Abstract
Smokers have substantial individual differences in quit success in response to current treatments for nicotine dependence. This observation may suggest that different underlying motivations for continued tobacco use across individuals and nicotine cessation may require different treatments in different individuals. Although most animal models of nicotine dependence emphasize the positive reinforcing effects of nicotine as the major motivational force behind nicotine use, smokers generally report that other consequences of nicotine use, including the ability of nicotine to alleviate negative affective states or cognitive impairments, as reasons for continued smoking. These states could result from nicotine withdrawal, but also may be associated with premorbid differences in affective and/or cognitive function. Effects of nicotine on cognition and affect may alleviate these impairments regardless of their premorbid or postmorbid origin (e.g., before or after the development of nicotine dependence). The ability of nicotine to alleviate these symptoms would thus negatively reinforce behavior, and thus maintain subsequent nicotine use, contributing to the initiation of smoking, the progression to dependence and relapse during quit attempts. The human and animal studies reviewed here support the idea that self-medication for pre-morbid and withdrawal-induced impairments may be more important factors in nicotine addiction and relapse than has been previously appreciated in preclinical research into nicotine dependence. Given the diverse beneficial effects of nicotine under these conditions, individuals might smoke for quite different reasons. This review suggests that inter-individual differences in the diverse effects of nicotine associated with self-medication and negative reinforcement are an important consideration in studies attempting to understand the causes of nicotine addiction, as well as in the development of effective, individualized nicotine cessation treatments.
Keywords: Nicotine, negative affect, cognitive impairment, negative reinforcement
Graphical abstract
Affective and Cognitive impairments, either premorbid in origin, or associated with nicotine withdrawal (as described above) contribute to nicotine dependence. Greater consideration of these processes, and the potential role of negative reinforcement, needs to be incorporated into animal models of nicotine dependence.
Introduction
Although the positively reinforcing effects of nicotine certainly play an important part in nicotine dependence, an accumulating literature implicates a role for negative reinforcement in nicotine dependence as well. Indeed, the most common responses of individuals when asked why they smoke usually involves some effect of nicotine that alleviates some negative state, either of cognition or affect. In many cases these negative states are certainly the result of nicotine dependence and acute withdrawal, but there is substantial evidence that in many cases these states may predate initial nicotine use. This possibility is rather difficult to determine in individual cases because smoking is initiated so early in life, often in early to mid-adolescence. However, the high degree of comorbidity of smoking with psychiatric disorders suggests that at least some portion of this self-medication may be true for premorbid conditions.
Nicotine addiction and dependence has a much higher prevalence in individuals with a psychiatric diagnosis and is associated with higher levels of smoking compared to psychiatrically healthy individuals (Lawrence et al., 2009). This relationship has been noted for numerous psychiatric disorders, including schizophrenia (de Leon et al., 1995), attention deficit hyperactivity disorder (ADHD) (Chen et al., 2012; McClernon and Kollins, 2008; Pomerleau et al., 1995), major depression (Breslau et al., 1991; Chen et al., 2012; Glassman et al., 1988), bipolar disorder (Waxmonsky et al., 2005), anxiety disorders (Breslau et al., 1991; Chen et al., 2012; Dickerson et al., 2009), post-traumatic stress disorder (PTSD) (Beckham et al., 1995; Chen et al., 2012; Dickerson et al., 2009; Koenen et al., 2005; Roberts et al., 2008), antisocial personality disorder (Chen et al., 2012; Dickerson et al., 2009), and obsessive-compulsive disorder (Grabe et al., 2001), as well as addiction to other substances (Chen et al., 2012; Dickerson et al., 2009; Grabe et al., 2001). These associations could be interpreted as support for the premise that psychological and neurobiological attributes that predispose individuals to nicotine addiction also predispose them to these other disorders (Paterson and Markou, 2007). While some nicotine use may reflect self-treatment for pre-existing psychiatric symptoms on the part of these individuals, other nicotine use could involve self-treatment for symptoms that emerge during, or are exacerbated by, nicotine withdrawal (Markou et al., 1998). The ability of nicotine to alleviate stress and anxiety, improve mood and cognition, promote wakefulness, etc., may account for higher rates of smoking in individuals with psychiatric diagnoses, the so-called self-medication hypothesis (Markou et al., 1998).
Self-treatment effects may also contribute to smoking in individuals without a psychiatric diagnosis, but with less extreme, sub-clinical alterations in mood, affect, or cognition. Indeed, a sample of smokers had a much higher lifetime incidence for mood, anxiety, and substance abuse disorders than non-smokers (Keuthen et al., 2000), prompting those authors to conclude that “subsyndromal” psychiatric symptoms may contribute substantially to the risk for nicotine dependence. Consistent with this idea, nicotine use is increased in non-psychotic siblings of individuals with schizophrenia (Smith et al., 2008). Moreover, greater anhedonia severity or cognitive dysfunction in individuals without a current diagnosis of major depression predicts increased risk for relapse to smoking (Cook et al., 2010; Leventhal et al., 2009; Patterson et al., 2010). The diversity of symptoms in these disorders, and the multitude of effects of nicotine, suggest that there is substantial heterogeneity in the underlying reasons for smoking across individuals.
Despite the evidence for self-medication in smokers, the vast majority of preclinical and clinical research into the biological basis of nicotine addiction has emphasized positive reinforcement (see Table 1 for a glossary of terms relevant to this review) as the major determinant of nicotine addiction liability (Glautier, 2004; Watkins et al., 2000a). Theoretical perspectives have been proposed that emphasize negative reinforcement at later stages of the addiction process (Koob, 2013; Koob and Le Moal, 2008; Watkins et al., 2000a). The pattern of psychiatric comorbidities discussed above however, and the early onset of smoking in most individuals, suggest that self-medication and negative reinforcement may also be important at earlier stages in some individuals. This review will discuss such premorbid possibilities, highlighting studies from both a clinical and preclincial modeling perspective.
Table 1.
Glossary
| Term | Definition |
|---|---|
| Positive Reinforcement | The strengthening of a conditioned response by the production of a rewarding, positive, or desirable state or feeling |
| Negative Reinforcement | The strengthening of a conditioned response by the elimination of an aversive, negative, or undesirable state or feeling |
| Precipitated withdrawal | A state that occurs after administration of an antagonist following a chronic period of drug administration (either experimenter administered or self-administered) resulting from the development of tolerance associated with chronic drug exposure. Many effects of precipitated withdrawal are generally in the opposite direction from those produced by the drug |
| Self-administration | Voluntary administration of a drug via oral consumption of drug solutions or direct intravenous injections controlled by operant contingencies (Note: drugs can also be self-administered intracerebrally) |
| Tolerance | Progressive reductions in responses to a drug resulting from neuroadaptations to high levels of drug exposure or drug self-administration; subsequent abstinence is associated with withdrawal |
| Spontaneous withdrawal | A state that occurs after voluntary or enforced cessation of drug administration, either experimenter administered or self-administered, resulting from the development of tolerance associated with chronic drug exposure; many of the effects are generally in the opposite direction from those produced by the drug |
| Incubation | The process by which, after a period of enforced drug abstinence, operant responding for stimuli associated with a reinforcer are progressively enhanced with longer periods of abstinence; does not correspond with the duration of withdrawal |
Premorbid conditions that may predispose individuals to nicotine dependence: Clinical findings
The relationship of psychiatric disorders to nicotine dependence suggests a potential mechanism - or mechanisms given the number of psychiatric conditions that are highly comorbid with smoking - that might underlie addiction liability for nicotine dependence. Understanding these processes may contribute substantially to the treatment of comorbid psychiatric conditions, as well as smoking, as the prognosis is poorer for psychiatric patients with comorbid addictions (Batel, 2000). For instance, individuals with a dual diagnosis of addiction and a psychotic disorder have more severe symptoms (Margolese et al., 2004), which may relate to both their poorer prognosis and greater use of addictive substances if those are taken in part for reasons of self-medication. However, one of the most fundamental questions surrounding these issues is the extent to which these psychiatric conditions are premorbid in origin, or develop after extended nicotine use. A prospective longitudinal study observed that teenage smoking was associated with an increased incidence of a range of psychiatric diagnoses (Sorensen et al., 2011). Of course, although smoking in this study predated the diagnoses of psychiatric disorders, this finding does not necessarily mean that psychiatric symptoms were not present prior to the actual diagnoses. It remains to be seen whether teenage smoking reflected self-medication prior to full onset of psychiatric conditions or if teenage smoking may have accelerated the development of those psychiatric conditions. Again, there need not be a single answer to this question; smoking, or subsequent withdrawal experiences, may exacerbate the development of some conditions but not others. As an indication of this sort of heterogeneity, it has been shown that smoking topography (total puffs, puffs per cigarette, inter-puff intervals and puff volumes) are quite different between equally dependent smokers with, or without, a concurrent diagnosis of schizophrenia (Tidey et al., 2005).
Adolescents with ADHD are more likely to initiate smoking (Hartsough and Lambert, 1987), which may reflect either a predisposition based upon some of the psychological characteristics of ADHD, such as impulsivity, or self-treatment for attentional or cognitive deficits. In either case, ADHD symptoms are associated with the progression from initial smoking experiences to later stages of nicotine addiction and dependence (Fuemmeler et al., 2007). Impairments in delayed discounting are observed in smokers (Doran et al., 2007; Field et al., 2007), which may represent a greater incidence of ADHD-like phenotypes in this population. Both smoking and nicotine agonist administration improve cognition in non-smokers as well, particularly in individuals with cognitive impairments associated with psychiatric and neurological conditions (see (Levin et al., 2006) for review). In a meta-analysis of studies examining the effects of nicotine and smoking in non-smokers and non-abstinent smokers, improvements were shown in fine motor performance, response time, alerting attention, orienting attention, short-term episodic memory and working memory (Heishman et al., 2010). Nicotine can improve attention in non-smokers with ADHD (Levin et al., 2000), which may suggest a reason for increased smoking in ADHD patients.
Smoking also normalizes sensory gating deficits in schizophrenia patients (Adler et al., 1993; George et al., 2006; Kumari et al., 2001). Although some cognitive deficits in schizophrenia patients may result from antipsychotic use itself (Levin et al., 1996), the initiation of smoking in schizophrenia patients appears to precede antipsychotic treatment. Indeed, some cognitive deficits in non-smoking schizophrenia patients can be ameliorated by nicotine (D'Souza and Markou, 2011; Jubelt et al., 2008; Young and Geyer, 2013), consistent with the self-medication hypothesis. It remains to be seen what proportion of smoking is driven by premorbid conditions versus the exacerbation of symptoms during nicotine withdrawal. Notably, poorer cognitive performance during an enforced period of abstinence predicts subsequent resumption of smoking (Patterson et al., 2010), suggesting that nicotine withdrawal-induced exacerbation of symptoms at least partially contributes to nicotine addiction.
Many other conditions may promote smoking behavior in individuals with other psychiatric co-morbidities. A history of major depression is associated with smoking cessation failure (Covey et al., 1993; Glassman et al., 1993; Glassman et al., 1988). This finding is consistent with either the hypothesis that depression and nicotine dependence share common underlying causes, or the self-medication hypothesis. Again however, there is an inter-relationship with smoking history as nicotine withdrawal can provoke a depressive episode in smokers with a dual diagnosis of major depression (Glassman, 1993). Antidepressant treatments can increase success in nicotine cessation (Hall et al., 1998), particularly if individuals have a diagnosis of depression (Hurt et al., 1997). Moreover, this success in quitting smoking has been associated with the ability of antidepressants to reduce negative affect during abstinence (Covey et al., 1997; Hall et al., 1998), or cognitive deficits associated with depression (Miller et al., 1995). Given this evidence, it might be suggested that some negative results of antidepressant trials for nicotine cessation might be the result of not separating individuals on the basis of psychiatric diagnoses (see discussion in (Glassman, 1998)). The specific mechanisms of action of the antidepressants may also be important in their ability to affect nicotine cessation.
A substantial portion of the association between psychiatric conditions and nicotine dependence is accounted for by shared genetic risk, such as the association between PTSD and nicotine dependence (Koenen et al., 2005). However, in a review of the literature on PTSD and nicotine dependence comorbidity it has been suggested that trauma and the development of PTSD precede nicotine dependence (Feldner et al., 2007). