Abstract
Osteocalcin is among the most abundant proteins in bone and is produced exclusively by osteoblasts. Initially believed to be an inhibitor of bone mineralization, recent studies suggest a broader role for osteocalcin that extends to the regulation of whole body metabolism, reproduction, and cognition. Circulating undercarboxylated osteocalcin, which is regulated by insulin, acts in a feed-forward loop to increase β-cell proliferation as well as insulin production and secretion, while skeletal muscle and adipose tissue respond to osteocalcin by increasing their sensitivity to insulin. Osteocalcin also acts in the brain to increase neurotransmitter production and in the testes to stimulate testosterone production. At least one putative receptor for osteocalcin, Gprc6a, is expressed by adipose, skeletal muscle, and the Leydig cells of the testes and appears to mediate osteocalcin’s effects in these tissues. In this review, we summarize these new discoveries, which suggest that the ability of osteocalcin to function both locally in bone and as a hormone depends on a novel post-translational mechanism that alters osteocalcin’s affinity for the bone matrix and bioavailability.
Keywords: Osteocalcin, Osteoblast, Metabolism, Diabetes
1. Introduction
Osteoblasts are specialized mesenchymal cells that are primarily responsible for the synthesis and deposition of the mineralized, collagen-rich matrix that composes bone tissue. Over the last decade, studies have elaborated an expanded biological function for the osteoblast that is focused on the actions of bone-derived osteocalcin (1, 2). Osteocalcin has routinely been used as a serum marker of osteoblastic bone formation and believed to act in the bone matrix to regulate mineralization, but new genetic and pharmacologic evidence now points to a hormonal role for the protein. These newly discovered actions link the energy demands of bone to global homeostasis (1, 3) and close a number of open endocrine loops associated with the impact of nutrient availability (4, 5), leptin (6, 7), adiponectin (8) and insulin (9) on skeletal metabolism. In this review, we summarize the current knowledge of osteocalcin function beginning with the initial suggestion that the protein inhibits mineralization and ending with evidence of the hormonal actions of osteocalcin in humans.
2. Structure and post-translational modifications of osteocalcin
Osteocalcin, also referred to as bone γ-carboxyglutamic acid (Gla) protein or BGP, is a 46–50 amino acid, 5.6 kDa secreted protein that is produced primarily by osteoblasts (10). Smaller amounts are also be produced by odontoblasts of the teeth and hypertrophic chondrocytes. The protein was first isolated by Price et al (11, 12) from bovine and human bone and shown to represent the major fraction of Gla containing protein in bone. A second Gla-protein, isolated later by the same group, was termed matrix Gla protein or MGP (13, 14). Together, these two proteins belong to a distinct subgroup of the larger vitamin K-dependent protein family, the constituents of which are primarily involved in coagulation.
The human osteocalcin gene, BGLAP, is located on chromosome 1 at 1q25-q31 (15) and encodes an 11kD, 98 amino acid pre-pro-protein (Figure 1). The mature peptide is generated by sequential cleavage events that remove an endoplasmic reticulum signal sequence and the pro-sequence followed by γ-carboxylation of three glutamic acid residues at positions 17, 21, and 24. All three of the glutamate carboxylation events occur during a single binding of the immature peptide to γ-glutamyl carboxylase (16). This enzyme utilizes CO2, O2, and vitamin K, supplied by the vitamin K cycle and circulation, as cofactors. With each γ-carboxylation cycle, vitamin K is converted to an epoxide, which is then reduced by vitamin K epoxide reductase to allow another round of carboxylation (17). The mature, carboxylated osteocalcin protein is packaged into intracellular vesicles for secretion into the bone matrix (10, 18).
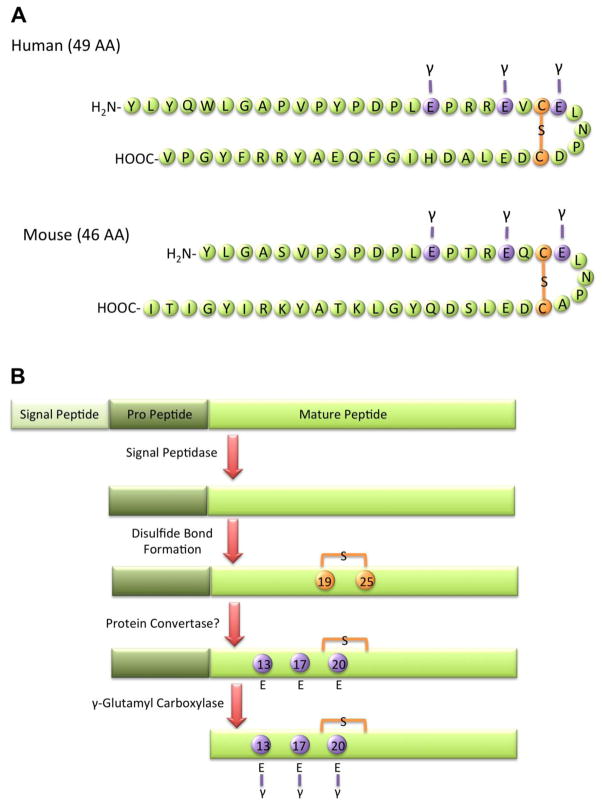
Figure 1. Osteocalcin structure, translation, and processing.
(A) The structure of osteocalcin is marked by three γ-carboxyglutamic acid residues and a disulfide bond. The human protein contains 49 amino acids with γ-carboxyglutamic acid residues at positions 17, 21, and 24 and a disulfide bond between cysteine residues at positions 23 and 29. The mouse protein is 3 amino acids shorter with γ-carboxyglutamic acid residues at positions 13, 17, and 20. (B) Osteocalcin is translated as a pre-pro-peptide. Following signal sequence cleavage the peptide translocates into the endoplasmic reticulum, where a disulfide bond forms. After processing in a potential protein convertase step, osteocalcin is γ-carboxylatedon three glutamic acid residues.
All vitamin K-dependent Gla proteins are thought to have evolved from a common ancestor because they and share both the ability to bind calcium and the same γ-carboxylase recognition sites (19, 20). The hepatic Gla proteins that function in blood coagulation bind to calcium-linked phospholipids whereas osteocalcin binds to calcium ions in hydroxyapatite (21).
