Abstract
Background
GPR88 is an orphan G protein coupled receptor (GPCR) highly enriched in the striatum, and previous studies have focused on GPR88 function in striatal physiology. The receptor is also expressed in other brain areas and here we examined whether GPR88 function extends beyond striatal-mediated responses.
Methods
We created Gpr88 knockout mice and examined both striatal and extra-striatal regions at molecular and cellular levels. We also tested striatum, hippocampus- and amygdala-dependent behaviors in Gpr88−/− mice using extensive behavioral testing.
Results
We found increased G protein coupling for delta (DOR) and mu opioid, but not other Gi/o coupled receptors, in the striatum of Gpr88 knockout mice. We also found modifications in gene transcription, dopamine and serotonin contents, and dendritic morphology inside and outside the striatum. Behavioral testing confirmed striatal deficits (hyperactivity, stereotypies, motor impairment in rotarod). In addition, mutant mice performed better in spatial tasks dependent on hippocampus (Y-maze, novel object recognition, dual solution cross-maze) and also showed markedly reduced levels of anxiety (elevated plus maze, marble burying, novelty suppressed feeding). Strikingly, chronic blockade of DOR using naltrindole partially improved motor coordination, and normalized spatial navigation and anxiety of Gpr88−/− mice.
Conclusion
We demonstrate that GPR88 is implicated in a large repertoire of behavioral responses that engage motor activity, spatial learning and emotional processing. Our data also reveal functional antagonism between GPR88 and DOR activities in vivo. The therapeutic potential of GPR88 therefore extends to cognitive and anxiety disorders, possibly in interaction with other receptor systems.
Keywords: GPR88 agonist-induced GTPγS binding, medium spiny neurons, ethological scoring of anxiety, gene clustering, spatial learning, psychiatric disorders
INTRODUCTION
The orphan G protein coupled receptor (GPCR) GPR88 is a striatal-enriched gene (1-5) whose expression is modified by neuropharmacological interventions (6-9). Within the striatum, GPR88 is homogenously distributed throughout dorsal (caudate putamen or CPu) and ventral (nucleus accumbens or NAc) areas. Gpr88 gene is detected in projection medium spiny neurons (MSNs) of both striatonigral and striatopallidal pathways, under the control of corticostriatal inputs (3). At present, only one synthetic agonist has been reported (10, 11) and functional studies of GPR88 have used genetic approaches (2, 12, 13). The analysis of mice lacking the Gpr88 gene demonstrates an essential role for GPR88 receptors in dopamine neurotransmission and striatal physiology. Mutant mice show altered basal dopamine and higher phosphoDARPP-32 levels in the striatum (2), as well as increased MSN excitability and firing rates (13). In addition, behavioral deficits evocative of striatal dysfunction were reported, including increased apomorphine and amphetamine effects on locomotor activity (2), or reduced motor coordination and altered cue-based learning (13). Finally, amphetamine locomotor effects were inhibited by local silencing of Gpr88 in the NAc (12).
Previous studies have focused on GPR88 function in the striatum, however Gpr88 expression is not confined to this brain structure. Extrastriatal Gpr88-expressing brain regions are discrete but widely distributed from cortical areas (layer IV) to inferior olive (Allen Brain Atlas; (2, 14)). GPR88 mRNA is absent in hippocampus but present in prefrontal cortex (PFC), septum and parasubiculum, which receive hippocampal inputs (15). Finally, GPR88 is abundant in the amygdala, prominently in the central nucleus (CeA) (14) and to a lesser extent in lateral, cortical and intercalated nuclei, as well as in the anterior part of the bed nucleus of the stria terminalis (BNST) (14). We therefore hypothesized that, beyond striatal-related responses, GPR88 may modulate a wide variety of behaviors, notably hippocampus- and amygdala-dependent behaviors.
We created a Gpr88 knockout mouse line and investigated the impact of Gpr88 gene deletion on several molecular and cellular endpoints in both striatal and extra-striatal regions. We also examined striatum, hippocampus- and amygdala-dependent behaviors in Gpr88−/− mice using an extensive set of behavioral tasks. Our data demonstrate that GPR88 activity regulates monoamine neurotransmission, influences neural connectivity inside and outside the striatum including hippocampus and amygdala, and is implicated in a vast repertoire of behavioral responses that engage cognitive and emotional processing. Intriguingly, most behavioral deficits in Gpr88 mutant mice were reversed by pharmacological blockade of delta opioid receptors (DOR), whose activity seems to oppose GPR88 function.
METHODS AND MATERIALS
Subjects
Male and female Gpr88+/+ and Gpr88−/− mice aged 8-10 weeks were bred in-house. Animals were group-housed (except during nest building test) and maintained on a 12hr light/dark cycle (lights on at 7:00 AM) at controlled temperature (22±1°C). Food and water were available ad libitum throughout all experiments, unless otherwise stated. All experiments were analyzed blind to genotypes. All experimental procedures were reviewed and approved by the local ethic comity (CREMEAS, 2003-10-08-[1]-58).
Generation of Gpr88−/− mice
Gpr88 floxed mice (Gpr88fl/fl) were generated at the Institut Clinique de la Souris using Cre-LoxP technology. Briefly, exon 2 was flanked by loxP sites and a Lox-FRT neomycin-resistance cassette was inserted downstream exon 2 using homologous recombination (Figure 1A and Supplement 1). F1 heterozygous Gpr88fl/+ mice were bred with CMV-Flip mice in order to remove the neomycin cassette, and produce a conditional Gpr88 floxed line. For this study, we further created constitutive knockout animals by breeding conditional animals with a general CMV-Cre driver line (16, 17). This led to germ-line deletion of Gpr88 exon 2 on a hybrid 50% C57BL/6J–50% 129Sv genetic background. Gpr88fl/fl × CMV-CreTg/+ and Gpr88+/+ × CMV-Cre0/+ were used as experimental (Gpr88−/− mice) and control (Gpr88+/+) animals, respectively.
