by ELISA and used to normalize pixel intensity to generate semi-quantitative measurements for the other secreted proteins. N=15 ± STD.
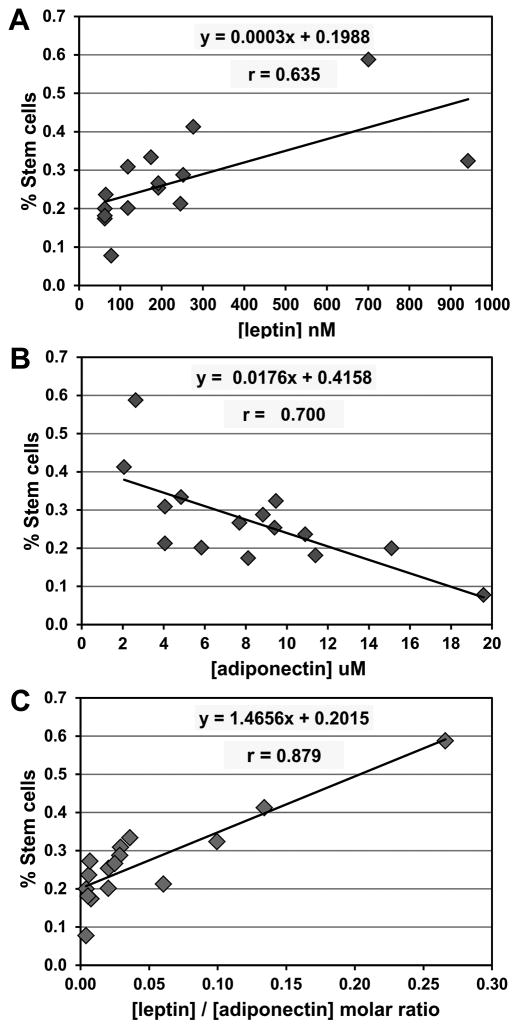
As mentioned above, primary mammosphere formation reflects the size of the stem cell population in breast tissue (34). Breast adipose explants were prepared from women undergoing elective reduction mammoplasty. Levels of leptin and adiponectin in conditioned media were correlated with the size of the stem cell population for each of these women. As shown in Figures 3A and 3B, we found a direct linear relationship between adipose-derived leptin and the estimated number of breast stem cells in vivo (X̄= 0.0029, r = 0.635), but an inverse linear relationship for adiponectin (X̄ = −0.0174, r = −0.700). The leptin/adiponectin molar ratio defined a better linear relationship with the number of stem cells in vivo (X̄ = 1.466, r = 0.879) than either of these factors alone, suggesting that these two hormones are sufficient to antagonistically modulate the size of the stem cell pool (Figure 3C).
Figure 3. Leptin and adiponectin in human breast adipose reflect the stem cell population in vivo.
A. Leptin concentration is positively correlated with the percentage of epithelial cells with stem-like features in vivo, whereas adiponectin is inversely correlated (B). C. The best linear relationship was seen between the leptin/adiponectin ratio and the stem cell population. A total of 15 subjects were evaluated.
Leptin promotes expansion of ALDH+ cells during mammosphere formation
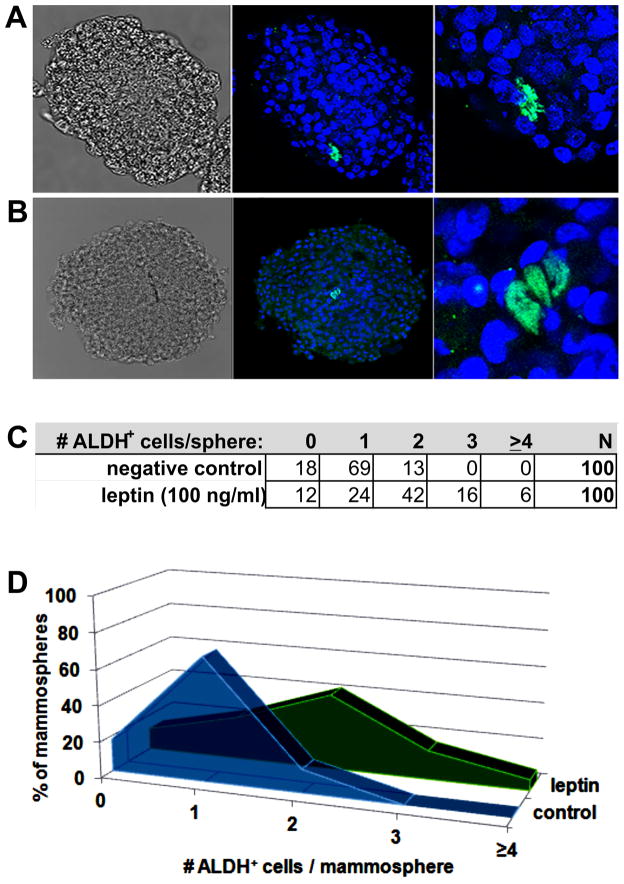
Wicha et al have previously identified aldehyde dehydrogenase (ALDH) as a marker of actively self-renewing mammary stem cells (37). Because we saw a direct relationship between the local leptin/adiponectin ratio and the in vivo mammary stem cell population based on the primary mammosphere formation assay, we hypothesized that leptin may directly promote expansion of the ALDH+ cell population. We treated primary mammary epithelial cells with leptin (100 ng/ml) during the period of primary mammosphere formation, and stained them with a monoclonal antibody to ALDH1A1. Panel A in Figure 4 shows a representative mammosphere grown in basal media (with vehicle control) with a single ALDH+ cell, while Panel B shows a representative mammosphere grown with basal media supplemented with recombinant leptin. The frequency of distribution of ALDH+ cells in the mammospheres are shown in Panels C and D. While not all mammospheres retained an ALDH+ cell after 10 days in culture and some untreated mammospheres had more than 1 ALDH+ cell, there was a trend toward multiple ALDH+ cells in the mammospheres treated with leptin compared to the control mammospheres (Figure 4C–D). This direct observation strengthens the hypothesis that leptin can directly stimulate mammary stem cell self-renewal.
Figure 4. Leptin promotes expansion of ALDH+ cell in mammospheres.
Primary human mammary epithelial cells were grown to form mammospheres in the presence of leptin (100 ng/ml), or vehicle control. After forming for 10 days, mammospheres were fixed and stained for ALDH1a1 (AlexFluor488, green) and DAPI to identify nuclei (blue). A. Representative mammosphere grown with vehicle control showing the presence of 1 ALDH+ cell. B. Representative mammosphere grown in the presence of leptin showing symmetric self-renewal of an ALDH+ cell. C–D. Frequency of distribution of number of ALDH+ cells per mammosphere in both conditions.
Leptin promotes, while adiponectin inhibits, breast epithelial stem cell self-renewal
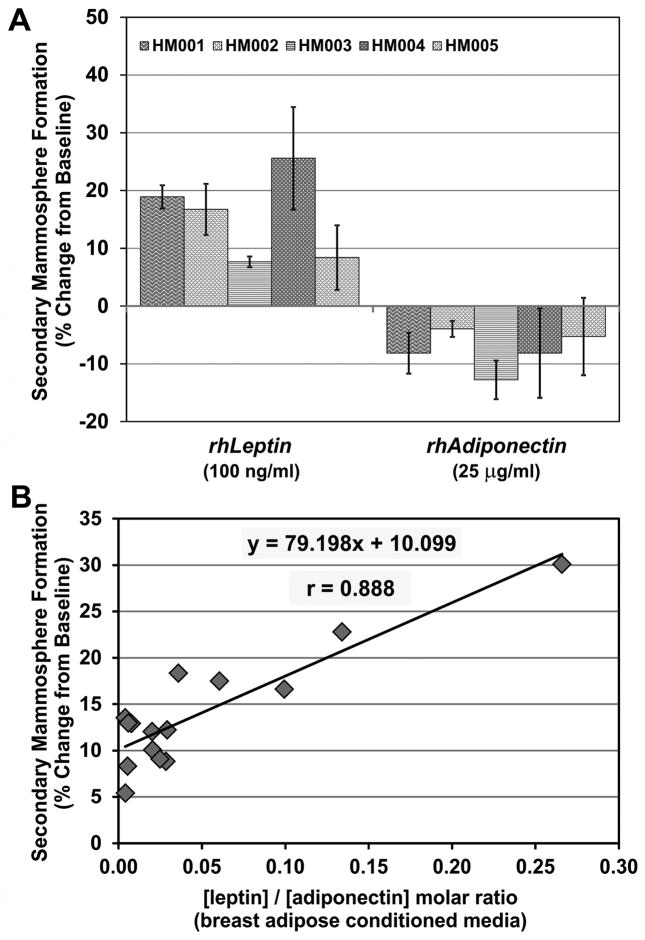