The osteocalcin and MGP genes appear to have diverged from an ancestral gene that first emerged 500 million years ago before the evolution of jawless fish (22). MGP appeared first with the development of cartilaginous structures, while a later genome duplication event coinciding with the emergence of bony structures led to the evolution of the osteocalcin gene. In most organisms, including humans, osteocalcin is encoded by a single gene that is highly conserved across species. However, mice contain a cluster of three osteocalcin genes, indicative of an additional duplication late in rodent evolution (23). Two of these genes, osteocalcin gene 1 (OG1) and osteocalcin gene 2 (OG2), are expressed predominantly in bone. The third gene, osteocalcin-related gene (ORG), is expressed primarily in the kidney. Although the exact function of ORG remains unknown (24), it has been suggested that ORG encodes nephrocalcin, a calcium-binding Gla protein important in calcium homeostasis (23).
3. Role of osteocalcin in mineralization
Mature osteocalcin is secreted into the bone micro-environment and then undergoes a conformational change that aligns its calcium-binding Gla residues with the calcium ions in hydroxyapatite. This property was initially proposed as a mechanism that enables osteocalcin to initiate the formation of hydroxyapatite crystals (12). However, subsequent work was more compatible with the notion that osteocalcin functions as an inhibitor of bone mineralization. In support of this idea, osteocalcin inhibits the precipitation of calcium salts from saturated solutions (25), and chronic treatment of rodents with warfarin, an inhibitor of vitamin K-dependent γ-carboxylation, results in over-mineralization and the premature closure of the growth plate (26).
To more fully study the role of osteocalcin in bone formation, Ducy et al. (27) created an osteocalcin null mouse lacking both osteocalcin genes expressed in bone. These mice exhibited an age-dependent increase in bone formation rate and bone mass compared to controls, without an impact on bone resorption. Subsequent analysis of the bone matrix using Fourier transform infrared microspectroscopy indicated that the mineral to matrix ratio was increased in older knockout animals. In addition, the hydroxyapatite crystal size was larger, suggesting that osteocalcin might regulating the rate of mineral maturation (28). However, the deposition of dentin by odontoblasts was normal in osteocalcin null mice (29), and analysis of mice overexpressing osteocalcin in bone revealed a relatively normal state of mineralization (30). Therefore, the precise role of osteocalcin within the bone matrix remains unclear, and osteocalcin’s inhibitory effect on bone mineralization is likely to be considerably lower than that of MGP since the removal of this gene results in the calcification of the aorta and the progressive mineralization of the growth plate (30, 31).
Other studies have led to the hypothesis that osteocalcin exerts a mechanical function within the bone matrix. As a result of its ability to tightly bind hydroxyapatite and form a complex with collagen through the matrix protein osteopontin (32–34), osteocalcin was proposed as means to bridge the matrix and mineral fractions of bone tissue. Such an arrangement is compatible with the formation of dilatational bands that are seen when bone fractures. In this situation, osteocalcin and osteopontin might serve to prevent crack growth by stretching and dissipating energy (35, 36). In accordance with this idea, fracture toughness is substantially reduced in osteocalcin null mice, osteopontin null mice, and double transgenic mice.
4. Regulation of glucose metabolism by osteocalcin
Recent work from several groups have now clearly demonstrated a role for osteocalcin in the regulation of glucose metabolism. Studies by the Karsenty group showed that mice lacking osteocalcin accumulate body fat and exhibit dramatic impairments in glucose metabolism (3, 27). Since osteocalcin is only produced by osteoblasts and can enter the circulation, Karsenty speculated that osteocalcin functions as a hormone in a manner analogous to leptin and adiponectin (Figure 2). Osteocalcin’s present in serum, expression exclusively in bone, and synthesis as a pre-pro-molecule are characteristic of such a function.
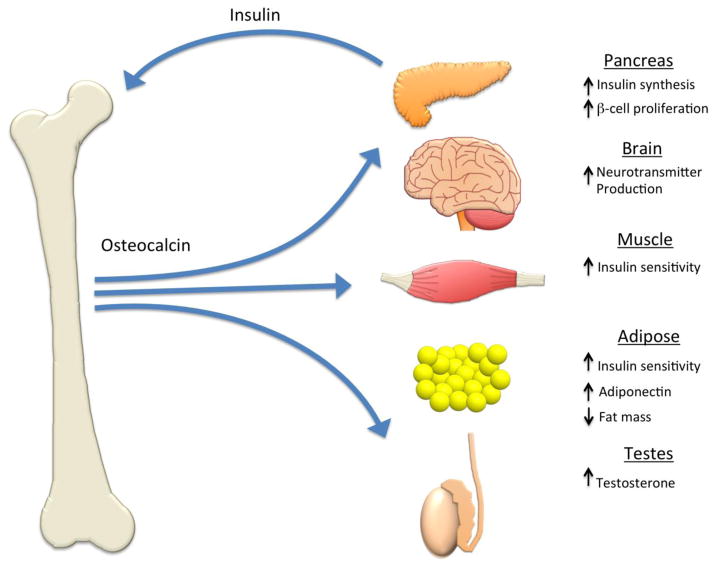
Figure 2. Hormonal effects of osteocalcin on whole body metabolism.
In response to insulin, undercarboxylated osteocalcin is produced by the osteoblast or released from the bone matrix by the low pH of the osteoclast resorption pit and enters the circulation where it acts as a hormone. Osteocalcin produces a widespread increase in insulin sensitivity, which may be partially mediated by the increased production of adiponectin by adipose tissue. In the pancreas, osteocalcin causes an increase in β-cell proliferation as well as increased insulin production. Additional studies have suggested that osteoclacin acts in the brain to stimulate neurotransmitter production and in the testes to increase testosterone production.
To test this idea, Lee and colleagues (3) performed a screen of osteoblast-enriched genes that could regulate osteocalcin levels in the serum. These analyses identified the Esp gene (also know as Ptprv), that encodes osteotesticular protein tyrosine phosphatase (OST-PTP), as a powerful regulator of glucose metabolism in mice. Mice lacking Esp exhibited a metabolic phenotype opposite of osteocalcin null mice, which was characterized by marked hypoglycemia and reduced fat mass secondary to hyperinsulinemia, increased β-cell proliferation, and increased insulin sensitivity. The effect on glucose metabolism was indeed so severe that a portion of Esp−/− mice did not survive until weaning. To demonstrate a functional linkage between osteocalcin and OST-PTP, Esp−/− mice were crossed with those deficient for osteocalcin. Removal of even a single allele of osteocalcin rescued the metabolic phenotypes of Esp−/− mice. While the mechanism was not known at this time (see below), OST-PTP appeared to regulate the carboxylation and bio-availability of the hormonal form of osteocalcin. Under-carboxylated osteocalcin increased the expression of adiponectin in adipose tissue and insulin and cyclin D1 expression in β-cells, but γ-carboxylated osteocalcin did not. In this way, bio-activation of osteocalcin appears to mimic the activation of pro-thrombin, which is also activated when its carboxylated residues are removed (37). However, work by another group have suggested that both carboxylated and undercarboxylated osteocalcin can stimulate a response in adipocytes and myoblasts (38).
