Abstract
Neural control of continence and micturition is distributed over a network of interconnected reflexes. These reflexes integrate sensory information from the bladder and urethra and are modulated by descending influences to produce different physiological outcomes based on the information arriving from peripheral afferents. Therefore, the mode of activation of primary afferents is essential in understanding the action of spinal reflex pathways in the lower urinary tract. We present an overview of sensory mechanisms in the bladder and urethra focusing on their spinal integration, identify the cardinal spinal reflexes responsible for continence and micturition, and describe how their functional role is controlled via peripheral afferent activity.
Keywords: neuroscience, reflex, sensory motor system, urinary tract, circuits, afferent
1. Introduction
The work of storing and expelling urine is done by the urinary bladder, which acts as a repository and pump, and the urethra, which functions as an outlet to transfer urine out of the body. These seemingly straightforward tasks, however, are controlled by numerous parallel neural systems that interact in local, spinal, and supraspinal organizations to produce a range of sophisticated behaviors. For example, the primary afferents of the lower urinary tract consists of at least four distinct types of fibers, may terminate in local ganglia to coordinate reflex integration, project to spinal interneurons that modulate numerous reflexes, or travel to executive centers to inform voluntary supervision. Further, these afferents can be differentially activated by pressure, stress, or toxins, be parasympathetic, sympathetic, or somatic, and even be subject to regulation by non-neural cells lining the bladder or urethra.
The complexity of the neural regulatory mechanisms controlling continence and voiding render the system vulnerable to a host of disorders that present with myriad and overlapping symptoms, making the identification of specific etiologies difficult. The principle challenge to neurourology is, therefore, to understand the mechanisms of the neural circuitry and associated regulators in sufficient detail to ascertain the causes of lower urinary tract diseases and identify potential treatments. This review examines our understanding of how primary afferents mediate the many reflexes that control the lower urinary tract, and how those reflexes affect the detrusor, urethral sphincters, and their synergistic cooperation.
2. Primary Afferent Nerves
2.1. Pelvic
The pelvic nerve carries “pressure-related” sensory information from the bladder to spinal centers, and conveys excitatory efferent signals to the detrusor. Early neurophysiological investigations discovered that distention of the detrusor muscle could reliably elicit afferent firing in in vivo preparations across animal models (Evans, 1936; Talaat, 1937). The pelvic afferents burst in response to onset of bladder filling, given that it occurs with sufficient pressure (Talaat, 1937), track intravesical pressure as it rises during artificial bladder filling with physiological saline, and persist after filling has ended, provided that bladder pressure remains high (Evans, 1936; Talaat, 1937). These early studies also showed that if filling is paused at low to mid volumes the bladder pressure slowly drops, along with pelvic afferent activity, but that at high volumes bladder pressure persists during pauses in filling. This provided indirect evidence that the bladder wall is elastic up to a point, and that pelvic afferents encode bladder pressure or detrusor stretch rather than fill volume. Corroborating studies have since shown directly that the completely denervated bladder does not exhibit a rise in intravesical pressure at low volumes and slow filling rates (Klevmark, 1977), and that passive isovolumetric stretching in vivo or stretching bladder strips in vitro also generate afferent responses (Iggo, 1955). This provides strong evidence that the bladder's intrinsic mechanical properties, and not inhibitory efferent neural control, are primarily responsible for pressure accommodation during urine storage, and enforces the supposition that pelvic afferents report stimuli related to detrusor stretch, as opposed to bladder volume, which is intimately tied to bladder elasticity and conformation (Sasaki, 1998).
More recently, this picture of pelvic afferent sensitivity has been complicated by single unit recordings from the nerve or its associated local ganglia. These data indicate that some neurons are activated selectively by different types of mechanical perturbation, while some respond exclusively to chemical irritation. Perhaps the clearest distinction is between the mechanosensitive and mechanoinsensitive afferent types. Electrophysiological studies of unmyelinated afferents located in the dorsal and ventral roots show that afferent fibers that were unresponsive to mechanical stimulation of the detrusor did respond to the chemical irritant mustard oil (Häbler, Janig, & Koltzenburg, 1990), capsaicin (Shea, Cai, Crepps, Mason, & Perl, 2000), or cold (Bors & Blinn, 1957). These afferents are commonly referred to as ‘silent’ because they are not active under physiological continence or micturition conditions. Complicating a bipartite distinction, however, is the fact that some unmyelinated chemosensitive fibers also respond selectively to high bladder pressures (Häbler et al., 1990), opening the possibility that these fibers could be multi-sensory in nature or that chemosensitive fibers can locally influence the action of mechanosensitive afferents at high pressures. More evidence against strictly separate classes of chemosensitive and mechanosensitive pelvic afferents emerged when it was found that following chemical irritation of the bladder with 300 mM KCl (and subsequent 0.9% NaCl cleansing) a subgroup of fibers that had previously not responded to either bladder distention or the KCl irritant became responsive to bladder distention (Shea et al., 2000). The finding is reproducible with mustard oil (Häbler et al., 1990) and other irritants (Rong, Spyer, & Burnstock, 2002), suggesting that irritants are able to induce latent mechanosensitivity in this subgroup of afferents. Therefore, a straightforward classification of activation modes of pelvic sensory neurons may not be possible and more research is needed to develop a complete picture of the response of these afferents. Such investigations could also inform theories of overactive bladder that posit a transformation in the role of capsaicin-sensitive pelvic afferents leading to chronic irritability of the bladder (Fowler, Griffiths, & de Groat, 2008; Yoshimura & de Groat, 1999).
Another set of pelvic afferents has been classified by the pressure or volume at which they become active, as opposed to the sensory modality of their activation. It has been noted that there is a relatively small subset of pelvic afferents (both myelinated and unmyelinated) that track bladder pressure in exclusively high pressure regimes, and are quiet at low pressures (Bahns, Halsband, & Janig, 1987; Häbler et al., 1990; Shea et al., 2000; Zagorodnyuk, Costa, & Brookes, 2006). The pressure regimes at which these so called “high-threshold” fibers respond (in cats) (Häbler et al., 1990) corresponds to the pressures at which humans report painful sensations (Torrens & Morrison, 1987), providing circumstantial evidence that these fibers are responsible for the sensation of pain associated with hyperdistention of the detrusor (Kanai, 2011). To show this conclusively, experiments are needed that directly link these fiber types with pain, either through recording in known pain centers during periods when the high threshold afferents are active or with other quantitative measures of visceral pain such as the visceral motor response (Ness & Gebhart, 1988). Another potential role for the range of pelvic afferent pressure thresholds is to ensure that bladder pressure is effectively conveyed to spinal centers across a full range of pressures, which may not be possible using homogeneous thresholds because afferent activity for some low threshold units plateaus at intermediate pressures (Shea et al., 2000).
