Abstract
Treatment of autoimmune diseases is still largely based on the use of systemically acting immunosuppressive drugs, which invariably cause severe side effects. Calcium/calmodulin-dependent protein kinase IV (CaMK4) is involved in the suppression of IL-2 and the production of IL-17. Its pharmacologic or genetic inhibition limits autoimmune disease in mice. Here we demonstrate that KN93, a small molecule inhibitor of CaMK4, targeted to CD4+ T cells via a nanolipogel delivery system, is markedly reduced experimental allergic encephalomyelitis (EAE) and 10x more potent than the free systemically-delivered drug in the lupus mouse models. The targeted delivery of KN93 did not deplete T cells, but effectively blocked Th17 cell differentiation and expansion as measured in the spinal cords and kidneys of mice developing EAE or lupus respectively. These results highlight the promise of cell-targeted inhibition of molecules involved in the pathogenesis of autoimmunity as a means of advancing the treatment of autoimmune diseases.
Introduction
T helper 17 (Th17) cells are defined by unique functional and developmental characteristics, producing IL-17A and IL-17F, which induce local chemokine production to recruit monocytes and neutrophils to sites of inflammation (1) . They are thought to play a crucial role in the development and pathogenesis of various autoimmune diseases including multiple sclerosis (MS), systemic lupus erythematosus (SLE), rheumatoid arthritis and psoriasis (2, 3).
Calcium/calmodulin-dependent protein kinase IV (CaMK4) is a multifunctional serine/threonine kinase that regulates several cellular processes(4) . We have previously reported that CaMK4 is increased in the nuclei of T cells from patients with SLE (5) and lupus-prone mice. CaMK4 can activate the repressor activity of cAMP response element modulator α (CREM-α) (5) and promote the AKT/mTOR signaling pathway. This induces the binding of CREM-α to the Il17 gene, driving epigenetic remodeling followed by Th17 cell differentiation (6). Furthermore, genetic or pharmacologic inhibition of CaMK4 ameliorates nephritis in MRL/lpr mice (7) and reduces the severity of EAE (6).
Nanoparticulate drug delivery systems have been designed to target small-molecule drugs or other agents into organs or cells using specific antibodies or peptides. These systems have been widely investigated to advance cancer therapies, improve vaccinations and have even been explored for the delivery of cytotoxic drugs in lupus-prone mice (8). Targeted delivery of such drugs to select immune cells should enhance the clinical efficacy of treatment efforts. Furthermore, targets like CaMK4 are expressed by several cells in various organs and systemic administration of drugs is bound to cause side effects, marking the importance of specific delivery to cells which contribute to disease pathology. We have found that KN93, an inhibitor of CaMK4, was effective in treating mice provoked to develop EAE and in lupus-prone mice when administered systemically. Here we demonstrate that by using nanolipogel (nlg) delivery of KN-93 to CD4+ cells, the same clinical effect can be achieved using one tenth of the systemically required dose. This is accomplished by decreasing IL17 production without affecting the numbers of CD4+ cells.
Materials and Methods
Nano Lipogels (nlg) preparations and characterization
KN-93 loaded Nanolipogels (nlg) were made as previously described (8). For in vitro proliferation studies, T cells from spleen were labeled with 0.5 μM CFSE (Invitrogen) in PBS for 10 minutes at 37°C at a concentration of 4 × 106 cells/ml, and then washed twice with PBS and stimulated with plate-bound goat anti-hamster antibodies, soluble anti-CD3(0.25 μg/ml, clone 145-2C11; Biolegend) and anti-CD28 (0.5 μg/ml, clone 37.51; Biolegend) in RPMI-1640 complete media (100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM β-mercaptoethanol with 10% heat-inactivated FBS) treated with either free-KN93 (10μM), nlg- KN93 or anti CD4 or CD8 antibody coated nlg- KN93 (5μM of KN93) for 72 hours, followed by flow cytometry. The percentages of proliferation were defined as the cells with low staining for CFSE of total cells in each population. To investigate their bio-distribution in vivo, ATTO 590, a fluorescent dye, loaded nlgs were also prepared and injected into B6 mice, followed by immunofluorescence analysis of each spleen 2 hours after administration.