To further examine the biological role of osteocalcin, Ferron et al (39) assessed the hormone’s influence on glucose metabolism in wild-type mice. In vitro, recombinant osteocalcin dose-dependently increased the expression of insulin and markers of proliferation in primary β-cells as well as cell models. At higher concentrations, osteocalcin treatment increased AdipoQ and Ppargc1a expression in white and brown adipose tissue. In vivo, infusion of recombinant osteocalcin via subcutaneous mini-pump improved glucose tolerance and insulin sensitivity. Several recent studies have confirmed this effect and demonstrated that daily injections (40) or oral administration (41, 42) of osteocalcin can abrogating the deleterious effects of a high fat diet on metabolism. The precise mechanism by which osteocalcin administration improves glucose metabolism is still not known but it may be related to the protein’s ability to stimulate the release of glucagon-like peptide-1, an incretin released by intestinal endocrine cells that stimulates insulin secretion (41, 42). Other beneficial actions of osteocalcin appear to involve its ability to reverse autophagic dysfunction and endoplasmic reticulum stress resulting from diet-induced obesity (43, 44).
5. Regulation of osteocalcin by insulin
The discovery of osteocalcin’s role in regulating glucose metabolism was independently established by Fulzele et al (9, 45) in the course of studies designed to examine insulin actions in osteoblasts. In these studies, mice lacking the insulin receptor specifically in osteoblasts (IRflox; Oc-Cre) developed a metabolic phenotype reminiscent of the osteocalcin null mice described by the Karsenty group (3). IR knockout mice accumulated body fat and exhibited hyperglycemia with reductions in serum insulin, insulin sensitivity, and glucose tolerance. Profiling of RNA from IR null osteoblasts, identified osteocalcin as a major insulin-responsive gene in osteoblasts, which supported the hypothesis that insulin receptor signaling in the osteoblast is required for osteocalcin production. Consistent with this idea, administration of osteocalcin to the insulin receptor mutant mice improved glucose metabolism, suggesting that insulin and osteocalcin form a bone-pancreas endocrine loop.
Ferron and colleagues (46) confirmed the existence of this feed-forward loop, but suggested a different mechanism for the bio-activation of osteocalcin. Structural analysis of the catalytic domain of OST-PTP revealed that it exhibits homology to other phosphatases like PTP1B (47, 48), that act to dephosphorylate the insulin receptor (46). Substrate trapping studies confirmed that the insulin receptor is indeed a target of OST-PTP and loss of OST-PTP function in Esp−/− osteoblasts accounted for enhanced insulin signaling. This increase in insulin signaling in Esp−/− mice was postulated to increase the activation of osteoclasts, which in turn decarboxylated martrix-embedded osteocalcin in the low pH of the resorption pit (49) to facilitate its release into the circulation. In support of this idea, osteopetrotic mice and humans that lack functional osteoclasts had lower levels of undercarboxylated osteocalcin and exhibit an impaired glucose tolerance. Considered together, the studies by Fulzele (9) and Ferron (46) suggest that insulin regulates the production of osteocalcin and also promotes its bio-availability by favoring decarboxylation.
The transcriptional mechanism through which insulin stimulates osteocalcin production has alsi been investigated. FoxOs are major transcriptional mediators of insulin action, and their phosphorylation and subsequent nuclear export is a primary response in many insulin target cells, including β-cells, adipocytes, and hepatocytes (50). Rached and colleagues identified three FoxO-binding sites in the osteocalcin gene and found that FoxO1 is a potent suppressor of osteocalcin expression (51). Mice rendered deficient for the transcription factor in osteoblasts (FoxO1flox; Collagen1-Cre) exhibited increased pancreatic β-cell proliferation, insulin secretion, and insulin sensitivity secondary to increased osteocalcin expression and bio-activation (51). The suppression of osteocalcin expression by FoxO1 may also be mediated by its interaction with the transcription factor Atf4 (52), which also suppresses osteocalcin bio-activation (53), and through its inhibition of Runx2 activity (54).
Tor signaling also provides additional control over osteocalcin production. Mice with unrestrained mTOR signaling in osteoblasts via the genetic ablation of the Tsc2 gene had 10-fold higher levels of undercaboxylated osteocalcin and ultimately became desensitized to the hormone (55). Whereas young mice had lower blood glucose levels and higher insulin levels, aged mice exhibited a reduction in β-cell area, glucose tolerance, and serum insulin and a downregulation of Gprc6a. The dramatic over-production of osteocalcin in these mice appears to be secondary to the inhibition of FoxO1 activity.
Because osteocalcin is produced by differentiated osteoblasts, the production of osteocalcin is also influenced in mouse models designed to interfere with osteoblast maturation. Two such examples include the Fra-2 (Fosl2) transcription factor and the vitamin K-dependent γ-glutamyl carboxylase (GGCX). Fra-2 plays an important role in coordinating osteoblast development (56) and also regulates osteocalcin production. Over-expression of Fra-2 in osteoblasts results in increased levels of osteocalcin, reduced body weight and reduced serum glucose with improved glucose tolerance and insulin sensitivity while knockouts have the opposite phenotype (57). GGCX should play a role in regulating osteocalcin bioavailability because it catalyzes the conversion of glutamic acid to γ-carboxyglutamic acid. Genetic removal of this gene in osteoblasts increased serum undercarboxylated osteocalcin levels concomitant with improved glucose tolerance and reduced fat mass (58, 59). A similar phenotype was observed in mice in which Vkorc1, the enzyme responsible for Vitamin K recycling, was ablated specifically in osteoblasts (59).
