Abstract
Background
Repeated exposure to cocaine or social stress leads to lasting structural and functional synaptic alterations in medium spiny neurons (MSNs) of nucleus accumbens (NAc). While cocaine- and stress-induced structural changes in dendritic spines have been well-documented, few studies have investigated functional consequences of cocaine and stress at the level of single spines.
Methods
We exposed mice to chronic cocaine or chronic social defeat stress (CSDS) and used two-photon laser scanning microscopy with glutamate photouncaging and whole-cell recording to examine synaptic strength at individual spines on two distinct types of NAc MSNs in acute slices following 24 hours of cocaine withdrawal and following CSDS.
Results
In animals treated with cocaine, average synaptic strength was reduced specifically at large mushroom spines of dopamine receptor type 1-expressing MSNs (D1-MSNs). In contrast, cocaine promoted a rightward shift in the distribution of synaptic weights toward larger synaptic responses in MSNs expressing dopamine receptor type 2 (D2-MSNs). Surprisingly, following CSDS, resilient animals displayed an upregulation of synaptic strength at large mushroom spines of D1-MSNs and a concomitant downregulation in D2-MSNs. Though susceptible mice did not exhibit a significant overall change in synaptic strength on D1 or D2-MSNs, we observed a slight leftward shift in cumulative distribution of large synaptic responses in both cell types.
Conclusions
This study provides the first functional cell type- and spine type-specific comparison of synaptic strength at a single spine level between cocaine- and stress-induced neuroadaptations and demonstrates that psychoactive drugs and stress trigger divergent changes in synaptic function in NAc.
Keywords: cocaine addiction, chronic social defeat stress, dendritic spine, nucleus accumbens, synaptic transmission, glutamate uncaging
Introduction
Chronic administration of psychostimulants, such as cocaine and amphetamine, or exposure to chronic social defeat stress (CSDS), induces robust structural plasticity of medium spiny neurons (MSNs) in the nucleus accumbens (NAc), a key part of the brain’s reward circuitry (1–10). Specifically, repeated exposure to either cocaine or stress is associated with an increase in the density of immature dendritic spines and restructuring of dendritic arbors of NAc MSNs (11–17). While cocaine or stress exposure also alters excitatory synaptic responses of these neurons measured by whole cell recordings (8; 15; 16; 18–21), it remains unclear whether the observed structural changes reflect functional alterations in synaptic strength at individual MSN synapses. For example, does exposure to cocaine or stress modulate synaptic transmission that can be detected at a single spine level? Furthermore, do both conditions employ similar mechanisms to regulate synaptic strength?
It is also becoming increasingly clear that cocaine and stress have diverging effects on the two major subtypes of NAc MSNs, D1 receptor-expressing neurons (D1-MSNs) versus D2 receptor-expressing neurons (D2-MSNs) (15; 17–20; 22; 23). This highlights the importance of tracking cell type-specific changes associated with drug or stress exposure. However, a direct comparison of the functional neuroadaptations of synaptic strength to chronic cocaine or chronic stress has not to date been performed at a single spine level in a cell type-specific manner.
To address these gaps in knowledge we exposed mice to a 7-day regimen of cocaine injections followed by a 24-hour withdrawal period or to a 10 day CSDS paradigm followed by social avoidance testing to identify susceptible and resilient individuals. We then performed ex-vivo studies using whole-cell electrophysiology combined with two-photon imaging and MNI-glutamate photouncaging to directly measure synaptic strength at single spines of D1- and D2-MSNs in the NAc. We aimed to find whether exposure to cocaine or stress induces similar or different functional adaptations on a single spine level and whether these adaptations are cell type-specific.
Materials and Methods
Animals
All experimental procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the Icahn School of Medicine at Mount Sinai. For both the cocaine and CSDS studies, we used 7–8 week old male Drd2-EGFP transgenic mice (GENSAT #RP23-161H15), which express EGFP under the control of a bacterial artificial chromosome containing the D2 dopamine receptor genomic locus to permit distinction between the D1 and D2 MSN subtypes. All animals were bred at Mount Sinai and maintained on a C57Bl/6J background. Male CD-1 mice (35–45 g; Charles River Laboratories), which were sexually experienced retired breeders at least 4 months of age, were used as aggressors for CSDS. For cocaine studies, animals were group housed and maintained on a 12:12 light:dark cycle with ad libidum access to food and water. For CSDS studies, the experimental animals were group housed before the CSDS and single housed after CSDS.
Cocaine injections and chronic social defeat stress
Animals received seven consecutive daily intraperitoneal injections, in their home cages, of either 20 mg/kg cocaine or saline as a control. All electrophysiological and two-photon imaging studies occurred 24 hours after the last injection.
CSDS was performed for 10 days as described previously (24); see Supplementary Methods. Twenty four hours after the last defeat episode, mice were screened with a social interaction test that identifies mice as susceptible versus resilient.
Electrophysiology
All experiments were performed blind to drug treatment or CSDS behavioral phenotype. Animals were perfused with ice-cold artificial cerebrospinal fluid (ACSF; see Supplementary Methods). Coronal slices (200 μm thick) containing NAc were cut with a microslicer, transferred for 1 hour to a holding chamber containing sucrose-ACSF (where 254 mM sucrose replaced NaCl) at 34°C and subsequently maintained at room temperature (20–22°C) until use. Individual slices were transferred to a recording chamber mounted on an upright microscope (Olympus BX61WI) and continuously perfused (2–3 ml per minute) with oxygenated ACSF also containing 3.5 mM MNI-glutamate (4-methoxy-7-nitroindolinyl-caged-L-glutamate; Tocris) and 10 μM D-serine (Sigma). Recordings were performed at room temperature. Whole-cell voltage-clamp recordings were made from D1-MSNs (EGFP-) and D2-MSNs (EGFP+) in the NAc shell region. Previous studies have established that EGFP− cells in Drd2-EGFP mice reliably represent D1 MSNs in dorsal striatum (25), although we recognize that a clean separation of these two cell types is more ambiguous in the NAc (26; 27). Cells were held at −70 mV. Recordings from EGFP+ and EGFP− cells were interleaved.
Two-photon imaging and photostimulation
Two-photon laser scanning microscopy and two-photon glutamate uncaging experiments were performed with an Olympus FV1000 MPE Twin System. For two-photon imaging 840 nm light from the Spectra Physics Maitai HP laser was used to excite Alexa-594. Reference frames were taken throughout the experiment to correct for any spatial drift of the preparation over time. Cells were filled with internal solution containing Alexa-594 for at least 10–15 min before imaging to allow clear visualization of single spines. Our studies were restricted to proximal dendritic regions; all spines were located on the secondary dendritic branches at an average distance of ~50 μm from the soma, with a range of ~30–70 μm. Spines were classified as large mushroom type or thin during the experiment based on the following criteria: mushroom spines were identified by a bright large spine head with a clearly visible neck and a spine head diameter >1 μm; thin spines were identified by a thin long faint neck with a small dim spine head with a diameter <0.3 μm. We did not record from stubby spines that lack a spine neck. However, we did not control for the neck length of the measured spines, which could introduce signal filtration variability (28). Only spines that clearly fit the mushroom vs. thin classification criteria were used. Extreme care was also taken to only use spines that were spatially well-isolated from each other to restrict photouncaging to a single spine.
Two-photon uncaging experiments were performed using a Specta Physics Maitai XF1 laser. MNI-glutamate was bath applied at 3.5 mM along with 10 μM D-serine to prevent NMDA receptor desensitization. Glutamate was uncaged using a brief (1 ms) pulse of 720 nm light. All spines studied were chosen to be at the same depth in the slice (within 30 μm from the surface) to minimize the depth effects on uncaging power (18; 29; 30). Several uncaging pulses around the spine head were delivered to find a hotspot with a maximal response. Subsequently, 5 uncaging pulses were delivered at 0.1 Hz and the average of 3–5 traces was used to measure the amplitude of unitary excitatory postsynaptic currents (uEPSCs).
