Abstract
The purpose of this study was to assess the feasibility of using the selective estrogen receptor modulator (SERM) acolbifene as a breast cancer prevention agent in premenopausal women. In order to do so we assessed change in proliferation in benign breast tissue sampled by random periareolar fine needle aspiration (RPFNA) as a primary endpoint, along with changes in other risk biomarkers and objective and subjective side effects as secondary endpoints. Twenty-five women with cytologic hyperplasia +/− atypia and ≥2% of breast epithelial cells staining positive for Ki-67, received 20 mg acolbifene daily for 6–8 months, and then had benign breast tissue and blood risk biomarkers re-assessed. Ki-67 decreased from a median of 4.6% (interquartile range, 3.1 – 8.5%) at baseline to 1.4% (IQR, 0.6 – 3.5%) after acolbifene (P<0.001; Wilcoxon signed rank test), despite increases in bioavailable estradiol. There were also significant decreases in expression (RT-qPCR) of estrogen inducible genes that code for pS2, ER-α, and PgR (p≤0.026). There was no significant change in serum IGF-1, IGFBP3, IGF-1:IGFBP3 ratio, or mammographic breast density. Subjective side effects were minimal with no significant increase in hot flashes, muscle cramps, arthralgias, or fatigue. Objective measures showed a clinically insignificant decrease in lumbar spine bone density (DEXA) and an increase in ovarian cysts but no change in endometrial thickness (sonography). In summary, acolbifene was associated with favorable changes in benign breast epithelial cell proliferation and estrogen inducible gene expression but minimal side effects, suggesting a Phase IIB placebo-controlled trial evaluating it further for breast cancer prevention.
Keywords: Breast Cancer Risk Biomarkers, SERM, RPFNA
Introduction
The selective estrogen receptor modulator (SERM) tamoxifen is the only FDA-approved agent for breast cancer risk reduction in premenopausal women 35 years of age or older with a Gail Model estimated 5-year risk of breast cancer of 1.66% or higher and/or atypical hyperplasia, or in situ cancer [1]. A recent meta-analysis suggests that use of tamoxifen in the chemopreventive setting reduced risk ~33% compared to placebo among high risk women [2]. Current ASCO guidelines suggest including tamoxifen in discussion of risk reduction strategies for high risk women [1] but tamoxifen uptake for risk eligible women remains low [3] primarily due to concerns about side effects and lack of demonstrated survival benefit [1, 3]. The benefit: risk ratio for tamoxifen used as primary prevention in high risk premenopausal women is generally seen as particularly favorable given the lack of significantly increased incidence of serious side effects in women < 50 years old in the NSABP P-1 trial [4, 5]. Yet the rate of tamoxifen uptake for premenopausal women attending high risk clinics has been cited as only 10% [6]. Concerns about induction of menstrual abnormalities and perimenopausal symptoms are the likely reasons that most younger women are reluctant to take tamoxifen. Tamoxifen can also result in an increase in size or number of ovarian cysts as well as bone density loss in premenopausal women [7, 8]. Currently, tamoxifen for primary prevention is regarded as a preference-sensitive decision [3, 6, 9]. A dramatic increase in uptake of endocrine therapy for primary prevention by premenopausal women is probably dependent on development of an agent with fewer uterine side effects and perimenopausal symptoms.
Acolbifene (EM-652.HCl) is a fourth generation SERM of the benzopyran class which has been found to have no estrogen agonist effects in either the breast or endometrium [10–13]. Acolbifene and its prodrug (EM-800) have been associated with reduction of growth of tumor xenografts [14] as well as the incidence of DMBA-induced rat mammary cancer [15]. The lack of estrogen agonist activity in the uterus of EM-800 as well as reported activity in tamoxifen-resistant metastatic disease [13] made it an attractive agent for assessment for treatment and prevention. Although efficacy has been reported in treatment trials of post-menopausal women, few pre-menopausal women have been treated in this setting.
The overall purpose of this pilot study was to assess the suitability of acolbifene as a prevention drug for breast cancer in premenopausal women as assessed by tolerability and favorable modulation of risk biomarkers for breast cancer. Tolerability encompassed subjective side effects relating to vasomotor symptoms, and quality of life, as well as objectively measured change in ovarian cysts, endometrial thickness, and by pelvic ultrasonography and bone density by dual energy x-ray absorption (DEXA). Primary biomarker assessment was to estimate the effect of acolbifene on proliferation assessed by Ki-67 in benign breast tissue acquired by random periareolar fine needle aspiration (RPFNA). Secondary objectives explored modulation of other breast cancer risk biomarkers in blood and breast tissue, including serum insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP3) and mammographic breast density. Exploratory markers included change in mRNA levels of selected estrogen response genes which code for estrogen receptor alpha, pS2, progesterone receptor, growth regulation by estrogen in breast cancer 1 (GREB-1), and splice variants for C-X-C motif chemokine 12 which codes for stromal cell-derived factor 1 (SDF-1).
Materials and Methods
Cohort and Trial Eligibility
Potential participants were required to be at increased risk for breast cancer as defined by any one or more of the following criteria: 1) five-year Gail predicted probability of breast cancer >1.67% or 5X that of an average woman of the same age [16]; 2) known deleterious BRCA1/2 mutation carrier or a family history consistent with hereditary breast cancer; 3) prior diagnosis of atypical hyperplasia, lobular or ductal carcinoma in situ; or 4) a prior random periareolar fine needle aspiration (RPFNA) showing hyperplasia with atypia [17]. A normal mammogram performed within three months of the baseline RPFNA on days 1–10 of the menstrual cycle was required with at least 5% estimated visual mammographic density. Participants were required to use birth control (hormonal, intrauterine device, or double barrier) for the duration of the study.
Protocols for screening RPFNA (HSC 4601; NCT00291096) and for the acolbifene intervention (HSC 10588; NCT00853996) were approved by the University of Kansas Medical Center Human Subjects Committee. Separate consents were utilized for the screening and interventional protocols. For entry into study, the RPFNA specimen must have exhibited cytologic evidence of hyperplasia with or without atypia [18] with a Masood cytology index score of ≥14 [19]; and have ≥2% positive staining for Ki-67.
RPFNA and Cytomorphology
RPFNA was performed (CJF) on two sites per breast under local anesthesia during the follicular phase (day 1–10) of the menstrual cycle. The first aspiration pass per site (four sites total) was placed in a 2-ml cryovial containing 0.5 ml PBS, immediately immersed in liquid nitrogen and transferred to a −80°C freezer within 12 hours for later use in gene expression assays. Remaining specimens were pooled from both breasts in a single 15 cc tube containing 9 cc of CytoLyt™ and 1 cc of 10% neutral buffered formalin. Cells were spun, washed and resuspended in PreservCyt™ after at least 24 hours in CytoLyt™. Aliquots were processed to slides using a ThinPrep™ (Hologic LP, Malborough, MA) Non-Gyn standard protocol. Slides for cytomorphology and Ki-67 were Papanicolaou-stained using an RNase-free technique.