6. A role for osteocalcin in fertility and cognition
The continued examination of the osteocalcin null mice by the Oury and Karsenty (60) led to the description of two additional hormonal functions for undercarboxylated osteocalcin. First, osteocalcin null mice were noted to be poor breeders and to produce litters of significantly smaller numbers of pups than wild-type counterparts. This observation led Oury and colleagues (61) to postulate that undercarboxylated osteocalcin acts to regulate fertility and possibly the production of sex steroid hormones. Estrogen and testosterone have a profound impact on skeletal growth and maintenance (62), but signaling in the reverse direction (ie bone to gonads) has not been examined. Consistent with this notion, osteoblast-conditioned medium and undercarboxylated osteocalcin increased testosterone production by the testes but did not affect estradiol or progesterone levels produced by ovarian explants. The mutant mice were also revealed to have low testosterone levels, oligospermia, and reductions in the weight of reproductive organs, and osteocalcin administration increased these parameters of male fertility. Intruigingly, Esp−/− mice that have increased insulin signaling in osteoblasts (46) were found to have enhanced fertility, which suggested the possibility that insulin signaling in the osteoblast regulates male fertility. This idea was confirmed in later work (63) that suggested insulin favors male fertility by stimulating bone turnover and osteoclast-mediated activation of osteocalcin. While these studies identified a putative receptor for osteocalcin (described below), it remains unclear what influence derangements in glucose metabolism in osteocalcin null mice have on fertility.
Second, behavioral abnormalities observed in osteocalcin null mice prompted additional studies that disclosed new actions for the protein in the brain. Routine handling of the osteocalcin null mice suggested that cognition in the mutant mice was impaired relative to control littermates, and more formal behavioral testing revealed an increase in behaviors associated with anxiety and depression (64). A previous study had indicated that osteocalcin is produced in the brain and might function as a neuropeptide (65). However, work by Oury and colleagues (64) demonstrated that circulating osteocalcin crosses the blood-brain barrier and acts in the brainstem, midbrain, and hippocampus to influence the synthesis of several neurotransmitters that favor learning and memory formation. In these studies, osteocalcin was shown to promote the expression of genes involved in the synthesis of monoamine neurotransmitters and inhibit those needed for γ-aminobutyric acid (GABA) synthesis, and corresponding changes in the levels of serotonin, dopamine, norepinephrine, and GABA were all noted. Consistent with the idea that bone-derived osteocalcin acts in the brain acute disruption of osteocalcin expression in the osteoblast resulted in a behavioral phenotype identical to null mice. Intriguingly, the maternal production of osteocalcin appears to be necessary for normal fetal brain development. Osteocalcin can be found in fetal blood before it is expressed during bone development, and pups born to osteocalcin null mothers have an increased level of neural apoptosis in the hippocampus. While considerable additional work will be required, these studies suggest the possibility that cognitive disabilities and defects in bone metabolism as in cleidocranial dysplasia could be linked.
7. Gprc6a: An osteocalcin receptor
The demonstration of specific functions for circulating undercarboxylated osteocalcin prompted studies to identify its receptor in target tissues. Gprc6a, a G protein coupled receptor with no previously known function, is expressed by a wide variety of cell types including those found to respond to circulating osteocalcin (66, 67), and is activated by a number of unrelated ligands including cations, like calcium and zinc, and amino acids such as L-Arg and L-Lys (68, 69). It is believed that this promiscuity allows Gprc6a and other receptors in its family to operate as sensors of protein nutrition (70), but two parallel lines of investigation ultimately led to the identification of Gprc6a as the receptor for osteocalcin. First, gene knockout studies demonstrated that the loss of Gprc6a function produces a phenotype nearly identical to osteocalcin null mice. These animals accumulate fat and exhibit hyperglycemia, glucose intolerance, and insulin resistance with reduced levels of testosterone that lead to demasculinization (71). Second, Oury and colleagues (61) narrowed the number of possible osteocalcin receptors by comparing the expression of receptors with unknown ligands in the testicular Leydig cell with those expressed by the non-responsive ovary and examined signaling pathways activated by osteocalcin treatment. Osteocalcin consistently produced an increase in cAMP levels in Leydig cells, and the pharmacokinetics of this response suggested that the receptor was G protein coupled. These analyses led to the identification of 4 possible receptors including Gprc6a, which was known to produce a fertility phenotype similar to osteocalcin null mice (61, 71).
The β cells of the pancreatic islets also express Gprc6a and the binding of osteocalcin by this receptor activates signaling through the MAP kinase pathway (72). Genetic inactivation of the receptor in the β-cell lineage (Gprc6aflox; Pdx1-Cre mice) results in hyperglycemia, hypoinsulinemia, and glucose intolerance owing to a defect in osteocalcin-stimulated β-cell proliferation (73). The development of an identical metabolic phenotype in mice lacking one allele of osteocalcin and one Gprc6a allele in the β-cell provides additional genetic evidence for the idea that osteocalcin and Gprc6a are in the same genetic cascade.
Based on the results of these studies, it appears likely that Gprc6a also mediates osteocalcin’s functions in other tissues including muscle, adipose and bone. Osteoblasts express Gprc6a and genetic ablation of the receptor results in osteopenia (71). Though this phenotype is likely to be influenced to a large degree by the metabolic defects and altered mineral homeostasis, null osteoblasts do exhibit differentiation defects in vitro (74). It is unclear if this represents an alteration in osteocalcin signaling in osteoblasts or is due to some other mechanism. It is likely that additional unidentified receptors for osteocalcin exist because the effect of osteocalcin deficiency on neurotransmitters and behavior is not recapitulated in GPRC6A-null mice (64).
8. Evidence of an endocrine function for osteocalcin in humans
Since the initial discovery that osteocalcin impacts metabolism in the mouse, a number of studies have attempted to address the function of osteocalcin in humans. To date, the vast majority of studies have used a cross-sectional design to examine the association of circulating levels of total and/or undercarboxylated osteocalcin with altered glucose metabolism. These studies indicate that the levels of osteocalcin are negatively correlated with fasting glucose, fasting insulin, HOMA-IR (a representation of insulin resistance), body mass index, and hyperlipidemia (75–79). Untreated diabetics and pre-diabetics have decreased levels of under-carboxylated osteocalcin, while higher serum levels were associated with higher HOMA-β, a representation of enhanced β-cell function (80, 81). Serum osteocalcin concentrations have also been reported to be significantly reduced in obese children and negatively related to leptin concentration and insulin resistance, but not to adiponectin (82–84). Moreover, substantial weight loss in these children was associated with an increased serum osteocalcin level, improved insulin sensitivity, and decreased serum leptin levels. Exercise also increases circulating osteocalcin levels and was correlated with an increase in insulin sensitivity and muscle strength (85–87).