Data acquisition and analysis
Membrane currents and potentials were amplified and low-pass filtered at 5 kHz using a Multiclamp 700B amplifier (Molecular Devices), digitized at 10 kHz (Digidata 1440) and acquired using pClamp 10 (Molecular Devices). Electrophysiology data was analyzed offline using pClamp. Averaged waveforms of 3–5 consecutive sweeps were used to obtain peak current amplitude. Detection threshold for EPSCs was set at 5 pA. High resolution stacks of representative dendritic segments were collected during the experiments and post-hoc using ImageJ (National Institutes of Health). Data (reported in text and figures as mean ± s.e.m.) were compared statistically using either a two-tailed unpaired Student’s t-test or one-way analysis of variance (ANOVA) for three group comparisons, followed by Newman-Keuls comparison between individual groups. Changes in percent of unresponsive thin spines were analyzed using a Chi-Square test. P values less than 0.05 were considered statistically significant.
Results
MNI-glutamate photouncaging reliably evokes uEPSCs at individual MSN spines
Our cocaine studies focused on investigating the impact of repeated cocaine exposure followed by a short withdrawal period, a regimen that reliably induces locomotor sensitization and CPP (conditional place preference) behavior, as well as a robust increase in formation of thin dendritic spines on NAc MSNs (1–3; 5; 11; 31). We restricted our analysis to MSN spines in the NAc medial shell (Figure 1A), a region known to undergo robust changes in dendritic spine density at a short withdrawal period after chronic cocaine exposure (12–14; 18; 32). A previous report from our group found a consistent increase in dendritic spine density following chronic cocaine administration on proximal dendritic segments of MSNs in the NAc shell (14). To maintain consistency we performed uncaging on large mushroom and thin spines within these proximal dendritic segments (Figure 1E).
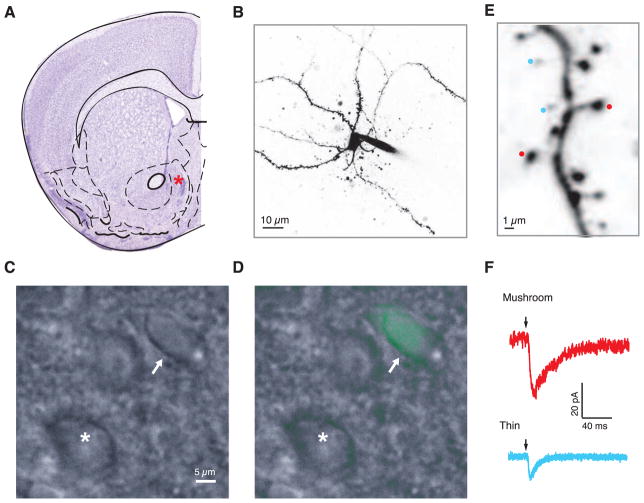
Figure 1.
Uncaging-evoked uEPSCs at single mushroom and thin spines of D1- and D2-MSNs. (A) Schematic of a coronal cross-section of the mouse brain with Nissl stain. A red star depicts the middle of the shell region of NAc where all of the recordings were made. (B) A representative two-photon image of an individual MSN (medium spiny neuron) in the NAc shell. (C) A DIC (differential interference contrast) image of putative MSNs in NAc shell. (D) The DIC image is overlaid with a D2-GFP image, where green fluorescence points to a GFP+/putative D2-MSN (white arrow), while a cell lacking green fluorescence is a putative D1-MSN (white asterisk). (E) A segment of an MSN proximal dendritic branch showing representative mushroom spines (red) and thin spines (blue). (F) Representative uncaging-evoked responses from mushroom and thin spines. Arrows indicate the uncaging pulse.
Using mice that express EGFP in D2-MSNs we were able to distinguish between the D1- and the D2-MSN population based on the presence or absence of green fluorescence (Figure 1C and 1D). We performed whole-cell patch clamp to fill D1 and D2 MSNs with Alexa-594, a red dye (Figure 1B), allowing us to readily visualize and identify spines as mushroom or thin (Figure 1E). We then reliably evoked uEPSCs by briefly uncaging MNI-glutamate at individual spatially well-isolated spines (Figure 1F). Using a representative subset of spines, we observed a positive correlation between spine head diameter and the amplitude of uEPSCs (R2 = 0.6). Amplitudes of uncaging-evoked currents from single spines were comparable to those observed previously in the NAc and ventral tegmental area (VTA) (19; 33).
Chronic cocaine bidirectionally modulates synaptic strength at D1- and D2-MSN spines
Using the amplitude of uncaging-evoked currents as a measure of synaptic strength, we next examined uEPSCs in dendritic spines of mice that underwent a standard regimen of repeated cocaine treatment for 7 days followed by a 24-hour withdrawal period (Figure 2A). Since earlier studies reported that a cocaine-induced increase in spine density was mainly due to appearance of new thin spines, (13; 34–36), we investigated the proportion of thin spines responsive to glutamate stimulation. Interestingly, we found that fewer than 20% of thin spines failed to produce a measurable uEPSC, and this percentage did not differ between the saline- and cocaine-treated groups (Figure 2B, P = 0.6, Chi-square test, saline: 24 spines total [4 unresponsive], 19 cells, 10 mice, cocaine: 30 spines total [7 unresponsive], 13 cells, 10 mice).
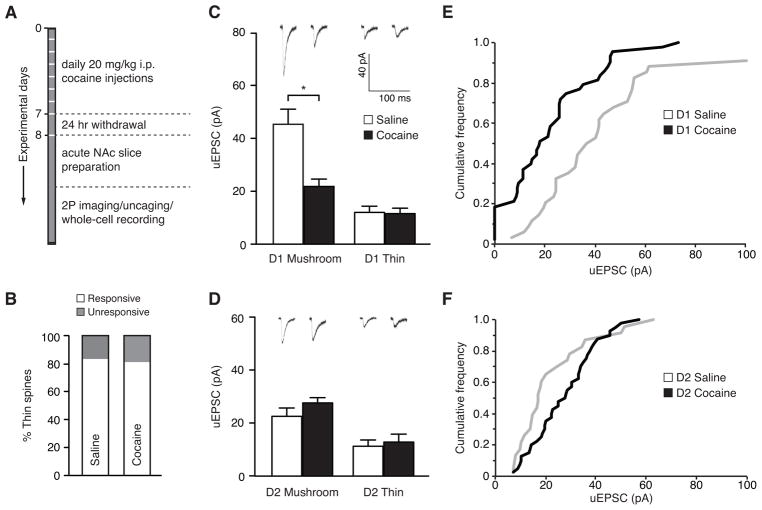
Figure 2.
Bidirectional effect of cocaine on evoked uEPSCs at single spines of D1- and D2-MSNs. (A) Schematic outlining the experimental timeline for cocaine administration and MNI-glutamate uncaging. (B) A summary plot of the effect of cocaine on the percent of responsive and unresponsive thin spines. (C, D) Representative traces with the summary of average data points of uncaging-evoked uEPSC amplitudes in mushroom and thin spines after saline or cocaine treatment in D1-MSNs (C) and D2-MSNs (D). (E) Cumulative frequency plot of uncaging-evoked uEPSCs from mushroom spines of D1-MSNs after a saline or a cocaine treatment. (F) Cumulative frequency plot of uncaging-evoked uEPSCs from mushroom spines of D2-MSNs after a saline or a cocaine treatment.
Next, we measured and compared the amplitude of uEPSCs evoked in D1-MSNs from mushroom and thin spines following cocaine or saline treatment. There was a striking reduction in synaptic strength at single mushroom spines after cocaine. This effect was observed in the average uEPSC amplitude (Figure 2C, saline: 45.3 ± 5.71 pA, cocaine: 21.8 ± 2.78 pA, P = 0.001, Student’s t-test, saline: 34 spines, 13 cells, 5 mice, cocaine: 43 spines, 8 cells, 5 mice), as well as a leftward shift in the cumulative frequency of synaptic strength across the entire range of spines tested (Figure 2E, P = 0.004, Kolmogorov-Smirnov test). There was no effect of cocaine on the uEPSC amplitude in either cell type at thin spines (Figure 2C, saline: 12.0 ± 2.34 pA, cocaine: 11.6 ± 2.05 pA, P > 0.05, Student’s t-test; saline: 10 spines, 13 cells, 5 mice, cocaine: 12 spines, 8 cells, 5 mice).
The same analysis in D2-MSNs revealed no difference in the mean uEPSC amplitude at either mushroom or thin spines in response to cocaine treatment (Figure 2C, 2D, saline: 22.6 ± 3.18 pA [mushroom], 11.3 ± 2.35 pA [thin], cocaine: 27.7 ± 2.0 pA [mushroom], 12.9 ± 3.0 pA [thin], P > 0.05, Student’s t-test, saline: 23 mushroom/14 thin spines, 6 cells, 5 mice, cocaine: 40 mushroom/18 thin spines, 5 cells, 5 mice). However, there was a small, but significant, rightward shift in the cumulative frequency plot in cocaine-treated mice compared to saline-treated mice, representing an enhancement in the number of spines with uEPSC amplitudes between ~10–40 pA (Figure 2F, P = 0.027, Kolmogorov-Smirnov test). Although on average the amplitude of uncaging-evoked currents at single mushroom spines did not change in D2-MSNs, this difference is interesting in two ways. First, it suggests that cocaine regulates synaptic function bidirectionally in D1- versus D2-MSNs. Secondly, a redistribution in synaptic weights might be indicative of circuit rewiring, particularly with respect to a change in the number, strength, or efficacy of specific inputs into NAc (18; 37–40).
CSDS bidirectionally regulates synaptic strength at single spines at D1- and D2-MSNs
CSDS, much like cocaine administration, results in structural and functional adaptations in NAc MSNs. However, modulation of synaptic strength by stress has never been examined at the level of single spines. Additionally, while previous studies suggest that cocaine and stress cause similar synaptic adaptations on VTA dopamine neurons (21), comparison of synaptic responses in D1- and D2-MSN by stress and cocaine has not yet been examined.
To address these questions in a cell type- and spine type-specific manner, we subjected D2-GFP mice to CSDS (Figure 3A) followed by social avoidance testing to identify animals that display either of two phenotypic responses: susceptibility versus resilience to the deleterious effects of the stress (24). These groups were compared to a control cohort that was not subjected to CSDS. Similar to cocaine exposure, social stress is associated with induction of immature spines on MSNs in the NAc shell region of susceptible mice only (6; 41). To test the functional concomitants of this morphological change, we first measured the ability to evoke uEPSCs with MNI-glutamate on thin spines of D1- and D2-MSNs following CSDS. As with cocaine treatment, we found no significant stress-induced change in the percentage of unresponsive thin spines between control, susceptible, and resilient phenotypes (Figure 3B, P = 0.8, Chi-square test, control: 27 spines total [1 unresponsive], 17 cells, 10 mice, susceptible: 24 spines total [1 unresponsive], 19 cells, 11 mice, resilient: 26 spines total [2 unresponsive], 13 cells, 8 mice).
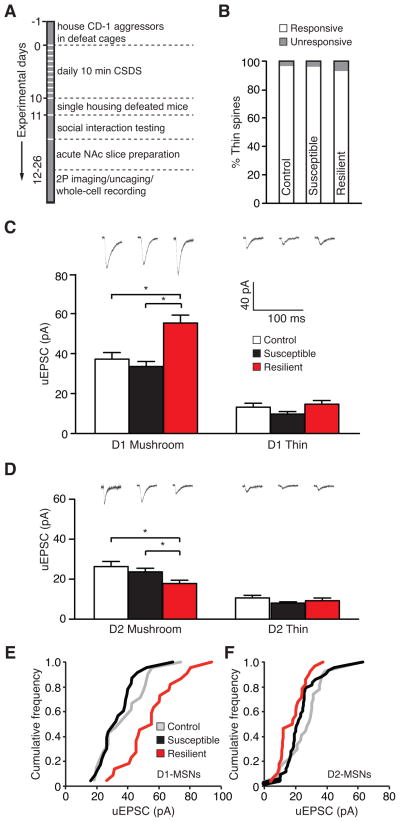
Figure 3.
Bidirectional effect of stress on evoked uEPSCs at single spines of D1-MSNs and D2-MSNs. (A) Schematic outlining the experimental timeline for CSDS and uncaging experiment. (B) A summary plot of the effect of stress on the percent of responsive and unresponsive thin spines. (C, D) Representative traces with the summary of average data points of uncaging-evoked uEPSC amplitudes in mushroom and thin spines after CSDS in D1-MSNs (C) and D2-MSNs (D). (E) Cumulative frequency plot of uncaging-evoked uEPSCs from mushroom spines of D1-MSNs in control and post-CSDS conditions. (F) Cumulative frequency plot of uncaging-evoked uEPSCs from mushroom spines of D2-MSNs in control and post-CSDS conditions.