Mice
Female MRL/MpJ-Tnfrsf6lpr (MRL/lpr), and male C57BL/6J (B6) mice were purchased from The Jackson Laboratory. MRL/lpr mice were treated with free- KN93 (10μg/week/mouse), anti CD4 antibody coated nlg- KN93 (10μg of KN93/week/mouse) or nlg- empty once a week from 8 weeks of age. B6 mice for EAE were immunized as previously described (6). EAE mice were treated with 4 different nlgs; empty nlg without antibody, empty nlg with anti CD4 antibody, KN93 loaded nlg (nlg- KN93) without antibody and nlg- KN93 with anti CD4 antibody. The dose of KN93 was either 4 μg per week for the disease prevention study or 10 μg per week for the disease treatment study of EAE. Each nlg was injected i.p. weekly from the beginning of the study for prevention study or from the day 12 after immunization for treatment study. Mice were maintained in an SPF animal facility (Beth Israel Deaconess Medical Center). All animal experiments were approved by the Institutional Animal Care Committee of Beth Israel Deaconess Medical Center.
Flow cytometry and ELISA
Infiltrating lymphocytes in spinal cords were isolated by the procedure as previously described(9). Flow cytometry and ELISA was performed as previously described (6).
Histological analysis
Spinal cords from EAE models on the day 14 were dissected and stained as described previously (6). Kidneys from MRL/lpr mice were fixed and stained with periodic acid-Schiff (PAS) and immunofluorescence analysis on frozen sections was performed as previously described(10).
Statistics
Statistical analyses were performed in GraphPad Prism version 5.0 software. P values of <0.05 were considered statistically significant.
Results and Discussion
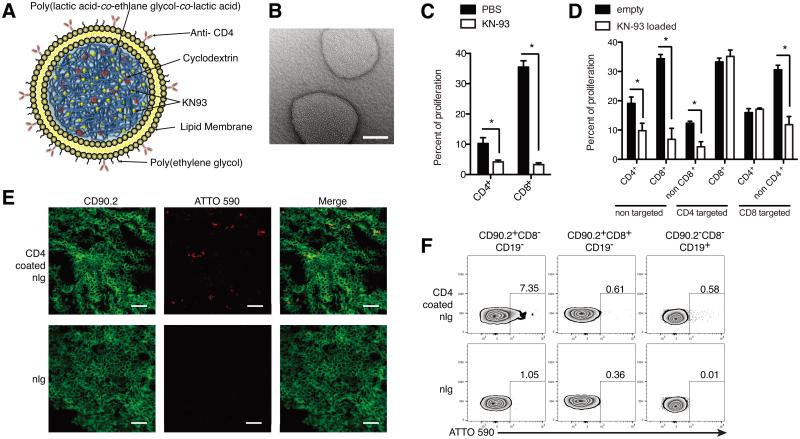
The nlgs were prepared as previously described (8) (Fig. 1A). A transmission electron microscopy image is shown in Fig. 1B. The average hydrodynamic diameter was 214.1 ± 87.3 nm and the average KN-93 load was approximately 20.83 ± 5.79 μg KN-93/mg nanoparticle (mean ± SD) with an encapsulation efficiency of approximately ~69%. To investigate the ability of antibody-coated nlg to target specific cells, we measured the proliferation of in vitro stimulated lymphocytes in their presence. Treatment of splenocytes with KN93 (free- KN93) suppressed cell proliferations of both CD4+ and CD8+ T cells (Fig. 1C). As seen in Fig. 1D, the presence of anti-CD4−coated KN93-loaded nlg selectively reduced the proliferation of non-CD8+ cells and the opposite took place when anti-CD8-coated K93-loaded nlg were present. Next, we injected ATTO590 labeled anti-CD4−coated nlg into mice and noted extensive localization in the spleen after 2 hours that was significantly greater than control nlg lacking antibody targeting (Fig. 1E). Flow cytometry showed that more than 7 % of CD90.2+CD8− T cells were positive for ATTO590 whereas no positive cells were observed in the spleens of mice injected with control nlg (Fig. 1F).