A few initial studies using relatively small sample sizes identified an association between polymorphisms in the human BGLAP locus and type 2 diabetes and obesity (88, 89), but there is some debate over whether this association is only present in select ethnic groups (90). This finding has now been substantiated by a more recent analysis using a larger cohort of European decent that linked genetic variations in BGLAP to alterations in body mass index (91). Likewise, Oury and colleagues (61) identified two unrelated individuals with T → A transversions in exon 4 of the gene encoding GPRC6A, which resulted in amino acid substitutions that produced a dominant negative protein. In addition to the low testosterone levels and oligospermia, both individuals presented with metabolic disturbances similar to osteocalcin null mice (3), including increased adiposity, dyslipidemia, and disturbances in glucose metabolism.
Pharmacologic modulators of bone metabolism have also been reported to influence circulating undercarboxylated osteocalcin levels. In a follow up analysis of samples collected in the PaTH study, in which osteoporotic postmenopausal women were treated with PTH(1–84) or alendronate, Schafer and colleagues (92) observed corresponding changes in undercarboxylated osteocalcin levels that supported the observations made in rodent models. Additionally, subjects with the largest increase in undercarboxylated osteocalcin levels exhibited the greatest decreases in weight and fat mass (92, 93). However, inhibition of bone resorption by alendronate, zoledronic acid, or denosumab treatment during the FIT, HORIZON-PFT, and FREEDOM trials was not associated with significant changes in fasting glucose levels or diabetes risk (93).
In line with this later finding, other cross-sectional studies examining the relationship between serum osteocalcin and metabolic disease have suggested a more limited role for osteocalcin in human glucose metabolism (94–96). In one study, serum osteocalcin was correlated with insulin resistance in post-menopausal women, but not in pre-menopausal women (97). Similarly, indices of insulin sensitivity of skeletal muscle but not that of the hepatocyte was reported to be positively associated with osteocalcin levels (98). Paradoxically, women with gestational diabetes have elevated osteocalcin levels that do not predict the development of this condition (99), and reducing undercarboxylated osteocalcin levels in postmenopausal women by vitamin K1 supplementation did not alter fasting glucose levels or insulin resistance (100). Taken together, data from these cross-sectional analyses, as well as those examining an association between osteocalcin and testosterone (101–103), indicate a more complex endocrine function for osteocalcin in humans.
To date, only one study has attempted to directly examine the effects of insulin on osteocalcin and bone turnover in humans. Basu and colleagues (104) analyzed serum samples collected during a hyperinsulinemic-euglycemic clamp in healthy patients and measured the effect of this procedure on serum total and undercarboxylated osteocalcin as well as other bone markers. No relationship between insulin levels and osteocalcin levels or bone turnover markers was apparent, but measures of insulin sensitivity, including glucose disposal rates, were positively correlated with serum levels of the C-terminal telopeptide of type 1 collagen, a bone resorption marker, suggesting that insulin might elicit factors that promote enhanced insulin sensitivity in the periphery.
9. Perspective
The studies reviewed herein on the unexpected functions of osteocalcin underscore a paradigm shift in our understanding of skeletal physiology. The notion that bone is primarily a hormone-responsive tissue should now be replaced by one in which bone actively communicates with other organ systems and coordinates mineral ion homeostasis with overall energy balance. Clearly, osteocalcin can now be viewed as a bone-derived factor that influences glucose metabolism, reproduction and cognition through endocrine loops between bone and the pancreas, brain and testes.
As this field moves forward, it will be important to determine whether or to what extent the hormonal actions of osteocalcin observed in animal models are also manifest in humans. Genetic mouse models do not always replicate human disease, and while cross-sectional clinical studies have revealed an association between metabolic disturbances and circulating osteocalcin, they do not prove causality. For instance, the increased energetic expense of maintaining body temperature in rodents relative to humans results in a higher metabolic rate and the possibility of additional regulators/modifiers of metabolism. The presence of a 3 osteocalcin gene cluster present in the mouse genome, while other species maintain a single gene, could indicate the evolution of novel functions specific to this species. Along similar lines, Esp, which governs osteocalcin carboxylation in the mouse, is a pseudogene in humans (105). If a similar regulatory mechanism exists in humans, another phosphatase, perhaps Ptpn2 (106), would have to assume the functions of OST-PTP.
Despite these caveats, the recent studies on the hormonal actions of osteocalcin prompt a number of additional questions regarding the nature of endocrine functions exerted by the osteoblast and the fuel requirements of bone cells. First, do acute increases in circulating osteocalcin associated with bone anabolism impact whole body metabolism? Second, do osteoblasts secrete other hormones in addition to osteocalcin and fibroblast growth factor-23? Genetic evidence points to at least one additional unknown factor that allows the osteoblast to contribute to whole body glucose metabolism (107). And finally, what fuel sources do osteoblasts require to produce and deposit new bone, and how is the cellular metabolism of osteoblasts regulated? Recent studies suggest that key osteoblastic developmental pathways also regulate the ability of bone cells to generate the energy necessary to sustain bone formation (108–110). The answers to these questions and those that follow will certainly expand our understanding of skeletal biology and its endocrine function.
Highlights.
Osteocalcin is a secreted factor influencing matrix mineralization and global metabolism.
Osteocalcin regulates glucose metabolism via a bone-pancreas endocrine loop.
New data suggests an additional role in cognition and male fertility.
Clinical studies suggest a more complex role for osteocalcin in human metabolism.
Acknowledgments
T.L. Clemens is supported by a Merit Review grant (BX001234) and is the recipient of a Research Career Scientist Award from the Veterans Administration. R.C. Riddle is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH (DK099134).