While direct visualization of ALDH+ cell replication in treated primary mammospheres lends strength to the argument that leptin promotes stem cell self-renewal, measurement of secondary mammosphere formation provides a high-throughput assay of stem cell self-renewal activity. Because a correlation was found between levels of leptin and adiponectin in adipose explant-derived conditioned media and primary mammosphere formation, additional explants were used to directly evaluate the effects of these adipokines on breast stem cells. When epithelial stem cells from 5 different human mammoplasty samples were grown in the presence of leptin, the number of secondary mammospheres formed increased by 7–25% (X̄ = 15.5 ± 7.5%) over the negative control (Figure 5A). This suggests that leptin was sufficient to expand the primary stem cell population in vitro. Conversely, treatment with adiponectin reduced the number of secondary mammospheres by 4–13% (X̄ = −7.7 ± 3.4%) in these same 5 human subjects suggesting that this hormone promotes either apoptosis, quiescence, or symmetric division/differentiation of the primary stem cells.
Figure 5. Locally-produced leptin and adiponectin modulate mammary stem cell self-renewal in vitro.
A. Primary mammospheres from 5 subjects were grown in the presence of recombinant leptin (100 ng/mL) or adiponectin (25 μg/mL) and counted. Spheres were dissociated and re-plated to form secondary mammospheres. The percent change from baseline is a measure of the rate of stem cell self-renewal. Leptin is sufficient to increase the rate of mammary stem cell self-renewal by 7–25%, whereas adiponectin inhibits self-renewal and decreases the stem cell population. N=4 replicates ± STD. B. Pooled primary mammospheres representing 12 random samples were grown in the presence of adipose conditioned media from 15 human specimens. Spheres were dissociated and re-plated to form secondary mammospheres. There was a strong linear relationship between the leptin/adiponectin ratio in adipose-conditioned media and human mammary stem cell self-renewal. Cultures were grown in quadruplicate, and the mean % change over negative control is shown.
In a separate series of experiments, a standardized pool of primary breast epithelial cells representing 12 random human specimens was treated with adipose conditioned media from 15 different human samples during primary mammosphere formation. Primary mammospheres were dissociated and re-plated to form secondary mammospheres to assess stem cell self-renewal. Leptin and adiponectin concentrations in the conditioned media were measured by ELISA and plotted against the change in stem cell self-renewal over the negative control. There was a linear relationship between the leptin/adiponectin molar ratio and breast stem cell self-renewal (X̄ = 76.049, r = 0.859) (Figure 5B).