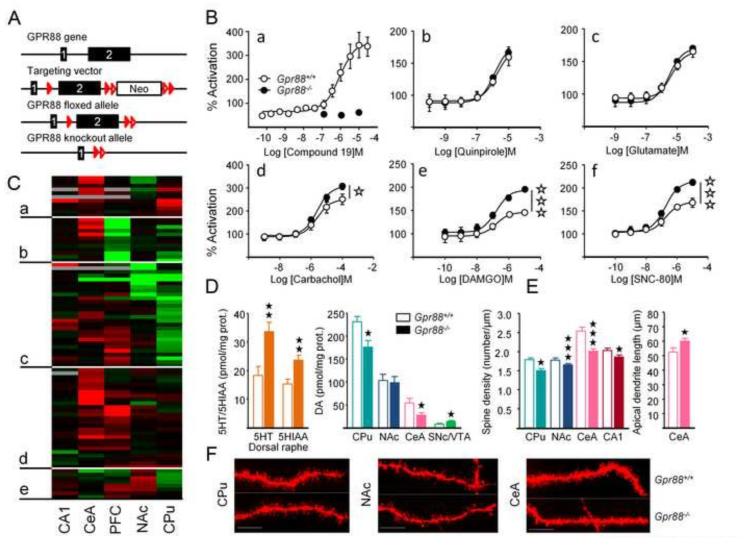
Figure 1. Deletion of the Gpr88 gene display modifies GPCR signaling in the striatum and monoamine neurotransmission at striatal and extrastriatal sites.
(A) Gene targeting strategy to generate a Gpr88−/− mouse line. (B) a: GPR88-mediated [35S]-GTPγS binding is abolished in the striatum of mutant animals; b-f: [35S]-GTPγS binding assay shows facilitated delta (SNC80) and mu (DAMGO) opioid receptor function in striatal membranes of Gpr88−/− mice, whereas signaling properties of Go/Gi coupled D2 dopamine (quinpirole), metabotropic glutamate (glutamate), muscarinic (carbachol) receptors are unchanged. (C) Clustering analysis of gene expression data across PFC, CPu, NAc, CeA and CA1 classifies genes in five clusters (a-e). Most genes with increased expression in the CeA and PFC of mutant mice gather in clusters (a) and (d); most genes with down-regulated expression in the PFC are found in (b), whereas cluster (c) groups genes with decreased expression in the CPu and NAc. Little regulation of gene expression is observed in cluster (e). In the CPu of Gpr88−/− mice, 19 genes are down-regulated, 7 being highly or specifically expressed D2R MSNs (Drd2, Adora2, Cnr1, Foxp1, Rgs4, Gpr6, Nr4a1, Penk) and 6 playing a role in glutamate transmission (gene names in Figure S2 (Supplement 1) and Table S3 (Supplement 2)). In the CeA, 24 over 27 genes with regulated expression show increased transcription, 11 of which coding for subunits of GABA-A receptors or main actors of glutamate signaling (most significant up-regulation for Grm5 and Homer3) and 8 coding for MSN-enriched proteins (Arpp21, Bcl11b, Cartpt, Gpr6, Hpca, Nr4a1, Oprk1, Oprm1, Pde10a, Wfs1). Examples of individual gene expression profiles are displayed in Figure S2B in Supplement 1. (D) Contents in serotonin (5HT) and its metabolite 5-hydroxyindoleacetic acid (5HIAA) are increased in the dorsal raphe of Gpr88−/− animals, while dopamine contents are increased in the midbrain of these animals (SNc/VTA) but decreased in the CPu and CeA (Table S4 in Supplement 2). (E) Spine density is reduced in the CPu, NAc, CeA and CA1 of Gpr88−/− mice. (F) Dil fluorescent terminal dendritic segments from MSNs in the CPu, NAc and CeA of Gpr88+/+ and Gpr88−/− mice. Scale bar: 10 µm. Data are presented as mean ± SEM. Open stars: *p < 0.05; **p< 0.01; *** p< 0.001 (Two-way ANOVA). Solid Stars: *p < 0.05, **p < 0.01, ***p < 0.001 (post-hoc: Newman–Keuls test). CA1: CA1 of the dorsal hippocampus; CeA: central nucleus of the amygdala; PFC: prefrontal cortex; CPu: caudate putamen; NAc: nucleus accumbens, SNc: substantia nigra, pars compacta; VTA: ventral tegmental area.
Drugs
Chemical compounds used for [35S]-GTPγS binding and in vivo pharmacology experiments, their doses and route of administration are described in Supplement 1.
[35S]-GTPγS binding
The assay was performed on membrane preparations from striatum and other brain regions and performed as described previously (21) (see dissection in Figure S1 and Methods in Supplement 1).
Real-time quantitative PCR analysis
Real-time quantitative PCR analysis was performed on brain samples as described previously (9, 19, 22) (see dissection in Figure S1 and Methods in Supplement 1).
Neurochemical assay for biogenic amine dosage
Monoamine levels across brain regions were measured by HPLC as described previously (22, 23) (see dissection in Figure S1 and Methods in Supplement 1).
Dil staining and quantification of dendritic spines
Dil labelling was performed on fixed slides, dendrites were then imaged using a Leica SP5 confocal laser scanning microscope and dendritic spine density was evaluated on 3D stacks using the plug-in AutoSpine of Neurolucida software package (Version 7.51) (full Methods in Supplement 1). Typical DiI fluorescent striatal MSN is displayed in Figure S2C (Supplement 1).
Behavioral experiments
Striatal-dependent behavioral responses were evaluated by evaluating locomotor activity in a novel environment, ability to build a nest, motor stereotypies, time to grasp a string and skill motor learning on the accelerating rotarod. Hippocampus-dependent behaviors were assessed using a continuous alternation paradigm in the Y-maze, a novel object recognition task and a dual solution cross-maze task followed by reversal. Anxiety-like behavior was evaluated by ethological scoring in the elevated plus-maze (EPM) and assessed in the marble burying and novelty-suppressed feeding (NSF) tests.
To assess the effects of chronic naltrindole administration, a first cohort of animals underwent successively EPM (day 8) and NSF (day 16) tests. A second cohort was trained on the accelerating rotarod (starting day 8), and then tested for Y-maze exploration (day 24) (detailed protocols in Supplement 1).
Statistical analyses
Statistical analyses were performed using Statistica 9.0 software (StatSoft, Maisons-Alfort, France). For all comparisons, values of p<0.05 were considered as significant. Statistical significance in behavioral experiments and monoamine dosage was assessed using two to five-way analysis of variance (genotype, gender, treatment, trial and session effects) followed by Newman-Keuls post-hoc test, except for nesting score, which significance was tested using the non-parametric Kruskal-Wallis’ analysis of variance. For [35S]-GTPγS binding, statistical significance was calculated using two-way analysis of variance (genotype and dose effects). Statistical and unsupervised clustering analysis of qPCR data were performed as previously described (9).