A mechanistic basis for the difference in sensitivity between high and low threshold afferents has yet to be established, and several observations suggest that the development of such an explanation will be challenging. Most notably, there are a range of thresholds in the high and low threshold fiber populations rather than two clearly separated groups, and unmyelinated and myelinated fiber types comprise both populations (Sengupta & Gebhart, 1994; Shea et al., 2000). Further, most chemical blocking studies of pelvic afferents, for example administering an extracellular ATP antagonist to inhibit ligand-gated ion channels in mechanosensitive afferents in the bladder (Rong et al., 2002), affects both populations, making it difficult to study each group in isolation. One study did observe that low-threshold afferents in TRPV1 knockout mice saturated at lower firing rates than in wild type mice, while there was no difference in firing rates between high threshold afferents in wild type and TRPV1 knockouts (Daly, Rong, Chess-Williams, Chapple, & Grundy, 2007). Although these data suggest a mechanistic difference between populations, the firing rates of high-threshold units in wild type and knockout mice were compared before their saturation points, a regime where there was also no distinction between firing rates of low-threshold units in the two groups. Moreover, another study in TRPV1 knockout mice found a 40% reduction in sensitivity to pain compared to wild type mice (Jones, Xu, & Gebhart, 2005), which would not be expected if high-threshold afferents are not responsive to TRPV1 and primarily code nociceptive states.
Classification of fibers by global bladder pressure thresholds is also confounded by the local stress to which their receptive fields are exposed. Relating intravesical pressure to afferent activation could generate the appearance of many activation thresholds because at any given pressure, fibers innervating different regions of the bladder would be exposed to different stresses. For instance, during bladder filling there will be significant distention of the bladder dome, moderate distention in the region of the bladder being fed by the ureters, and little distention near the bladder neck, which remains largely undeformed across physiologic bladder volumes. The geometry of this distention over the course of a filling cycle has been observed in vivo using magnetic resonance imaging (Lotz et al., 2005), and modeled in three-dimensions using a completely stationary bladder neck position (Fig. 1C) (Tziannaros, Glavin, & Smith, 2013). Therefore, afferents innervating the dome will register large distention, while, at the same pressure there will be little distention of the bladder neck, potentially resulting in minimal discharge from afferents innervating the bladder neck. Further, an in vivo study that characterized the receptive fields of mechanosensitive pelvic afferents by probing the bladder with a glass rod revealed that the distributions of their pressure activation thresholds decreased with their proximity to the bladder dome, as shown in Fig. 1 (Shea et al., 2000). Therefore, average pressure may not be an accurate way to classify afferent neurons, and high-pressure responding afferents may be encoding small local distention rather than noxious pressures. Finally, it should be noted that in vitro studies do not find a similar correspondence between receptive field location and activation threshold (Xu & Gebhart, 2008). However, after the bladder is excised, cut open, pinned flat and stretched it is difficult to know what the local distention and stress are at any given receptive field location as compared with the morphologically intact bladder.
Fig. 1.
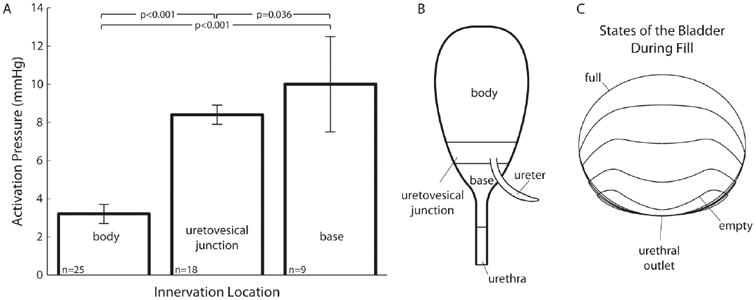
Apparent bladder pressure thresholds of pelvic afferents correlate with location of bladder innervation. A) Minimum global bladder pressures at which afferents begin firing are separated according to the location of their receptive fields. Data show that afferents with receptive fields in locations that undergo the most distention during filling are activated at the lowest pressures, indicating local distention rather than average bladder pressure may determine when afferents respond. The p-values are calculated using one-tailed paired t-tests assuming unequal group variances and the null hypotheses are that group means are lower from left to right. Error bars are standard errors. Analysis is from data reported in Shea et al. 2000. The number of receptors found in each region (listed in the lower right of each bar) reflect the distribution of pelvic afferents throughout the LUT. B) A diagram showing the location of each region to which receptive fields were assigned (adapted from Shea et al. 2000). C) Three-dimensional mathematical model of bladder shape viewed from the coronal plane at discrete times during filling. The diagram emphasizes the large changes in the body compared to the stable shape of the bladder at the base near the urethra. The urethra meets the bladder base at the point marked ‘urethral outlet’ (adapted from Tziannaros et al., 2013).
The complexity of developing a framework for understanding signaling by pelvic afferents continues to grow as detailed investigations discover new apparent sensory modes. For example, the existence of “volume receptors” - receptors that respond to distention but not contraction - has been proposed (Morrison, 1997; Shea et al., 2000), as well as “mucosal mechanoreceptors” that respond to stroking of the mucosal lining of the bladder body (or probing normal to the bladder surface) but do not respond to detrusor stretch (Xu & Gebhart, 2008; Zagorodnyuk et al., 2006). However, some investigators have expressed concern that differences in afferent response during passive distention versus active isovolumetric contraction are confounded by differences in the regions where the detrusor is active (Floyd, Hick, & Morrison, 1976; Lapides, Hodgson, Boyd, Shook, & Lichtwardt, 1958). Ultimately, it is very difficult to attribute causally particular afferent responses to a single mode of activation because the dynamic and kinematic variables of the bladder, with which it is natural to attempt to associate afferent activity, such as strain, volume, pressure, tension, or stress, are themselves highly correlated, especially at low volumes (le Feber, van Asselt, & van Mastrigt, 2004), and are affected by nonlinear processes, such as hysteresis (Downie & Armour, 1992). More work is required to develop a quantitative relationship between primary stimuli in the bladder and the sensitivity of individual afferents as well as the aggregate sensory activity in the pelvic nerve.