All slides were assessed by a single cytopathologist (CMZ) who assigned a categorical assessment of non-proliferative, hyperplasia, borderline hyperplasia with atypia, or hyperplasia with atypia [18]; as well as a Masood semi-quantitative index score [19].
Ki-67 Immunocytochemistry
Only slides having >500 epithelial cells visible by Papanicolaou-staining were further processed for Ki-67. After de-staining, antigen retrieval was performed with a 10 mM citrate buffer (pH 6) in a Biocare (Walnut Creek, CA) decloaking chamber for two minutes at 120°C. Slides were stained with a MIB-1 monoclonal antibody (M7240 Dako Cytomation, Carpenteria, CA) at a 1:20 dilution using a Dako autostainer [20]. Hyperplastic clusters were preferentially assessed and the number of cells with unequivocal nuclear staining out of a total of 500 cells were assessed manually by two technicians and a consensus score recorded [20].
Gene Expression by RT-qPCR
Total RNA was extracted from frozen RPFNA samples using Trizol® LS according to the manufacturer’s instructions. The RNA collected was thus reflective of adipocytes, stroma, and epithelial cells. RNA was amplified using MessageAmp™II aRNA amplification kit (Life Technologies Grand Island NY) and reverse transcribed to cDNA using SMARTScribe™ Reverse Transcriptase (Clontech Laboratories, Inc., Mountain View, CA) and random nonamer primers. Real-time PCR (qPCR) was performed in the Breast Cancer Prevention Laboratory via 5′ nuclease assay using hydrolysis probes as previously described [21].
Reference transcripts were β-glucuronidase (GUSB), β-actin (ACTB), cyclophilin A (peptidylprolyl isomerase A, PPIA), hypoxanthine phosphoribosyltransferase 1 (HPRT1), cytokeratin 19 (KRT19), and E-cadherin (CDH1). Tested transcripts were estrogen receptor 1 (ESR1) for estrogen receptor alpha (ERα), trefoil factor 1 (TFF1) for pS2, two splice variants of C-X-C motif chemokine 12 (CXCL12) which code for stromal cell derived factor 1 (SDF1α) and 2 (SDF-1β), growth regulation by estrogen in breast cancer 1 (GREB1), steroid sulfatase (STS), progesterone receptor (PGR), cyclin D1 (CCND1), insulin-like growth factor 1 receptor (IGF1R), and keratin 5 (KRT5). Baseline and post-intervention specimens were assessed together. PCR reactions were run on an Applied Biosystems Prism 7000 Sequence Detection System. The quantity of each biomarker transcript in a sample is expressed relative to the level of the reference transcript HPRT1 which showed the least change between paired specimens. Further normalization by epithelial cell markers (cytokeratin 19 and E-Cadherin) was not indicated based on lack of significant directional change in these markers; but if done, the results of statistical analysis for the tested transcripts were nor materially altered.
Hormones, IGF-1, and IGFBP-3
Blood was obtained for analysis of estradiol and sex hormone binding globulin (SHBG) during the follicular phase (day 1–10) of the menstrual cycle, at the time of RPFNA. Fasting blood for progesterone, SHBG, testosterone, insulin-like growth factor-1 (IGF-1) and its binding protein IGFBP-3 was obtained at days 20–24 of the menstrual cycle. Samples were stored frozen at −80°C until analysis. Commercial kits from R&D Systems, Inc. (Minneapolis MN) were used for enzyme-linked immunoabsorbent assay (ELISA) of IGF-1 (DG100) and IGFBP-3 (DGB300). Commercial kits from Diagnostics Biochem Canada (Dorchester ONT, Canada) were used for enzyme immunoassay of estradiol (CAN-E-430), progesterone (CAN-P-305), testosterone (CAN-TE-250) and ELISA of SHBG (CAN-SHBG-4010). Baseline and post-intervention specimens were run together with pooled serum controls to assess batch variation. Bioavailable estradiol and testosterone were calculated according to standard formulae [22, 23].
Mammographic Breast Density
Digital mammograms were converted to a common, de-identified format for breast density assessments. The left cranial caudal view was generally used for assessments by a single reviewer (CJF) using the Cumulus software program developed by Boyd and Yaffee [24]. Breast density was calculated as percent dense area compared to the entire breast area. Baseline and post-intervention mammographic images were assessed together in a blinded fashion [25].
Adverse Events and Quality of Life
Subject-reported adverse events were recorded using NCI common toxicity criteria (version 3.0). Subjects were contacted monthly for adverse events reporting. For quantitative assessment of quality of life aspects, specific information was collected monthly regarding the frequency and severity of muscle cramps and hot flashes. The Health Assessment Questionnaire II (HAQ-II) and the Brief Fatigue Inventory (BFI) questionnaire were also completed at baseline and post-intervention.
Safety Assessments by Pelvic Sonography and DEXA
To monitor for possible side effects that might relate to administration of a SERM, pelvic sonography and dual energy x-ray absorption (DEXA) bone density assessments were performed at baseline and post-intervention on all subjects. Number and size of ovarian cysts and endometrial thickness were recorded by the evaluating radiologist. From DEXA, the T-score was used to evaluate bone mineral density for both the femur and lumbar spine.
Study Agent
Acolbifene was provided by Endorecherche, Inc. (Quebec City, Quebec, Canada) as 20 mg capsules. Subjects were instructed to take one capsule orally each day.
Sample Size and Statistical Analysis
Our planned accrual was 44 subjects, anticipating a 10% dropout rate. With 40 evaluable subjects, there would be at least 80% power to detect an effect size (defined as the mean change divided by the standard deviation of change) of 0.45 or greater for change in Ki-67 (as percent of cells staining positive) at a two-sided level of 0.05 in a one-sample t-test. After 25 subjects had been accrued (and 9 completed study) a technical problem with the Ki-67 immunocytochemistry staining was identified and accrual was temporarily suspended. By the time the staining problem was resolved (see Supplemental File 1), all 25 subjects had completed study (no dropouts) and had post-study RPFNA specimens evaluable for the primary endpoint. A decision was made to not re-open the study for further accrual but to stop at 25 subjects. With this number of evaluable subjects, there would still be 80% power to detect an effect size of 0.59.
For the primary endpoint of change in Ki-67, which did not appear normally distributed, the non-parametric Wilcoxon signed-rank test was used to assess if acolbifene had any effect on this marker. Similarly, for the secondary endpoints, serum hormones, IGF-1 and IGFBP3 levels, IGF-1: IGFBP-3 ratio, breast density, and gene expression, the Wilcoxon test was also used. For qualitative dichotomous outcomes, McNemar’s test was used. Two-sample comparisons were made using non-parametric Mann-Whitney test. All analyses were conducted by IBM, SPSS, version 20. As these secondary analyses were all considered exploratory, no corrections were made for multiple comparisons.
Results
Screening and Enrollment
A total of 75 high risk women were screened by RPFNA, of whom three (4%) were not medically eligible and 39 (52%) were not eligible on the basis of Ki-67 <2.0% and/or cytomorphology. A total of eight elected not to participate in the intervention, leaving 25 (33%) who enrolled and received acolbifene. The first subject started in March 2009, the last started in December 2009, and the last subject completed study in July 2010.
Demographic and Risk Information
Demographic and risk information for the 25 premenopausal women enrolled is shown in Table 1. All subjects were Caucasian, with one self-identified as Hispanic. Seven subjects (28%) were taking oral contraceptives.
Table 1.
Demographic variables for 25 enrolled participants.
| Variable | Mean ± SD | Median | Range |
|---|---|---|---|
| Age, years | 42.8 ± 5.2 | 43 | 33 – 52 |
| Height (in) | 65 ± 2 | 65 | 61 – 70 |
| Weight (lb) | 155 ± 29 | 153 | 109 – 224 |
| BMI (kg/m2) | 25.8 ± 4.8 | 25.3 | 18.9 – 34.1 |
| 5-Year Gail Risk, % | 3.6 ± 4.4 | 2.3 | 0.4 – 16.5 |
| Age First Live Birth, years (2 non-parous) | 28 ± 4 | 29 | 18 – 37 |
Retention and Compliance
All 25 women enrolled completed the intervention, met the study definition of compliance, had a repeat RPFNA, and provided paired biomarker data for assessment of change over the study period. The minimum value for compliance was 81% of prescribed agent, and the median compliance was 95%, based on subject-maintained logs and returned pill counts. The median duration on study agent was 204 days (range 182 to 243). Per protocol, the nominal 6 months of study agent could be extended to 8 months for purposes of scheduling RPFNA with menstrual cycle.
Changes in Ki-67 and Cytomorphology in Benign Breast Tissue
The median level of Ki-67 staining at baseline was 4.6% (range 2.4% to 21.8%; (interquartile range (IQR), 3.1 – 8.5%); post-intervention, the median was 1.4% (range 0% to 6.6%; IQR, 0.6 – 3.5%) (Table 2). The median change was −3.0% (range −20.2% to 2.8%; IQR, −7.1 – −2.0%), which corresponded to a relative change of −77% with a range of −100% (zero staining post-intervention) to 117% (IQR, −88% – −53%) (p<0.001, Wilcoxon). Despite increases in serum bioavailable estradiol (see below), a decrease in Ki-67 positive staining was noted in 23/25 (92%) subjects (Figure 1). There were no differences between the seven oral contraceptive (OC) users and 18 non-users for expression of Ki-67: baseline (p=0.93), post-intervention (p=1.0), change (p=0.88), or relative change (p=0.69) (Mann-Whitney test). Both OC users and non-users showed decreases (5/7, p=0.091; 18/18, p=0.001) with intervention.
Table 2.
Changes in a) cytomorphology and Ki-67 in RPFNA specimens and b) mammographic breast density over the course of the study. Median, range, [interquartile range], and mean, ± standard deviation are shown for the 25 subjects, except mammographic density where only 24 comparisons were available.
| Biomarker | Baseline | Post-Intervention | Absolute Change | Relative Change | P-Value |
|---|---|---|---|---|---|
| Masood Score | 15 14 – 17 [14 – 15] 14.8 ± 1.0 |
14 9 – 17 [13 – 16] 14.0 ± 2.2 |
−1 −5 – 2 [−2 – 1] −0.8 ± 2.0 |
−7% −36% –14% [−14% – 7%] −5% ± 14% |
0.10 |
| 14 decrease 9 increase |
|||||
| Atypia | 13 (52%) | 12 (48%) | 4 “gain” atypia 5 “lose” atypia |
1.00 | |
| Estimated Epithelial Cell Number per slide | 10 decrease 2 increase |
0.012 | |||
| 1–5 × 102 | 0 (0%) | 4 (16%) | |||
| 5–10 × 102 | 2 (8%) | 5 (20%) | |||
| 1–5 × 103 | 18 (72%) | 12 (48%) | |||
| >5 × 103 | 5 (20%) | 4 (16%) | |||
| Ki-67, percent | 4.6 2.4 – 21.8 [21.1 – 60.8] 6.6 ± 4.8 |
1.4 0.0 – 6.6 [18.5 – 48.8] 2.1 ± 1.9 |
−3.0 −20.2 – 2.8 [−10.0 – 0.9] −4.5 ± 4.7 |
−77% −100% –117% [−23% – 4%] −57 ± 52% |
<0.001 |
| 23 decrease 2 increase |
|||||
| Mammographic Breast Density | |||||
| Mammographic breast density (percent area at increased density) | 35.8 2.9 – 76.3 [3.1 – 8.5] 40.5 ± 22.5 |
35.0 3.6 – 70.8 [0.6 – 3.5] 36.3 ± 20.2 |
−3.9 −31.4 –16.2 [−7.1 – −2.0] −4.2.± 10.3 |
−11% −49% – 71% [−88% – −52%] −5 ± 30% |
0.067 |
| 16 decrease 8 increase |
|||||
Figure 1.
Ki-67 expression (percent of cells staining positive) post-intervention as a function of baseline value. Baseline aspiration values are shown on the x-axis; repeat aspiration on the y-axis. The line represents no change in value; triangles above the line denote an increase and diamonds below the line a decrease.
There were no significant changes in cytomorphology over the course of the intervention, either by a categorical descriptor or by Masood score (Table 2). Thirteen of the 25 women exhibited hyperplasia with atypia at baseline versus 12 at study conclusion. Similarly, for the semi-quantitative Masood cytomorphology score, median score was 15 at baseline and 14 at second RPFNA, with a median 1 point decrease (p=0.10, Wilcoxon).
Changes in Gene Expression in Benign Breast Tissue
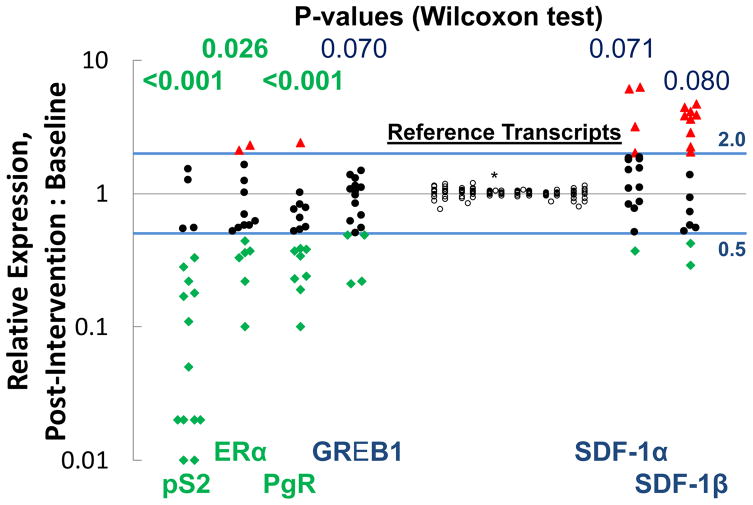
Seventeen paired specimens (baseline and post-intervention) were available for RT-qPCR analysis for levels of mRNA. Specimens from eight women were excluded from analysis because either the baseline or post-intervention specimen was grossly bloody. Significant decreases (p<0.05) were noted for transcripts for three estrogen inducible genes that code for pS2, ERα, and PgR. There were also borderline significant decreases for GREB1 and borderline significant increases for SDF-1α and SDF-1β (Figure 2). There were no changes noted for keratin 5, insulin-like growth factor 1 receptor, cyclin D1, or steroid sulfatase.