Circulating obesity-related adipokines modulate human breast stem cell self-renewal
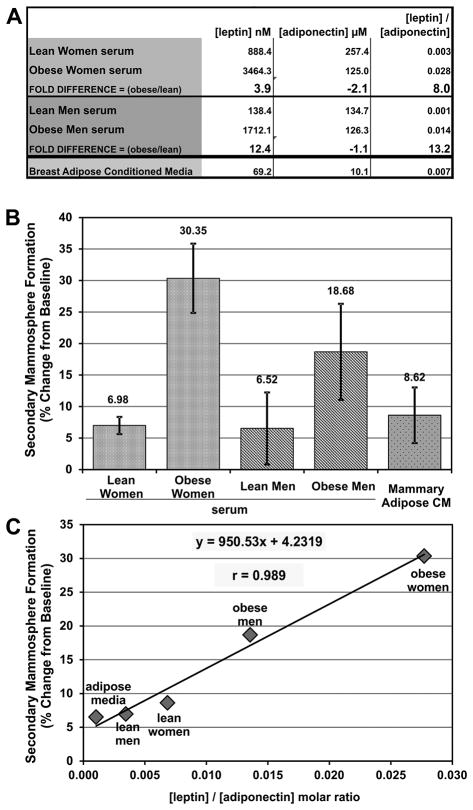
Obesity-related changes in circulating levels of both leptin and adiponectin have been suggested to contribute to the pathogenesis of breast cancer. Hence, to complement our studies of locally produced leptin and adiponectin, we next evaluated the effects of circulating leptin and adiponectin. Concentrations of leptin and adiponectin were measured in pooled serum from lean and obese post-menopausal women. As previously reported (40), obesity in women is associated with a marked increase in the circulating leptin level, but a decrease in adiponectin. Comparable measurements were carried out on pooled sera from obese vs. age-matched lean men. Shifts in leptin and adiponectin molar concentration was calculated as: . In obese men, leptin levels rose >12-fold, while adiponectin levels were essentially unchanged. Driven mostly by differences in leptin levels, the leptin/adiponectin ratio in serum was dramatically higher in obese than lean women (8.0-fold) and men (13.2-fold) (Figure 6A).
Figure 6. Circulating leptin and adiponectin modulate mammary stem cell self-renewal in vitro.
A. Leptin and adiponectin concentrations were measured by ELISA in pooled serum from lean (N=9) and obese (N=10) women, and lean (N=10) and obese (N=10) men and shown with the leptin/adiponectin molar ratio and the fold difference between lean and obese samples. Obese serum is enriched for leptin compared to lean samples, whereas adiponectin is decreased in obese women but not men. Mostly driven by changes in leptin concentration, the leptin/adiponectin ratio is dramatically increased in obese women and men. Pooled breast adipose conditioned media was used as a control. B. Primary human mammospheres were grown in the presence of 2% pooled serum from lean (N=9) and obese (N=10) post-menopausal women, and lean (N=10) and obese (N=10) men. Pooled breast adipose explant conditioned media was used as a control. Primary mammospheres were counted, dissociated, and re-plated to form secondary mammospheres. The percent change from baseline is a measurement of stem cell self-renewal. Cultures were grown in quadruplicate and the mean ± STD is shown. Circulating factors present in serum from obese women and men more strongly increased mammary stem cell self-renewal than serum from lean humans C. Leptin and adiponectin concentrations in the serum and conditioned media were measured by ELISA and plotted against the changes in stem cell self-renewal. There was a strong linear relationship between the circulating leptin/adiponectin ratio and mammary stem cell self-renewal in these samples.
When pooled primary human mammary epithelial cells were treated with human serum during primary mammosphere formation, stem cell self-renewal was increased over the negative control by about 7% for lean women and lean men (Figure 6B). By contrast, serum from obese women caused about a 30% increase in stem cell self-renewal, while serum from obese men produced a nearly 19% increase. These results suggest that factors enriched in the serum of obese humans stimulate breast epithelial stem cell self-renewal. Similarly, treatment with pooled breast adipose explant-derived conditioned media caused an almost 9% increase in stem cell self-renewal. Given the strong evidence that both leptin and adiponectin can modulate breast epithelial stem cell self-renewal, we also used these five treatment conditions to determine if a correlation existed between the leptin/adiponectin ratio and stem cell self-renewal. Interestingly, the leptin/adiponectin ratio had a very strong linear relationship with breast stem cell self-renewal, as indicated by secondary mammosphere formation (X̄ = 950.529, r = 0.989) (Figure 6C). Taken together, these data also strongly support the antagonistic roles of leptin and adiponectin in breast stem cell self-renewal.
DISCUSSION
Obesity is an independent risk factor for the development of breast cancer, but the mechanisms underlying this are uncertain (41). The leptin-adiponectin axis has been of particular interest due to these hormones’ opposing direct effects on cancer cells in vitro. While those results support the role of these factors in cancer progression, they do not address the question of how obesity might promote cancer initiation. Here, we demonstrate that locally-produced adipokines, including leptin and adiponectin, modulate the normal breast stem cell pool. Evidence is also presented that circulating adipokines may also be determinants of breast stem cell self-renewal.
