Abstract
This study was conducted to determine the safety and efficacy of the green-tea derived Polyphenon E (Poly E) in patients with Barrett’s Esophagus (BE). Subjects were randomized to a 6-month, twice daily (BID) oral treatment of placebo or Poly E (200 mg, 400 mg, or 600 mg). Endoscopic evaluation, including biopsies, was performed before and after treatment. The primary objective was to demonstrate safety; secondary objectives investigated catechin accumulation and effects in clinical specimens. Of the 44 enrolled subjects, 11 received placebo, and 33 received Poly E. No dose-limiting toxicities were encountered, and a maximum tolerated dose (MTD) was not reached. The recommended phase 2 dose was 600 mg BID. The most common treatment-related adverse events (AEs) in Poly E-treated subjects were grade 1–2 nausea, grade 1 belching, and grade 1 LDH elevation. No treatment-related AEs were reported in placebo-treated subjects, aside from grade 1 laboratory abnormalities. Pill counts and subject diaries were not consistently collected, and compliance was difficult to determine. However, based on an intention-to-treat analysis there was a significant relationship between Poly E dose and esophageal EGCG level – mean changes (pmol/g) of 0.79 (placebo), 6.06 (200 mg), 35.67 (400 mg), and 34.95 (600 mg); p=0.005. There was a possible relationship between Poly E dose and urine PGE-M concentration. In conclusion, Poly E was well-tolerated, and treatment with Poly E (400 mg and 600 mg) but not Poly E (200 mg) or placebo resulted in clinically relevant and detectable EGCG accumulation in the target organ, esophageal mucosa.
Keywords: Barrett’s Esophagus, Polyphenon E, Chemoprevention, Green tea, Esophageal cancer
INTRODUCTION
Population studies have demonstrated an inverse relationship between green tea consumption and incidence and mortality rates for a variety of human malignancies, including those of the breast, esophagus, stomach, and hematologic system (1, 2). In addition, there is an extensive literature demonstrating the anti-cancer activity of several green tea-derived compounds or catechins, including epigallocatechin gallate (EGCG), the major biologically active component of green tea, in a wide variety of cell culture (including esophageal cancer) and animal models (1, 3–5). Clinical trials of green tea preparations have demonstrated clinical responses in patients with chronic lymphocytic leukemia (CLL) and precancerous lesions of the cervix, prostate, and colon (6–9).
Polyphenon E (Poly E) is a standardized botanical drug substance that is extracted from green tea leaves and contains a defined mixture of catechins (80–98% total catechins by weight) – EGCG (50–75%) and other catechins (≤ 12%), including epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and gallocatechin gallate (GCG). Each 200 mg capsule of Poly E contains 200 mg of EGCG. Poly E and EGCG have been formulated into capsules by the Chemoprevention Agent Development Research Group at the National Cancer Institute (NCI) and are undergoing active clinical investigation for the prevention and treatment of a variety of cancers (9–10).
MATERIALS AND METHODS
STUDY DESIGN
This was a randomized, double-blinded, multicenter phase 1b clinical trial that enrolled patients with BE with or without low-grade dysplasia (LGD) from Columbia University Medical Center (CUMC; New York, NY), the Weill-Cornell Medical Center (New York, NY), and the MD Anderson Cancer Center (MDACC; Houston, TX). Subjects were randomized to a 6-month treatment course of either placebo or Poly E (200 mg BID, 400 mg BID, 600 mg BID; Figure 1). Endoscopic evaluation including biopsy was performed at baseline and after 6 months of treatment, and subjects were followed for an additional six months after completing treatment.
Figure 1. Study Schema.
Subjects were randomized to a 6-month, twice daily (BID) oral treatment of either placebo or Poly E (200 mg, 400 mg, 600 mg dose cohorts). Endoscopic evaluation, including multiple biopsies, was performed at baseline and after treatment.
The protocol [MDA03-1-01] was approved by the Institutional Review Board at each participating institution and was conducted in accordance with the ethical principles of the Declaration of Helsinki. All study subjects provided written informed consent before participating in the trial. This clinical trial was registered at ClinicalTrials.gov with accession number NCT00233935.
The trial was designed jointly by the academic authors and representatives of the NCI Division of Cancer Prevention. The corresponding academic author prepared an initial draft of the manuscript, and all authors contributed to subsequent drafts and made the decision to submit the manuscript for publication.
ELIGIBILITY
Eligibility criteria included age 18 years or older; histologically-confirmed, Barrett’s metaplasia (with or without LGD or indeterminate for dysplasia); willingness to abstain from tea consumption during treatment; and adequate marrow and organ (including hepatic) function. Subjects with high-grade dysplasia, invasive carcinoma of the esophagus, or other active malignancy within 5 years were not eligible for enrollment. Central pathology review of all biopsies by 2 independent pathologists (HHH and HR, CUMC) was required for enrollment. All cases for which there was disagreement regarding the presence of LGD were reviewed by a third pathologist (HR, CUMC).