RESULTS
Molecular and cellular alterations are detected throughout Gpr88−/− mouse brains
We first verified the absence of receptor expression in Gpr88−/− mice. The Gpr88 transcript was undetectable in mutant brains by in situ hybridization (not shown). Further, the GPR88 agonist Compound 19 (24) elicited strong [35S]-GTPγS binding in striatal membranes from control mice (EC50=940.6±7.12 nM and Bmax=361.3±18.0) but was ineffective in Gpr88−/− samples, demonstrating lack of GPR88 receptor signaling in mutant animals (Figure 1B).
Using the [35S]-GTPγS assay, we also examined whether the absence of GPR88 in mutant mice modifies signaling efficacy of other Go/Gi coupled receptors expressed in the striatum. We selected best-studied GPCRs, including dopamine D2, glutamate metabotropic, muscarinic acetylcholine (m2/m4), mu opioid (MOR) and delta opioid (DOR) receptors (Figure 1B). Quinpirole, glutamate and carbachol dose-dependently increased [35S]-GTPγS binding in striatal membrane preparations, with efficacies and potencies similar in GPR88 mutants and controls. Intriguingly, carbachol and the two opioid agonists DAMGO (MOR agonist) and SNC-80 (DOR agonist) induced a slightly but significantly stronger [35S]-GTPγS response in mutant samples (statistics in Table S2 in Supplement 2). Gpr88 ablation therefore leads to enhanced signaling for muscarinic and opioid receptors.
We then quantified the expression of 89 genes in PFC, CPu, NAc, CeA and CA1 using qRT-PCR (Table S3 in Supplement 2). Genes were selected as key actors of GABA, glutamate and monoamine signaling pathways or were striatal gene markers. Cluster analysis of qRT-PCR data identified groups of genes sharing similar expression profiles (Figures 1C and S2A in Supplement 1), organized qRT-PCR data in five main clusters. Genes with increased expression gathered in clusters (a) and (d); most genes with down-regulated expression in PFC clustered in (b), whereas cluster (c) grouped genes with decreased expression in CPu and NAc. Limited regulation of gene expression was observed in cluster (e). This analysis reveals that Gpr88 deletion modifies gene transcription in regions where the receptor is abundant (CPu, NAc, CPF and CeA), and also in areas with no detectable expression (CA1). Gene regulation appeared region-specific, with most down-regulated expression observed in striatal regions and up-regulation detected in PFC and CeA (see examples of individual gene expression profiles in Figure S2B in Supplement 1).
We examined the impact of Gpr88 deletion on monoamines in areas of high receptor density (CPu, NAc and CeA), as well as in other brain regions where the receptor is weakly expressed (PFC) or undetectable (dorsal and ventral hippocampus or HPCd and HPCv, dorsal raphe or DRN, ventral tegmental area/substantia nigra pars compacta or VTA/SNc). Levels of DA and 5HT, and their metabolites are shown in Figure 1D and Table S4 in Supplement 2. In agreement with previous findings (2) (but see (13)), we found decreased DA levels in CPu, but not NAc of mutant mice. DA content was also lower in CeA, and higher in SNc/VTA. DA was otherwise unchanged in other brain regions (PFC, HPCd and HPCv), and the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) was not modified in any sample (Table S4 in Supplement 2). 5HT and its metabolite 5-hydroxyindoleacetic acid (5HIAA) were higher in the DRN of Gpr88−/− mice. No significant modification was detected in other brain structures. Monoamine levels are increased at their site of synthesis (DA in SNc/VTA and 5HT in DRN).
We compared dendritic spine morphology in the CPu, NAc, CeA and CA1 of Gpr88−/− mice (Figure 1E, F). Spine density was significantly reduced in CPu, NAc and CeA, as well as in hippocampus CA1.
Striatal-dependent behaviors are impaired in Gpr88−/− mice
Similar to previous investigators (3, 5), we tested behaviors involving striatal circuits. We first assessed locomotor activity in a novel environment (Figure 2A; Table S5 in Supplement 2). Gpr88−/− animals and controls were exposed to an open-field for 5 daily consecutive 30-min sessions. As observed earlier (5), mutant animals displayed increased forward locomotion and this hyperactivity failed to habituate over days of testing.
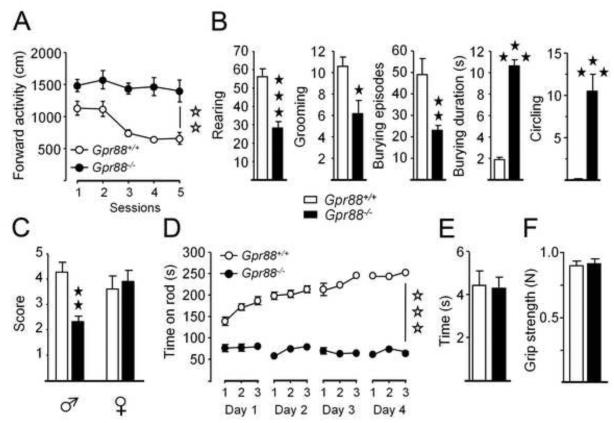
Figure 2. Gpr88−/− mice display impaired striatal dependent behaviors.
(A) Gpr88−/− mice travel a longer distance in the open field as compared to controls and this hyperactivity fails to habituate over sessions. (B) Mutant animals display less rearing, grooming and burying episodes but spend longer time burying and make more circles than Gpr88+/+ mice. (C) Male, and not female, mice lacking Gpr88 fail to build a nest. (D) Gpr88 deletion impairs motor coordination and skill learning in the accelerating rotarod test, while forelimb motor coordination (E) and strength (F) are preserved. Data are presented as mean ± SEM. Open stars: **p < 0.01, *** p< 0.001 (genotype effect). Solid Stars: *p < 0.05, **p < 0.01, ***p < 0.001 (Newman–Keuls post-hoc test or Kruskal Wallis’ analysis of variance).
Altered striatal function often results in the development of spontaneous motor stereotypies. Gpr88−/− mice displayed decreased number of rearing, grooming, and burying episodes (Figure 2B; Table S5 in Supplement 2) and also spent more time burying due to markedly longer episodes than controls, as well as increased frequency of circling, indicating altogether that motor stereotypies develop as a consequence of Gpr88 deletion. We also used the nest-building test to further evaluate striatal function (25). Male, but not female, Gpr88−/− mice showed impaired ability to build a nest (Figure 2C; Table S5 in Supplement 2).