Figure 2.
Effects of acolbifene on relative change (post-intervention:baseline) in expression (by RT-qPCR) of genes that code for relevant proteins. There was minimal change for the six reference transcripts assessed (Cytokeratin 19, E-cadherin, HPRT1, Cyclophilin A, β-actin, β-glucuronidase). HPRT1 (*) was used for normalization purposes.
Changes in Mammographic Breast Density
There was no statistically significant change in mammographic breast density, expressed as percent of breast area with increased density, from baseline (median 35.8%) to post-intervention (median 35.0%), with an average of 9 months between mammograms (Table 2). Breast density was statistically significantly (p<0.001) lower at baseline and post-intervention for women with higher BMI (dichotomized at the median of 25 Kg/m2); but there was no difference for either absolute or relative change in density.
Change in Serum Hormones and IGF-1 and IGFBP3
Follicular phase (days 1–10 of cycle) estradiol and bioavailable estradiol increased by medians of 78% and 110%, respectively, relative to baseline (p≤0.002) (Table 3). Both OC users and non-users exhibited significant (p=0.018; p=0.044) increases in bioavailable estradiol. For OC users this was due not only to an increase in estradiol but also a significant decrease (7/7 subjects; p=0.018) in SHBG. Luteal phase total testosterone increased by a median of ~30% relative to baseline (p=0.002). Bioavailable testosterone did not change for the 18 OC non-users (p=0.91); but did increase in each of the seven OC users (p=0.018), in part due to significant (7/7; p=0.018) decreases in SHBG. OC users also had lower levels of bioavailable testosterone at baseline than non-users (median 2.3 nM vs 17.2 nM; p=0.046). There were no statistically significant changes for progesterone, IGF-1, IGFBP3, or the IGF-1:IGFBP3 molar ratio (Table 3),
Table 3.
Change in serum hormones and growth factors from baseline to post-intervention. Median, range, [interquartile range], and mean ± standard deviation are shown for the 25 subjects.
| Variable or Biomarker | Baseline | Post-Intervention | Change | P-value |
|---|---|---|---|---|
| Collected at time of RPFNA (day 1–10 of menstrual cycle) | ||||
| SHBG (with E2), nM | 82 18 – 221 [38 – 124] 90 ± 56 |
68 25 – 154 [41 – 92] 73 ± 37 |
1 −140 – 43 [−54 – 18] −18 ± 51 |
0.46 |
| Estradiol, pg/ml | 88 <20* – 615 [56 – 131] 112 ± 115 |
190 <20* – 312 [120 – 224] 174 ± 76 |
80 * −481 – 247 [35 – 129] 62 ± 136 |
0.001 |
| Serum Estradiol, nM | 0.32 <0.07* – 2.28 [0.21 – 0.49] 0.41 ± 0.43 |
0.70 <0.07* – 1.15 [0.45 – 0.83] 0.64 ± 0.28 |
0.29 * −1.79 – 0.92 [0.14 – 0.48] 0.23 ± 0.51 |
|
| Bioavailable (Free) Estradiol, pM | 4.5 0.7 – 31.1 [1.4 – 6.4] 5.3 ± 6.2 |
8.0 1.2 – 15.7 [5.3 – 10.8] 7.8 ± 3.4 |
3.5 * −25.5 – 10.1 [1.0 – 7.0] 2.6 ± 7.0 |
0.002 |
| Collected at day 20–24 of menstrual cycle | ||||
| IGF-1, ng/ml | 129 72 – 223 [107 – 167] 139 ± 42 |
146 71 – 220 [103 – 173] 141 ± 41 |
3 −41 – 66 [−15 – 18] 2 ± 27 |
0.74 |
| IGF-1, nM | 16.8 9.4 – 29.0 [13.9 – 21.7] 18.1 ± 5.4 |
19.0 9.3 – 28.6 [13.4 – 22.6] 18.3 ± 5.3 |
0.4 −5.4 – 8.6 [−1.9 – 2.3] 0.3 ± 3.5 |
|
| IGFBP-3, ng/ml | 2350 1505–3683 [2061 – 3025] 2504 ± 609 |
2667 1497–3556 [2249 – 3006] 2618 ± 539 |
111 −307–1239 [−62 – 246] 114 ± 267 |
0.051 |
| IGFBP-3, nM | 82.3 52.7 – 128.9 [72.1 – 105.9] 87.6 ± 21.3 |
93.4 52 – 124 [78.7 – 105.2] 92 ± 19 |
3.9 −10.7 –32.6 [−2.2 – 8.6] 4.0 ± 9.3 |
|
| IGF-1:IGFBP-3 Molar Ratio | 0.21 0.10 – 0.37 [0.16 – 0.25] 0.21 ± 0.06 |
0.19 0.10 – 0.42 [0.16 – 0.24] 0.21 ± 0.07 |
−0.01 −0.12 – 0.11 [−0.03 – 0.02] −0.01 ± 0.05 |
0.41 |
| Progesterone, ng/ml | 3.5 0.4 – 26.2 [0.9 – 3.5] 4.4 ± 5.5 |
2.8 0.5 – 36.3 [0.7 –9.7] 7.1 ± 10.0 |
0.0 −10.7 – 30.1 [−2.6 – 1.9] 2.7 ± 9.7 |
0.78 |
| Progesterone, nM | 11.0 1.1 – 83.3 [2.7 – 18.8] 14.0 ± 17.3 |
8.9 1.5 – 115.3 [2.1 – 30.7] 22.6 ± 31.7 |
−0.1 −34.1 – 95.8 [−8.3 – 6.1] 8.6 ± 30.7 |
|
| SHBG, nM | 87 22 – 276 [59 – 193] 117 ± 80 |
85 34 – 179 [48 – 135] 93 ± 46 |
6 −227 – 60 [−45 – 18] −24 ± 73 |
0.53 |
| Testosterone, ng/ml | 0.40 <0.08* – 2.59 [0.18 – 0.83] 0.63 ± 0.64 |
0.58 0.19 – 3.52 [0.29 – 0.83] 0.85 ± 0.91 |
0.16 −0.21 – 2.33 [0.02 – 0.24] 0.22 ± 0.48 |
0.002 |
| Testosterone, nM | 1.37 <0.28* – 9.00 [0.63 – 2.87] 2.18 ± 2.21 |
2.0 0.64 – 12.21 [1.00 – 2.86] 2.93 ± 3.15 |
0.53 −0.74 – 8.09 [0.06 – 0.80] 0.75 ± 1.66 |
|
| Bioavailable (Free) Testosterone, pM | 13.4 1.1 – 120.2 [4.6 – 35.1] 25.1 ± 30.7 |
17.3 3.8 – 194.4 [9.5 – 38.3] 32.4 ± 41.6 |
4.1 −26.9 – 118.2 [−2.1 – 8.5] 7.3 ± 26.8 |
0.13 |
One woman had estradiol levels below limit of detection at both times and was imputed to have no change. Another women had testosterone levels below limit of detection only at baseline and was considered to have exhibited an increase.