2.2. Hypogastric
The hypogastriac nerve densely innervates the bladder near the ureters and near the bladder neck, with much fewer endings projecting to the dome than the pelvic nerve (Uemura, Fletcher, Dirks, & Bradley, 1973; Xu & Gebhart, 2008). With few exceptions (Talaat, 1937) studies indicate that hypogastric afferents signal a wide range of bladder pressure and/or tension information (Bahns et al., 1987; de Groat & Lalley, 1972; Floyd et al., 1976; Weaver, 1985), similar to the pelvic nerve. The aggregate sensory activity over many units of the hypogastric nerve closely tracks average intravesical pressure without saturating across a wide range of pressures, and is sensitive to small changes in average pressure (Winter, 1971) or tension caused by intrinsic detrusor contractions (McCarthy et al., 2009). As in the pelvic nerve, no clear distinction can be made between individual fiber conduction velocity or myelination type and the average bladder pressure at which the unit begins to respond (Winter, 1971). Because the afferent signals in the pelvic and hypogastric nerves qualitatively appear to encode similar information, there is no obvious way to dissociate their respective functions during continence and voiding based solely their afferent activity.
Another issue complicating study of the hypogastric nerve in isolation is that some pelvic afferents travel in the hypogastric nerve as proximal as the inferior mesenteric ganglion, making pelvic contamination of signals in the distal hypogastric nerve a possibility (Langley & Anderson, 1895; Talaat, 1937). The distribution of fiber types in hypogastric and pelvic afferents has been reported in the mouse, with the main difference being that many more hypogastric fibers exist that were not responsive to stretch or urothelial stroking, but that instead were responsive to blunt probing (Xu & Gebhart, 2008). However, in general, nearly all fibers respond to mechanical perturbations at the site of their receptive field (Floyd et al., 1976; Xu & Gebhart, 2008).
Given the apparent similarity of signaling qualities, the most likely important difference between afferent signals in the pelvic and hypogastric nerves are the spinal circuits to which their respective information is conveyed. In rats hypogastric afferents project to the inferior mesenteric ganglion and subsequently to the spinal cord in a broad spatial distribution centered at L1 and L2, while pelvic afferents project to the major pelvic ganglion and then predominantly to L6, with lower density projections as rostral as L2 (Vera & Nadelhaft, 1992). The functional difference is most notable when examining the micturition reflex, but is apparent in other circuits as well. For example, overdistension of the bladder causes a significant increase in blood pressure in dogs under chloralose anesthesia. This link persists after transection of the pelvic afferents but is greatly attenuated or completely abolished after hypogastric transection (Talaat, 1937), indicating that sensory information from these nerves is processed in different spinal and/or supraspinal centers. In awake behaving cats, chronic pelvic transection resulted in low voiding efficiency and erratic micturition behavior, while hypogastric transection had little effect (Barrington, 1914). Although primary afferent signals from the hypogastric and pelvic nerves carry similar information they have different roles in micturition, as outlined below (Fig. 2).
Fig. 2.
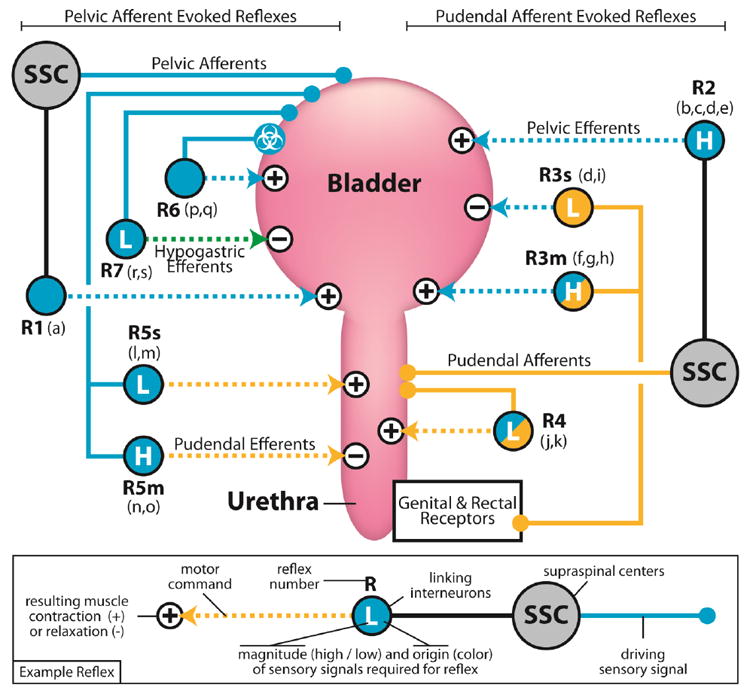
Reflexes controlling storage of urine and micturition with an emphasis on physiological effects on the detrusor and external urethral sphincter. Reflexes are numbered for reference (as referred to in the text) and a suffix is added if the reflex mediates different responses during storage (s) or micturition (m). Reflexes that generate contractions are labeled with “+” on the target muscle, or “-” for relaxation of the muscle. Each reflex is labeled at the junction connecting afferent signals and efferent commands. Junctions are colored according to the afferent signals required for their activation (pudendal, pelvic, or both) and the “H” or “L” indicates that the corresponding sensory activity must be high or low, respectively. Reflexes that are abolished by high-level spinal cord transection are shown passing through supraspinal centers (SSC). a) Bladder distention evokes bladder contraction, which is silenced by spinal transection but persists after pudendal and hypogastric nerve transections: cat (Barrington, 1914). b) Passing fluid through the urethra with volume in the bladder evokes bladder contraction, which is reduced by spinal transection: cat (Barrington, 1914). c) Peng et al. 2008: Interventions reducing urethral afferent output decreases bladder contraction magnitude and voiding efficiency: rat (Peng, Chen, Chang, de Groat, & Cheng, 2006). d) Electrical stimulation of distal sensory branches of the pudendal nerve evoked reflex detrusor contractions at high bladder volumes, some of which were abolished by spinal transection: cat (Yoo et al., 2008a). e) Volume dependence of pudendal mediated supraspinal reflex contraction of detrusor: cat (McGee & Grill, 2014). f) Electrical stimulation of pudendal afferents evokes detrusor contractions at high bladder volumes and persists following hypogastric transection: cat (Woock et al., 2011). g) Electrical stimulation of pudendal nerve reflexively evokes detrusor contractions, and persists following spinal transection: rat (Chen et al., 2011). h) Electrical stimulation of pudendal afferents produce detrusor contraction only when intravesical pressure is high: cat (Bradley & Teague, 1972). i) Low frequency electrical stimulation of pudendal afferents inhibit detrusor contraction, an effect that persists following hypogastric transection and is abolished by local pharmacological blockade in the sacral cord: cat (McGee et al., 2014). j) Passing fluid through the urethra evokes external urethral sphincter relaxation, which persists following hypogastric and pelvic nerve transections: cat (Barrington, 1931). k) Electrical stimulation of proximal transected pudendal afferents evokes contralateral pudendal efferent activation at low frequency, an effect which can be inhibited with pelvic shock conditioning: cat (Bradley & Teague, 