STUDY OBJECTIVES
The primary objective of this study was to demonstrate the safety (i.e., determine the maximum tolerated dose (MTD)) of using Poly E over a six-month period in patients with BE with or without LGD.
Secondary objectives investigated the pharmacodynamic properties of Poly E (i.e., accumulation of catechin constituents (e.g., EGCG) in esophageal tissue) and dose-related efficacy – reducing metaplasia and dysplasia; modulating PGE-M in urine; and regulating protein expression in esophageal mucosa.
SAFETY
Safety was assessed by monitoring clinical and laboratory parameters at baseline, every 2 weeks during the first month, then monthly for the duration of the study.
Adverse events (AEs), weight, vital signs (heart rate, blood pressure, respiratory rate, temperature), and clinical laboratory changes (hematology, biochemistry, liver and renal laboratory tests, and urinalysis) were recorded at each clinic visit.
Toxicities were graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (11).
Toxicities were managed generally by interrupting treatment until resolution to grade 1 or baseline. Study drug may have been held or discontinued, but dose reductions were not allowed. For grade 1 elevations of liver laboratory tests (total bilirubin, alkaline phosphatase, AST (SGOT), ALT (SGPT)), study drug was held until recovery to normal. For grade 2–4 elevations, study drug was discontinued and laboratory tests were monitored until resolution to normal. For all other grade 3–4 toxicities, study drug was held, and the decision for resumption or discontinuation was at the discretion of the Study Investigator.
EFFICACY
Surveillance endoscopy was performed at baseline and after 6 months of Poly E treatment. Standard four-quadrant biopsies were taken every 2 cm throughout the longest segment of BE using standard biopsy forceps (approximately 3 mm by 1 mm) and were processed by routine methods (i.e., formalin fixation and paraffin embedding) for histological evaluation (including characterization of the degree of dysplasia). (For patients with “short-segment” BE (≤ 3 cm), biopsies were taken every 1 cm.) An additional two biopsies were taken from each level, frozen immediately in liquid nitrogen in snap-cap vials, and stored at −70°F.
Esophageal tissue levels of catechins
Fresh frozen esophageal tissue was collected at baseline and after treatment to measure levels of Poly E catechin constituents (EGCG, EGC, EC). Tissue specimens were embedded in cryopreservation medium, placed in snap-capped tubes, and then frozen immediately in liquid nitrogen. Samples were analyzed using a validated method as previously described (12). Briefly, sample extracts were prepared using methanol/ethyl acetate and analyzed on a high-performance liquid chromatography (HPLC) system comprised of an ESA coulochem electrode array system. Catechin concentrations were assayed and interpreted by an observer blinded to the treatment (placebo versus Poly E) using previously described methods (12).
Endoscopic measurements and pathological classification
Visual endoscopic measurements were recorded during the baseline and Month 6 endoscopies. The length of Barrett’s epithelium was measured from the squamocolumnar junction to the esophago-gastric junction at the top of gastric folds. Biopsies were evaluated for the presence of metaplasia and dysplasia using currently accepted guidelines. Efficacy was evaluated by measuring histological characteristics in esophageal mucosa (disease regression, stabilization, or progression) obtained in sequential biopsy specimens. The pathologist was blinded to the treatment (placebo versus Poly E).
Urine PGE-M measurement
Urine was collected during the Month 3 or 4 clinic visit, and then stored at −70°F. PGE-M concentrations were measured in the Eicosanoid Core Laboratory at Vanderbilt University using previously described methods (13). Briefly, urine samples were treated with methyloxime HCl to convert PGE-M to the O-methyloxime derivative. The methoximated PGE-M was extracted, applied to a C-18 Sep-Pak, and eluted with ethyl acetate. Liquid chromatography was performed using a Waters Acquity UPLC fitted with an Acquity BEH C18 UPLC column (2.0 × 50mm, 1.7 microm) coupled to a Thermo Scientific Quantum Vantage triple quadrupole mass spectrometer. Quantification of PGE-M was calculated by radiometric determinations of unlabeled:labeled peak areas corresponding to both precursor and product ions. Urinary creatinine levels were measured using a test kit from Enzo Life Sciences, Inc. (Farmingdale, NY). The urinary PGE-M level in each sample was normalized using the urinary creatinine level of the sample and expressed in ng/mg creatinine. PGE-M concentrations were assayed and interpreted by an observer blinded to the treatment (placebo versus Poly E).
Reverse Phase Protein Arrays
Protein and phospho-protein expression were measured in paraffin-embedded esophageal mucosal tissue using functional proteomics (Reverse Phase Protein Arrays; RPPA) in collaboration with the Functional Proteomics RPPA Core Facility at the MDACC (Houston, TX). Protein from paraffin blocks or immunoblanks were extracted at CUMC and combined (ng protein lysates) according to procedures provided by the RPPA Shared Resource (14). Protein lysates were submitted to the RPPA Core Facility for analysis.
