Abstract
A variety of decellularized materials have been developed that have demonstrated potential for treating cardiovascular diseases and improving our understanding of cardiac development. Of these biomaterials, decellularized myocardial matrix hydrogels have shown great promise for creating cellular microenvironments representative of the native cardiac tissue and treating the heart after a myocardial infarction. Decellularized myocardial matrix hydrogels derived from porcine cardiac tissue form a nanofibrous hydrogel once thermally induced at physiological temperatures. Use of isolated cardiac extracellular matrix in 2D and 3D in vitro platforms has demonstrated the capability to provide tissue specific cues for cardiac cell growth and differentiation. Testing of the myocardial matrix hydrogel as a therapy after myocardial infarction in both small and large animal models has demonstrated improved left ventricular function, increased cardiac muscle, and cellular recruitment into the treated infarct. Based on these results, steps are currently being taken to translate these hydrogels into a clinically used injectable biomaterial therapy. In this review, we will focus on the basic science and preclinical studies that have accelerated the development of decellularized myocardial matrix hydrogels into an emerging novel therapy for treating the heart after a myocardial infarction.
Keywords: myocardial infarction, biomaterial, hydrogel, tissue engineering, regenerative medicine
Graphical Abstract
1. Introduction
Advancements in cardiac tissue engineering have demonstrated great promise in the pursuit of regenerative medicine for the treatment of cardiovascular diseases. Ischemic heart disease leading to myocardial infarction (MI) and subsequent heart failure (HF) is both the leading cause of death in the western world [1] and worldwide with a projected increase to 13.4% of overall annual deaths by 2030 [2]. After suffering a MI, the adult human heart lacks the regenerative capabilities to restore damaged myocardium leading to progressive pathophysiological remodeling such as extracellular matrix (ECM) degradation by matrix metalloproteinases (MMPs), fibrosis by increased collagen deposition, hypertrophy, and tissue necrosis that ultimately leads to HF [3–5]. Currently, no treatment is available that can prevent HF post-MI and options for end-stage HF are limited to whole heart transplant and left ventricular (LV) assist devices. However, these procedures put heavy strain on patients’ quality of life and medical resources [1]. In response, research in cardiac tissue engineering is focused on investigating whether restoration of function and possibly regeneration of the damaged myocardium can be achieved by novel methods of therapy.
An advancement that has particularly accelerated the field of cardiac tissue engineering is the isolation of the underlying ECM scaffold from native cardiac tissue first pioneered by decellularization techniques used by Ott et al. [6]. The ECM provides cells with tissue specific biochemical cues which has been demonstrated to be important for cellular migration [7,8], differentiation [9,10], and development [11]. Later work by Singelyn et al. first demonstrated that decellularized porcine cardiac tissue could be processed into an injectable biomaterial therapy that forms a nanofibrous porcine myocardial matrix hydrogel when thermally induced or injected in vivo [12]. Application of the hydrogel in post-MI animal models has resulted in improved LV function, increased cardiac muscle, and induced cellular recruitment into the treated infarct [13,14]. By creating a biomaterial representative of the native cardiac tissue, decellularized myocardial matrix hydrogels have quickly demonstrated their promising applications as a novel cell study platform and biomaterial therapy.