A growing body of data supports the concept that normal stem cells can potentially undergo a carcinogenic event to become the cell of cancer origin (37, 42, 43). Assuming that stem cells or a stem cell progenitor is indeed the target cell of carcinogenesis, then it follows that increases in the size of the stem cell pool would increase risk of transformation by increasing the number of potential targets for mutation and tumor formation (32). Moreover, interventions that reduce the number of stem cells might protect against the increased incidence of breast cancer in obese post-menopausal women. Here, we have shown that among a variety of locally-acting adipokines coming from adipose directly adjacent to breast epithelium, the leptin/adiponectin ratio is directly proportional to the size of the stem cell population in vivo. We have also provided evidence that leptin alone is sufficient to stimulate human breast epithelial stem cell self-renewal, leading to significant increases in the stem cell population. In contrast, un-opposed adiponectin decreases the size of the mammary stem cell pool in vitro. Based on these findings, future studies are warranted to elucidate the signal transduction pathways that mediate these effects of leptin and adiponectin.
A limitation in this study is the use of mammary stem cells from de-identified women, whose menopausal status is unknown. Epidemiologic data demonstrate a possible protective effect of obesity in regard to pre-menopausal breast cancer, whereas it is an independent risk factor for breast cancer in post-menopausal women. In our study, we used reduction mammoplasty tissue from a convenience population of self-selected and de-identified women presenting for elective surgery. The women who’s tissue was used in this study ranged in age from 18 to 48 years (X̄ = 32.8 ± 9.5 years), while the median age of menopause in the United States is 51 years. One could reasonably expect that the majority of these women are pre-menopausal based on their age, but we are unable to confirm menopausal status in these women. While one would expect that differences in circulating hormones and other factors between pre- and post-menopausal women might impact breast epithelial stem cell biology, no data to date have addressed this question. Our data, using mammary stem cells from presumably pre-menopausal women, may partially explain the increased risk of breast cancer associated with obesity seen in post-menopausal women. Obesity, and its associated aberrant adipokine signaling, earlier in adult life may allow expansion of the mammary stem cell pool during a critical period and creating an environment rich in potential targets for carcinogenesis after menopause.
As a functional assay of stem cell activity, one limitation of the primary mammosphere formation assay is that it can only measure the actively cycling stem cells and does not measure those in a quiescent state. However, if the stem cell hypothesis of cancer origin is correct, the cycling sub-population is expected to be of clinical importance, making the functional mammosphere assay clinically meaningful with the ability to directly test the efficacy of targeted agents on stem cell activity (44, 45).
A recent population-based nested case-control study of risk factors for breast cancer in post-menopausal women found that elevated serum leptin levels account for 10% of the obesity-related breast cancer risk, while low adiponectin levels contribute 9% of the risk (19). Our study provides mechanistic insights that can explain these population-based findings. Our findings are also consistent with the results of a small prospective clinical trial by Santillan-Benitez et al, who measured serum leptin, adiponectin, CA15-3, and BMI in women prior to undergoing a routine screening mammogram (46). They calculated that a tetrad of factors including total leptin concentration and the leptin/adiponectin ratio was predictive of breast cancer with 83.3% sensitivity, 80% specificity, a positive predictive value of 83.3%, and a negative predictive value of 80%. This is in accordance with a nested case-controlled study from the MultiEthnic Cohort (MEC) study, which demonstrated that circulating leptin and the leptin/adiponectin ratio is associated with post-menopausal breast cancer risk (OR 1.94 and 1.91, respectively), and these associations remained after adjustment for BMI (14). Future studies are warranted to determine whether circulating factors in addition to leptin and adiponectin can modulate breast stem cell self-renewal. While the leptin/adiponectin ratio may be a surrogate marker for body mass, one shortcoming of the current study using de-identified tissue is the lack of information regarding our subjects’ body mass index and other anthropometric data. Future studies are warranted and should include identified subjects, anthropometric data including body mass index (BMI) and waist-hip ratios, as well as direct measurement of body fat percentage.