STATISTICAL ANALYSIS
Subjects were enrolled in a staggered fashion, and randomization was conducted in blocks of 4 subjects, such that 1 out of every 4 consecutive subjects was randomized to the placebo, and the other 3 subjects received one of the Poly E doses (200 or 400 or 600 mg) according to the time-to-event continual reassessment method (TITE-CRM; 15). The initial seven recruited subjects would be assigned to either placebo or the lowest dose of Poly E. Therefore, a total of 30 subjects were planned to receive a dose of Poly E. This sample size would ensure that estimates of any binary variable, including incidence of toxicity, would have a 95% confidence interval (CI) of width < 0.36.
The MTD was defined as a dose that causes 25% dose-limiting toxicity (DLT) that occurs during the six-month treatment. A DLT was defined according to the NCI CTCAE (Version 3.0): any grade 3 or higher toxicity, or any grade 2 or higher hepatic or renal toxicity. The majority of DLTs were expected to occur during the first month of treatment. However, if any DLT were observed after the first month, the TITE-CRM would automatically consider this DLT and de-escalate the treatment dose assigned to the next recruited subject. The decision for escalation was made after every 5 subjects were evaluated for toxicity at a single dose. Every subject was followed for the entire 6-month treatment period for toxicity evaluation.
All subjects were evaluable for toxicity from the time of their first dose of study drug (Poly E or placebo). All subjects included in the study were also assessed for response to intervention. The placebo group provided the background rate of lower grade toxicities as well as important reference levels for all biomarkers. Due to the small sample size, parameter estimation was not considered in the analysis of the efficacy endpoints. Dose-response relationships were explored in an estimation framework. Generalized linear models were used to establish the dose-response relationship of each outcome. Logistic regression was used for binary outcomes.
For the dose escalation and DLT (primary) endpoints, subjects who demonstrated at least 80% compliance with the planned dose intervention were included in the analysis. For the efficacy (secondary) endpoints, all non-compliant subjects were analyzed according to the intent-to-treat principle. Compliance was defined as completion of the entire 6-month treatment course. Measures of compliance included completion dates of planned dose intervention, pill counts determined from pharmacy records, and daily subject diaries. Subjects in the 600 mg cohort who were not evaluable based on incompletion of the planned dose intervention were replaced by additional recruitment.
Reverse Phase Protein Arrays
A Linear Models for Microarray Data (LIMMA) moderated t-statistic was used to test the null hypothesis that the difference between the change in protein concentration upon treatment with Poly E (600 mg) versus placebo: (600time2-600time1)=(0time2-0time1) (16–17). P-values were corrected for multiple testing using the false discovery rate (FDR) according to the method of Benjamini and Hochberg (18).
RESULTS
Between November 2005 and November 2009, 261 subjects were screened for study enrollment at 3 centers – CUMC, Weill-Cornell Medical Center, and the MDACC. Sixty-six subjects were consented, and 44 were randomized to treatment with either placebo or Poly E. The target sample size was 40 evaluable subjects for dose escalation (i.e., 80% compliance with the intended treatment course). During the study, 5 subjects were not evaluable for dose escalation – 2 subjects (200 mg cohort – pregnancy, incomplete course), 1 subject (400 mg cohort – elevated lipase), and 4 subjects (600 mg cohort – 2 subjects with persistent grade 1 ALT elevation, 1 lost to follow-up, and 1 subject with nausea). Thus, 4 subjects in the 600 mg cohort were replaced. No study-related procedures were begun in any subject prior to signing of an informed consent document. Pill counts and daily subject diaries were not consistently collected, and compliance was difficult to determine for the majority of subjects.
A total of 44 subjects were enrolled in a staggered fashion and randomization was conducted in blocks of 4 subjects, such that 1 in each block received placebo and 3 received Poly E (200 mg, 400 mg, or 600 mg) according to the TITE-CRM. Of the 44 randomized subjects, 11 subjects received placebo, and 33 subjects received Poly E – 200 mg (6 subjects), 400 mg (7 subjects), 600 mg (20 subjects).
SUBJECT CHARACTERISTICS
Table 1 shows the baseline characteristics of the 44 randomized subjects. The majority of subjects were male (75%), Caucasian (100%), and non-Hispanic (93%). More subjects in both treatment groups did not consume green tea, and the majority of subjects in both groups had metaplasia only and no evidence of esophagitis at baseline endoscopy. There were no significant differences in any baseline characteristic between Poly E- and placebo-treated subjects.
Table 1.