Figure 1.
Characterization of KN93 loaded nanolipogels (nlg). (A) The schema of the structure of KN93 loaded nlg used in this work. The nlg contain streptavidin on their surface and were coated with anti- CD4 antibody conjugated with biotin just before the injection in each experiment. (B) Scanning electron microscope image of nlg. Scale bar represents 100 nm. The mean diameter on the nlg was 214.1 ± 87.3 nm. (C and D) Proliferation assays to elucidate the targeting ability of KN-93-loaded nlg. T cells from C57BL/6 (B6) mice were labeled with CFSE and treated with either free- KN93 (C) or anti CD4 or CD8 antibody coated KN-93 nlg (D). The cells were stimulated in the presence of plate bound anti CD3 and CD28 antibodies for 72 hours, followed by measuring the proliferation of T cells by flow cytometry. Data are representative of 3 independent experiments. Error bars represent the mean ± SEM. *P<0.05 by unpaired Student’s t-test. (E and F) Immuno-cytochemical and flow cytometric analysis of MRL/lpr mice (12 weeks female) treated with anti CD4 antibody coated nlg- ATTO590. The nlg for these experiments were fabricated by loading ATTO590 dye and analyzed 2 hours after injections. (E) CD90.2 expression is in green and ATTO590 loaded nlg in red. Upper panels are the mice spleen treated with anti CD4 antibody coated nlg and lower with non- coated nlg. Scale bar represents 20μm. (F) CD8−/+ T cells (CD90.2+CD19−) or B cells (CD90.2−CD19+) from spleen in each treated mouse were analyzed by flow cytometry. The number in the panel represents the frequency of ATTO590 expressing cells in each group.
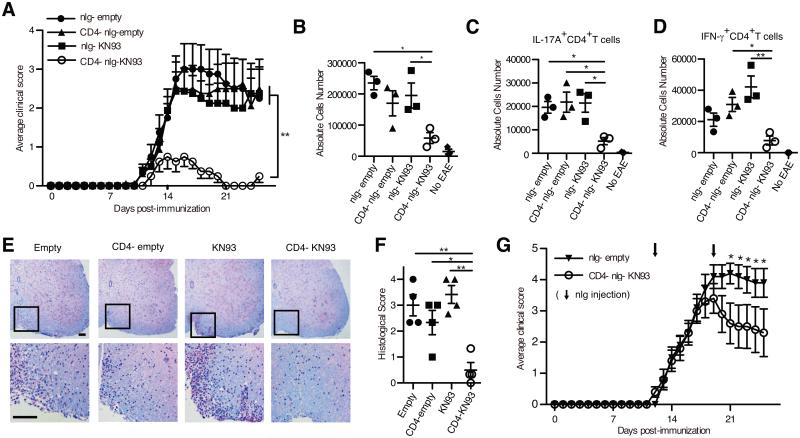
To determine the efficacy of anti-CD4 antibody coated, KN93 loaded nlg (CD4− nlg- KN93) in preventing disease mice we immunized mice with myelin oligodendrocyte glycoprotein (MOG35–55) to induce EAE (11). Mice which received CD4− nlg- KN93, but not other nlg formulations, displayed dramatically reduced clinical disease progression (n= 8; Fig. 2A and Supplemental Fig. S1E). The numbers and ratio of CD4+ to CD8+ T cells in the spleen were not affected in the spleen (Supplemental Fig. S1A and B), indicating that CD4− nlg- KN93 do not deplete CD4+ T cells. On day 14, the peak of EAE progression, the absolute number of cells infiltrating the spinal cord of mice treated with CD4− nlg- KN93 were decreased (Fig. 2B) as were the number of IL-17A+ CD4+ T cells (Fig. 2C) and IFN-γ+ CD4+ T cells (Fig. 2D). Interestingly, IL-17A and IFN-γ producing CD4+ T cells in the spleen were not affected (Supplemental Fig. S1C and D). Histological analysis of spinal cords revealed significant suppression of both inflammation and demyelination only in mice treated with CD4− nlg- KN93 (Fig. 2E and F).