We evaluated procedural abilities of mutant using a motor skill-learning task on the accelerating rotarod (26, 27). Mice were trained to run for 3 trials a day during 4 consecutive daily sessions. In both mutant and control groups, females performed better than males. Consistent with previous report (13), Gpr88−/− mice performed poorly in this test. Moreover, mutants strikingly failed to ameliorate over trials and sessions, suggesting a marked impairment of motor skill learning (Figure 2D; Table S5 in Supplement 2). Mutant mice achieved similar performance as controls in the string task, suggesting that motor coordination was not affected in this test (Figure 2E). Muscular strength was not altered in Gpr88−/− animals, as shown in the grip test (Figure 2F).
Together, these data indicate that striatal-dependent behaviors are impaired in Gpr88−/− mice.
Hippocampus-dependent behaviors are facilitated in Gpr88−/− mice
Hippocampal and striatal activities compete to drive behavior during learning and memory tasks (28). Altered striatal function in Gpr88−/− mice may thus influence hippocampal-dependent behaviors. We performed three behavioral tests to tackle hippocampal function. First, hippocampal-dependent navigation was evaluated by scoring spontaneous alternation in a Y-maze (Figure 3A; Table S5 in Supplement 2). Consistent with locomotor hyperactivity, Gpr88−/− mice entered more often into arms of the maze during a 5-min exploration session, males being more active. Mutant mice showed a trend towards higher spontaneous alternation and returned significantly less into the same arm, indicative of less perseverative errors, while alternate arms returns were unchanged.
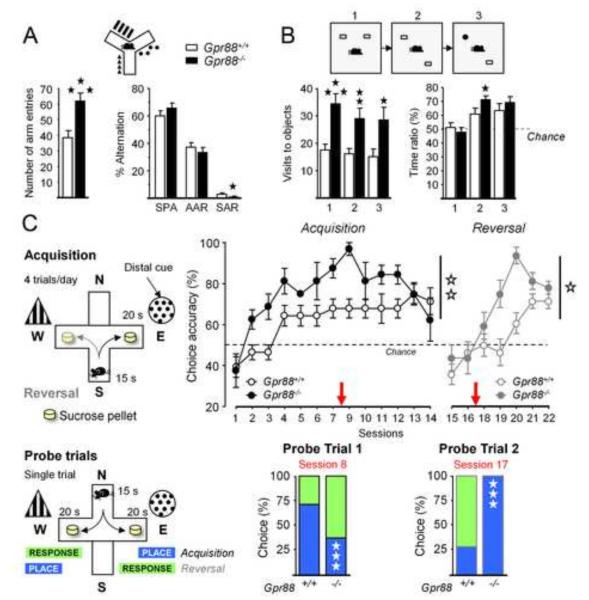
Figure 3. Gpr88−/− animals show facilitated hippocampus-dependent behaviors.
(A) When exploring a Y-maze, mutant mice displayed more arm entries, evoking hyperactivity, and made less perseverative arm reentries. (B) In the novel object recognition test, Gpr88 null mice visited the objects more often and spent more time exploring the moved object during phase 2 (place phase). (C) Mutant animals acquired earlier and better a dual solution cross-maze task using distal extra-maze cues, shifted sooner to a response strategy to solve the task (probe trial 1), and reacquired more rapidly this task after spatial reversal than Gpr88+/+ controls, by shifting sooner to an allocentric strategy (probe trial 2). More parameters are displayed in Figure S3 in Supplement 1. Data are presented as mean ± SEM. Solid stars: *p < 0.05, **p < 0.01, ***p < 0.001 (post-hoc: Newman–Keuls test). Open stars: *p < 0.05; **p< 0.01 (genotype effect). See Figure S2 in Supplement 1 for more parameters.
Second, we used a novel object recognition task to assess the ability to discriminate either novel objects or their spatial location (19). In trial 1, mice were presented two copies of an unfamiliar object in an open field (familiarization phase); in trial 2, one of the two objects was displaced to a novel location (place phase); in trial 3, the unmoved object was replaced by a novel object (object phase). Mutant animals explored the objects more often, but not longer, than controls across phases, further demonstrating hyperactivity (Figure 3B; Table S5 in Supplement 2). During place phase (Trial 2), knockout animals spent more time exploring the displaced object compared to controls, suggesting facilitated hippocampal-mediated recognition of object location (Figures 3B and S3 in Supplement 1).
Lastly, we tested mutants in a dual-solution cross-maze task, which specifically addresses hippocampal/striatal balance in learning. In this task, performance at early stages of acquisition and reversal rely preferentially on hippocampus-mediated allocentric strategy (place), and then switches to striatal-dependent egocentric strategy (response) at later stages as habitual behavior develops (see Supplement 1). Mutant animals acquired the task more rapidly and reached higher levels of performance than controls (Figure 3C; Figure S3 in Supplement 1; Table S5 in Supplement 2), although they initially displayed longer choice latencies. A probe trial performed after 8 training sessions revealed that Gpr88−/− mice had already shifted towards an egocentric strategy to solve the task, whereas Gpr88+/+ animals still used an allocentric strategy at this stage (χ2=23.27, p<0.0001). Reversal confirmed higher levels of performance in mutant mice, which acquired the novel rule more rapidly than controls. A probe trial performed after 2 reversal sessions indicated that mutant mice had consistently re-shifted to an allocentric strategy (χ2=111.00, p<0.0001).
Altogether, our results concur to demonstrate that hippocampus-dependent behaviors are facilitated in mice lacking GPR88.
Levels of anxiety are diminished in Gpr88−/− mice
GPR88 is densely expressed in the CeA and, further, Gpr88 mutant mice showed significant modifications of transcriptional activity, dendritic spines morphology and dopamine levels in this region, suggesting potential effects on anxiety-like behavior. We used three tests to address different aspects of anxiety.