We next measured uncaging-evoked uEPSC amplitude at mushroom and thin spines in D1- and D2-MSNs of control, susceptible (SI scores: 0.34–0.84), and resilient (SI scores: 1.1–1.6) mice. Surprisingly, we observed no effect of stress on the average strength of mushroom or thin spines of D1- or D2-MSNs in susceptible mice (Figure 3C and 3D, One-way ANOVA, P > 0.05, D1-MSNs: control: 37.2 ± 3.31 pA [mushroom]/13.2 ± 2.02 pA [thin], 21 mushroom/14 thin spines, 12 cells, 5 mice, susceptible: 33.5 ± 2.54 pA [mushroom]/9.73 ± 1.24 pA [thin], 23 mushroom/11 thin spines, 11 cells, 6 mice; D2-MSNs: control: 26.3 ± 2.53 pA [mushroom]/10.6 ± 1.28 pA [thin], 27 mushroom/13 thin spines, 5 cells, 5 mice, susceptible: 23.7 ± 1.77 pA [mushroom]/8.01 ± 0.65 pA [thin], 43 mushroom/13 thin spines, 8 cells, 5 mice). In stark contrast, when we compared uEPSC amplitudes of resilient mice to those evoked from control and susceptible animals, we noticed a strong upregulation in the mean synaptic strength at mushroom spines of D1-MSNs (Figure 3C, One-way ANOVA, P < 0.0001; P > 0.05 for control vs. susceptible and P < 0.0001 for resilient vs. control or susceptible, Newman-Keuls Multiple Comparison Test; resilient: 55.3 ± 3.98 pA, 22 spines, 8 cells, 4 mice) and a significant downregulation of mean synaptic strength at mushroom spines of D2-MSNs (Figure 3D, One-way ANOVA, P < 0.01; P > 0.05 for control vs. susceptible and P < 0.01 for resilient vs. control or susceptible, Newman-Keuls Multiple Comparison Test; resilient: 17.8 ± 1.63 pA, 28 spines, 5 cells, 4 mice). No difference was observed in the strength of synapses on thin spines in either cell type in resilient animals (Figure 3C and 3D, One-way ANOVA, P > 0.05, D1-MSNs: 14.7 ± 1.85 pA, 12 spines, 8 cells, 4 mice, D2-MSNs: 9.21 ± 1.39 pA, 14 spines, 5 cells, 4 mice).
Plotting the data as a cumulative frequency distribution confirmed the observed result in resilient animals, showing a strong increase (rightward shift in the cumulative frequency function) in synaptic strength across the entire population of mushroom spines tested in D1-MSNs (Figure 3E, P = 0.026, Kolmogorov-Smirnov test for control vs. resilient and P = 0.002, Kolmogorov-Smirnov test for susceptible vs. resilient) as well as a decrease (leftward shift of the cumulative frequency function) in synaptic function across the experimental mushroom spine population in D2-MSNs (Figure 3F, P = 0.008, Kolmogorov-Smirnov test for control vs. resilient). Also consistent with our previous observation, there was no change in the distribution of synaptic weights at D1-MSN mushroom spines of susceptible animals (Figure 3E, P = 0.47, Kolmogorov-Smirnov test for control vs. susceptible). Interestingly, examination of cumulative frequency plots from susceptible animals revealed that stress significantly decreased synaptic strength of individual spines with amplitudes in the range of ~20–40 pA (Figure 3F, P = .027, Kolmogorov-Smirnov test for control vs. susceptible). However, the reduction in individual spine strength in the D2-MSN mushroom spines of susceptible animals was very small and not nearly as strong as in the equivalent spine population of resilient mice (Figure 3F, P = 0.019, Kolmogorov-Smirnov test for susceptible vs. resilient). Despite this modest reduction in synaptic strength at a very defined spine population, it should be noted that susceptible mice exhibit a marked increase in the frequency of miniature EPSCs (mEPSCs), suggesting that overall excitatory input to D2-MSNs might be increased in susceptibility (20).