Baseline demographic and endoscopy characteristics
| Characteristic | Total (n=44) | Poly E (n=33) | Placebo (n=11) | p-value | |
|---|---|---|---|---|---|
| Age | Median (range) | 61.5 (36,81) | 61 (36,81) | 62 (50,72) | 0.98 |
| Gender | Female | 11 | 10 (30%) | 1 (9%) | 0.24 |
| Male | 33 | 23 (70%) | 10 (91%) | ||
| Exposures | Never Smokers | 28 | 23 (70%) | 5 (45%) | 0.17 |
| Ever Smokers | 16 | 10 (30%) | 6 (55%) | ||
| Green Tea – No | 36 | 28 (85%) | 8 (73%) | 0.39 | |
| Green Tea – Yes | 8 | 5 (15%) | 3 (27%) | ||
| Baseline EGD | Esophagitis | 1 | 0 | 1 (9%) | 0.25 |
| No Esophagitis | 43 | 33 (100%) | 10 (91%) | ||
| Metaplasia only | 40 | 31 | 9 | ||
| Dysplasia | 4 | 2 | 2 |
SAFETY
No DLTs were encountered in any subject during the trial, and a MTD was not reached. A total of 631 AEs were reported among the 44 randomized, Poly E-treated (451 AEs) and placebo-treated (180 AEs) subjects. These included AEs that were present during the baseline visit, before the start of study treatment (data not included). Conservative reporting of AEs (including those at baseline) was followed, due to the nature of the study indication (i.e., cancer prevention), as opposed to the more routine reporting of AEs in chemotherapy trials (i.e., AEs that were absent at baseline prior to the initiation of study treatment or present that worsened in either intensity after initiation of study treatment; i.e., “non-baseline AEs”). There was no difference in the type or severity of total AEs (including baseline abnormalities and on-study drug abnormalities) as compared to those AEs that occurred after starting study drug (non-baseline AEs; data not included). The large majority of reported AEs were liver, pancreas, and renal laboratory abnormalities and were reported in both Poly E-treated and placebo-treated subjects. The most common non-laboratory, clinical AEs were abdominal pain/discomfort, belching, diarrhea, loss of energy, nausea, and upper respiratory infection. There was only 1 grade 3 AE (elevated lipase; 400 mg cohort) and 1 grade 4 AE (non-small cell lung cancer; placebo; onset 1 year after study treatment). Both were assessed as unrelated to study drug. The lipase measurement was repeated 15 days later and was within normal limits. All other AEs were grade 1 or 2. One case of persistent nausea (600 mg cohort) and 2 cases of persistent grade 1 elevated ALT (both in 600 mg cohort) led to study drug discontinuation. One subject (200 mg) developed pregnancy during the trial, which led to study drug discontinuation. None of the remaining subjects, including 20 subjects who were treated in the 600 mg cohort, discontinued Poly E for an AE.
The most common AEs reported in Poly E-treated (vs placebo-treated) subjects were laboratory abnormalities – low glucose (58% vs 18%), low chloride (42% vs 54%), and elevated BUN (39% vs 9%). All were grade 1 in severity. Grade 1 elevations in creatinine were reported in 18% of Poly E-treated subjects and no placebo-treated subjects. There were no grade 2 or higher elevations in either BUN or creatinine. The most common non-laboratory AEs reported in Poly E-treated (vs placebo-treated) subjects were gastrointestinal (GI) – nausea (21% vs 0%), abdominal pain/discomfort (15% vs 9%), and diarrhea (12% vs 9%). Elevations in ALT (grade 1) were reported in 9% of Poly E-treated and 17% of placebo-treated subjects; elevations in AST (all grade 1) were reported in 9% of Poly E-treated and 27% of placebo-treated subjects.
A total of 26 AEs in 13 subjects were considered as possibly or probably related to study treatment (Table 2). The most common treatment-related AEs reported in Poly E-treated subjects were nausea (15%) and abdominal pain (including discomfort and gas; > 9%). No treatment-related AEs were reported in placebo-treated subjects, aside from grade 1 laboratory abnormalities. Treatment-related AEs (GI symptoms, fatigue, liver laboratory abnormalities; all grade 1) were reported in only 3 of the 20 subjects (15%). Thus, although we did not achieve a MTD, the recommended phase 2 dose (RP2D) was determined to be the highest dose tested – Poly E 600 mg BID.
Table 2.