Figure 2.
Anti-CD4 antibody coated nlg- KN93 suppresses EAE. (A) (Prevention study) Average clinical score of the mice treated with nlg- empty, anti CD4 antibody coated nlg- empty (CD4− nlg- empty), nlg- KN-93 (4 μg/week) and CD4− nlg- KN-93 (4 μg/week) after immunization with MOG35-55 in CFA (n = 8 mice in each group). The nlg were injected into the peritoneum weekly from day 0. **P<0.01 by 2way ANOVA. Mice were monitored daily and clinical scores were given as follows: 1, limp tail; 2, hind-limb paresis; 3, hind-limb paralysis; 4, tetraplegia; 5, moribund. (B - D) Intracellular staining of IFN-γ and IL-17A in spinal cord- derived cells from the treated mice 14 days after immunization or the normal B6 mice (no EAE) for negative control. Absolute cell numbers of whole infiltrating cells (B), IL-17A (C) and IFN-γ producing CD4+ T cells (D) in spinal cord in each treatment group. (E) Spinal cord sections from each treated mouse was obtained at 14 days after immunization. Sections were stained with luxol fast blue to assess inflammation and myelin content. Each scale bar represents 100 μm. Data are representative of 3 independent experiments. (F) Quantitative data of histological score. (B - D, F) Each Symbol represents an independent experiment. Error bars represent the mean ± SEM. *P < 0.05, **P<0.01 by 1way ANOVA with Bonferroni’s post-test. (G) Average clinical score of mice treated with CD4− nlg- KN-93 (10 μg / week) or control nlg from day 12 after immunization (n = 10 mice in each group). The nlg were injected weekly from day 12. *P<0.05 by 2way ANOVA with Bonferroni’s post-test.
CD4− nlg- KN93 improved clinical EAE scores by suppressing the differentiation of pathogenic Th17 cells. IL-17A+ and IFN-γ+ CD4+ T cells in the draining lymph nodes from the mice treated with CD4− nlg- KN93 were slightly decreased on FACS analysis performed on day 7 (Supplemental Fig. S1F - H). Further stimulation of draining lymph node lymphocytes with MOG35–55 for 72 hours in the presence of CD4− nlg- KN93, but not other nlg formulations, yielded significantly suppressed production of IL-17A (Supplemental Fig. S1I and J). IFN-γ production was also suppressed but to a lesser extent. This confirms previous observations that CaMK4 inhibition preferentially suppresses IL-17A production over the Th1-specific cytokines (6).
We next explored whether treatment with KN93-loaded CD4+-targeted nlg could suppress disease after it had started. Accordingly, we initiated the administration of CD4− nlg- KN93 on day 12 after immunization with MOG35–55. As demonstrated in Fig. G and Supplemental Fig. S1K, KN93-loaded CD4+-targeted nlg suppressed effectively EAE in progress.