1972). l) Low frequency electrical stimulation of pelvic afferents activates the urethral sphincter, and persists following high-level spinal transection, suggesting a continence reflex at low pelvic afferent outflow: cat (Karicheti et al., 2010). m) Electrical stimulation of pelvic afferents readily induces a urethral sphincter contraction, and persists following spinal transection: cat (Bradley & Teague, 1972). n) Substantial bladder distention evokes urethral relaxation: cat (Barrington, 1914). o) High frequency electrical stimulation of pelvic afferents inhibits urethral sphincter activation, and persists following spinal transection: cat (Karicheti et al., 2010). p) Following recovery from spinal transection and hypogastric and/or pudendal nerve transection, leak point bladder pressure is increased by transecting the pelvic nerve, indicating that there is an internal pelvic spinal pathway promoting micturition: cat (Barrington, 1931). q) Following spinal transection bladder distention evoked detrusor contractions re-emerge in chronic conditions that can be abolished by capsaicin, which is known to block selectively unmyelinated afferent fibers carrying information about noxious bladder stimuli: cat (de Groat et al., 1990). r) High frequency electrical stimulation of pelvic afferents inhibits this hypogastric efferent reflex, thereby promoting detrusor contraction, and persists following spinal transection, whereas low frequency stimulation activates hypogastric efferents: cat (Karicheti et al., 2010). s) Onset, but not persistence, of spontaneous bladder contractions under isovolumentric conditions evoke a hypogastric efferent response that persists following spinal transection: cat (de Groat & Lalley, 1972).
2.3. Pudendal
The afferents of the pudendal nerve are perhaps the least well characterized of the fibers of the lower urinary tract. Pudendal afferents gather sensory information from the urethra to coordinate continence and voiding across many species, and are also responsible for transmission of local cutaneous, rectal, and genital sensation (Garry & Garven, 1957; McKenna & Nadelhaft, 1986; Thor, Morgan, Nadelhaft, Houston, & Degroat, 1989; Yoo, Woock, & Grill, 2008b). The urethra is comprised of layers of smooth muscle, likely innervated by the pelvic nerve, and a surrounding striated sphincter predominantly innervated by the pudendal nerve (Barrington, 1931; Creed, Van der Werf, & Kaye, 1998; Wang, Bhadra, & Grill, 1999). It remains unknown precisely which fiber types and sensory organs innervate the urethra. Histological studies have not discovered muscle spindles or Golgi tendon organs, and only cursory qualitative evidence has been shown for the presence of Pacinian corpuscles (Gosling, Dixon, Critchley, & Thompson, 1981; Todd, 1964). Moreover, the distribution of fast and slow motor fibers innervating the striated sphincter differs across species and experiments (Praud, Sebe, Mondet, & Sebille, 2003). Despite ambiguities in the physiology, evidence suggests that the striated urethral sphincter plays a larger role in maintaining continence than smooth muscle; the largest urethral pressures coincide spatially with striated muscle density (Wang et al., 1999) and selectively blocking urethral striated muscle reduces urethral pressure to a much greater extent than smooth muscle blockade in all conditions except a resting state with low intravesical pressure (Gosling et al., 1981; Thind, 1995).
Passing fluid through the urethra, irrespective of direction, causes activation of pudendal afferents, and during this stimuli the pelvic and hypogastric nerves remains completely silent, with slight hypogastric activity becoming apparent only at the highest pressures. It was also found that the afferent signals would accommodate to sustained mechanical distention (Talaat, 1937). Holding urethral pressure constant without passing fluid along its axis, either via mechanical distention or filling the urethra while clamping the outlet, generates less activity that is more transient than activity evoked by similar pressures during flow, although these patterns are somewhat inconsistent across studies (le Feber, Van Asselt, & Van Mastrigt, 1997; Talaat, 1937; Todd, 1964). A consistent initial volley of activity in response to flow onset, the magnitude of which is also modulated by flow rate, followed by a decay in activity is observed in single pudendal afferents (Snellings, Yoo, & Grill, 2012) and aggregate activity (le Feber et al., 1997). It has also been observed that there is a low level of background activity in the pudendal afferents (Talaat, 1937).
The differences across studies combined with limited electrophysiological data make it challenging to determine the precise sensory mode of activation of pudendal afferents. It has been proposed that pudendal afferents respond to pressure only, because afferent activity is equivalent in response to equal pressures regardless if they are generated by static or dynamic fluids (le Feber et al., 1997), however, not all studies confirm this (Talaat, 1937). Further, it has been found that passing air through the urethra (which generates lower average pressures than saline) evokes greater responses than saline, which was attributed to the turbulent nature of the air flow (Todd, 1964). Yet, it should be noted that the turbulent flow effects cannot be isolated from rapid fluctuations in urethral wall distention, as this was not measured in the study, and passing air through the urethra causes repeated undulations and movement of the meatus in female rats. Moreover, the urothelium is actively involved in neuronal signaling (de Groat, 2004), and may be sensitive to flow, turbulence, distention, pressure, derivatives of any of these dynamic variables, or other factors. It is also likely that afferents in the external urethral sphincter, which surrounds the urethra, play a role in sensory signaling, and, if these afferents resemble those in the anal sphincter, will be comprised of multiple adapting subtypes (Lynn & Brookes, 2011). Despite the limited and sometimes inconsistent data, it appears that the primary function of pudendal afferents is to report the pressure and rate of flow in the urethra. However, more studies are needed to develop a precise map from urethral stimuli to pudendal afferent output.