Self-Reported Adverse Events
Five (20%) of 25 subjects reported no adverse events, 11 (44%) subjects reported only grade 1 events, seven (28%) subjects reported a grade 2 event, and two (8%) subjects reported a grade 3 event. One subject reported at the post-intervention visit that grade 3 hot flashes had begun approximately two months earlier (after 4 months on study agent). One subject reported a grade 3 dizziness that began approximately 6 weeks after starting study agent; this was considered to be unrelated to study agent. A total of 71 adverse events (50 grade 1, 19 grade 2, two grade 3) were self-reported, with only half being attributed by the protocol chair (CJF) as possibly or probably related to study agent. Most common reported AEs (percent of subjects) included irregular menses (32%), leg/muscle cramps (25%), diarrhea (16%), and hot flashes (16%). No Serious Adverse Events were reported. Nor did any subject drop out of the study due to AEs.
Quantitative Assessment of Hot Flashes, Menstrual Irregularities, Musculoskeletal Symptoms, and General Quality of Life
Consistent with the low incidence of study-related moderate or severe adverse events, no significant changes were observed for the quantitative assessments of quality of life.
Problems with hot flashes were assessed for average number per day and intensity. Only six women reported mild to moderate hot flashes prior to starting drug, and only for two were these as frequent as daily. Five of the six with initial hot flashes did not report hot flashes at their post-intervention visit; for the sixth, there was a slight increase in number and intensity. Five other participants with no hot flashes at baseline reported infrequent hot flashes post-intervention. Overall, there was no effect of acolbifene use on symptoms associated with hot flashes.
The Health Assessment Questionnaire II (HAQ-II) measures interference in daily activities from arthralgias and joint pain. No woman reported a score above zero at baseline and only one had a non-zero score (1.0) post-intervention. Thus, acolbifene use was not associated with joint discomfort or disability. Similar results were obtained for self-reported incidence, frequency, and severity of muscle cramps. Only three women reported mild muscle cramps prior to starting drug. For two, no muscle cramps were reported post-intervention; for the third, there was no change in any aspect of muscle cramp symptoms. For three other women with no muscle cramps at baseline, there were mild muscle cramps reported post-intervention. There was thus no adverse effect of acolbifene use on symptoms associated with muscle cramps.
The Brief Fatigue Inventory (BFI) measures intensity of fatigue and interference with daily activities. BFI scores at baseline and post-intervention were similar, reflecting no change overall (p=0.82, Wilcoxon test). Medians were 9 and 10; ranges were 0 – 54 and 0 – 44; and means/standard deviations were 12.8 ± 13.2 and 12.9 ± 13.5, respectively. For change over the study, there was a median of 1, range of −13 – 13, and mean of −0.2 ± 7.6.
Gynecologic Parameters Assessed by Pelvic Ultrasonography
Endometrial thickness was unchanged over the course of the study (Table 4). In contrast, the number of women in whom ovarian cysts could be visualized increased from 15 (60%) to 23 (82%) (p=0.011, McNemar’s test). The largest diameter of ovarian cysts increased from a median of 12 mm at baseline to 23 mm post-intervention (p<0.001).
Table 4.
Change in quantitative measures assessed by pelvic sonography and DEXA from baseline to post-intervention. For quantitative measures, median, range, [interquartile range], and mean ± standard deviation are shown for the 25 subjects. For categorical indices, the number and percentage are shown.
| Variable or Biomarker | Baseline | Post-Intervention | Change | P-value |
|---|---|---|---|---|
| Pelvic Ultrasonography | ||||
| Endometrial thickness, mm | 6 2 – 26 [4 – 9] 7.6 ± 5.5 |
6 1 – 17 [3.5 – 8] 6.3 ± 4.0 |
0.0 −11 – 5 [−3 – 2] −0.7 ± 3.9 |
0.40 |
| Endometrial evaluation as Abnormal | 0% | 0% | 0% | 1.00 |
| Ovarian Cyst largest diameter, mm | 12 0 – 26 [0 – 19] 10.5 ± 9.5 |
23 0 – 54 [16 – 35.5] 25.6 ± 14.4 |
14 −19 – 49 [3.5 – 24.5] 15.1 ± 15.4 |
<0.001 |
| Ovarian Cysts Present Any | 15 (60%) | 23 (92%) | 8 (32%) | 0.011 |
| > 30 mm | 0 (0%) | 9 (36%) | 9 (36%) | 0.003 |
| Fibroid largest diameter, mm | 0 0 – 16 [0 – 0] 2.3 ± 5.5 |
0 0 – 25 [0 – 0] 1.8 ± 6.3 |
0 −15 – 9 [0 – 0] −0.52 ± 4.4 |
0.47 |
| Fibroids Present | 4 (16%) | 2 (8%) | 2 (8%) | 0.16 |
| DEXA | ||||
| Lumbar Spine Bone Mineral Density, g/cm2 | 1.28 1.09 – 1.48 [1.19 – 1.37] 1.28 ± 0.11 |
1.24 1.07 – 1.42 [1.17 – 1.30] 1.24 ± 0.09 |
−0.05 −0.11 – 0.03 [−0.07 – −0.02] −0.05 ± 0.04 |
<0.001 |
| Lumbar Spine T-Score | 0.70 −0.8 – 2.3 [0 – 1.4] 0.69 ± 0.84 |
0.30 −1.2 – 1.9 [−0.25 – 0.9] 0.30 ± 0.78 |
−0.40 −1.1 – 0.3 [−0.6 – −0.2] −0.39 ± 0.30 |
<0.001 |
| Femur Bone Mineral Density, g/cm2 | 0.99 0.80 – 1.28 0.94[− 1.15] 1.04 ± 0.13 |
1.03 0.88 – 1.35 [0.95 – 1.18] 1.05 ± 0.14 |
−0.01 −0.06 – 0.36 [−0.02 – 0.01] 0.016 ± 0.08 |
0.48 |
| Femur T-Score | −0.2 −1.7 – 2.2 [−0.6 – 1.1] 0.25 ± 1.03 |
−0.1 −1.1 – 2.7 [−0.5 – 1.4] 0.36 ± 1.10 |
−0.1 −0.60 – 2.90 [−0.2 – 0.1] 0.11 ± 0.69 |
0.68 |
| Percent Body Fat | 35.8 16.7 – 54.2 [31.3 – 46.1] 37.0 ± 9.4 |
37.5 19.3 – 54.7 [31.2 – 46.7] 37.8 ± 9.9 |
0.3 −4.0 – 5.8 [−1.7 – 2.4] 0.3 ± 2.6 |
0.76 |
Bone Density Assessed by DEXA
From DEXA assessments (Table 4), there was a statistically significant (p<0.001) but minor decrease in lumbar spine bone density measurements. Median changes were −0.04 g/cm2 (range −0.11 to +0.03) and −0.40 (range −1.10 to +0.30) for T-score. Only one participant showed a clinically significant T-score decrease of at least one unit, from −0.1 to −1.2. There was no observable effect on femur bone density or percent body fat measured.
Discussion
This is the first report of the effect of the SERM acolbifene on benign breast tissue of healthy premenopausal women. There was favorable modulation of the risk biomarker Ki-67 as well as expression of several estrogen responsive genes including pS2 and PgR despite dramatic increases in serum estradiol levels. There were no subjects who discontinued use because of side effects and no increase in endometrial thickness. Clinically insignificant decreases in lumbar spine bone density were observed following 6–8 months exposure, as well as an asymptomatic increase in ovarian cysts. Overall, acolbifene appears to modulate tissue risk biomarkers in a similar fashion as tamoxifen; but in this single arm study hot flashes and other perimenopausal symptoms were not increased as would be expected with tamoxifen. Nor was there an increase in endometrial thickness.
Ki-67 was selected as the primary risk biomarker endpoint of this study because proliferation is permissive for cancer development. In observational studies, Ki-67 was higher in foci of hyperplasia and atypical hyperplasia of women who subsequently developed breast cancer than in those that did not [26, 27]. Women with ≥2% of cells in atypical foci labeling for Ki-67 had a 4-fold increased risk for breast cancer [27]. By eligibility criterion, a minimum baseline Ki-67 of 2% was required in clusters of cells judged to be hyperplastic by cytologic criteria. The reduction in Ki-67 observed was statistically significant and almost universal (23/25 paired specimens), consistent with the well-known effects induced by tamoxifen in early breast cancer in short-term window of opportunity trials and premenopausal benign breast tissue [28–30]. In neo-adjuvant cancer treatment studies, reduction of or low post-tamoxifen Ki-67 in tumor tissue is associated with superior recurrence-free survival [31]. However, a serial biopsy study reported by Moshin and Allred in 2005 did not show an effect of 1 year of tamoxifen vs control on Ki-67 in a small number of women with benign hyperplastic foci generally without atypia [32].
We did not see any reduction in cytologic evidence of atypia after 6 months of acolbifene use. Cytologic evidence of atypia by RPFNA, like atypical hyperplasia in diagnostic biopsies, is associated with increased risk [17], but there is no evidence that short-term use of a SERM, including tamoxifen, will significantly change morphology in benign breast tissue [32, 33].
Reduction observed at the transcript (mRNA) level of the estrogen inducible genes for pS2 and PgR is qualitatively similar for acolbifene as that observed with tamoxifen [34, 35]. Expression of the gene for ERα was reduced dramatically and GREB1 which is an estrogen response gene associated with proliferation [34, 36] was slightly reduced. There was no clear effect on the chemokine stromal cell-derived factor 1 (SDF-1) which is important for viability of stem cells and has been implicated in ligand-independent phosphorylation of the estrogen receptor and tamoxifen resistance [37–39] Tamoxifen has been variably associated with increases in SDF-1 [34, 40, 41]. A recent report assessing the short term effects of several SERMs and fulvestrant on a large number of genes in mammary cancer from ovariectomized mice suggests that acolbifene reverses the effect of estradiol on more estrogen inducible genes than tamoxifen, raloxifene, or fulvestrant [42].
The risk biomarkers of serum IGF-1 to IGFBP3 ratio [43] and mammographic breast density [24] are known to be modulated by tamoxifen [33, 44, 45]. In IBIS-1 tamoxifen reduced mammographic breast density in premenopausal women with baseline density of >10% by a mean of 13% compared to placebo [45]. The median absolute decrease in mammographic density of 3.9% after 6 months of acolbifene was not statistically significant although a greater numerical effect is likely had the drug been given longer [46] and might have reached significance had more subjects been entered into the trial. Although acolbifene did not significantly modulate either IGF-1 or breast density, this does not necessarily mean that acolbifene is a less effective anti-estrogen than tamoxifen. Aromatase inhibitors modulate neither IGF-1 nor mammographic breast density but have demonstrated efficacy in prevention and are generally viewed as more effective than tamoxifen in a low estrogen environment [46, 47].
Acolbifene was associated with an increase in serum estradiol levels and ovarian cysts. Increased mid-cycle and luteal levels of estrogen and increased ovarian cysts have been observed for tamoxifen where the prevalence in asymptomatic premenopausal women undergoing regular pelvic ultrasound monitoring has been reported as up to 80% without regard to cyst size and approximately 30% for cysts of 30 mm or greater diameter [7, 48–51]. In the absence of symptoms, these cysts are probably of little clinical significance and are likely due to prolonged elevation of FSH in the follicular phase combined with elevated mid-cycle or luteal estradiol for both tamoxifen and acolbifene [7]. Increase in ovarian cyst formation after acolbifene tended to resolve shortly after the drug was stopped (data not shown). We found no change in SHBG or free testosterone. Information on SHBG and free testosterone in premenopausal women without breast cancer is limited but SHBG is generally increased and free testosterone generally reduced in postmenopausal women after tamoxifen [52]. There was a minimal reduction in premenopausal bone density similar to that observed with tamoxifen [53]. Finally, there was a statistically significant, but clinically insignificant, decrease in white blood cells and platelets (data not shown), similar to what has been observed with tamoxifen [33].
Importantly, from the standpoint of uptake of a prevention agent by premenopausal women, there was no evidence of worsening of hot flashes, other perimenopausal or musculoskeletal symptoms, or overall quality of life; nor was there any evidence of endometrial thickening. In contrast in the NSABP P-1 trial, hot flashes were reported by 81% of individuals randomized to 5 years of tamoxifen vs 65% of those randomized to placebo [54] and endometrial thickening is commonly observed in premenopausal women taking tamoxifen in the absence of concomitant Goserelin [55, 56].
The ability of a SERM to act as an agonist or antagonist depends on hormone levels and the specific tissue as activator/repressor levels vary by tissue type. Acolbifene differs from tamoxifen in that it blocks the co-activator SRC-1 expressed in high amounts in uterine but not breast tissue [12], thus explaining acolbifene’s lack of agonist effect on the uterus. Acolbifene has the potential to be more effective than tamoxifen as it inhibits both the AF-1 and AF-2 functions of both ERα and ERβ, while the inhibitory action of tamoxifen is limited to AF-2. SERMs which block only AF2 are likely to have partial estrogen agonist activity [reviewed in 57]. A SERM’s relative potency depends on many factors including its bioavailability, serum and tissue half-life, affinity for the estrogen receptor, and rate of ubiquination of the ligand-ER complex [58]. The plasma half-life of tamoxifen is ~7 days [59] whereas that of acolbifene is ~24 hours [Personal communication, Fernand Labrie, Endorecherche, Inc., Quebec, Canada].
Limitations of this study include the small number of subjects and lack of a control (placebo) arm. Because of the eligibility criterion of Ki-67 ≥2%, and the resulting baseline mean Ki-67 of our cohort being higher than the population mean, there is a risk that the apparent decrease in Ki-67 was the result of a regression to the mean artifact. Without a parallel placebo arm one cannot conclusively distinguish between this possibility and a true effect of acolbifene. In addition, the large number of variables considered, without correction for multiple comparisons, increases the risk of type I error for the exploratory biomarkers. None-the-less, a number of factors were identified as potential pharmacodynamic effect markers or that might assist in elucidation of mechanisms of action; these can be evaluated further in future trials.
In conclusion, acolbifene was associated with a favorable side effect profile, and an apparent favorable modulation of risk biomarkers including proliferation as well as the estrogen response genes for pS2, ERα, and PgR. Given the lack of demonstrated increase in hot flashes and other subjective symptoms, acolbifene should be compared to placebo (2 arms) or placebo and tamoxifen (3 arms) in a Phase IIB trial for premenopausal women with modulation of benign breast tissue proliferation and vasomotor symptoms as co-primary endpoints.
Supplementary Material
Acknowledgments
Financial Support: Supported by Subcontract 938N232 from the University of Wisconsin Cancer Consortium for “Phase I and Phase II Clinical Trials of Cancer Chemopreventive Agents” (PI: Howard H. Bailey, M.D.); NO1-CN-35153, National Cancer Institute, National Institutes of Health. Study agent was provided by Endorecherche, Inc. of Quebec, Canada.
Footnotes
Conflicts of Interest: None
References
- 1.Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–62. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–34. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: A systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–5. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Brest and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC. Estrogen, selective estrogen receptor modulation, and coronary heart disease. Something or nothing. J Natl Cancer Inst. 2001;93:2–4. doi: 10.1093/jnci/93.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly LS, Evans DG, Wiseman J, Fox J, Greenhalgh R, Affen J, et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br J Cancer. 2014;110:1681–7. doi: 10.1038/bjc.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen I, Figer A, Tepper R, Shapira J, Altaras MM, Yigael D, et al. Ovarian overstimulation and cystic formation in premenopausal tamoxifen exposure: comparison between tamoxifen-treated and nontreated breast cancer patients. Gynecol Oncol. 1999;72:202–7. doi: 10.1006/gyno.1998.5201. [DOI] [PubMed] [Google Scholar]
- 8.Buijs C, Willemse PH, de Vries EG, Ten Hoor KA, Boezen HM, Hollema H, et al. Effect of tamoxifen on the endometrium and the menstrual cycle of premenopausal breast cancer patients. Int J Gynecol Cancer. 1996;19:677–81. doi: 10.1111/IGC.0b013e3181a47cbe. [DOI] [PubMed] [Google Scholar]
- 9.Melnikow J, Paterniti D, Azari R, Kuenneth C, Birch S, Kuppermann M, et al. Preferences of women evaluating risks of tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103:1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 10.Labrie F, Labrie C, Belanger A, Simard J, Gauthier S, Luu-The V, et al. EM-652 (SCH 57068), a third generation SERM acting as pure antiestrogen in the mammary gland and endometrium. J Steroid Biochem Mol Biol. 1999;69:51–84. doi: 10.1016/s0960-0760(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 11.Labrie F, Simard J, Labrie C, Bélanger A. EM-652 (SCH 57068), a pure SERM in the mammary gland and endometrium. Références en Gynécologie Obstétrique. 2001;8:331–6. [Google Scholar]
- 12.Labrie F, Labrie C, Belanger A, Simard J, Giguere V, Tremblay A, et al. EM-652 (SCH57068), a pure SERM having complete antiestrogenic activity in the mammary gland and endometrium. J Steroid Biochem Mol Biol. 2001;79:213–25. doi: 10.1016/s0960-0760(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 13.Labrie F, Champagne P, Labrie C, Roy J, Laverdière J, Provencher L, et al. Activity and safety of the antiestrogen EM-800, the orally active precursor of acolbifene, in tamoxifen-resistant breast cancer. J Clin Oncol. 2004;22:864–71. doi: 10.1200/JCO.2004.05.122. [DOI] [PubMed] [Google Scholar]
- 14.Roy J, Couillard S, Gutman M, Labrie F. A novel pure SERM achieves complete regression of the majority of human breast cancer tumors in nude mice. Breast Cancer Res Treat. 2003;81:223–9. doi: 10.1023/A:1026118602273. [DOI] [PubMed] [Google Scholar]
- 15.Luo S, Stojanovic M, Labrie C, Labrie F. Inhibitory effect of the novel anti-estrogen EM-800 and medroxyprogesterone acetate on estrone-stimulated growth of dimethylbenz(a) anthracene-induced mammary carcinoma in rat. Int J Cancer. 1997;73:580–6. doi: 10.1002/(sici)1097-0215(19971114)73:4<580::aid-ijc20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–46. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 17.Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Kamel S, Zeiger S, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000;92:1217–27. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 18.Zalles C, Kimler BF, Kamel S, McKittrick R, Fabian CJ. Cytologic patterns in random aspirates from women at high and low risk for breast cancer. Breast J. 1995;1:343–9. [Google Scholar]
- 19.Masood S, Frykberg ER, McLellan GL, Scalapino MC, Mitchum DG, Bullard JB. Prospective evaluation of radiologically directed fine-needle aspiration biopsy of nonpalpable breast lesions. Cancer. 1990;66:1480–7. doi: 10.1002/1097-0142(19901001)66:7<1480::aid-cncr2820660708>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Petroff BK, Khan QJ, et al. Reduction in Ki-67 in benign breast tissue of high risk women with the lignan secoisolariciresinol diglycoside (SDG) Cancer Prev Res. 2010;3:1342–50. doi: 10.1158/1940-6207.CAPR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips TA, Fabian CJ, Kimler BF, Petroff BK. Assessment of RNA in human breast tissue sampled by random periareolar fine needle aspiration and ductal lavage and processed as fixed or frozen specimens. Reprod Biol. 2013;13:75–81. doi: 10.1016/j.repbio.2013.01.179. [DOI] [PubMed] [Google Scholar]
- 22.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prev. 2003;12:1457–61. [PubMed] [Google Scholar]
- 23.Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab. 1992;74:939–42. doi: 10.1210/jcem.74.4.1548361. [DOI] [PubMed] [Google Scholar]
- 24.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 25.Stone J, Gunasekara A, Martin LJ, Yaffe M, Minkin S, Boyd NF. The detection of change in mammographic density. Cancer Epidemiol Biomarkers Prev. 2003;12:625–30. [PubMed] [Google Scholar]
- 26.Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol. 2002;160:597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santisteban M, Reynolds C, Barr Fritcher EG, Frost MH, Vierkant RA, Anderson SS, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. 2010;121:431–7. doi: 10.1007/s10549-009-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–90. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 29.DeCensi A, Guerrieri-Gonzaga A, Gandini S, Serrano D, Cazzaniga M, Mora S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22:582–7. doi: 10.1093/annonc/mdq427. [DOI] [PubMed] [Google Scholar]
- 30.de Lima GR, Facina G, Shida JY, Chein MB, Tanaka P, Dardes RC, et al. Effects of low dose tamoxifen on normal breast tissue from premenopausal women. Eur J Cancer. 2003;39:891–8. doi: 10.1016/s0959-8049(02)00530-0. [DOI] [PubMed] [Google Scholar]
- 31.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11(2 Pt 2):951s–8s. [PubMed] [Google Scholar]
- 32.Mohsin SK, Allred DC, Osborne CK, Cruz A, Otto P, Chew H, Clark GM, Elledge RM. Morphologic and immunophenotypic markers as surrogate endpoints of tamoxifen effect for prevention of breast cancer. Breast Cancer Res Treat. 2005;94:205–11. doi: 10.1007/s10549-005-4896-1. [DOI] [PubMed] [Google Scholar]
- 33.Euhus D, Bu D, Xie XJ, Sarode V, Ashfaq R, Hunt K, et al. Tamoxifen downregulates ets oncogene family members ETV4 and ETV5 in benign breast tissue: implications for durable risk reduction. Cancer Prev Res. 2011;4:1852–62. doi: 10.1158/1940-6207.CAPR-11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92:141–9. doi: 10.1007/s10549-005-1483-4. [DOI] [PubMed] [Google Scholar]
- 35.Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–33. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000;60:6367–75. [PubMed] [Google Scholar]
- 37.Rhodes LV, Short SP, Neel NF, Salvo VA, Zhu Y, Elliott S, et al. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71:603–13. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubrovska A, Hartung A, Bouchez LC, Walker JR, Reddy VA, Cho CY, et al. CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling. Br J Cancer. 2012;107:43–52. doi: 10.1038/bjc.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–45. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 40.Kubarek Ł, Jagodzinski PP. Epigenetic up-regulation of CXCR4 and CXCL12 expression by 17 beta-estradiol and tamoxifen is associated with formation of DNA methyltransferase 3B4 splice variant in Ishikawa endometrial adenocarcinoma cells. FEBS Lett. 2007;581:1441–8. doi: 10.1016/j.febslet.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 41.Pietkiewicz PP, Lutkowska A, Lianeri M, Jagodzinski PP. Tamoxifen epigenetically modulates CXCL12 expression in MCF-7 breast cancer cells. Biomed Pharmacother. 2010;64:54–7. doi: 10.1016/j.biopha.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 42.Calvo E, Luu-The V, Belleau P, Martel C, Labrie F. Specific transcriptional response of four blockers of estrogen receptors on estradiol-modulated genes in the mouse mammary gland. Breast Cancer Res Treat. 2012;134:625–47. doi: 10.1007/s10549-012-2104-7. [DOI] [PubMed] [Google Scholar]
- 43.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 44.Bonanni B, Johansson H, Gandini S, Guerrieri-Gonzaga A, Torrisi R, Sandri MT, et al. Effect of low dose tamoxifen on the insulin-like growth factor system in healthy women. Breast Cancer Res Treat. 2001;69:21–7. doi: 10.1023/a:1012241505717. [DOI] [PubMed] [Google Scholar]
- 45.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 46.Cigler T, Richardson H, Yaffe MJ, Fabian CJ, Johnston D, Ingle JN, et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat. 2011;126:453–61. doi: 10.1007/s10549-010-1322-0. [DOI] [PubMed] [Google Scholar]
- 47.Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 48.Premkumar A, Venzon DJ, Avila N, Johnson DV, Remaley AT, Forman MR, Eng-Wong J, Zujewski J, Stratton P. Gynecologic and hormonal effects of raloxifene in premenopausal women. Fertil Steril. 2007;88:1637–44. doi: 10.1016/j.fertnstert.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 49.Shushan A, Peretz T, Uziely B, Lewin A, Mor-Yosef S. Ovarian cysts in premenopausal and postmenopausal tamoxifen-treated women with breast cancer. Am J Obstet Gynecol. 1996;174(1 Pt 1):141–4. doi: 10.1016/s0002-9378(96)70386-1. [DOI] [PubMed] [Google Scholar]
- 50.Inal MM, Incebiyik A, Sanci M, Yildirim Y, Polat M, Pilanci B, Nayki C, Camuzcuoğlu H. Ovarian cysts in tamoxifen-treated women with breast cancer. Eur J Obstet Gynecol Reprod Biol. 2005;120:104–6. doi: 10.1016/j.ejogrb.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Berliere M, Duhoux FP, Dalenc F, Baurain JF, Dellevigne L, Galant C, Van Maanen A, Piette P, Machiels JP. Tamoxifen and ovarian function. PLoS One. 2013;8:e66616. doi: 10.1371/journal.pone.0066616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kostoglou-Athanassiou I, Ntalles K, Gogas J, Markopoulos C, Alevizou-Terzaki V, Athanassiou P, Georgiou E, Proukakis C. Sex hormones in postmenopausal women with breast cancer on tamoxifen. Horm Res. 1997;47:116–20. doi: 10.1159/000185445. [DOI] [PubMed] [Google Scholar]
- 53.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual energy x-ray absorptionmetry in health premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 54.Day R. Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. National Surgical Adjuvant Breast and Bowel Project. Ann N Y Acad Sci. 2001;949:143–50. [PubMed] [Google Scholar]
- 55.Chang J, Powles TJ, Ashley SE, Iveson T, Gregory RK, Dowsett M. Variation in endometrial thickening in women with amenorrhea on tamoxifen. Breast Cancer Res Treat. 1998;48:81–5. doi: 10.1023/a:1005999008736. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Zong X, Yu Y, Shao G, Zhang L, Qian C, Bian Y, Xu X, Sun W, Meng X, Ding X, Chen D, Zou D, Xie S, Zheng Y, Zhang J, He X, Sun C, Yu X, Ni J. Combined effects of goserelin and tamoxifen on estradiol level, breast density, and endometrial thickness in premenopausal and perimenopausal women with early-stage hormone receptor-positive breast cancer: a randomised controlled clinical trial. Br J Cancer. 2013;109:582–8. doi: 10.1038/bjc.2013.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabian CJ, Kimler BF. Selective estrogen receptor modulators for primary prevention of breast cancer. J Clin Oncol. 2005;23:1644–55. doi: 10.1200/JCO.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437–52. doi: 10.2147/CIA.S66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordan VC. New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer. Steroids. 2007;72:829–842. doi: 10.1016/j.steroids.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.