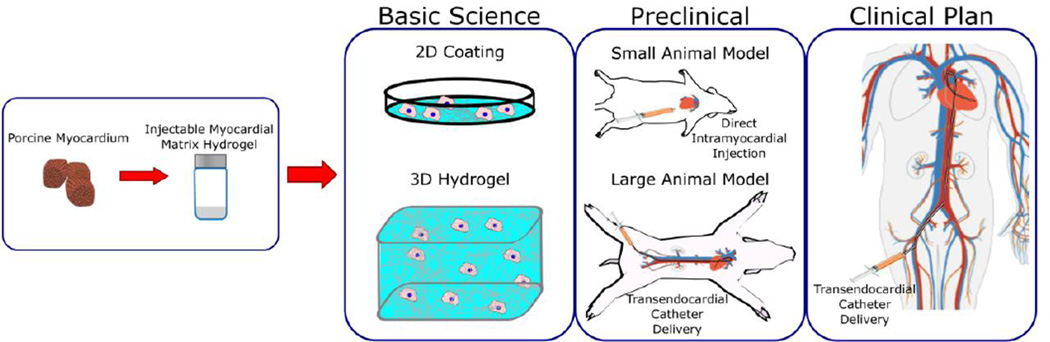
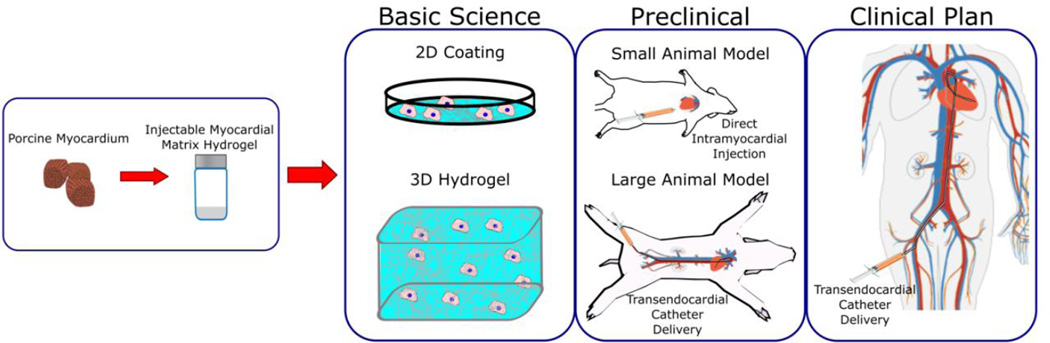
In this review, we will describe characteristics of decellularized myocardial matrix hydrogels, methods to modulate their material properties, demonstrated uses in creating in vitro models of the cardiac microenvironment, and significant progress in preclinical studies that have demonstrated their therapeutic potential (Fig. 1). Additional studies, which utilize the native architecture of the decellularized cardiac tissue scaffold either as a whole heart construct [6,15–18] or as pieces of cardiac ECM [19–27], are not covered since this method provides a spatial and mechanical environment that has distinct advantages and disadvantages compared to hydrogel methods.
Fig. 1.
Summary of the use of injectable decellularized myocardial matrix hydrogels for basic science and preclinical studies along with future plans for translation. Selected images reprinted from [28].
2. Generation and Characterization of Decellularized Myocardial Matrix Hydrogels
2.1. Generation of Decellularized Myocardial Matrix Hydrogels
To create decellularized myocardial matrix hydrogels, myocardium is first cut into smaller pieces and decellularized by agitation in a 1% sodium dodecyl sulfate (SDS) solution [12]. Other decellularization techniques using mechanical, chemical, and enzymatic methods [29] have also been described for the heart [6,15,29]. Following complete decellularization and water rinses to remove detergent, the decellularized ECM is lyophilized and milled into a fine powder. ECM powder is re-suspended and partially digested with pepsin at a tenth the ECM concentration for 48 hours in 0.1 M HCl solution. After enzymatic treatment, pepsin is inactivated by increasing the pH to 7.4 with NaOH and diluted to a desired concentration in a 1× PBS solution. The digested ECM can then be lyophilized, stored at −80°C, and simply re-suspended with sterile water prior to use [30].
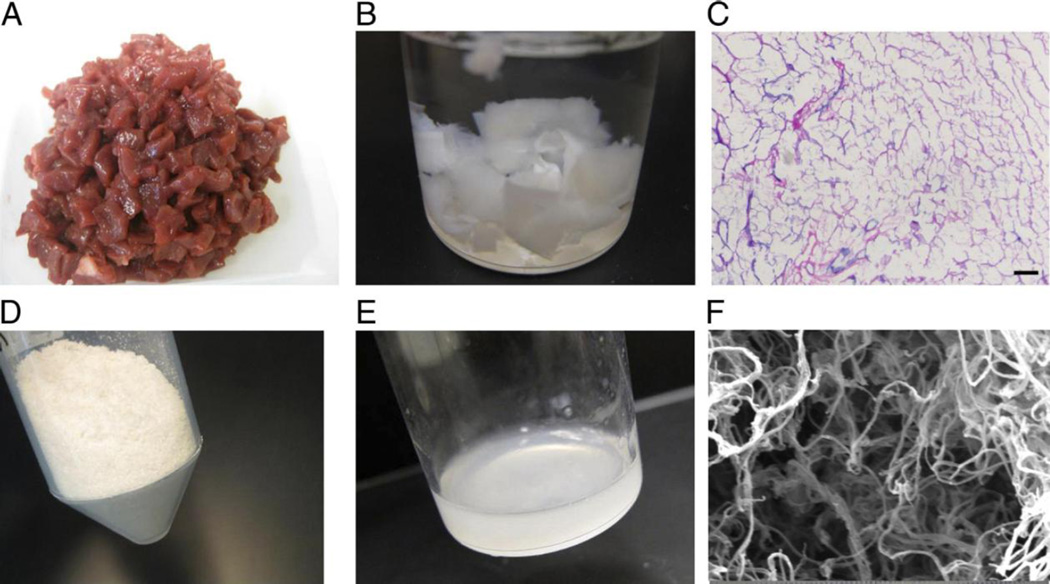
Depending on the source material, additional processing might be required to isolate the ECM such as isopropyl alcohol rinses for removing excess lipid content, which was needed for processing human heart tissue [31]. Material from multiple hearts should be collected into a single batch to reduce batch–to-batch variability. After generating a new batch of material, characterization should be performed to confirm quality of processing and consistency between each batch. These characterization steps include hematoxylin and eosin (H&E) staining to observe lack of nuclei indicating successful decellularization, DNA isolation and quantification to further confirm removal of cellular content, protein separation by gel electrophoresis to verify a similar pattern of ECM peptides between batches, a dimethylmethylene blue assay (DMMB) assay to determine sulfated glycosaminoglycan (sGAG) content, and a demonstration of material in vitro gelation [30]. Images of material generation are displayed in Figure 2 and video instruction demonstrating pericardial tissue processing into a decellularized injectable hydrogel can be found online [32]. Although the pericardial tissue has different material properties such as ECM composition [33], the processing is identical to the porcine myocardial matrix procedure with the exception of the time necessary for decellularization and digestion.
Figure 2.
Images of the flow-through for porcine-derived decellularized myocardial matrix hydrogel biomaterial generation. (A) Pieces of left ventricular tissue isolated from a pig heart and cut into small pieces. (B) Decellularized myocardium after continuous agitation with sodium dodecyl sulfate. (C) Hematoxylin and eosin stain of decellularized tissue to confirm lack of cellular content. (D) Lyophilized and milled extracellular matrix. (E) Extracellular matrix powder partially digested with pepsin into an injectable liquid. (F) Self-assembled, nanofibrous porcine myocardial matrix hydrogel structure imaged by scanning electron microscopy. Images were reprinted from [12,13] with permission from Elsevier.
2.2. Properties of Decellularized Myocardial Matrix Hydrogels
Decellularized cardiac ECM composition by mass spectroscopy has shown retention of an array of ECM proteins such as collagen, elastin, and fibronectin [28,31], and analysis by the DMMB assays has indicated retention of sGAGs. Based on complex viscosity analysis, liquid myocardial matrix is shear thinning, which supports its capability to be successfully injected through a catheter. Gelation occurs in less than 30 minutes when incubated at 37°C or injected in vivo [12]. Scanning electron microscopy of a formed hydrogel has demonstrated self-assembly into a nanofibrous scaffold with fiber diameters ranging from 40–100 nm [12]. With the breakdown of the native scaffold, myocardial matrix hydrogels are much softer than the native myocardium. Storage modulus of myocardial matrix hydrogels was determined to be around 5 Pa when measured by parallel plate rheology [34,35]. Johnson et al. demonstrated that changing ECM and ionic salt concentration of the gel raised the storage modulus to around 10 Pa and increased gelation kinetics [35]. However for in vivo use, there is a limited range these factors can be manipulated since increasing ECM concentration raises the complex viscosity and lowering salt concentrations makes the liquid hypotonic to in vivo conditions.
2.3. Methods to Alter Myocardial Matrix Hydrogel Properties
As discussed in Section 2.2, the processing steps described in Section 2.1 create decellularized myocardial matrix hydrogels with specific material properties. However, characteristics such as hydrogel composition, mechanical properties, and degradability might be limiting for certain experimental and therapeutic design parameters. Several methods have been developed to modify such properties, which could enable more tailored formulations for a broad range of applications. For example, alternative formulations may be better for directing cell survival, differentiation, and/or behavior in vitro, while others may be more appropriate for in vivo applications such as the delivery of cells or other therapeutics. There will be greater room for modifying properties in vitro, while tailoring properties for in vivo applications will be more limited if catheter deliverability is to be maintained [36]. Modifications and hybrid hydrogels that have been developed to date are discussed below.
Singelyn et al. demonstrated that using a cross-linking agent, glutaraldehyde, at 0.05% and 0.01% could increase the storage modulus by an order of magnitude. Gel degradation was slowed when measured by a ninhydrin assay, and cell migration into the gel was also slowed although not hindered overall [34]. However, glutaraldehyde toxicity was a concern since it could negatively affect the cellular response. Other studies have created composite hydrogels combining cardiac ECM with synthetic materials for greater control of mechanical properties. Gershalak et al. and Sullivan et al. used 2D culture substrates consisting of crosslinking digested rat myocardial matrix on polyacrylamide gels with elastic moduli ranging from 9–50 kPa [37,38]. Grover et al. tested incorporation of poly(ethylene glycol) (PEG) in porcine myocardial matrix hydrogels by amidation and radical conjugation methods. Radical conjugation of star PEG-acrylate resulted in the greatest increase in storage modulus ranging from 127 to 719 Pa at 1 Hz while other mechanisms led to storage moduli similar to the untreated or glutaraldehyde-treated myocardial matrix. All PEG-myocardial matrix hybrid gels had increased fiber diameter and slower enzyme degradation [39].
Hybrid myocardial matrix gels combined with naturally derived materials have also been demonstrated. Duan et al. created porcine myocardial matrix-collagen gels consisting of 25% or 75% cardiac ECM with storage moduli of around 59.3 Pa and 8.17 Pa at 1 Hz, respectively. Both hydrogels formed within 30 minutes allowing uniform encapsulation of human embryonic stem cells (hESCs) [40]. Pok et al. combined digested cardiac ECM with chitosan into a cardiac patch containing a polycaprolactone (PCL) core. Patches made with 33% ECM had the highest elastic modulus at approximately 49.2 kPa with stiffness diminishing with decreasing ECM content. Decreasing ECM content also raised hydrogel volume, pore diameter, and liquid uptake [41]. Williams et al. created rat myocardial matrix-fibrin hybrid gels cross-linked with transglutaminase. Although pure fibrin and untreated myocardial matrix-fibrin gels had similar stiffness, treatment with 120 µg/mL transglutaminase increased elastic modulus up to around 32 kPa. Greater amounts of cross-linking agent also decreased liquid uptake and lessened observed network formation [42].
3. In Vitro Studies
3.1. Cellular Responses to 2D Culture on Cardiac ECM Coated Substrates
Although not in hydrogel form, we will briefly review studies using digested cardiac ECM coated surfaces since similar biochemical cues are presented to cells. Several studies have focused on encouraging cardiomyocyte differentiation and maturation of stem cells or immature cardiac cells cultured on myocardial matrix coatings. DeQuach et al. studied the maturation of hESCs preconditioned towards a cardiomyocyte lineage on porcine myocardial matrix coatings compared to standard differentiation protocols on gelatin coatings. hESCs cultured to 112 days on the matrix showed significantly greater organized cell clustering and intercalated disc formation at lateral cell ends characteristic of mature cardiomyocytes [28]. French et al. also demonstrated elevated gene expression of early and late cardiomyocyte markers in cardiac progenitor cells on porcine myocardial matrix [43]. Proliferation, viability, and adhesion also increased. These shifts were not produced on adipose ECM coatings suggesting the tissue specific ECM provided a more ideal cell environment. Greater gene expression of ECM, MMPs, and adhesion proteins also suggested induced remodeling of the microenvironment [43]. Johnson et al. found that culture of human fetal cardiomyocyte progenitor cells on porcine derived, but not human derived myocardial matrix had greater gene expression of early cardiac markers compared to gelatin. However, proliferation of endothelial cells on human myocardial matrix was greater compared to the porcine derived matrix. These differences could have arisen from compositional shifts in the older human ECM [31]. A study by Williams et al. showed that primary neonatal rat cardiac cells had significantly greater percentages of cardiomyocyte adherence and prolonged proliferation on fetal rat myocardial matrix in serum-free media compared to adult matrix and poly-L-lysine coatings with or without serum. Results on neonatal rat myocardial matrix were generally similar, but to a lesser degree compared to fetal ECM and did not always reach significance compared to other conditions [44].
3.2. Cellular Responses to 2D Culture on Composite Cardiac ECM Hydrogels
Considering influences of substrate stiffness on stem cell behavior [45], composite hydrogels presented in Section 2.3. have been used to study the combined effect of ECM and stiffness. Gershlak et al. cultured rat mesenchymal stem cells on myocardial matrix-polyacrylamide gels incorporating different ECM compositions from fetal, neonatal or adult rat hearts with stiffness representative of the heart tissue at these developmental stages. In this study, cell spread area, and traction force generally increased with increasing substrate stiffness except for on adult ECM, though it had the greatest increase in traction stress at lower stiffness [37]. Gershlak et al. later investigated the stable cell traction force on adult ECM despite different substrate stiffness, which contrasted with cell behavior on collagen. This investigation determined that the β1 integrin subunit and certain ECM components, fibronectin and laminin, at compositional ratios mimicking the adult ECM composition play a role in attenuating cellular responses to different substrate stiffness [46]. Sullivan et al. utilized rat myocardial matrix-polyacrylamide gels mimicking the healthy and infarct cardiac microenvironment to investigate the effects on the mesenchymal stem cell phenotype. Conditions representative of the diseased condition shifted expression of early cardiac markers, and secretion of certain growth factors was increased, supporting hypotheses that therapeutic effects of stem cell treatment are achieved by a reparative paracrine mechanism [38,47].
3.3. Cellular Reponses in 3D Composite Cardiac Hydrogels
In hybrid porcine myocardial matrix-collagen scaffolds, Duan et al. encapsulated hESCs embryoid bodies and compared their differentiation toward a cardiomyocyte phenotype to protocols with pure collagen gels and additives mimicking early cardiac growth factor patterns. Gels with higher percentages of ECM had both significantly greater and more prolonged gene expression of cardiomyocyte markers compared to both growth factor regimens and pure collagen gels. Beating populations, contraction amplitudes, and gap junction organization were also increased [40]. Interestingly, combined treatment with ECM and growth factor additives seemed to hinder cardiac phenotype, though the regimen was likely not optimized to work with an ECM-coated substrate. Williams et al. investigated the development of human c-kit+ cells in neonatal and adult rat myocardial matrix-fibrin gels cross-linked with transglutaminase. Looking at the combined effect of stiffness and different ECM compositions, cell viability and proliferation were enhanced in lower stiffness gels. Additionally modulation of endothelial, smooth muscle, and cardiac muscle cell markers were observed on different stiffness and ECM combinations suggesting synergistic effects [42]. Pok et al. showed that neonatal rat cardiomyocyte development on porcine myocardial matrix-chitosan cardiac patches versus gelatin-chitosan patches had significantly greater gene expression of cardiomyocyte markers and gap junction presence. Further analysis of cell function determined faster conduction velocity and greater contractile force [41]. Overall, these studies demonstrate that the biochemical cues in the myocardial matrix promote mature cardiac cell lineages and could support similar mechanisms in vivo.
4. Preclinical Research
4.1. Assessment of Decellularized Myocardial matrix Hydrogels as a Post-MI Therapy
Application of decellularized myocardial matrix hydrogels in both small and large animal models has demonstrated its potential as a post-MI therapy. Singelyn et al. demonstrated that porcine myocardial matrix encouraged vessel infiltration based on endothelial and smooth muscle cell migration into the injected tissue with significant increases in arteriole density 11 days post-injection [12]. Following this proof-of-concept, Singelyn et al. tested the injectable hydrogel treatment in a rat MI model, injecting the material 2 weeks post-MI, and found greater areas of surviving cardiomyocytes in the infarct area [13]. Low numbers of c-kit+ cells, and proliferative cardiomyocytes and myofibroblasts were also detected. Although no significant difference in M2 macrophage presence was observed, there was a trend towards an increase in this cell type, implying a pro-remodeling response. Evaluation of the treatment efficacy of the myocardial matrix pre-injection (1 week post-MI) and 4 weeks post-injection (6 weeks post-MI), as quantified by magnetic resonance imaging, showed preservation of ejection fractions (EF), end-diastolic-volume (EDV), and end-systolic-volume (ESV) unlike saline injected controls, which had a significant decrease in EF and increase in LV volumes [13].
Moving on from small animal models, delivery of the myocardial matrix hydrogel via a transendocardial catheter to 15–25 injection sites was demonstrated in both healthy and infarcted pigs [13]. In a subsequent functional study in a porcine MI, Seif-Naraghi et al. determined by echocardiography that pigs treated with the hydrogel 2 weeks post-MI via transendocardial delivery had significantly improved EF, EDV, and ESV compared to controls. In addition to improvements in global cardiac function, regional cardiac function, as measured by the global wall motion index, was also significantly improved, suggesting the matrix improved contractility of the ventricle. Evaluation of the cardiac tissue by histology and NOGA mapping also showed significantly greater cardiac muscle, lesser collagen deposition, reduced infarct expansion, and higher neovascularization indicating mitigated fibrosis, and preservation and/or regeneration of cardiac tissue as displayed in Figure 3 [14]. Overall, this series of studies demonstrates the potential of the myocardial matrix hydrogel alone as an effective post-MI therapy.
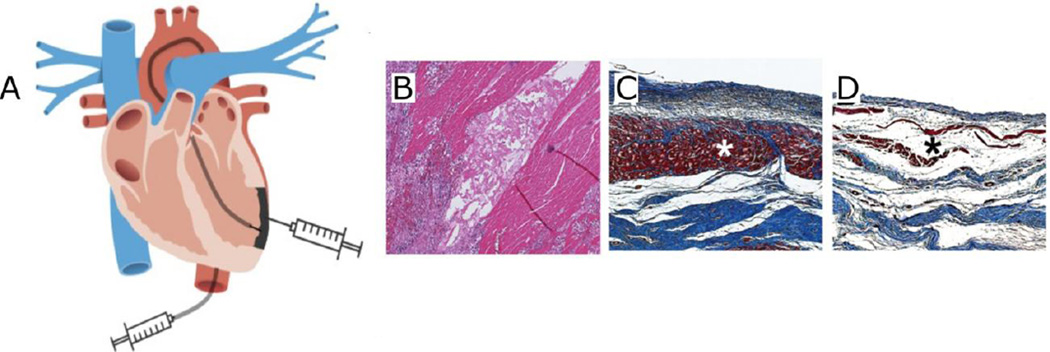
Fig. 3.
Method of injection of the myocardial matrix into the heart with representative histological images of matrix-treated and control porcine hearts. (A) Diagram for delivery of decellularized myocardial matrix hydrogel therapy into the heart post-myocardial infarction by direct injection or transendocardial catheter. (B) Hematoxylin and eosin stain of decellularized myocardial matrix hydrogel after injecting into the porcine myocardium. (C) Masson’s trichrome stain of matrix-injected heart with thick endocardium layer (red stain) as denoted by an asterisk taken 3 months post-injection. (D) Non-injected control group with loose fibrillar layer and thin endocardium denoted by an asterisk at the same time point. Images were taken and modified from [13,14,48] with permission.
4.2. Safety Assessment of Decellularized Myocardial matrix Hydrogel Therapy
For new therapies, safety is critical to assess and has been addressed by various methods for the porcine myocardial matrix. Since injectable therapies could disrupt cardiac rhythm and surrounding tissue [49], Singelyn et al. induced ventricular tachycardia by electrode pacing in porcine myocardial matrix-injected rats. Results did not significantly differ relative to saline controls demonstrating that matrix treatment is not pro-arrhythmic [13]. Similarly, Seif-Naraghi et al. found no major abnormalities in cardiac rhythm and waveform morphology in ECG recordings taken over 3 months in MI pigs treated with the hydrogel [14].
Biocompatibility and biodegradability allow for biomaterials to properly integrate into the host tissue and promote safe tissue repair. In rat myocardium, Seif-Naraghi et al. compared the immune response to the porcine myocardial matrix, saline, and a non-decellularized version of the porcine matrix over several time points out to 112 days post-injection, with the latter material used to create a strongly negative response. The non-decellularized material resulted in foreign body giant cells and signs of chronic inflammation after 2–4 weeks. In contrast, the decellularized porcine myocardial matrix was completely degraded by 28 days post-injection and had a biocompatible response without signs of rejection or a chronic response [14].
Consequences due to leakage during catheter delivery of the biomaterial were also assessed as a possible safety concern. Rats were injected with porcine myocardial matrix directly into their LV lumen and survived for 6 hours [14]. The rats showed no signs of distress during this period, and histological analysis showed no signs of thromboembolism or ischemia in satellite tissues. In the functional porcine MI study, a pathologist blinded to the identity of each study group further found satellite organ condition and blood composition to be normal. Hemocompatibility was also tested by mixing the liquid myocardial matrix with human plasma. Results showed no shifts in prothrombin time or activated partial thromboplastin time, and no clinically relevant effects were observed for platelet activation [14].
4.3. Method of Repair
Although the therapeutic effect of the myocardial matrix and other injectable biomaterials has been demonstrated, the exact mechanism of repair has not been determined. Several hypotheses have arisen based on the results of current in vitro and in vivo studies. After gelling, the material forms a new physical scaffold with appropriate pore size and fiber diameter to promote endogenous cell infiltration. From the in vitro studies described in Section 3, the myocardial matrix provides tissue specific cues that promote differentiation of cardiac stem/progenitor cells towards a cardiomyocyte lineage [40,43] and a more mature cardiomyocyte phenotype [28,41]. Attraction of vascular cells has also been demonstrated [12]. Corresponding results have been observed from application in both the rat and porcine MI models as described in Section 4.1 such as enhanced neovascularization and increased cardiac muscle. This increase in cardiac muscle could be a result of reducing borderzone cardiomyocyte apoptosis, as suggested in the rat MI model, since increased areas of cardiomyocytes were found at only 1 week post injection [13]. Other contributions to increased cardiac muscle could also occur from proliferating cardiomyocytes, which were seen in small numbers in the rat model, or differentiation of progenitor cells. Previous research has shown there are greater numbers of cardiac progenitors in the heart post-MI [50], and myocardial matrix therapy might provide an environment that promotes their development into new cardiomyocytes. Decellularized ECM scaffolds have been shown to promote a M2 and Th2 pro-remodeling response [51–53], and it is also likely that this contributes to the cardiac repair response observed with the myocardial matrix.
5. Conclusion
Overall, great progress has been made in demonstrating the clinical potential and feasibility of decellularized myocardial matrix hydrogels to promote repair post-MI. With the results from preclinical studies, there are currently plans to apply the myocardial matrix hydrogel therapy in clinical trials (Clinicaltrials.gov identifier NCT02305602). There are, however, still several potential studies that warrant their continued use in basic science and preclinical studies. For example, utilizing the ECM hydrogel in combination with other therapeutics could yield greater benefits in cardiac function. While a biomaterial alone therapy has numerous practical advantages such as quicker and cheaper manufacturing, off-the-shelf availability, and reduced regulatory concerns compared to a combination product, there are still numerous co-delivery possibilities with drugs, growth factors, and/or cells that could provide worthwhile improvements. Previous studies by Seif-Naraghi et al. and Sonnenberg et al. have demonstrated retention of heparin binding growth factors, basic fibroblast growth factor and engineered hepatocyte growth factor, by sGAG interactions in ECM hydrogels, allowing for prolonged release profiles and enhanced therapeutic effects [54,55]. Previous research with other injectable materials has also supported that combining cells with injectable materials improves the overall therapeutic effect [48]. In addition to further preclinical studies, the myocardial matrix is also a promising in vitro platform to provide a cardiac specific microenvironment for cardiac stem cell and development studies [28,39,40].