Discussion
Our findings provide important and novel insight into the regulation of synaptic strength at single spines of NAc D1- and D2-MSNs in response to cocaine or stress. We discovered that both conditions bidirectionally modulate synaptic function at mushroom spines and that these adaptations of synaptic strength are cell type-specific and non-overlapping between the two models.
We demonstrate a cocaine-induced downregulation of uEPSC responses at mushroom spines of D1-MSNs, which can be observed both as a decrease in the average amplitude of the uncaging-evoked currents and a shift toward smaller evoked currents across the entire population of spines tested. This is consistent with previous findings of decreased AMPA/NMDA (α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/N-Methyl-D-aspartate) ratio and mEPSC amplitude in D1-MSNs following cocaine, albeit under different exposure conditions (18; 40; 42). Curiously, some studies show an opposite effect with an increase in AMPA/NMDA ratios and higher mESPC amplitudes at D1-MSNs (18; 19; 23). This discrepancy is likely due to differences in cocaine administration paradigms, NAc subregion tested, input specificity, and mouse strain. It is clear that regulation of the NAc network, including its inputs and outputs, is very complex, but it is noteworthy that many studies reveal strong regulation of synaptic transmission specifically at D1-MSNs.
In our experiments we were able to unmask a much subtler effect that cocaine has on D2-MSNs in that there is no change in the mean uESPC amplitude, but instead a redistribution of synaptic weights with a portion of spines exhibiting greater uncaging-evoked uEPSC amplitude compared to saline controls. Although the effect is small, it is interesting that there is an opposing regulation of the two MSN subtypes. Given that D1- and D2-MSNs have diverging roles in regulating a range of behaviors and generally project to different output structures within the basal ganglia, this type of bidirectional regulation is not surprising and has been described before (43–45). Most previous studies that investigate the effect of cocaine on MSN subtypes report little or no change in D2-MSN function, which may reflect no change in the total synaptic strength of the cell. Our method of probing synaptic strength at a single spine level allowed us to uncover a previously unobserved effect of cocaine on D2-MSN synapses. The subtlety of this finding may suggest that a prolonged withdrawal period following cocaine may be required for changes in the strength of D2-MSN synapses to become more pronounced or that changes in synaptic transmission at these synapses might be input-specific and could be masked when inputs are not discriminated. This last point could be particularly pertinent to recent studies that demonstrate opposing cocaine-driven regulation of synaptic strength stemming from diverging synaptic inputs to the NAc (18; 40).
A common effect of psychostimulants and stress on dopamine neurons in the VTA (21) led us to examine whether synaptic strength at individual D1- and D2-MSN synapses is also regulated similarly by chronic cocaine administration and by chronic social stress. Though we found that stress also regulates synaptic function bidirectionally at D1- and D2-MSNs, this occurred most robustly in stress resilient mice and was opposite to what we observed with cocaine. We found that, in mice resilient to the effects of stress, the amplitude of uEPSCs is dramatically upregulated at mushroom spines of D1-MSNs and significantly downregulated at mushroom spines of D2-MSNs. This result is consistent with a recent study that demonstrates that activation of D1-MSNs induces resilience, while activation of D2-MSNs promotes susceptibility, to stress (20). The changes we observe in resilient animals could represent a novel and active adaptation that allows animals to better cope with the effects of social stress. Though we do not observe a strong regulation of synaptic strength at individual spines in susceptible animals, previous data suggest that susceptible mice exhibit an increase in the frequency of EPSCs that may reflect an increase in the total number of spines on D2-MSNs rather than a shift in the individual strength of each synapse (41; 46). Alternatively, another explanation could be that D1-MSN synapses of susceptible animals are resistant to adaptation, which, in turn, could lead to maladaptive behavior. It is important to note that, despite different changes in the strength of synaptic transmission driven by exposure to cocaine versus stress, the two conditions (which are highly comorbid) could lead to shared downstream mechanisms, which should be the focus of future investigations.