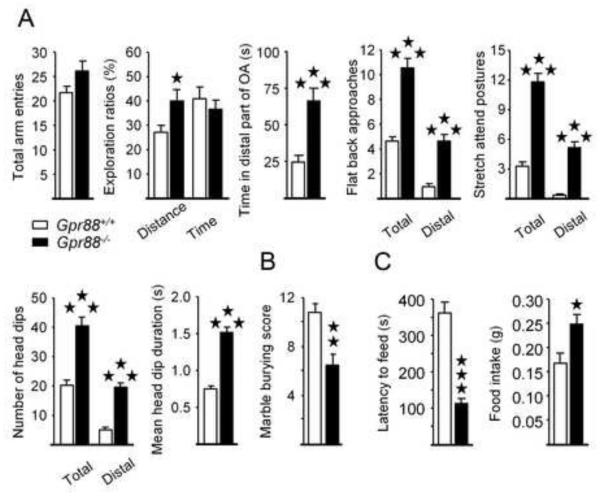
In the EPM, Gpr88−/− mice travelled a longer distance in the open arms, and spent more time in their distal parts (Figure 4A; Table S5 in Supplement 2). Moreover, ethological measures (29) revealed increased exploration in mutant mice, as shown by more frequent flat back approaches, stretched attend postures and head-dips than controls, specifically in the distal part of the open arms. Also, individual head-dips lasted longer in Gpr88−/− mice, suggesting altogether that GPR88 receptor deletion reduces anxiety levels in this test. In the anxiety-induced marble-burying test, Gpr88−/− mice buried less marbles than Gpr88+/+ animals (Figure 4B), consistent with lower anxiety. Finally, we exposed Gpr88−/− to the novelty-suppressed feeding (NSF) test, a conflict task challenging approach/avoidance behavior (30). Mutant mice needed less time to start feeding in the center of the arena, and ate more chow when returned to their home cage compared to controls (Figure 4C), suggestive of decreased conflict anxiety.
Figure 4. Gpr88 null mice display low levels of anxiety.
(A) During the elevated-plus maze test, if Gpr88−/− mice display similar number of arm entries as Gpr88+/+ controls, they travel a longer distance in the open arms, and spend more time in the distal part of these arms than Gpr88+/+ animals; moreover knockout mice display more frequent flat back approaches, stretched attend postures and head-dips than controls, specifically in the distal part of the open arms, with individual head-dips lasting longer than in Gpr88+/+ counterparts.(B) Mutant animals bury less marbles than controls in the marble burying test. (C) In the novelty-suppressed feeding test, Gpr88 null mice display shorter latencies to start eating in the center of the arena compared to Gpr88+/+ animals, and eat more when placed back in their home cage. Data are presented as mean ± SEM. Solid stars: *p < 0.05, **p < 0.01, ***p < 0.001 (post-hoc: Newman–Keuls test).
Together, these data indicate that disruption of the Gpr88 gene results in decreased anxiety-like behaviors in mice.
Chronic blockade of delta opioid receptors partly normalizes behavioral phenotypes of Gpr88−/− mice
We noticed that behavioral features of Gpr88−/− animals remarkably oppose several aspects of behavioral phenotypes that we previously identified in mice lacking DORs. In contrast to GPR88 knockout mice, DOR knockout mutants show facilitated striatal-dependent behaviors and impaired hippocampal-dependent responses in the dual-solution cross-maze task (19), as well as higher levels of anxiety in the elevated plus maze (31). In addition, [35S]-GTPγSdata indicate excessive DOR signaling in GPR88 mutant mice (Figure 1B). We therefore tested the hypothesis that GPR88 and DOR activities compete in vivo. To this aim, we examined whether chronic inhibition of DORs (to mimic gene invalidation as closely as possible) using the antagonist naltrindole (NTI, 0.3 mg/kg s.c.) would normalize behavioral phenotypes of Gpr88−/− animals in the rotarod, Y-maze, EPM and NSF tests.
Chronic NTI administration transiently reduced motor skill deficits in Gpr88 mutant mice. Overall, control mice performed better than mutants in this test, and this effect varied across sessions (Figure 5A; Table S6 in Supplement 2). When analyzing rotarod data for each session separately, we found that NTI significantly improved motor performance of Gpr88−/− animals in session 2, suggesting a transient effect during early skill learning. During Y-maze exploration, chronic NTI normalized spontaneous alternation in Gpr88−/− mice, by increasing significantly the number of same arm returns, without effect on arm entries (Figure 5B; Figure S4A in Supplement 1; Table S6 in Supplement 2).
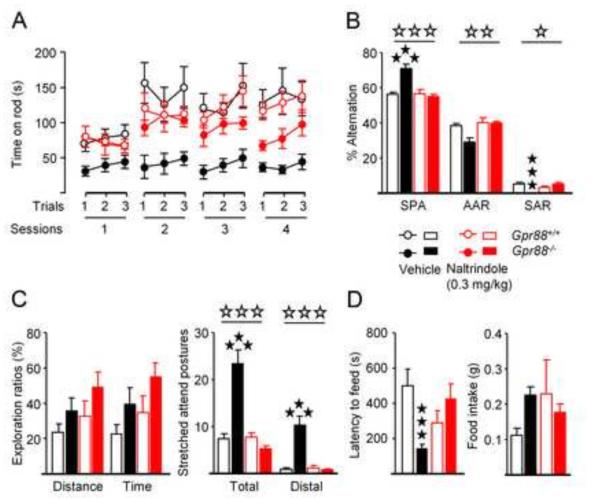
Figure 5. Chronic blockade of delta opioid receptor activity normalizes behavior in mice lacking GPR88 receptors.
(B) Chronic naltrindole administration (0,3mg/kg) transiently (session 2) restores motor skill learning deficit in mice lacking GPR88 receptors. (C) In the Y-maze, this treatment increases significantly their number of same arm returns. (D) Naltrindole had no effect on exploration ratios during the elevated plus-maze test in mutants, but normalized their number of stretched attend postures, (E) as well as their latency to feed in the center of the open field in the novelty-suppressed feeding test. More parameters are displayed in Figure S4 in Supplement 1. Data are presented as mean ± SEM. Open stars: *p < 0.05; **p< 0.01; ***p < 0.001 (upper panel: genotype effect; lower panel: treatment effect). Solid stars: *p < 0.05, **p < 0.01, ***p < 0.001 (post-hoc: Newman– Keuls test).
The chronic NTI treatment also normalized anxiety levels of Gpr88−/− animals in two behavioral tests. In the EPM (Figure 5C; Figure S4B in Supplement 1; Table S6 in Supplement 2), NTI failed to modify exploration ratios, but normalized ethological exploration parameters in mutant mice. The total number of flat back approaches (FBA) and stretched attend postures (SAP), as well as the number of distal FBA, SAP and head-dips, which are remarkably higher in mutant mice, were reduced to control levels upon NTI treatment. In the NSF test, chronic NTI administration increased latency to feed of mutant mice to control level, without effect on food intake (Figure 5D; Table S6 in Supplement 2).