3. Continence and Micturition Reflexes
The afferent signals of the lower urinary tract provide state information to spinal and supraspinal centers that promote continence and mediate micturition through a network of reflexes. The reflexes are broadly organized so as to synergistically contract the detrusor and relax the urethra to expel urine, and contract the urethra and relax the detrusor to prevent leaking during storage. Part of the difficulty in developing a complete theory of neural control of the lower urinary tract is the large number of interdependent reflexes (Fig. 2). Here we build upon previous attempts (Barrington, 1931; Mahony, Laferte, & Blais, 1977) to synthesize the functional roles of the involuntary component of these reflexes and how they are mediated by afferent signals to promote continence and voiding behavior. Evidence is discussed for each pathway and it is given a reference number associated with the summary diagram (figure 2).
When the bladder is filled and sufficiently distended, a coordination of reflexes causes contraction of the detrusor synergistically coupled to the relaxation of the urethral sphincter and expulsion of the contents of the bladder (Barrington, 1914; Elliott, 1907) [R1]. This behavior requires intact afferent and efferent signals from the pelvic and pudendal nerves, but persists after transection of the hypogastric nerve (Barrington, 1931; Sasaki, 1998). The primary reflex causing detrusor contraction during physiological voiding occurs via pelvic efferents and is driven by pelvic afferents that travel through supraspinal centers, as reflex contraction persists following both pudendal and hypogastric transection but is completely abolished following acute high-level spinal transection (Barrington, 1914) [R1]. Retrograde labeling studies have identified many preganglionic neurons that relay sensory information to key supraspinal centers in the brainstem and hypothalamus (Birder, Roppolo, Erickson, & de Groat, 1999), such as the pontine micturition center or the nucleus raphe magnus (Vizzard, Erickson, Card, Roppolo, & Degroat, 1995). Electrical stimulation of ascending pelvic afferents shows involvement of the periaqueductal grey and laterodorsal tegmental nucleus in this micturition reflex, and stimulation of brainstem centers directly, such as the dorsal pontine tegmentum, also evokes activation of these afferents (Noto, Roppolo, Steers, & de Groat, 1991). Broad reviews of the voluntary control of micturition reflexes and the location of associated centers in the central nervous system are available (de Groat & Wickens, 2013).
Additional reflexes coordinate urethral relaxation with detrusor contraction during micturition. When pelvic afferents are electrically stimulated at high frequencies, presumably emulating a state of significant bladder distention, urethral sphincter activity is inhibited through a short latency spinal reflex (Karicheti, Langdale, Ukai, & Thor, 2010) [R5m]. Stimulation of pelvic afferents at low frequencies, however, evokes reflex contraction of the urethral sphincter (Bradley & Teague, 1972; Karicheti et al., 2010), indicating that low levels of pelvic afferent firing during the storage phase engage this reflex to promote continence [R5s]. Taken together, this suggests that the strength of pelvic afferent activity can toggle the pelvic afferent-to-pudendal efferent reflex between continence and voiding behavior, and that supraspinal descending commands to pelvic efferents are primarily responsible for detrusor contraction.
Reflexes promoting detrusor-urethra synergies also run in the opposite direction, that is, when fluid is passed through the urethra, afferent activity in the pudendal nerve triggers a pelvic efferent mediated contraction of the bladder (Barrington, 1914, 1931). Accumulating evidence suggests that pudendal mediated bladder activation is separated into two pathways, a local and supraspinal reflex [R3m, R2]. Bladder contractions evoked via electrical stimulation of distal branches of the pudendal nerve (responsive to high frequencies) survive spinal transection [R3] while those generated by proximal branch stimulation (responsive to low frequencies) do not (Yoo, Woock, & Grill, 2008a) [R2]. A clinical study in persons with spinal cord injury revealed qualitatively different bladder responses when intra-urethral stimulation was delivered to proximal versus distal locations (Yoo, Horvath, Amundsen, Webster, & Grill, 2011). Collectively, these results point to two distinct reflex pathways.
A strong dependence on bladder volume, a proxy measure for pelvic afferent activity, also exists in both of these reflexes, and high bladder volumes (70-90% of the volume needed for a pelvic distention evoked reflex detrusor contraction [R1]) are required for pudendal afferent stimulation to elicit detrusor contractions (McGee & Grill, 2014; Yoo et al., 2008a), and this volume dependence is not merely a product of the length-tension properties of the detrusor (Boggs, Wenzel, Gustafson, & Grill, 2005). The frequency of pudendal afferent stimulation can also selectively elicit inhibitory [R3s] or excitatory [R3m] detrusor responses, both of which persist following transection of the hypogastric nerve (McGee, Danziger, Bamford, & Grill, 2014; Woock, Yoo, & Grill, 2011).
An internal pudendal reflex has also been identified where pudendal afferents activate pudendal efferents [R4]. This was demonstrated first in awake behaving cats where, following recovery from pelvic transection, urethral relaxation was induced by applying sustained pressure to the bladder that forced fluid through the urethra. If the flow was maintained urethral relaxation persisted, but once flow was reduced by alleviating external pressure on the bladder, the urethra closed reflexively (Barrington, 1914). This pudendal-pudendal reflex also appears to be modulated by pelvic afferent activity (and possibly chemical irritants (Yang, Dolber, & Fraser, 2010)). Following pelvic transection, low frequency stimulation of the proximal transected pudendal afferents evokes a response in contralateral pudendal efferents, an effect that can be inhibited by a large conditioning shock or high frequency stimulation of the proximal pelvic nerve (Bradley & Teague, 1972; Karicheti et al., 2010). Because pelvic afferent activity depresses spontaneous pudendal efferent activity (Evans, 1936), reflexively relaxes the urethral sphincter at high stimulation frequencies, and inhibits the internal pudendal reflex, it is possible that afferent information is integrated from many sources and a single command is subsequently issued to many pudendal efferents, although additional studies are needed to confirm this.