Since exposure to cocaine and stress are associated with an increase in thin/immature spines (5; 6), we were curious to see whether the thin spines in our preparations were generally responsive to glutamate. We found this to be true following either chronic cocaine administration or CSDS. There is an extensive body of work showing that cocaine induces silent synapses that likely reside on the newly formed thin spines (12; 34; 47–49). It might appear that our findings contradict this hypothesis. However, we do not believe this to be the case because glutamate photouncaging cannot be spatially restricted to the center of the synapse and likely activates extra- and peri-synaptic AMPA receptors. Future studies will need to address which receptor subtypes shape the uncaging-evoked response from thin spines. Nevertheless, our finding is important because it suggests that the newly formed spines can actively contribute to alterations in synaptic transmission, which might serve to trigger adaptations in synaptic strength that we report in this study.
In considering the interpretations of our findings, it is important to remember that both cocaine and stress affect the brain shortly after exposure as well as after prolonged withdrawal (17; 19; 23; 31; 34; 40; 50). The effects are highly dependent on the subregion of NAc investigated as well as dose and duration of cocaine or stress administration (19; 40; 51), among many other factors. We focused in the present study on synaptic neuroadaptations that are predominant in NAc shell at short withdrawal times. In the future it would be important to examine the effects of cocaine and stress on synaptic transmission at single spines after longer withdrawal periods, different cocaine and stress treatment regimens alone and in combination, and within various subregions of the NAc. It would also be interesting to examine single spine neuroadaptations in the NAc core, as many previous studies have found opposing effects of cocaine in the core versus shell (14; 16; 32; 52).
The results of our study shed light onto several key aspects of the effect of chronic cocaine exposure and CSDS on NAc circuitry. Both paradigms induce rapid, cell type- and spine type-specific adaptations in synaptic strength that can be detected at the level of a single spine. In both cases the effects on D1- and D2-MSNs are bidirectional. We reveal a potentially novel mechanism of resilience to stress, which opens exciting avenues for exploration to enhance natural resilience and promote positive coping. Finally, we demonstrate that the regulation of synaptic neuroadaptations by cocaine and stress is divergent and non-overlapping, a finding that might prove to be an important consideration in therapeutic approaches, given the high comorbidity of addiction with stress disorders in humans.
Supplementary Material
Acknowledgments
The authors thank members of Russo, Nestler, and Slesinger laboratories for technical support and helpful discussions. We thank Drs. Adam Carter and Graham Ellis-Davies for consultation and insightful comments on the manuscript. This work was supported by US National Institutes of Health Grants R01 MH090264 (to S.J.R.), R01 MH104559 (to S.J.R.), R01 DA014133 (to E.J.N.), and R01 MH051399 (to E.J.N.).
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lena A. Khibnik, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai
Michael Beaumont, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai.
Marie Doyle, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai.
Mitra Heshmati, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai.
Paul A. Slesinger, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai
Eric J. Nestler, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai
Scott J. Russo, Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai.
References
- 1.Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 3.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–8. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–76. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–49. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–20. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–74. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–6. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, et al. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32:6957–66. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Park B-H, Lee JH, Park SK, Kim J-H. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–34. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–83. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K-W, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type– and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17:1198–207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–8. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chase Francis T, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. Nucleus Accumbens Medium Spiny Neuron Subtypes Mediate Depression-Related Outcomes to Social Defeat Stress. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 22.Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tritsch NX, Ding JB, Sabatini BL. Nature. Vol. 490. Nature Publishing Group, a division of Macmillan Publishers Limited; 2012. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA; pp. 262–6. All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau J-M, Valjent E, et al. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault J-A. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–85. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–40. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–62. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–54. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 32.Martin BJ, Naughton BJ, Thirtamara-Rajamani K, Yoon DJ, Han DD, Devries AC, Gu HH. Dopamine transporter inhibition is necessary for cocaine-induced increases in dendritic spine density in the nucleus accumbens. Synapse. 2011;65:490–6. doi: 10.1002/syn.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mameli M, Bellone C, Brown MTC, Lüscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–6. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- 34.Lee BR, Ma Y-Y, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–51. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–93. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–8. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto Y, Grace AA. Neuron. Vol. 47. Elsevier Inc; 2005. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization; pp. 255–266. [DOI] [PubMed] [Google Scholar]
- 39.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–64. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 41.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–44. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 43.Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Nature. Vol. 466. Nature Publishing Group; 2010. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry; pp. 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–50. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. I B kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–21. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–7. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61:1060–9. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–62. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen H, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–84. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–9. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.