DISCUSSION
The present study reports that Gpr88 gene knockout in mice broadly modifies brain physiology and behavior. We show transcriptional, anatomical and neurochemical modifications in several brain areas. We confirm striatal dysfunction and further demonstrate that Gpr88 knockout facilitates hippocampus-dependent learning and decreases levels of anxiety. We finally provide evidence that excessive DOR signaling contributes to this behavioral profile. Whether molecular and behavioral phenotypes observed in Gpr88 knockout mice arise from developmental compensations or lack of tonic GPR88 receptor activity in adult mutant mice, or both, deserves further investigation. In a previous report, virally mediated inactivation of Gpr88 in the NAc of adult rats reduced amphetamine-induced hyperlocomotion (12), demonstrating a tonic role of GPR88 activity for this particular behavior. Future studies will involve time-controlled genetic inactivation, as well as pharmacology with existing (10) or novel GPR88 drugs to tease out developmental from adult GPR88 activities.
Consistent with previous reports (2, 13), we found impaired striatal function in mutant mice. Knockout animals show non-habituating hyperactivity in a novel environment, a primary landmark of striatal dysfunction (32), and increased activity in several other tests (Y-maze, novel object recognition). Increased circling and duration of burying episodes suggest stereotypic behavior and difficulty ending a behavioral sequence, both major features of striatal deficit (33). Male Gpr88−/− mice failed in the building nests, a behavior normally developing in males for thermoregulation purposes (34) and sensitive to striatal deficits (25, 35). Note that this phenotype may be linked to hyperactivity (36-38). Finally, severe deficit in procedural learning on the rotarod points towards dorsal striatal (CPu) dysfunction (39, 40). Notably also, mutant mice show transcriptional, cellular and neurochemical modifications in the striatum, mostly in the CPu, including down-regulated D2R-MSN and glutamate synapse gene markers, low dendritic spine density and DA contents (as previously shown using microdialysis (2)). Meanwhile, DA levels were higher in the midbrain, suggesting defective release. Similar findings were reported after dopamine-depleting lesions mimicking Parkinson's disease (41, 42) with modifications occurring preferably in D2R-MSNs at least in early stages (41, 43). Whether hyperactivity, motor skill learning deficit and increased exploration in Gpr88 mutants arise from alterations of either D2R-MSN or D1R-MSN activities, or both (44), in the CPu, remains to be clarified using cell-specific Gpr88 gene knockout.
An interesting finding is that, in contrast to striatal-mediated behaviors, hippocampus-dependent responses are enhanced in Gpr88 knockout mice. We found less perseverative arm reentries (Y-maze), indicative of enhanced hippocampus-dependent working memory (45), and higher preference for the displaced object in a task (novel object recognition) that requires hippocampal integrity (46-48). In the cross-maze, mutant mice acquired the dual solution task, requiring distal extra-maze cues, earlier than controls, and shifted sooner after spatial reversal, two behavioral landmarks of improved hippocampal function (49-52). Gpr88−/− mice display modified spine density and gene expression in the hippocampus, whereas Gpr88 expression is undetectable in this region (Allen Brain Atlas; (2, 14)). In mutant mice therefore, lack of GPR88 receptor activity in hippocampal inputs and outputs where the receptor is normally expressed (striatum, PFC, septum, amygdala, parasubiculum) likely contributes to anatomical changes in the hippocampus and, possibly, to improved spatial navigation. Alternatively, functional competition between striatum and hippocampus may account for this behavioral phenotype. Indeed, hippocampal lesion/inactivation facilitates dorsal striatal function (51, 53, 54), although striatal lesion/inactivation fails to conversely facilitate hippocampal-dependent spatial learning (55-57), possibly due to differential implication of MSN subpopulations. Previous studies showed that striatal deletion of the adenosine A2a receptor (Adora2a) gene decreases excitability of D2R-MSNs and facilitates spatial learning (58-60). Reduced A2a receptor expression in the CPu of Gpr88 null mice (Figure S2 in Supplement 1), or decreased D2R-MSN excitability by other mechanisms, may thus have facilitated hippocampal-dependent behaviors in Gpr88−/− animals.
We report for the first time decreased anxiety levels in Gpr88−/− mice. Both classical parameters and ethological measures suggest that levels of anxiety are lower in the EPM. Decreased marble burying in mutant mice can also be interpreted as a sign of lower anxiety (61) or may result from hyperactivity (38, 62, 63). Mutant animals also showed reduced conflict anxiety (NSF) and together the data demonstrate that Gpr88 knockout leads to hyperactive exploratory behavior when mice are exposed to a novel and potentially hostile environment. Altered gene transcription, neuronal morphology and DA levels in the CeA, where Gpr88 is highly expressed, likely contributed to this phenotype (64-67). Gpr88 expression, however, is also detectable in several brain regions involved in regulating anxiety and approach (30), including ventral striatum (NAc and olfactory tubercles), anterior BNST and lateral septum. In the future, site-specific Gpr88 gene knockout should clarify brain sites for anxiolytic activity of GPR88 receptors.
Because of the remarkable opposing phenotypes of Gpr88 and DOR knockout mice in several tests, we probed the GPR88/DOR interaction behaviorally and the partial normalization of Gpr88−/− mice phenotype by chronic DOR blockade was striking. Chronic NTI alleviated motor skill learning deficit in Gpr88−/− mice at an early stage, when motor learning engages dorsal striatal D2R-MSNs (44), suggesting that excessive DOR activity in Gpr88−/− mice could contribute to this process, possibly at the level of D2R-MSNs. In the Y-maze, DOR blockade decreased spontaneous alternation of Gpr88−/− mice back to control levels, consistent with impaired hippocampal-dependent navigation in DOR knockout mice (19). Finally, NTI normalized low anxiety levels in Gpr88−/− mice in both EPM and NSF, in accordance with previously reported anxiogenic effects of DOR gene knockout and blockade (31, 68), notably in the CeA (69). We therefore suggest that GPR88 activity normally acts as a brake on DOR activity to regulate these behaviors. Restoration of normal behaviors in double GPR88/DOR null mutants may confirm this remarkable GPR88/DOR balance in the future. Functional interactions between GPR88 and DOR revealed in this study could operate at systems levels, through functional competition at the level of downstream effectors within neurons, or via physical interactions between the two receptors, and all these possibilities will need to be tested in future studies. Our observation that DOR, muscarinic and MOR coupling to Gi/Go proteins is enhanced in striatal membranes from Gpr88−/− mice (GTPγS assay) supports the notion that GPR88 receptors inhibit DOR, MOR and muscarinic receptor function, and possibly signaling of other GPCRs, at cellular level. The analysis of potential cellular interactions in the striatum, including down-regulated expression of Rgs4 (present study, (13)), that was shown to repress opioid receptor signaling (70, 71), will deserve further investigations.