Treatment-related Adverse Events
| Treatment | Subject | AE (grade) |
|---|---|---|
| Placebo | 1 | ELEVATED SERUM AST (1) |
| 2 | MILDLY ELEVATED AST (1) | |
| 3 | ELEVATED SERUM ALT (1), ELEVATED SERUM ALT (1), ELEVATED SERUM AMYLASE (2) | |
| 4 | ELEVATED SERUM AST (1) | |
| Poly E 200 mg | 5 | NAUSEA (1), HYPOTHYROIDISM (2) |
| 6 | BELCHING (1) | |
| 7 | BELCHING (1) | |
| Poly E 400 mg | 8 | NAUSEA (2), VOMITING (2) |
| 9 | ELEVATED SERUM ALK PHOS (1), | |
| 10 | ELEVATED SERUM LDH (1), NAUSEA (1) | |
| Poly E 600 mg | 11 | DIARRHEA (1), GAS (1), NAUSEA (1; 2 AEs), SOUR TASTE (1; 2 AEs), STOMACH DISCOMFORT (1), STOMACH PAIN (1), LOSS OF ENERGY (2) |
| 12 | ELEVATED SERUM AST (1) | |
| 13 | ELEVATED SERUM LDH (1) |
EFFICACY
Poly E catechin accumulation
Fresh frozen esophageal tissue was collected at baseline and after treatment to measure levels of Poly E catechin constituents (EGCG, EGC, EC). The study required that study treatment should continue until the day of the post-treatment endoscopy. However, in 15 subjects, treatment completion occurred prior to the post-treatment endoscopy (< 2 weeks for 9 subjects and > 2 weeks for 6 subjects) for scheduling and other reasons that were not reported. Thus, continued exposure to study treatment was absent prior to the post-treatment endoscopy. In addition, post-treatment endoscopy specimens were missing for 10 subjects.
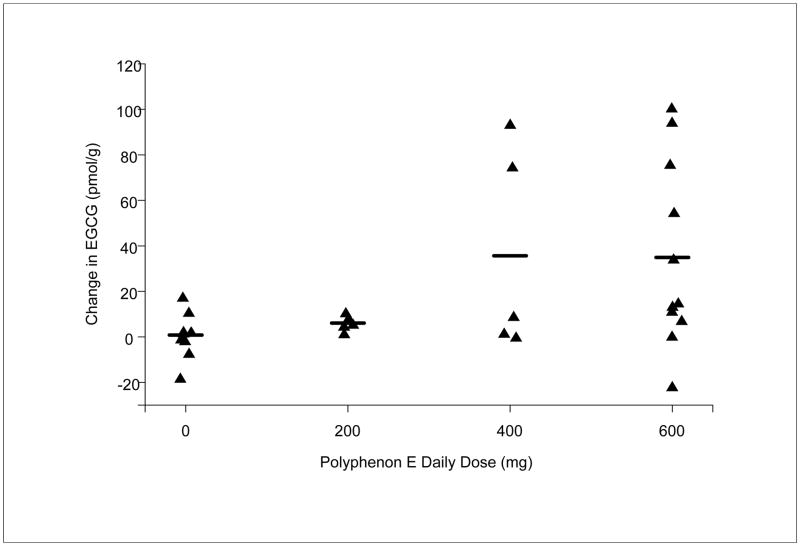
Regardless of these missing and delayed endoscopy specimens and based on an intention-to-treat analysis, there was a dose-exposure relationship between the dose of Poly E and mean change in EGCG concentration (pmol/g) between the baseline and post-treatment specimens – 0.79 (placebo; n=10), 6.06 (200 mg; n=5), 35.67 (400 mg; n=5), and 34.95 (600 mg, n=11); p=0.005, Spearman correlation test (Figure 2). Thus, treatment with Poly E (400 mg and 600 mg) but not Poly E (200 mg) or placebo resulted in detectable EGCG accumulation in the target organ, esophageal mucosa. Of note, mean EGCG concentrations (range; standard deviation) at baseline and post-treatment were 7.54 (0–18.87; 6.50; placebo), 1.70 (0–3.96; 1.71; 200 mg), 2.52 (1.27–5.16; 1.59; 400 mg), 3.70 (0–21.96; 6.65; 600 mg) and 8.33 (0–23.21; 7.14; placebo), 7.76 (3.3–12.19; 3.63; 200 mg), 38.19 (1.59–94.99; 45.41; 400 mg), 38.65 (0–110.25; 39.40; 600 mg), respectively.
Figure 2. Mean EGCG concentrations (pmol/g) in esophageal tissue specimens.
Fresh frozen esophageal tissue was collected at baseline and after 6 months of treatment with Placebo or Poly E (200 mg, 400 mg, 600 mg). ECGC concentration was measured by high-performance liquid chromatography. EGCG concentration was assayed and interpreted by an observer blinded to the treatment (placebo versus Poly E).
There were 6 subjects who demonstrated high accumulations of Poly E (i.e., greater than 50 pmol/g) – 2 subjects in the 400 mg cohort and 4 subjects in the 600 mg cohort. Of note, the post-treatment endoscopy of 4 of the 6 high-accumulating subjects occurred on the same day as the last treatment dose; for the 2 other subjects, the post-treatment endoscopy occurred 20 days before and 9 days after the last treatment dose. Intervals between the dates of the last Poly E dose and post-treatment endoscopy (i.e., “days off” of Poly E prior to biopsy) were determined to investigate if an imbalance in continued Poly E exposure contributed to the observed dose-exposure relationship. The mean days off (+/− standard deviations) were greater in the placebo (20.7 +/− 62.31) and 600 mg (13.64 +/− 35.03) cohorts compared with the 200 mg (4.2 +/− 7.26) and 400 mg (5.2 +/− 7.66) cohorts. However, decreased EGCG with lack of continued Poly E (i.e., days off) administration was not observed in the 600 mg cohort. These results suggest that study drug accumulation can occur in the target organ despite interrupted drug exposure.