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton JG, Patel S, Lacey JH, White S. A prospective study of changes in bone turnover and bone density associated with regaining weight in women with anorexia nervosa. Osteoporos Int. 2005;16:1955–1962. doi: 10.1007/s00198-005-1972-7. [DOI] [PubMed] [Google Scholar]
- 5.Nussbaum M, Baird D, Sonnenblick M, Cowan K, Shenker IR. Short stature in anorexia nervosa patients. J Adolesc Health Care. 1985;6:453–455. doi: 10.1016/s0197-0070(85)80052-8. [DOI] [PubMed] [Google Scholar]
- 6.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 7.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 8.Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo XE, Mann JJ, Karsenty G. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell metabolism. 2013;17:901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 11.Price PA, Poser JW, Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci U S A. 1976;73:3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976;73:1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price PA, Urist MR, Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983;117:765–771. doi: 10.1016/0006-291x(83)91663-7. [DOI] [PubMed] [Google Scholar]
- 14.Price PA, Williamson MK. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J Biol Chem. 1985;260:14971–14975. [PubMed] [Google Scholar]
- 15.Puchacz E, Lian JB, Stein GS, Wozney J, Huebner K, Croce C. Chromosomal localization of the human osteocalcin gene. Endocrinology. 1989;124:2648–2650. doi: 10.1210/endo-124-5-2648. [DOI] [PubMed] [Google Scholar]
- 16.Morris DP, Stevens RD, Wright DJ, Stafford DW. Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate. J Biol Chem. 1995;270:30491–30498. doi: 10.1074/jbc.270.51.30491. [DOI] [PubMed] [Google Scholar]
- 17.Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3:1873–1878. doi: 10.1111/j.1538-7836.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 18.Gundberg CM, Clough ME. The osteocalcin propeptide is not secreted in vivo or in vitro. J Bone Miner Res. 1992;7:73–80. doi: 10.1002/jbmr.5650070111. [DOI] [PubMed] [Google Scholar]
- 19.Rice JS, Williamson MK, Price PA. Isolation and sequence of the vitamin K-dependent matrix Gla protein from the calcified cartilage of the soupfin shark. J Bone Miner Res. 1994;9:567–576. doi: 10.1002/jbmr.5650090417. [DOI] [PubMed] [Google Scholar]
- 20.Sunnerhagen M, Drakenberg T, Forsen S, Stenflo J. Effect of Ca2+ on the structure of vitamin K-dependent coagulation factors. Haemostasis. 1996;26 (Suppl 1):45–53. doi: 10.1159/000217240. [DOI] [PubMed] [Google Scholar]
- 21.Willems BA, Vermeer C, Reutelingsperger CP, Schurgers LJ. The realm of vitamin K dependent proteins: Shifting from coagulation toward calcification. Mol Nutr Food Res. 2014;58:1620–1635. doi: 10.1002/mnfr.201300743. [DOI] [PubMed] [Google Scholar]
- 22.Laize V, Martel P, Viegas CS, Price PA, Cancela ML. Evolution of matrix and bone gamma-carboxyglutamic acid proteins in vertebrates. J Biol Chem. 2005;280:26659–26668. doi: 10.1074/jbc.M500257200. [DOI] [PubMed] [Google Scholar]
- 23.Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem. 1994;269:1183–1190. [PubMed] [Google Scholar]
- 24.Petrucci M, Paquette Y, Leblond FA, Pichette V, Bonnardeaux A. Evidence that the mouse osteocalcin-related gene does not encode nephrocalcin. Nephron Exp Nephrol. 2006;104:e140–146. doi: 10.1159/000094965. [DOI] [PubMed] [Google Scholar]
- 25.van de Loo PG, Soute BA, van Haarlem LJ, Vermeer C. The effect of Gla-containing proteins on the precipitation of insoluble salts. Biochem Biophys Res Commun. 1987;142:113–119. doi: 10.1016/0006-291x(87)90458-x. [DOI] [PubMed] [Google Scholar]
- 26.Price PA, Williamson MK, Haba T, Dell RB, Jee WS. Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc Natl Acad Sci U S A. 1982;79:7734–7738. doi: 10.1073/pnas.79.24.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 28.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 29.Bronckers AL, Price PA, Schrijvers A, Bervoets TJ, Karsenty G. Studies of osteocalcin function in dentin formation in rodent teeth. Eur J Oral Sci. 1998;106:795–807. doi: 10.1046/j.0909-8836.1998.eos106306.x. [DOI] [PubMed] [Google Scholar]
- 30.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 32.Ritter NM, Farach-Carson MC, Butler WT. Evidence for the formation of a complex between osteopontin and osteocalcin. J Bone Miner Res. 1992;7:877–885. doi: 10.1002/jbmr.5650070804. [DOI] [PubMed] [Google Scholar]
- 33.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 34.Hauschka PV, Carr SA. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982;21:2538–2547. doi: 10.1021/bi00539a038. [DOI] [PubMed] [Google Scholar]
- 35.Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D. Dilatational band formation in bone. Proc Natl Acad Sci U S A. 2012;109:19178–19183. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikel O, Laurencin D, McCallum SA, Gundberg CM, Vashishth D. NMR investigation of the role of osteocalcin and osteopontin at the organic-inorganic interface in bone. Langmuir. 2013;29:13873–13882. doi: 10.1021/la403203w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 38.Hill HS, Grams J, Walton RG, Liu J, Moellering DR, Garvey WT. Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm Metab Res. 2014;46:341–347. doi: 10.1055/s-0034-1368709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2011;50:568–575. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, Hirata M. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One. 2013;8:e57375. doi: 10.1371/journal.pone.0057375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizokami A, Yasutake Y, Higashi S, Kawakubo-Yasukochi T, Chishaki S, Takahashi I, Takeuchi H, Hirata M. Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone. 2014;69C:68–79. doi: 10.1016/j.bone.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Zhou B, Li H, Liu J, Xu L, Zang W, Wu S, Sun H. Intermittent injections of osteocalcin reverse autophagic dysfunction and endoplasmic reticulum stress resulting from diet-induced obesity in the vascular tissue via the NFkappaB-p65-dependent mechanism. Cell Cycle. 2013;12:1901–1913. doi: 10.4161/cc.24929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-kappaB signaling pathway. Endocrinology. 2013;154:1055–1068. doi: 10.1210/en.2012-2144. [DOI] [PubMed] [Google Scholar]
- 45.Fulzele K, DiGirolamo DJ, Liu Z, Xu J, Messina JL, Clemens TL. Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem. 2007;282:25649–25658. doi: 10.1074/jbc.M700651200. [DOI] [PubMed] [Google Scholar]