In conclusion, this study confirms that GPR88 receptors are a promising target in pathologies involving deficient striatal function, such as Parkinson Disease or Attention Deficit/Hyperactivity Disorder, as facilitating GPR88 activity is expected to alleviate striatal dysfunction. Furthermore, our work extends implications of receptor function to areas of cognitive and anxiety disorders where, on the contrary, inhibiting GPR88 signaling could exert beneficial effects on spatial leaning and working memory, or improve some aspects of anxiety-related conditions. The therapeutic potential of GPR88 as a target, therefore, is broad, likely complex, and mechanistically highly novel.
Supplementary Material
Acknowledgements
We thank the Mouse Clinic Institute (Illkirch, France) for the generation of the Gpr88fl/fl mouse line. We thank D. Filliol for technical assistance. We thank M.A. Ayoub for providing reagents and material and for critical analysis of experimental results. We thank A. Matifas, G. Duval and D. Memetov for animal care. This work was supported by the Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), Université de Strasbourg, Institut National de la Recherche Agronomique (INRA) and Université François Rabelais de Tours. We thank the ATHOS Consortium, including the Fonds Unique Interministériel (FUI), the Région Alsace and our partners, Domain Therapeutics (Illkirch, France) and Prestwick Chemicals (Illkirch, France), as well as Région Centre (ARD2020 Biomédicament – GPCRAb), for critical support in this project. A.C.M. acknowledges doctoral fellowship from Fondation Française pour la Recherche Médicale (FRM: FDT20140930830). L.P. Pellissier acknowledges postdoctoral support from the Marie-Curie/AgreenSkills Program. We thank the National Institutes of Health (NIH-NIAAA #16658 and NIH-NIDA #005010) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
A.C.M., J.L.M., B.L.K. and J.A.J.B. designed the experiments. A.C.M. and J.A.J.B. performed and analyzed behavioral and pharmacological experiments. A.C.M. performed and analyzed [35S]-GTPγS binding experiments. L.P. Pellissier performed in vitro pharmacological experiments. J.A.J.B. performed and analyzed qRT-PCR. D.C. performed and analyzed HPLC dosage of monoamine levels in brain tissue. J.D. performed and analyzed Dil staining and quantification of dendritic spines. A.C.M., J.L.M., B.L.K. and J.A.J.B. interpreted the results. A.C.M., J.L.M., B.L.K. and J.A.J.B. wrote the article. B.L.K. and J.A.J.B. contributed equally to this work. All authors discussed the results and commented on the manuscript.
Disclosure/conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Ghate A, Befort K, Becker JA, Filliol D, Bole-Feysot C, Demebele D, et al. Identification of novel striatal genes by expression profiling in adult mouse brain. Neuroscience. 2007;146:1182–1192. doi: 10.1016/j.neuroscience.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Logue SF, Grauer SM, Paulsen J, Graf R, Taylor N, Sung MA, et al. The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Molecular and cellular neurosciences. 2009;42:438–447. doi: 10.1016/j.mcn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Massart R, Guilloux JP, Mignon V, Sokoloff P, Diaz J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. The European journal of neuroscience. 2009;30:397–414. doi: 10.1111/j.1460-9568.2009.06842.x. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima K, Miyamoto Y, Tsukahara F, Hirai M, Sakaki Y, Ito T. A novel G-protein-coupled receptor gene expressed in striatum. Genomics. 2000;69:314–321. doi: 10.1006/geno.2000.6340. [DOI] [PubMed] [Google Scholar]
- 5.Van Waes V, Tseng KY, Steiner H. GPR88 - a putative signaling molecule predominantly expressed in the striatum: Cellular localization and developmental regulation. Basal ganglia. 2011;1:83–89. doi: 10.1016/j.baga.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, et al. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–2984. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- 7.Bohm C, Newrzella D, Herberger S, Schramm N, Eisenhardt G, Schenk V, et al. Effects of antidepressant treatment on gene expression profile in mouse brain: cell type-specific transcription profiling using laser microdissection and microarray analysis. Journal of neurochemistry. 2006;97(Suppl 1):44–49. doi: 10.1111/j.1471-4159.2006.03750.x. [DOI] [PubMed] [Google Scholar]
- 8.Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Molecular psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 9.Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, et al. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addiction biology. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 10.Jin C, Decker AM, Huang XP, Gilmour BP, Blough BE, Roth BL, et al. Synthesis, pharmacological characterization, and structure-activity relationship studies of small molecular agonists for the orphan GPR88 receptor. ACS chemical neuroscience. 2014;5:576–587. doi: 10.1021/cn500082p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JX, Thorn DA, Jin C. The GPR88 receptor agonist 2-PCCA does not alter the behavioral effects of methamphetamine in rats. European journal of pharmacology. 2013;698:272–277. doi: 10.1016/j.ejphar.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Ingallinesi M, Le Bouil L, Faucon Biguet N, Do Thi A, Mannoury la Cour C, Millan MJ, et al. Local inactivation of Gpr88 in the nucleus accumbens attenuates behavioral deficits elicited by the neonatal administration of phencyclidine in rats. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.92. [DOI] [PubMed] [Google Scholar]
- 13.Quintana A, Sanz E, Wang W, Storey GP, Guler AD, Wanat MJ, et al. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nature neuroscience. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JA, Befort K, Blad C, Filliol D, Ghate A, Dembele D, et al. Transcriptome analysis identifies genes with enriched expression in the mouse central extended amygdala. Neuroscience. 2008;156:950–965. doi: 10.1016/j.neuroscience.2008.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. The Journal of comparative neurology. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 16.Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 18.Dzierba CD, Hartz RA, Bi Y, Ahuja VT, Bronson JJ, Carson K, et al. Modulators of g protein-coupled receptor 88. 2011 Patent number WO2011044225 A1; USA: Google Patents. [Google Scholar]
- 19.Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL. Impaired Hippocampus-Dependent and Facilitated Striatum-Dependent Behaviors in Mice Lacking the Delta Opioid Receptor. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PloS one. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker JA, Clesse D, Spiegelhalter C, Schwab Y, Le Merrer J, Kieffer BL. Autistic-Like Syndrome in Mu Opioid Receptor Null Mice is Relieved by Facilitated mGluR4 Activity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzierba CD, Bi Y, Dasgupta B, Hartz RA, Ahuja V, Cianchetta G, et al. Design, synthesis, and evaluation of phenylglycinols and phenyl amines as agonists of GPR88. Bioorg Med Chem Lett. 2015;25:1448–1452. doi: 10.1016/j.bmcl.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Sager TN, Kirchhoff J, Mork A, Van Beek J, Thirstrup K, Didriksen M, et al. Nest building performance following MPTP toxicity in mice. Behav Brain Res. 2010;208:444–449. doi: 10.1016/j.bbr.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci U S A. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 28.Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 29.Sorregotti T, Mendes-Gomes J, Rico JL, Rodgers RJ, Nunes-de-Souza RL. Ethopharmacological analysis of the open elevated plus-maze in mice. Behav Brain Res. 2013;246:76–85. doi: 10.1016/j.bbr.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 30.Aupperle RL, Paulus MP. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci. 2010;12:517–531. doi: 10.31887/DCNS.2010.12.4/raupperle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nature genetics. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 32.Do J, Kim JI, Bakes J, Lee K, Kaang BK. Functional roles of neurotransmitters and neuromodulators in the dorsal striatum. Learning & memory. 2012;20:21–28. doi: 10.1101/lm.025015.111. [DOI] [PubMed] [Google Scholar]