Endoscopic measurements and pathological classification
Treatment with Poly E or placebo for 6 months did not reduce the length of Barrett’s Epithelium. The median difference in length was 0 cm in all four cohorts. Furthermore, there were no significant changes in histological characteristics between the dose cohorts, although the large majority of baseline specimens contained metaplasia only (Table 1). In one subject (placebo), high-grade dysplasia was observed in the post-treatment endoscopy specimen. The length of Barrett’s epithelium for this subject was 2 cm.
Urine PGE-M measurement
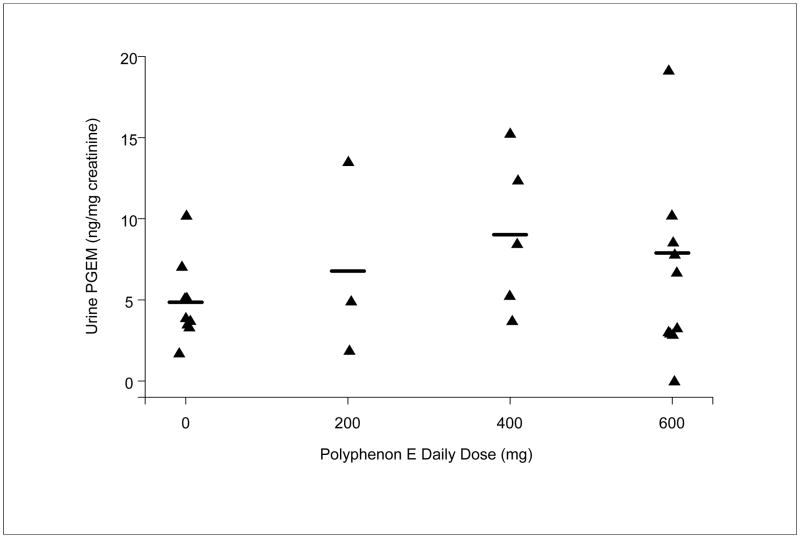
Urine specimens were collected for each subject during the Month 3 or 4 clinic visit. Urine specimens were not collected from 15 subjects. The mean urine PGE-M concentrations (ng/mg creatinine) with treatment were 4.86 (placebo; n=9), 6.78 (200 mg; n=3), 9.02 (400 mg; n=5), and 7.89 (600 mg; n=11), and there was a possible relationship between the dose of Poly E and urine PGE-M concentration (Figure 3). This trend was not statistically significant (data not included). There were 5 subjects with high concentrations of PGE-M (i.e., greater than 10 ng/mg creatinine).
Figure 3. Mean PGE-M concentrations (ng/mg creatinine) in urine specimens.
Urine specimens were collected after 3–4 months of treatment with Placebo or Poly E (200 mg, 400 mg, 600 mg). PGE-M concentration was measured by liquid chromatography. PGE-M concentration was assayed and interpreted by an observer blinded to the treatment (placebo versus Poly E).
Reverse Phase Protein Arrays
Protein was extracted from formalin-fixed, paraffin-embedded specimens from all enrolled subjects. However, amounts of protein adequate for array hybridization were obtained from only subjects enrolled at CUMC and the MDACC (data not included). Furthermore, unsupervised clustering demonstrated that samples obtained at CUMC clustered separately from those obtained at the MDACC (data not included), suggestive of a “batch effect” in the analysis. Thus, only samples from CUMC-enrolled subjects, which provided a greater sample size than those from MDACC-enrolled subjects, were included in the comparative proteomic analysis (3 placebo, 8 Poly E 600 mg) to avoid detection of wide signaling distributions between the 2 enrolling site cohorts. Treatment with Poly E or placebo did not lead to any statistically significant changes in mucosal protein expression.
DISCUSSION
Patients with BE have an increased risk of developing esophageal cancer, and there are currently no proven approaches for preventing cancer in this population (19). Thus, patients must undergo aggressive surveillance endoscopy involving multiple biopsies.
We conducted a phase 1b study of Poly E in subjects with BE with or without LGD. BE patients often have chronic gastroesophageal reflux, including symptoms of nausea, vomiting, and abdominal pain. Thus, we designed a double-blinded, placebo-controlled, randomized trial to differentiate GI side effects that are commonly observed with other green tea preparations (20) from those existing at baseline. While nausea and abdominal pain/discomfort were observed in this study, they were generally mild or moderate and did not lead to treatment discontinuation. There were few AEs that were assessed as related to Poly E; however, persistent grade 1 nausea and persistent grade 1 ALT elevations did lead to treatment discontinuation. Thus, persistent low-grade toxicities are certainly relevant in the non-cancer population who would be expected to take chemoprevention treatment chronically.