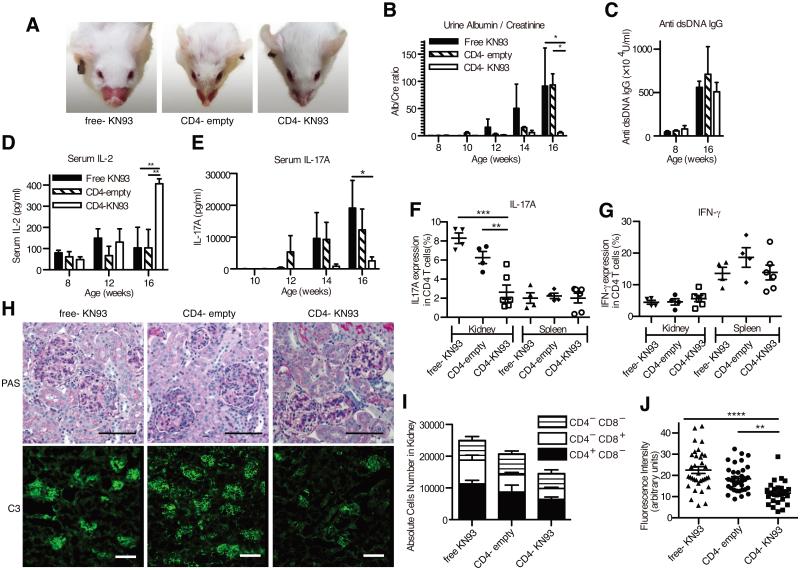
Next, we investigated whether CD4− nlg- KN93 can control disease in the lupus-prone mouse. We administered the various nlg formulations to mice, at a KN93 dose of 10 μg / mouse, weekly during weeks 8 – 16. Treatment with CD4− nlg- KN93 decreased the size of the spleens (Supplemental Fig. S2A and B) but none of the treatments altered T cell subsets in the spleen, including double negative T cells (Supplemental Fig. S2C - E). Similarly, the distribution of effector memory, central memory and naïve CD4+ T cells in the peripheral blood was not affected (Supplemental Fig. S2F - H). Yet, at 16 weeks of age, the mice treated with CD4− nlg- KN93, in contrast to the other groups of mice, showed very few or no facial skin rash (Fig. 3A). More importantly, administration of CD4− nlg- KN93 eliminated proteinuria (Fig. 3B). Interestingly, and unlike our previous experience in treating the same mice with high doses of free KN93(12), administration of CD4− nlg- KN93 did not reduce the titers of anti-DNA antibodies (Fig. 3C), suggesting that targeted delivery of KN93 to CD4+ cells does not affect B cell function. The serum levels of IL-2 from the mice treated with CD4− nlg- KN93 was higher than those of control mice (Fig. 3D), a finding consistent with our previous report (13). Although we did not observe differences in the numbers of IFN-γ+ CD4+ T cells in the spleen or the kidney (Fig. 3G), we noted a significant reduction of IL-17A+ CD4+ T cells in the kidneys and of IL-17A in serum of mice treated with CD4− nlg- KN93 (Fig. 3E and F). We did not find any significant differences in the presence of regulatory T cells (Treg) in the peripheral blood or the spleen (Supplemental Fig. S2I and J). Histological and immunofluorescent analysis revealed reduced inflammation and complement 3 (C3) deposition only in the kidneys of mice treated with CD4− nlg- KN93 (Fig. 3H - J, and Supplemental Fig. S2K - M).
Figure 3.
Anti-CD4 antibody coated nlg- KN93 suppresses disease progressions in MRL/lpr mice. (A) The representative pictures of facial skin rush from mice (16 weeks of age) treated with free- KN-93 (10μg/week/mouse), anti CD4 antibody coated nlg- empty (CD4− nlg- empty) and CD4− nlg- KN-93 (10μg of KN93 /week/mouse). The treatment was started from 8 weeks of age to 16 weeks (i.p. injections; 8 times in total). (B) The mean ratio of urine albumin and creatinine from the mice of each treatment group. Urine samples were obtained biweekly and determined by albumin and creatinine ELISA. (C - E) The mean titer of anti- dsDNA antibody (C), IL-2 (D) or IL-17A (E) in serum in each group on indicated weeks determined by ELISA. (F and G) The frequencies of IL-17A- (F) and IFN-γ- (G) producing CD4+ T cells in kidneys or in spleen by Flow cytometry using intra cellular cytokine staining. (H) Representative images of glomerular areas from 16-week-old MRL/lpr mice with each treatment. PAS staining are shown in the upper panels and C3 deposition detected by immunofluorescence are in the lower. Each scale bar represents 100 μm. (I) The cumulative numbers of infiltrating T lymphocyte in the kidney from the indicated treatment group at 16 weeks of age. The numbers of each T lymphocyte are divided into 3 groups; CD4+CD8−, CD4−CD8+ and CD4−CD8− T cells. (H) The fluorescence intensity of C3 deposition measured in each glomeruli from the mice of the indicated treatment group. Representative data of 3 independent experiments are shown (n=4-6 mice per group) in B-E and I and each symbol represents an independent sample in F, G and J. Error bars represent the mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by 1way ANOVA (F and J) or 2way ANOVA(B, D, E and F) with Bonferroni’s post-test.