The inhibitory action of hypogastric efferents on the detrusor is mainly mediated by reflex activation driven by pelvic afferents [R7]. Low levels of pelvic afferent discharge caused by bladder distention, or the rising edge of a bladder contraction under isovolumetric conditions, elicits activity in hypogastric efferents (de Groat & Lalley, 1972). This reflex has also been demonstrated using low frequency electrical stimulation of the pelvic afferents, which evokes a hypogastric response (Karicheti et al., 2010). High frequency pelvic afferent stimulation (Karicheti et al., 2010) or large bladder distention (de Groat & Lalley, 1972) inhibits this reflex. This reflex inhibition appears to receive additional drive from descending supraspinal commands that are generated by pelvic afferents, as evidenced by the fact that spinal transection attenuated reflex inhibition at very high intravesical pressures (de Groat & Lalley, 1972) and reduced the pelvic stimulation frequency at which reflex inhibition was observed (Karicheti et al., 2010). This indicates that both spinal and supraspinal drive, mediated by pelvic afferents, may contribute to inhibition of the hypogastric reflex. Therefore, the pelvic afferent-to-hypogastric efferent reflex inhibits detrusor activity at low bladder volumes, apparently to remain continent, and is suppressed at large volumes to promote efficient voiding.
Barrington noted an additional phenomenon in chronic high-level spinal cord injured cats, which had either pudendal nerves, hypogastric nerves, or both transected concurrently with spinal transection, in which leak point bladder pressure increased following transection of the pelvic nerves (Barrington, 1931). This led to the conclusion that there exists an internal pelvic micturition-promoting spinal reflex [R6]. It is now known that the ‘silent’ bladder afferent fibers, which normally engage a bladder-contracting reflex following noxious stimuli, become sensitive to physiological states of bladder distention following chronic spinal cord injury, thereby developing a distention evoked spinal reflex that compensates for the loss of the natural pelvic-afferent mediated supraspinal reflex (de Groat et al., 1981) [R1]. Corroborating evidence for this theory was obtained by using capsaicin to block selectively the unmyelinated fibers driving the noxious response, while leaving the myelinated fibers driving the supraspinal pelvic reflex intact, which abolished the micturition reflex in spinal animals but not in intact animals (de Groat et al., 1990). Because reflex voiding following chronic spinal transection is inefficient due to lack of urethral sphincter relaxation in concert with detrusor contraction i.e., detrusor sphincter dyssynergia (Fowler et al., 2008), it is likely that the silent pelvic afferent pathway is distinct from the pelvic afferent mediated reflexes that evoke urethral sphincter relaxation.
A summary diagram of the reflexes described in this section is presented in figure 2. Reflexes evoked by pelvic afferent signals (solid blue) emanating from the bladder are shown on the left and reflexes evoked by pudendal afferent signals (solid orange) are shown on the right. The circle junctions (representing interneurons) linking afferent signals (solid) and efferent commands (dashed) indicate additional requirements on sensory activity necessary to activate the reflex (orange for pudendal and blue for pelvic, plus for high magnitude and minus for low magnitude). The diagram highlights the complex modulatory role that afferent signals play. In fact, all reflexes are gated by pelvic or pudendal afferent activity with only two exceptions: R6, which is a pelvic mediated response to chemical irritation (chemical symbol), and R1, which is the crucial detrusor contraction reflex that transmits pelvic afferent signals to supraspinal centers (SSC) and, in turn, facilitates pelvic efferent mediated detrusor contraction via signals descending to spinal interneurons. Notably, certain reflex pathways (R3 and R5) have their effect on the target muscle reversed based on modulatory afferent inputs, either contracting or relaxing the muscle depending on the state of the bladder and urethra. This reinforces the importance of sensory feedback effects on the web of reflexes controlling micturition and storage.
4. Conclusions
The neural control of voiding and continence is maintained by a wide spectrum of afferent signals regulating multiple reflexes that synergistically couple detrusor relaxation and urethral sphincter contraction to maintain continence and reverse this action to promote micturition. Because the enormous array of afferent signals in the lower urinary tract are essential to effective reflex function (Gillespie, van Koeveringe, de Wachter, & de Vente, 2009) it is critical that studies continue to investigate what physiological stimuli activate these afferents and to quantify the frequency and magnitude of their responses. To develop a complete understanding of the reflex architecture and its control, it will be necessary to build a clearer picture of how afferent information is shared between reflexes. A large body of work exists investigating the spinal and supraspinal centers where information is integrated for individual reflex control (Birder et al., 2009), however, information regarding inter-reflex coordination, especially under pathophysiological conditions, is still lacking. Advancing our understanding of afferent control of reflexes will ultimately uncover new treatments and new therapeutic targets for many lower urinary tract dysfunctions.
Highlights.
We review the sensory regulation of reflexes in the lower urinary tract.
Sensory signals of peripheral nerves exhibit complex responses across many stimuli.
Relating pelvic sensory responses to global bladder pressure is too simplistic.
A framework is developed outlining the functional interaction of spinal reflexes.