Since this was a chemoprevention and not cancer treatment study, investigators were guided to report all AEs and laboratory abnormalities as AEs, including those present before study treatment and any laboratory result that was higher than the upper limit of normal. Thus, the very large number of grade 1 laboratory abnormalities in all cohorts, including placebo, were the most common class of AEs reported in this trial. No significant differences in AEs were apparent between Poly E- or placebo-treated subjects, although this study may not have been powered to detect differences between cohorts due to small sample sizes.
We did not encounter any DLTs in this study, and therefore a MTD was not reached. Poly E was tolerable up to the maximum dose tested (600 mg BID), in which treatment-related AEs (all grade 1 GI symptoms, fatigue or liver laboratory abnormalities) were reported in only 3 of 20 subjects (15%). Thus, although we did not achieve a MTD, the RP2D was determined to be 600 mg BID. We would not recommend a higher dose that would potentially lead to a higher frequency and/or grade of GI symptoms and liver test abnormalities. In a similar phase Ib trial of breast cancer patients, which included the same placebo-controlled study design and a higher dose of Poly E (800 mg BID), several DLTs were observed – GI bleeding, weight gain, insomnia, indigestion, and grade 3 liver test abnormalities (10). In this study, the RP2D was also determined to be 600 mg BID. Interestingly, nausea and indigestion appeared to be more frequent, which could be related to the common use of proton pump inhibitors among BE patients, as well as differences in AE reporting between the trials. Both trials contained small sample sizes (40 patients in the breast cancer trial), which would make comparisons of safety and tolerability difficult.
Treatment with Poly E or placebo did not reduce the length of Barrett’s Epithelium and did not significantly alter the expression of any potential protein biomarker. This could be related to the duration of therapy (i.e., 6 months), completion of study treatment prior to post-treatment endoscopy (15 subjects), and issues with compliance. Pill counts and daily subject diaries were not consistently collected in this trial, and compliance was difficult to determine for the majority of subjects.
Despite these limitations in trial conduct, treatment with Poly E (400 mg and 600 mg) but not Poly E (200 mg) or placebo resulted in detectable EGCG accumulation in the target organ, esophageal mucosa, and high accumulation in 6 subjects (i.e., > 50 pmol/g). In contrast, in a recent trial of Poly E in men with prostate cancer, treatment with a lower dose (800 mg) and shorter course (3–6 weeks) resulted in low to undetectable green tea polyphenol levels in prostatectomy tissue, and levels of EGC were detectable in prostate tissue (range 17.77 to 59.67 pmol/g) in 5 of 15 subjects (21). There are no reported studies which have measured EGCG in human esophageal tissue. In the current study, EGCG concentrations of baseline specimens were < 10 pmol/g (within the “undetectable” range of the prostate cancer study) in all except 3 of the subjects – 2 in the placebo and 1 in the 600 mg cohort; and post-treatment EGCG concentrations were in the undetectable range in the following subjects – 5 of 10 (placebo), 3 of 5 (200 mg), 2 of 5 (400 mg), and 3 of 11 (600 mg). Furthermore, mean post-treatment EGCG concentrations were > 10 pmol/g in only the 400 mg and 600 mg cohorts. Perhaps if study treatment were extended to a year or longer, continued exposure could lead to even higher achievable tissue concentrations and consequently reduction in the length and/or severity of dysplasia of Barrett’s epithelium and/or significant changes in protein expression.
This trial also demonstrated a non-significant trend between Poly E dose and PGE-M concentration. Cyclooxygenase-2 (COX-2) and prostaglandin E2 receptors are upregulated in BE, and Poly E has been shown to decrease COX-2 in colonic mucosa in animal models (22–23). Measurement of PGE2 in tissues is invasive, and PGE-M (24) has been shown to be an index of systemic PGE2 production (25–27). Thus, urine PGE-M may be a useful and non-invasive biomarker for assessing chemoprevention activity. The current cohort of 28 subjects was likely not powered to detect a relationship between urine PGE-M levels and Poly E treatment, given the normal inter-subject variation in baseline PGE-M levels and presence of confounders, such as gender, smoking status and use of non-steroidal, anti-inflammatory drugs (28).
This study demonstrated the feasibility of conducting phase 1b studies in BE using endoscopic biopsy specimens and paraffin-embedded specimens for proteomic analysis. There did not appear to be differences in the size of forceps, number of collected samples, or methods of specimen processing and storage between the 3 enrolling sites, and the reason for lower extracted protein concentrations in some specimens was not clear. The reason for the distinct unsupervised, institution-dependent clustering was also not clear, since protein extraction of all paraffin specimens was conducted at CUMC, and sample preparation and array hybridization occurred at the same time and during the same run at the RPPA facility. Methods should be investigated further and standardized if similar exploratory studies are planned across multiple institutions.
In a recent study, 98 subjects with cervical intraepithelial neoplasia were treated with Poly E (800 mg daily) or placebo for 4 months (29). There were no reported differences in efficacy, possibly due to the lower dose and short duration of treatment. Conversely, a recent phase 2 trial of Poly E was reported, in which 42 patients with CLL were treated with a higher dose of Poly E (2000 mg BID) for up to 6 months (9). In this study, biological responses were seen in the majority (69%) of patients, and Poly E was well tolerated. AEs consisted of GI events, liver test elevations, and fatigue. It may be difficult to investigate this higher dose in BE patients, given their baseline GI symptoms, unless if perhaps investigated in combination with proton pump inhibitors.
In conclusion, we completed a prospective, phase 1b, placebo-controlled trial of Poly E in patients with BE. Poly E was well-tolerated at all dose levels, and the RP2D dose (600 mg BID) is consistent with the recently reported Poly E breast cancer study (10). Poly E treatment had measurable biological effects in both esophageal tissue and urine.
Acknowledgments
Supported by NCI, DCP Contract N01-CN-035159 to the UT MD Anderson Early Phase Chemoprevention Consortium (P.H. Brown/S.M. Lippman)
This trial was supported by the National Cancer Institute Division of Cancer Prevention – NCI, DCP Contract N01-CN-035159 to the UT MD Anderson Early Phase Chemoprevention Consortium. We would like to acknowledge additional investigators at Columbia University Medical Center (Katherine Crew, Heidrun Rotterdam, Helen Remotti, Shing Lee, Bin Cheng, Kevin Bukowski, Victor Grann, M. Mansukhani), the Weill-Cornell Medical Center (Andrew J. Dannenberg), the MD Anderson Cancer Center (Lana Vornik, Sherri Patterson, Carolyn Paraguya. Christine Fark, Tawana Castile), and the National Cancer Institute (James Crowell, Levy Kopelovich). We wish to dedicate this manuscript to the memory of I. Bernard Weinstein, who provided invaluable advice during the design of this study.
We thank the study teams (including research nurses and data coordinators), for their contributions to this study. We appreciate the commitment of all the patients who were screened for and enrolled in this trial.
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436:69–97. doi: 10.1016/s1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 3.Borgovan T, Bellistri JP, Slack KN, Kopelovich L, Desai M, Joe AK. Inhibition of BCL2 expression and activity increases H460 sensitivity to the growth inhibitory effects of polyphenon E. J Exp Ther Oncol. 2009;8:129–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–73. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song S, Krishnan K, Liu K, Bresalier RS. Polyphenon E inhibits the growth of human Barrett’s and aerodigestive adenocarcinoma cells by suppressing cyclin D1 expression. Clin Cancer Res. 2009;15:622–31. doi: 10.1158/1078-0432.CCR-08-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–90. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–5. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 8.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 9.Shanafelt TD, Call TG, Zent CS, Leis JF, LaPlant B, Bowen DA, et al. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119:363–70. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res. 2012;5:1144–54. doi: 10.1158/1940-6207.CAPR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE) doi: 10.1200/JOP.2015.006106. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [DOI] [PMC free article] [PubMed]
- 12.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 13.Mohebati A, Milne GL, Zhou XK, Duffield-Lillico AJ, Boyle JO, Knutson A, et al. Effect of zileuton and celecoxib on urinary LTE4 and PGE-M levels in smokers. Cancer Prev Res. 2013;6:646–55. doi: 10.1158/1940-6207.CAPR-13-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The University of Texas, MD Anderson Cancer Center. RPPA Core Facility - Functional Proteomics. Available at: http://www.mdanderson.org/education-and-research/resources-for-professionals/scientific-resources/core-facilities-and-services/functional-proteomics-rppa-core/index.html.
- 15.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–82. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 16.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 17.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate; A practical and powerful approach to multiple testing. J Roy Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 19.American Gastroenterological Association. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 21.Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, et al. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res. 2012;5:290–8. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez P, Piazuelo E, Cebrian C, Ortego J, Strunk M, García-Gonzalez MA, et al. Prostaglandin EP2 receptor expression is increased in Barrett’s oesophagus and oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2010;31:440–51. doi: 10.1111/j.1365-2036.2009.04172.x. [DOI] [PubMed] [Google Scholar]
- 23.Shirakami Y, Shimizu M, Tsurumi H, Hara Y, Tanaka T, Moriwaki H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol Med Rep. 2008;1:355–61. [PubMed] [Google Scholar]
- 24.Hamberg M, Samuelsson B. The structure of the major urinary metabolite of prostaglandin E2 in man. J Am Chem Soc. 1969;91:2177–8. doi: 10.1021/ja01036a092. [DOI] [PubMed] [Google Scholar]
- 25.Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (1) N Engl J Med. 1988;319:689–98. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- 26.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–75. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, et al. Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res. 2009;2:322–9. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, et al. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer Prev Res. 2013;6:428–36. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia FA, Cornelison T, Nuño T, Greenspan DL, Byron JW, Hsu CH, et al. Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2014;132:377–82. doi: 10.1016/j.ygyno.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]