- 46.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delibegovic M, Bence KK, Mody N, Hong EG, Ko HJ, Kim JK, Kahn BB, Neel BG. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol. 2007;27:7727–7734. doi: 10.1128/MCB.00959-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engelke JA, Hale JE, Suttie JW, Price PA. Vitamin K-dependent carboxylase: utilization of decarboxylated bone Gla protein and matrix Gla protein as substrates. Biochim Biophys Acta. 1991;1078:31–34. doi: 10.1016/0167-4838(91)90088-h. [DOI] [PubMed] [Google Scholar]
- 50.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 51.Rached MT, Kode A, Silva BC, Jung DY, Gray S, Ong H, Paik JH, DePinho RA, Kim JK, Karsenty G, Kousteni S. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120:357–368. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kode A, Mosialou I, Silva BC, Joshi S, Ferron M, Rached MT, Kousteni S. FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis. J Biol Chem. 2012;287:8757–8768. doi: 10.1074/jbc.M111.282897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119:2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, Fransceschi RT, Dong HH, Xiao G. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J Biol Chem. 2011;286:19149–19158. doi: 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riddle RC, Frey JL, Tomlinson RE, Ferron M, Li Y, DiGirolamo DJ, Faugere MC, Hussain MA, Karsenty G, Clemens TL. Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol Cell Biol. 2014;34:1850–1862. doi: 10.1128/MCB.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozec A, Bakiri L, Jimenez M, Schinke T, Amling M, Wagner EF. Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production. J Cell Biol. 2010;190:1093–1106. doi: 10.1083/jcb.201002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bozec A, Bakiri L, Jimenez M, Rosen ED, Catala-Lehnen P, Schinke T, Schett G, Amling M, Wagner EF. Osteoblast-specific expression of Fra-2/AP-1 controls adiponectin and osteocalcin expression and affects metabolism. J Cell Sci. 2013;126:5432–5440. doi: 10.1242/jcs.134510. [DOI] [PubMed] [Google Scholar]
- 58.Shiba S, Ikeda K, Azuma K, Hasegawa T, Amizuka N, Horie-Inoue K, Inoue S. gamma-Glutamyl carboxylase in osteoblasts regulates glucose metabolism in mice. Biochem Biophys Res Commun. 2014;453:350–355. doi: 10.1016/j.bbrc.2014.09.091. [DOI] [PubMed] [Google Scholar]
- 59.Ferron M, Lacombe J, Germain A, Oury F, Karsenty G. GGCX and VKORC1 inhibit osteocalcin endocrine functions. J Cell Biol. 2015;208:761–776. doi: 10.1083/jcb.201409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karsenty G, Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol Cell Endocrinol. 2014;382:521–526. doi: 10.1016/j.mce.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 63.Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, Srinivas P, Chamouni A, Lugani F, Lejeune H, Kumar TR, Plotton I, Karsenty G. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123:2421–2433. doi: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, Huang YY, Lee H, Srinivas P, Gao XB, Suyama S, Langer T, Mann JJ, Horvath TL, Bonnin A, Karsenty G. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patterson-Buckendahl P, Sowinska A, Yee S, Patel D, Pagkalinawan S, Shahid M, Shah A, Franz C, Benjamin DE, Pohorecky LA. Decreased sensory responses in osteocalcin null mutant mice imply neuropeptide function. Cell Mol Neurobiol. 2012;32:879–889. doi: 10.1007/s10571-012-9810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem. 2005;93:383–391. doi: 10.1111/j.1471-4159.2005.03025.x. [DOI] [PubMed] [Google Scholar]
- 67.Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conigrave AD, Hampson DR. Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol Metab. 2006;17:398–407. doi: 10.1016/j.tem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Conigrave AD, Hampson DR. Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther. 2010;127:252–260. doi: 10.1016/j.pharmthera.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Clemmensen C, Smajilovic S, Wellendorph P, Brauner-Osborne H. The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol. 2014;171:1129–1141. doi: 10.1111/bph.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, Faber P, Brunden KR, Harrington JJ, Quarles LD. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–1683. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63:1021–1031. doi: 10.2337/db13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pi M, Zhang L, Lei SF, Huang MZ, Zhu W, Zhang J, Shen H, Deng HW, Quarles LD. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res. 2010;25:1092–1102. doi: 10.1359/jbmr.091037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2009;24:785–791. doi: 10.1359/jbmr.081234. [DOI] [PubMed] [Google Scholar]
- 76.Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, Norman PE. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. European Journal of Endocrinology. 2010;163:265–272. doi: 10.1530/EJE-10-0414. [DOI] [PubMed] [Google Scholar]
- 77.Hwang YC, Jee JH, Jeong IK, Ahn KJ, Chung HY, Lee MK. Circulating osteocalcin level is not associated with incident type 2 diabetes in middle-aged male subjects: mean 8.4-year retrospective follow-up study. Diabetes Care. 2012;35:1919–1924. doi: 10.2337/dc11-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chin KY, Ima-Nirwana S, Mohamed IN, Ahmad F, Ramli ES, Aminuddin A, Ngah WZ. Serum osteocalcin is significantly related to indices of obesity and lipid profile in Malaysian men. Int J Med Sci. 2014;11:151–157. doi: 10.7150/ijms.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X, Wu Y, Liu L, Tian H, Yu X. Osteocalcin is inversely associated with glucose levels in middle-aged Tibetan men with different degrees of glucose tolerance. Diabetes/Metabolism Research and Reviews. 2014;30:476–482. doi: 10.1002/dmrr.2509. [DOI] [PubMed] [Google Scholar]
- 80.Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes/Metabolism Research and Reviews. 2009;25:768–772. doi: 10.1002/dmrr.1045. [DOI] [PubMed] [Google Scholar]
- 81.Sarkar PD, Choudhury AB. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 2013;17:1631–1635. [PubMed] [Google Scholar]
- 82.Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. International Journal of Obesity. 2010;34:852–858. doi: 10.1038/ijo.2009.282. [DOI] [PubMed] [Google Scholar]