Acknowledgements
The authors would like to thank the NIH (R01HL113468) for supporting this work. Dr. Christman is co-founder, board member, and holds equity interest in Ventrix, Inc.
Abbreviations
- DMMB
dimethylmethylene blue assay
- ECM
extracellular matrix
- EDV
end-diastolic-volume
- EF
ejection fraction
- ESV
end-systolic-volume
- H&E
hematoxylin and eosin
- hESC
human embryonic stem cell
- HF
heart failure
- LV
left ventricular
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- PCL
polycaprolactone
- PEG
poly(ethylene glycol)
- SDS
sodium dodecyl sulfate
- sGAG
sulfated glycosaminoglycan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: A temporal and spatial window. Cardiovasc. Res. 2006;69:604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Cleutjens JPM, Creemers EEJM. Integration of concepts: Cardiac extracellular matrix remodeling after myocardial infarction. J. Card. Fail. 2002;8:S344–S348. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- 5.Rubin SA, Fishbein MC, Swan HJC. Compensatory hypertrophy in the heart after myocardial infarction in the rat. J. Am. Coll. Cardiol. 1983;1:1435–1441. doi: 10.1016/s0735-1097(83)80046-1. [DOI] [PubMed] [Google Scholar]
- 6.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 7.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daub JT, Merks RMH. A Cell-Based Model of Extracellular-Matrix-Guided Endothelial Cell Migration During Angiogenesis. Bull. Math. Biol. 2013;75:1377–1399. doi: 10.1007/s11538-013-9826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz SA, Chen CS. Emergence of Patterned Stem Cell Differentiation Within Multicellular Structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 11.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, et al. Catheter-Deliverable Hydrogel Derived From Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, et al. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Sci. Transl. Med. 2013;5:173ra125. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The Quest for an Optimized Protocol for Whole-Heart Decellularization: A Comparison of Three Popular and a Novel Decellularization Technique and Their Diverse Effects on Crucial Extracellular Matrix Qualities. Tissue Eng. Part C: Methods. 2011;17:915–926. doi: 10.1089/ten.TEC.2011.0210. [DOI] [PubMed] [Google Scholar]
- 16.Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K, So P, Korkmaz S, et al. Development and Evaluation of a Perfusion Decellularization Porcine Heart Model. Circ. J. 2011;75:852–860. doi: 10.1253/circj.cj-10-0717. [DOI] [PubMed] [Google Scholar]
- 17.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of Cardiac Extracellular Matrix from an Intact Porcine Heart. Tissue Eng. Part C: Methods. 2010;16:525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasui H, Lee J-K, Yoshida A, Yokoyama T, Nakanishi H, Miwa K, Naito AT, Oka T, et al. Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials. 2014;35:7839–7850. doi: 10.1016/j.biomaterials.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 19.Eitan Y, Sarig U, Dahan N, Machluf M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility. Tissue Eng Part C Methods. 2010;16:671–683. doi: 10.1089/ten.TEC.2009.0111. [DOI] [PubMed] [Google Scholar]
- 20.Sarig U, Au-Yeung GC, Wang Y, Bronshtein T, Dahan N, Boey FY, Venkatraman SS, Machluf M. Thick acellular heart extracellular matrix with inherent vasculature: a potential platform for myocardial tissue regeneration. Tissue Eng Part A. 2012;18:2125–2137. doi: 10.1089/ten.tea.2011.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarig U, Nguyen EB, Wang Y, Ting S, Bronshtein T, Sarig H, Dahan N, Gvirtz M, et al. Pushing the envelope in tissue engineering: ex vivo production of thick vascularized cardiac extracellular matrix constructs. Tissue Eng Part A. 2015;21:1507–1519. doi: 10.1089/ten.tea.2014.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulte JB, Simionescu A, Simionescu DT. The acellular myocardial flap: a novel extracellular matrix scaffold enriched with patent microvascular networks and biocompatible cell niches. Tissue Eng Part C Methods. 2013;19:518–530. doi: 10.1089/ten.tec.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Borazjani A, Tahai M, Curry AL, Simionescu DT, Guan J, To F, Elder SH, et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J. Biomed. Mater. Res. A. 2010;94:1100–1110. doi: 10.1002/jbm.a.32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godier-Furnémont AFG, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7974–7979. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Tedder ME, Perez CE, Wang G, de Jongh Curry AL, To F, Elder SH, Williams LN, et al. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J. Mater. Sci. Mater. Med. 2012;23:1835–1847. doi: 10.1007/s10856-012-4660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higuchi S, Lin Q, Wang J, Lim TK, Joshi SB, Anand GS, Chung MC, Sheetz MP, et al. Heart extracellular matrix supports cardiomyocyte differentiation of mouse embryonic stem cells. J. Biosci. Bioeng. 2013;115:320–325. doi: 10.1016/j.jbiosc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momtahan N, Sukavaneshvar S, Roeder BL, Cook AD. Strategies and Processes to Decellularize and Recellularize Hearts to Generate Functional Organs and Reduce the Risk of Thrombosis. Tissue Eng. Part B. Rev. 2014;21:115–132. doi: 10.1089/ten.TEB.2014.0192. [DOI] [PubMed] [Google Scholar]
- 28.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and High Yielding Method for Preparing Tissue Specific Extracellular Matrix Coatings for Cell Culture. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Ungerleider JL, Johnson TD, Rao N, Christman KL. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods. 2015 doi: 10.1016/j.ymeth.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson TD, DeQuach JA, Gaetani R, Ungerleider J, Elhag D, Nigam V, Behfar A, Christman KL. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014;2 doi: 10.1039/C3BM60283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seif-Naraghi S, Singelyn J, DeQuach J, Schup-Magoffin P, Christman K. Fabrication of Biologically Derived Injectable Materials for Myocardial Tissue Engineering. J. Vis. Exp. 2010 doi: 10.3791/2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and Characterization of an Injectable Pericardial Matrix Gel: A Potentially Autologous Scaffold for Cardiac Tissue Engineering. Tissue Eng. Part A. 2010;16:2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singelyn JM, Christman KL. Modulation of Material Properties of a Decellularized Myocardial Matrix Scaffold. Macromol. Biosci. 2011;11:731–738. doi: 10.1002/mabi.201000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson TD, Lin SY, Christman KL. Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/49/494015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2013;10:59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 37.Gershlak JR, Resnikoff JIN, Sullivan KE, Williams C, Wang RM, Black LD., Iii Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem. Biophys. Res. Commun. 2013;439:161–166. doi: 10.1016/j.bbrc.2013.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res. Ther. 2014;5 doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grover GN, Rao N, Christman KL. Myocardial matrix–polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology. 2014;25 doi: 10.1088/0957-4484/25/1/014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid Gel Composed of Native Heart Matrix and Collagen Induces Cardiac Differentiation of Human Embryonic Stem Cells without Supplemental Growth Factors. J. Cardiovasc. Transl. Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pok S, Benavides OM, Hallal P, Jacot JG. Use of Myocardial Matrix in a Chitosan-Based Full-Thickness Heart Patch. Tissue Eng. Part A. 2014;20:1877–1887. doi: 10.1089/ten.tea.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, Black LD., Iii Cardiac extracellular matrix–fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 2015;14:84–95. doi: 10.1016/j.actbio.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, Davis ME. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8:4357–4364. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams C, Quinn KP, Georgakoudi I, Black LD. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 2014;10:194–204. doi: 10.1016/j.actbio.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Gershlak JR, Black LD., Iii Beta 1 integrin binding plays a role in the constant traction force generation in response to varying stiffness for cells grown on mature cardiac extracellular matrix. Exp. Cell Res. 2015;330:311–324. doi: 10.1016/j.yexcr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ungerleider JL, Christman KL. Concise Review: Injectable Biomaterials for the Treatment of Myocardial Infarction and Peripheral Artery Disease: Translational Challenges and Progress. Stem Cells Transl. Med. 2014:1090–1099. doi: 10.5966/sctm.2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolettis TM. Arrhythmogenesis after cell transplantation post-myocardial infarction. Four burning questions–And some answers. Cardiovasc. Res. 2006;69:299–301. doi: 10.1016/j.cardiores.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, et al. Increased Cardiac Myocyte Progenitors in Failing Human Hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage Phenotype as a Determinant of Biologic Scaffold Remodeling. Tissue Eng. Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 52.Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage Participation in the Degradation and Remodeling of Extracellular Matrix Scaffolds. Tissue Eng. Part A. 2009;15:1687–1694. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic Extracellular Matrix Grafts Elicit A Th2-Restricted Immune Response1. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 54.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8:3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonnenberg SB, Rane AA, Liu CJ, Rao N, Agmon G, Suarez S, Wang R, Munoz A, et al. Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative LV remodeling post-myocardial infarction. Biomaterials. 2015;45:56–63. doi: 10.1016/j.biomaterials.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]