Current treatment of autoimmune diseases relies heavily on the systemic administration of corticosteroids and cytotoxic drugs which, although effective, cause significant side effect-related morbidity. The development of drugs that target molecules involved in the pathogenesis of disease is also hampered by the fact that these molecules are expressed by several cell types and tissues and their indiscriminate blockade or inhibition can cause unwanted side effects. Here we demonstrate that we can target the kinase CaMK4, which we have found to promote IL-17 production in patients with SLE and mice prone to lupus, only in CD4+ cells in a way that selectively disrupts their ability to produce IL-17 without depleting them or affecting other functions like helping B cells to produce autoantibodies. Hence the difficulty of targeting autoreactive cells still remains in this model. The nlgs that we used deliver only 10% of the KN93 dose required to achieve equivalent immunosuppression if given systemically in lupus- prone mice. The nanoparticle system described in this work facilitates a localized and increased concentration profile of the released drug. In previous studies, Fahmy and Laboswky, described an in silico model demonstrating how the nature and profile of this concentration gradient near a cell surface or embedded in a cell develops over time and space. Specifically, it was shown that an encapsulated drug can accumulate to several orders of magnitude above systemic free doses within the target of interest and dependent on the proximity of the nanoparticle to the target (14). Such a targeted delivery system, if used in humans, could significantly curtail the side effect profile associated with these drugs and improve patient quality of life, in addition to increasing efficacy.
In conclusion, we demonstrate that pharmacological inhibition of CaMK4 in CD4+ T cells suppresses IL17 production and is clinically efficacious in limiting EAE progression and skin / kidney disease in lupus-prone mice. Targeted delivery of small molecule inhibitors using the described nlg system is therefore a promising approach to improve treatment of chronic autoimmune conditions.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01AR064350 (to G. C. Tsokos) and grant from 2013 SENSHIN Medical Research Foundation (to K. Otomo).
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 3.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 4.Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends in immunology. 2008;29:600–607. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. The Journal of clinical investigation. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA, Rauen T, Crispin JC, Tsokos GC. CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. The Journal of clinical investigation. 2014;124:2234–2245. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichinose K, Rauen T, Juang YT, Kis-Toth K, Mizui M, Koga T, Tsokos GC. Cutting edge: Calcium/Calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. Journal of immunology. 2011;187:5500–5504. doi: 10.4049/jimmunol.1102357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, Craft J, Fahmy TM. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. The Journal of clinical investigation. 2013;123:1741–1749. doi: 10.1172/JCI65907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen HX, Beck KD, Anderson AJ. Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga T, Mizui M, Yoshida N, Otomo K, Lieberman LA, Crispin JC, Tsokos GC. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3(+) regulatory T cells in MRL/lpr mice. Autoimmunity. 2014;47:445–450. doi: 10.3109/08916934.2014.915954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. Journal of autoimmunity. 2013;45:31–39. doi: 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose K, Juang YT, Crispin JC, Kis-Toth K, Tsokos GC. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis and rheumatism. 2011;63:523–529. doi: 10.1002/art.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga T, Ichinose K, Mizui M, Crispin JC, Tsokos GC. Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. Journal of immunology. 2012;189:3490–3496. doi: 10.4049/jimmunol.1201785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labowsky M, Lowenthal J, Fahmy TM. An in silico analysis of nanoparticle/cell diffusive transfer: Application to nano-artificial antigen-presenting cell:T-cell interaction. Nanomedicine : nanotechnology, biology, and medicine. 2015;11:1019–1028. doi: 10.1016/j.nano.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.