Acknowledgments
NIH F32-DK098904 and NIH R01 NS050514
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahns E, Halsband U, Janig W. Responses of Sacral Visceral Afferents from the Lower Urinary-Tract, Colon and Anus to Mechanical Stimulation. Pflugers Archiv-European Journal of Physiology. 1987;410(3):296–303. doi: 10.1007/Bf00580280. [DOI] [PubMed] [Google Scholar]
- Barrington FJF. The Nervous Mechanism of Micturition. Experimental Physiology. 1914;8:99–71. [Google Scholar]
- Barrington FJF. The component reflexes of micturition in the cat. Parts I and II. Brain. 1931;54:177–188. doi: 10.1093/brain/54.2.177. [DOI] [Google Scholar]
- Birder LA, Drake M, De Groat WC, Fowler CJ, Mayer E, Morrison J, et al. Thor K. Neural Control. In: A P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 4th. Health Publication Ltd; 2009. pp. 167–253. [Google Scholar]
- Birder LA, Roppolo JR, Erickson VL, de Groat WC. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Research. 1999;834(1-2):55–65. doi: 10.1016/S0006-8993(99)01546-2. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. Journal of Neurophysiology. 2005;93(5):2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- Bors EH, Blinn KA. Spinal Reflex Activity from the Vesical Mucosa in Paraplegic Patients. Archives of Neurology and Psychiatry. 1957 Oct;78:339–354. doi: 10.1001/archneurpsyc.1957.02330400013002. [DOI] [PubMed] [Google Scholar]
- Bradley WE, Teague CT. Electrophysiology of Pelvic and Pudendal Nerves in Cat. Experimental Neurology. 1972;35(2):378–&. doi: 10.1016/0014-4886(72)90162-8. [DOI] [PubMed] [Google Scholar]
- Chen SC, Grill WM, Fan WJ, Kou YR, Lin YS, Lai CH, Peng CW. Bilateral pudendal afferent stimulation improves bladder emptying in rats with urinary retention. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed KE, Van der Werf BA, Kaye KW. Innervation of the striated muscle of the membranous urethra of the male dog. Journal of Urology. 1998;159(5):1712–1716. doi: 10.1097/00005392-199805000-00099. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. Journal of Physiology-London. 2007;583(2):663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64(6 Suppl 1):7–11. doi: 10.1016/j.urology.2004.08.063. Review. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. Roppolo JR. Mechanisms Underlying the Recovery of Urinary-Bladder Function Following Spinal-Cord Injury. Journal of the Autonomic Nervous System. 1990;30:S71–S78. doi: 10.1016/0165-1838(90)90105-R. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Lalley PM. Reflex Firing in Lumbar Sympathetic Outflow to Activation of Vesical Afferent Fibers. Journal of Physiology-London. 1972;226(2):289–&. doi: 10.1113/jphysiol.1972.sp009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the Sacral Parasympathetic Reflex Pathways to the Urinary-Bladder and Large-Intestine. Journal of the Autonomic Nervous System. 1981;3(2-4):135–160. doi: 10.1016/0165-1838(81)90059-X. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiologica. 2013;207(1):66–84. doi: 10.1111/Apha.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie JW, Armour JA. Mechanoreceptor Afferent Activity Compared with Receptor Field Dimensions and Pressure Changes in Feline Urinary-Bladder. Canadian Journal of Physiology and Pharmacology. 1992;70(11):1457–1467. doi: 10.1139/y92-206. [DOI] [PubMed] [Google Scholar]
- Elliott TR. The innervation of the bladder and urethra. Journal of Physiology-London. 1907;33(5/6):367–445. doi: 10.1113/jphysiol.1907.sp001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP. Observations on the nerves of supply to the bladder and urethra of the cat, with a study of their action potentials. J Physiol. 1936;86(4):396–414. doi: 10.1113/jphysiol.1936.sp003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K, Hick VE, Morrison JFB. Mechanosensitive Afferent Units in Hypogastric Nerve of Cat. Journal of Physiology-London. 1976;259(2):457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466. doi: 10.1038/nrn2401. Research Support, N.I.H., Extramural Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry RC, Garven HSD. The Ganglia, Afferent Nerve-Endings and Musculature of the Urethra in the Cat. Journal of Physiology-London. 1957;139(3):P1–P2. [PubMed] [Google Scholar]
- Gillespie JI, van Koeveringe GA, de Wachter SG, de Vente J. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int. 2009;103(10):1324–1333. doi: 10.1111/j.1464-410X.2009.08377.x. Research Support, Non-U.S. Gov't Review. [DOI] [PubMed] [Google Scholar]
- Gosling JA, Dixon JS, Critchley HOD, Thompson SA. A Comparative-Study of the Human External Sphincter and Periurethral Levator Ani Muscles. British Journal of Urology. 1981;53(1):35–41. doi: 10.1111/j.1464-410X.1981.tb03125.x. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RCW, Xu LJ, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. Journal of Neuroscience. 2005;25(47):10981–10989. doi: 10.1523/Jneurosci.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai AJ. Afferent mechanism in the urinary tract. Handb Exp Pharmacol. 2011;(202):171–205. doi: 10.1007/978-3-642-16499-6_9. Review. [DOI] [PubMed] [Google Scholar]
- Karicheti V, Langdale CL, Ukai M, Thor KB. Characterization of a spinal, urine storage reflex, inhibitory center and its regulation by 5-HT1A receptors in female cats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2010;298(5):R1198–R1208. doi: 10.1152/ajpregu.00599.2009. [DOI] [PubMed] [Google Scholar]
- Klevmark B. Motility of the urinary bladder in cats during filling at physiological rates. II. Effects of extrinsic bladder denervation on intramural tension and on intravesical pressure patterns. Acta Physiol Scand. 1977;101(2):176–184. doi: 10.1111/j.1748-1716.1977.tb05996.x. [DOI] [PubMed] [Google Scholar]
- Langley JN, Anderson HK. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapides J, Hodgson NB, Boyd RE, Shook EL, Lichtwardt JR. Further Observations on Pharmacologic Reactions of the Bladder. Journal of Urology. 1958;79(4):707–713. doi: 10.1016/S0022-5347(17)66327-4. [DOI] [PubMed] [Google Scholar]
- le Feber J, Van Asselt E, Van Mastrigt R. Neurophysiological modeling of voiding in rats: bladder pressure and postganglionic bladder nerve activity. Am J Physiol. 1997;272(1 Pt 2):R413–421. doi: 10.1152/ajpregu.1997.272.1.R413. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- le Feber J, van Asselt E, van Mastrigt R. Afferent bladder nerve activity in the rat: a mechanism for starting and stopping voiding contractions. Urological Research. 2004;32(6):395–405. doi: 10.1007/s00240-004-0416-8. [DOI] [PubMed] [Google Scholar]
- Lotz HT, van Herk M, Betgen A, Pos F, Lebesque JV, Remeijer P. Reproducibility of the bladder shape and bladder shape changes during filling. Medical Physics. 2005;32(8):2590–2597. doi: 10.1118/1.1992207. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Brookes SJ. Pudendal afferent innervation of the guinea pig external anal sphincter. Neurogastroenterol Motil. 2011;23(9):871–e343. doi: 10.1111/j.1365-2982.2011.01741.x. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Mahony DT, Laferte RO, Blais DJ. Integral storage and voiding reflexes. Neurophysiologic concept of continence and micturition. Urology. 1977;9(1):95–106. doi: 10.1016/0090-4295(77)90297-7. [DOI] [PubMed] [Google Scholar]
- McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ. Spontaneous Contractions Evoke Afferent Nerve Firing in Mouse Bladders With Detrusor Overactivity. Journal of Urology. 2009;181(3):1459–1466. doi: 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MJ, Danziger ZC, Bamford JA, Grill WM. A spinal GABAergic mechanism is necessary for bladder inhibition by pudendal afferent stimulation. American Journal of Physiology-Renal Physiology. 2014;307(8):F921–F930. doi: 10.1152/ajprenal.00330.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MJ, Grill WM. Selective Co-Stimulation of Pudendal Afferents Enhances Bladder Activation and Improves Voiding Efficiency. Neurourology and Urodynamics. 2014;33(8):1272–1278. doi: 10.1002/Nau.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248(4):532–549. doi: 10.1002/cne.902480406. Research Support, U.S. Gov't, Non-P.H.S. [DOI] [PubMed] [Google Scholar]
- Morrison JFB. The physiological mechanisms involved in bladder emptying. Scandinavian Journal of Urology and Nephrology. 1997;31:15–18. [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal Distension as a Noxious Visceral Stimulus - Physiologic and Pharmacologic Characterization of Pseudaffective Reflexes in the Rat. Brain Research. 1988;450(1-2):153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Noto H, Roppolo JR, Steers WD, de Groat WC. Electrophysiological analysis of the ascending and descending components of the micturition reflex pathway in the rat. Brain Res. 1991;549(1):95–105. doi: 10.1016/0006-8993(91)90604-t. Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25(4):388–396. doi: 10.1002/nau.20229. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Praud C, Sebe P, Mondet F, Sebille A. The striated urethral sphincter in female rats. Anat Embryol (Berl) 2003;207(2):169–175. doi: 10.1007/s00429-003-0340-7. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541(Pt 2):591–600. doi: 10.1113/jphysiol.2001.013469. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M. Bladder motility and efferent nerve activity during isotonic and isovolumic recording in the cat. J Physiol. 1998;510(Pt 1):297–308. doi: 10.1111/j.1469-7793.1998.297bz.x. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72(5):2420–2430. doi: 10.1152/jn.1994.72.5.2420. Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the Rat's urinary bladder. J Neurophysiol. 2000;84(4):1924–1933. doi: 10.1152/jn.2000.84.4.1924. Research Support, U.S. Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- Snellings AE, Yoo PB, Grill WM. Urethral flow-responsive afferents in the cat sacral dorsal root ganglia. Neurosci Lett. 2012;516(1):34–38. doi: 10.1016/j.neulet.2012.03.045. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat M. Afferent impulses in tide nerves supplying the urinary bladder. Journal of Physiology-London. 1937;89(1):1–13. doi: 10.1113/jphysiol.1937.sp003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind P. The significance of smooth and striated muscles in the sphincter function of the urethra in healthy women. Neurourology and Urodynamics. 1995;14(6):585–618. doi: 10.1002/nau.1930140602. [DOI] [PubMed] [Google Scholar]
- Thor KB, Morgan C, Nadelhaft I, Houston M, Degroat WC. Organization of Afferent and Efferent Pathways in the Pudendal Nerve of the Female Cat. Journal of Comparative Neurology. 1989;288(2):263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- Todd JK. Afferent Impulses in the Pudendal Nerves of the Cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258–267. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- Torrens M, Morrison JFB. The Physiology of the lower urinary tract. London ; New York: Springer-Verlag; 1987. [Google Scholar]
- Tziannaros M, Glavin SE, Smith FT. Three-dimensional effects in the lower urinary tract. Ima Journal of Applied Mathematics. 2013;78(4):729–749. doi: 10.1093/imamat/hxt019. [DOI] [Google Scholar]
- Uemura E, Fletcher TF, Dirks VA, Bradley WE. Distribution of Sacral Afferent Axons in Cat Urinary-Bladder. American Journal of Anatomy. 1973;136(3):305–313. doi: 10.1002/aja.1001360305. [DOI] [PubMed] [Google Scholar]
- Vera PL, Nadelhaft I. Afferent and Sympathetic Innervation of the Dome and the Base of the Urinary-Bladder of the Female Rat. Brain Research Bulletin. 1992;29(5):651–658. doi: 10.1016/0361-9230(92)90134-J. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erickson VL, Card JP, Roppolo JR, Degroat WC. Transneuronal Labeling of Neurons in the Adult-Rat Brain-Stem and Spinal-Cord after Injection of Pseudorabies Virus into the Urethra. Journal of Comparative Neurology. 1995;355(4):629–640. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- Wang BQ, Bhadra N, Grill WM. Functional anatomy of the male feline urethra: Morphological and physiological correlations. Journal of Urology. 1999;161(2):654–659. doi: 10.1016/S0022-5347(01)61989-X. [DOI] [PubMed] [Google Scholar]
- Weaver LC. Organization of Sympathetic Responses to Distension of Urinary-Bladder. American Journal of Physiology. 1985;248(2):R236–R240. doi: 10.1152/ajpregu.1985.248.2.R236. [DOI] [PubMed] [Google Scholar]
- Winter DL. Receptor Characteristics and Conduction Velocities in Bladder Afferents. Journal of Psychiatric Research. 1971;8(3-4):225–&. doi: 10.1016/0022-3956(71)90021-5. [DOI] [PubMed] [Google Scholar]
- Woock JP, Yoo PB, Grill WM. Mechanisms of reflex bladder activation by pudendal afferents. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R398–407. doi: 10.1152/ajpregu.00154.2010. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LJ, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. Journal of Neurophysiology. 2008;99(1):244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZG, Dolber PC, Fraser MO. Differential vulnerabilities of urethral afferents in diabetes and discovery of a novel urethra-to-urethra reflex. American Journal of Physiology-Renal Physiology. 2010;298(1):F118–F124. doi: 10.1152/ajprenal.00281.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. J Urol. 2011;185(2):737–743. doi: 10.1016/j.juro.2010.09.079. Comparative Study Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008a;212(1):218–225. doi: 10.1016/j.expneurol.2008.04.010. Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Woock JP, Grill WM. Somatic innervation of the feline lower urinary tract. Brain Research. 2008b;1246:80–87. doi: 10.1016/j.brainres.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. Journal of Neuroscience. 1999;19(11):4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Costa M, Brookes SJH. Major classes of sensory neurons to the urinary bladder. Autonomic Neuroscience-Basic & Clinical. 2006;126:390–397. doi: 10.1016/j.autneu.2006.02.007. [DOI] [PubMed] [Google Scholar]