- 33.Lewis M, Kim SJ. The pathophysiology of restricted repetitive behavior. Journal of neurodevelopmental disorders. 2009;1:114–132. doi: 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauce B, de Brito RA, Peripato AC. Genetic architecture of nest building in mice LG/J × SM/J. Frontiers in genetics. 2012;3:90. doi: 10.3389/fgene.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo C, Renoir T, Hannan AJ. Ethological endophenotypes are altered by elevated stress hormone levels in both Huntington's disease and wildtype mice. Behav Brain Res. 2014;274:118–127. doi: 10.1016/j.bbr.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 36.Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, et al. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barkus C, Dawson LA, Sharp T, Bannerman DM. GluN1 hypomorph mice exhibit wide-ranging behavioral alterations. Genes, brain, and behavior. 2012;11:342–351. doi: 10.1111/j.1601-183X.2012.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox MA, Panessiti MG, Hall FS, Uhl GR, Murphy DL. An evaluation of the serotonin system and perseverative, compulsive, stereotypical, and hyperactive behaviors in dopamine transporter (DAT) knockout mice. Psychopharmacology (Berl) 2013;227:685–695. doi: 10.1007/s00213-013-2988-x. [DOI] [PubMed] [Google Scholar]
- 39.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature neuroscience. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 42.Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism & related disorders. 2007;13(Suppl 3):S251–258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian X, Kai L, Hockberger PE, Wokosin DL, Surmeier DJ. MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Molecular and cellular neurosciences. 2010;44:94–108. doi: 10.1016/j.mcn.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. The EMBO journal. 2012;31:640–653. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon GM, Qu X, Marcus JN, Dodart JC. Excitotoxic lesions restricted to the dorsal CA1 field of the hippocampus impair spatial memory and extinction learning in C57BL/6 mice. Neurobiology of learning and memory. 2008;90:426–433. doi: 10.1016/j.nlm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 47.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learning & memory. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deipolyi AR, Fang S, Palop JJ, Yu GQ, Wang X, Mucke L. Altered navigational strategy use and visuospatial deficits in hAPP transgenic mice. Neurobiology of aging. 2008;29:253–266. doi: 10.1016/j.neurobiolaging.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 50.Kleinknecht KR, Bedenk BT, Kaltwasser SF, Grunecker B, Yen YC, Czisch M, et al. Hippocampus-dependent place learning enables spatial flexibility in C57BL6/N mice. Frontiers in behavioral neuroscience. 2012;6:87. doi: 10.3389/fnbeh.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packard MG. Exhumed from thought: basal ganglia and response learning in the plus-maze. Behav Brain Res. 2009;199:24–31. doi: 10.1016/j.bbr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of learning and memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 53.Middei S, Geracitano R, Caprioli A, Mercuri N, Ammassari-Teule M. Preserved fronto-striatal plasticity and enhanced procedural learning in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe. Learning & memory. 2004;11:447–452. doi: 10.1101/lm.80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroeder JP, Wingard JC, Packard MG. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus. 2002;12:280–284. doi: 10.1002/hipo.10024. [DOI] [PubMed] [Google Scholar]
- 55.Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Leonibus E, Pascucci T, Lopez S, Oliverio A, Amalric M, Mele A. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology (Berl) 2007;194:517–525. doi: 10.1007/s00213-007-0862-4. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson TK, Gruenbaum BF, Markus EJ. Extensive training and hippocampus or striatum lesions: effect on place and response strategies. Physiology & behavior. 2012;105:645–652. doi: 10.1016/j.physbeh.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 58.Wang JH, Ma YY, van den Buuse M. Improved spatial recognition memory in mice lacking adenosine A2A receptors. Experimental neurology. 2006;199:438–445. doi: 10.1016/j.expneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, et al. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learning & memory. 2011;18:459–474. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, et al. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain research. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 61.Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50978. 50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moy SS, Riddick NV, Nikolova VD, Teng BL, Agster KL, Nonneman RJ, et al. Repetitive behavior profile and supersensitivity to amphetamine in the C58/J mouse model of autism. Behav Brain Res. 2014;259:200–214. doi: 10.1016/j.bbr.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158:1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Progress in neurobiology. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Hebb AL, Robertson HA, Denovan-Wright EM. Phosphodiesterase 10A inhibition is associated with locomotor and cognitive deficits and increased anxiety in mice. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008;18:339–363. doi: 10.1016/j.euroneuro.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Luuk H, Plaas M, Raud S, Innos J, Sutt S, Lasner H, et al. Wfs1-deficient mice display impaired behavioural adaptation in stressful environment. Behav Brain Res. 2009;198:334–345. doi: 10.1016/j.bbr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. British journal of pharmacology. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fourla DD, Papakonstantinou MP, Vrana SM, Georgoussi Z. Selective interactions of spinophilin with the C-terminal domains of the delta- and mu-opioid receptors and G proteins differentially modulate opioid receptor signaling. Cellular signalling. 2012;24:2315–2328. doi: 10.1016/j.cellsig.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Georgoussi Z, Leontiadis L, Mazarakou G, Merkouris M, Hyde K, Hamm H. Selective interactions between G protein subunits and RGS4 with the C-terminal domains of the mu- and delta-opioid receptors regulate opioid receptor signaling. Cellular signalling. 2006;18:771–782. doi: 10.1016/j.cellsig.2005.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.