Based on published reports, an elevated leptin/adiponectin ratio may be a reasonable surrogate measure of obesity (47). Here we tested the hypothesis that obesity, through alteration in the leptin/adiponectin ratio, promotes increased breast stem cell self-renewal leading to a larger number of stem cells. Based on our findings, long-term prospective studies should be carried out to determine if the size of the stem cell population is also a marker for breast cancer risk. Taken together, the findings from this study and others strongly support the notion that the leptin/adiponectin ratio is a significant marker for post-menopausal breast cancer risk, and may indeed be a driving factor through direct actions on normal breast stem cells. This raises the intriguing possibility that interventions (e.g. weight reduction) that modulate the leptin/adiponectin ratio in vivo may effectively decrease breast cancer risk, especially in the obese subpopulation. Importantly, efforts are underway to develop pharmaceutical leptin antagonists and adiponectin receptor agonists to overcome some of the effects of obesity, including cancer progression (48–50). Behavioral, dietary or pharmacological interventions that modulate the leptin/adiponectin ratio or related signaling pathways may prove to be useful in reducing the risk of breast cancer.
Acknowledgments
Grant Support: This work was supported by The Kutsche Memorial Chair in Internal Medicine (to D.E. Brenner); The Cancer Center Thomas Fund for Cancer Prevention (to D.E. Brenner); NCI T32 Training Grant: 5 T32 CA 9357-32 (to Kathleen Cooney); grant #UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS, NIH Clinical and Translational Science (CTSA) program and cosponsored by the Center for Basic and Translational Research on Disorders of the Digestive System at Rockefeller University); grant NIH/NCI R01CA154481, the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick), the Breast Cancer Research Foundation (to A.J. Dannenberg), and the Sackler Center for Biomedicine and Nutrition Research (to P.R. Holt).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Bhaskaran K, Douglas I, Forbes H, Dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014 doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. International journal of cancer Journal international du cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 3.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 4.Brisken C, Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem cell reviews. 2007;3:147–56. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 5.Inadera H. The usefulness of circulating adipokine levels for the assessment of obesity-related health problems. International journal of medical sciences. 2008;5:248–62. doi: 10.7150/ijms.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci R, Bevilacqua F. The potential role of leptin and adiponectin in obesity: a comparative review. Veterinary journal. 2012;191:292–8. doi: 10.1016/j.tvjl.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Gnerlich JL, Yao KA, Fitchev PS, Goldschmidt RA, Bond MC, Cornwell M, et al. Peritumoral expression of adipokines and fatty acids in breast cancer. Annals of surgical oncology. 2013;20(Suppl 3):S731–8. doi: 10.1245/s10434-013-3274-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 10.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 11.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:1447–53. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. International journal of cancer Journal international du cancer. 2006;118:1414–9. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 14.Ollberding NJ, Kim Y, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer prevention research. 2013;6:188–95. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocrine-related cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Q, Hursting SD, Reizes O. Leptin regulates cyclin D1 in luminal epithelial cells of mouse MMTV-Wnt-1 mammary tumors. Journal of cancer research and clinical oncology. 2012;138:1607–12. doi: 10.1007/s00432-012-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, et al. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocrine-related cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. The Journal of clinical endocrinology and metabolism. 2004;89:1102–7. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 19.Gross AL, Newschaffer CJ, Hoffman-Bolton J, Rifai N, Visvanathan K. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:1319–24. doi: 10.1158/1055-9965.EPI-12-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian YF, Chu CH, Wu MH, Chang CL, Yang T, Chou YC, et al. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocrine-related cancer. 2007;14:669–77. doi: 10.1677/ERC-06-0089. [DOI] [PubMed] [Google Scholar]
- 21.Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, et al. Plasma adiponectin concentrations and risk of incident breast cancer. The Journal of clinical endocrinology and metabolism. 2007;92:1510–6. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:5699–704. [PubMed] [Google Scholar]
- 23.Jarde T, Caldefie-Chezet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, et al. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocrine-related cancer. 2009;16:1197–210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine reviews. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. British journal of cancer. 2008;98:370–9. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, et al. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PloS one. 2009;4:e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Rosen JM. Stem/progenitor cells in mouse mammary gland development and breast cancer. Journal of mammary gland biology and neoplasia. 2005;10:17–24. doi: 10.1007/s10911-005-2537-2. [DOI] [PubMed] [Google Scholar]
- 28.Fu N, Lindeman GJ, Visvader JE. The mammary stem cell hierarchy. Current topics in developmental biology. 2014;107:133–60. doi: 10.1016/B978-0-12-416022-4.00005-6. [DOI] [PubMed] [Google Scholar]
- 29.Shah M, Allegrucci C. Keeping an open mind: highlights and controversies of the breast cancer stem cell theory. Breast cancer. 2012;4:155–66. doi: 10.2147/BCTT.S26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wicha MS, Schwartz SJ, Sun D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. The Journal of nutritional biochemistry. 2011;22:799–806. doi: 10.1016/j.jnutbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu SM. Origin of cancers. Clinical perspectives and implications of a stem-cell theory of cancer. Cancer treatment and research. 2010;154:v–239. doi: 10.1007/978-1-4419-5968-3_1. [DOI] [PubMed] [Google Scholar]
- 32.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell proliferation. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw FL, Harrison H, Spence K, Ablett MP, Simoes BM, Farnie G, et al. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. Journal of mammary gland biology and neoplasia. 2012;17:111–7. doi: 10.1007/s10911-012-9255-3. [DOI] [PubMed] [Google Scholar]
- 35.Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogenesis: implications for prevention and treatment. Stem cell reviews. 2005;1:207–13. doi: 10.1385/SCR:1:3:207. [DOI] [PubMed] [Google Scholar]
- 36.Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. Journal of mammary gland biology and neoplasia. 2005;10:75–86. doi: 10.1007/s10911-005-2542-5. [DOI] [PubMed] [Google Scholar]
- 37.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem cell reports. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Kirkham JC, McCormack MC, Medina MA, Nicholls AM, Randolph MA, et al. A novel approach to adipocyte analysis. Plastic and reconstructive surgery. 2012;129:380–7. doi: 10.1097/PRS.0b013e31823aea29. [DOI] [PubMed] [Google Scholar]
- 40.Liuzzi A, Savia G, Tagliaferri M, Lucantoni R, Berselli ME, Petroni ML, et al. Serum leptin concentration in moderate and severe obesity: relationship with clinical, anthropometric and metabolic factors. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23:1066–73. doi: 10.1038/sj.ijo.0801036. [DOI] [PubMed] [Google Scholar]
- 41.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA oncology. 2015;1:611–21. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diehn M, Cho RW, Clarke MF. Therapeutic implications of the cancer stem cell hypothesis. Seminars in radiation oncology. 2009;19:78–86. doi: 10.1016/j.semradonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. Journal of cellular and molecular medicine. 2009;13:2236–52. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montales MT, Rahal OM, Kang J, Rogers TJ, Prior RL, Wu X, et al. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33:652–60. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- 45.Grudzien P, Lo S, Albain KS, Robinson P, Rajan P, Strack PR, et al. Inhibition of Notch signaling reduces the stem-like population of breast cancer cells and prevents mammosphere formation. Anticancer research. 2010;30:3853–67. [PubMed] [Google Scholar]
- 46.Santillan-Benitez JG, Mendieta-Zeron H, Gomez-Olivan LM, Torres-Juarez JJ, Gonzalez-Banales JM, Hernandez-Pena LV, et al. The tetrad BMI, leptin, leptin/adiponectin (L/A) ratio and CA 15-3 are reliable biomarkers of breast cancer. Journal of clinical laboratory analysis. 2013;27:12–20. doi: 10.1002/jcla.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alikasifoglu A, Gonc N, Ozon ZA, Sen Y, Kandemir N. The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. Journal of clinical research in pediatric endocrinology. 2009;1:233–9. doi: 10.4274/jcrpe.v1i5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otvos L, Jr, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, et al. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC biotechnology. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otvos L, Jr, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. European journal of cancer. 2011;47:1578–84. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Otvos L, Jr, Kovalszky I, Scolaro L, Sztodola A, Olah J, Cassone M, et al. Peptide-based leptin receptor antagonists for cancer treatment and appetite regulation. Biopolymers. 2011;96(2):117–25. doi: 10.1002/bip.21377. [DOI] [PubMed] [Google Scholar]