- 83.Kim GS, Jekal Y, Kim HS, Im JA, Park JY, Chu SH. Reduced serum total osteocalcin is associated with central obesity in Korean children. Obes Res Clin Pract. 2014;8:e201–298. doi: 10.1016/j.orcp.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Wang JW, Tang QY, Ruan HJ, Cai W. Relation between serum osteocalcin levels and body composition in obese children. J Pediatr Gastroenterol Nutr. 2014;58:729–732. doi: 10.1097/MPG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 85.Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, Ahn CW. The Effects of Aerobic Exercise Training on Serum Osteocalcin, Adipocytokines, and insulin resistance on Obese Young Males. Clin Endocrinol (Oxf) 2014 doi: 10.1111/cen.12601. [DOI] [PubMed] [Google Scholar]
- 86.Levinger I, Jerums G, Stepto NK, Parker L, Serpiello FR, McConell GK, Anderson M, Hare DL, Byrnes E, Ebeling PR, Seeman E. The Effect of Acute Exercise on Undercarboxylated Osteocalcin and Insulin Sensitivity in Obese Men. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2285. [DOI] [PubMed] [Google Scholar]
- 87.Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, Herrmann M, Ebeling PR, Sanders KM. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. 2014;64:8–12. doi: 10.1016/j.bone.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Das SK, Elbein SC. The search for type 2 diabetes susceptibility loci: the chromosome 1q story. Curr Diab Rep. 2007;7:154–164. doi: 10.1007/s11892-007-0025-3. [DOI] [PubMed] [Google Scholar]
- 89.Xu H, Xiao W, Luo D, Liu YM, Zou L, Kuang HB. Association analysis of genetic polymorphisms and potential interaction of the osteocalcin (BGP) and ER-alpha genes with body mass index (BMI) in premenopausal Chinese women. Acta Pharmacol Sin. 2010;31:455–460. doi: 10.1038/aps.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Das SK, Sharma NK, Elbein SC. Analysis of osteocalcin as a candidate gene for type 2 diabetes (T2D) and intermediate traits in Caucasians and African Americans. Dis Markers. 2010;28:281–286. doi: 10.3233/DMA-2010-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korostishevsky M, Malkin I, Trofimov S, Pei Y, Deng HW, Livshits G. Significant association between body composition phenotypes and the osteocalcin genomic region in normative human population. Bone. 2012;51:688–694. doi: 10.1016/j.bone.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, Bilezikian JP, Shoback DM, Black DM. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study) J Clin Endocrinol Metab. 2011;96:E1982–1989. doi: 10.1210/jc.2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwartz AV, Schafer AL, Grey A, Vittinghoff E, Palermo L, Lui LY, Wallace RB, Cummings SR, Black DM, Bauer DC, Reid IR. Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J Bone Miner Res. 2013;28:1348–1354. doi: 10.1002/jbmr.1865. [DOI] [PubMed] [Google Scholar]
- 94.Caglar GS, Ozdemir ED, Kiseli M, Demirtas S, Cengiz SD. The association of osteocalcin and adiponectin with glucose metabolism in nondiabetic postmenopausal women. Gynecol Obstet Invest. 2014;77:255–260. doi: 10.1159/000358826. [DOI] [PubMed] [Google Scholar]
- 95.Liatis S, Sfikakis PP, Tsiakou A, Stathi C, Terpos E, Katsilambros N, Makrilakis K. Baseline osteocalcin levels and incident diabetes in a 3-year prospective study of high-risk individuals. Diabetes Metab. 2014;40:198–203. doi: 10.1016/j.diabet.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 96.Mori K, Emoto M, Motoyama K, Lee E, Yamada S, Morioka T, Imanishi Y, Shoji T, Inaba M. Undercarboxylated osteocalcin does not correlate with insulin resistance as assessed by euglycemic hyperinsulinemic clamp technique in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:53. doi: 10.1186/1758-5996-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim S, Lee JY, Im JA, Kim DW, Lee HS, Kim SH, Lee JW. Association between serum osteocalcin and insulin resistance in postmenopausal, but not premenopausal, women in Korea. Menopause. 2013;20:1061–1066. doi: 10.1097/GME.0b013e31828838e8. [DOI] [PubMed] [Google Scholar]
- 98.Gower BA, Pollock NK, Casazza K, Clemens TL, Goree LL, Granger WM. Associations of Total and Undercarboxylated Osteocalcin With Peripheral and Hepatic Insulin Sensitivity and beta-Cell Function in Overweight Adults. J Clin Endocrinol Metab. 2013;98:E1173–1180. doi: 10.1210/jc.2013-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tabatabaei N, Giguere Y, Forest JC, Rodd CJ, Kremer R, Weiler HA. Osteocalcin is Higher Across Pregnancy in Caucasian Women with Gestational Diabetes Mellitus. Can J Diabetes. 2014 doi: 10.1016/j.jcjd.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 100.Kumar R, Binkley N, Vella A. Effect of phylloquinone supplementation on glucose homeostasis in humans. Am J Clin Nutr. 2010;92:1528–1532. doi: 10.3945/ajcn.2010.30108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cui R, Su B, Sheng C, Cheng X, Yang P, Bu L, Li H, Wang J, Sheng H, Qu S. Total osteocalcin in serum predicts testosterone level in male type 2 diabetes mellitus. Int J Clin Exp Med. 2014;7:1145–1149. [PMC free article] [PubMed] [Google Scholar]
- 102.Kirmani S, Atkinson EJ, Melton LJ, 3rd, Riggs BL, Amin S, Khosla S. Relationship of testosterone and osteocalcin levels during growth. J Bone Miner Res. 2011;26:2212–2216. doi: 10.1002/jbmr.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanazawa I, Tanaka K, Ogawa N, Yamauchi M, Yamaguchi T, Sugimoto T. Undercarboxylated osteocalcin is positively associated with free testosterone in male patients with type 2 diabetes mellitus. Osteoporos Int. 2013;24:1115–1119. doi: 10.1007/s00198-012-2017-7. [DOI] [PubMed] [Google Scholar]
- 104.Basu R, Peterson J, Rizza R, Khosla S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. J Clin Endocrinol Metab. 2011;96:1450–1455. doi: 10.1210/jc.2010-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cousin W, Courseaux A, Ladoux A, Dani C, Peraldi P. Cloning of hOST-PTP: the only example of a protein-tyrosine-phosphatase the function of which has been lost between rodent and human. Biochem Biophys Res Commun. 2004;321:259–265. doi: 10.1016/j.bbrc.2004.06.137. [DOI] [PubMed] [Google Scholar]
- 106.Zee T, Settembre C, Levine RL, Karsenty G. T-cell protein tyrosine phosphatase regulates bone resorption and whole-body insulin sensitivity through its expression in osteoblasts. Mol Cell Biol. 2012;32:1080–1088. doi: 10.1128/MCB.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshikawa Y, Kode A, Xu L, Mosialou I, Silva BC, Ferron M, Clemens TL, Economides AN, Kousteni S. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26:2012–2025. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell metabolism. 2013;17:745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc Natl Acad Sci U S A. 2014;111:8673–8678. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, Wolfgang MJ, Clemens TL, Riddle RC. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015 doi: 10.1128/MCB.01343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]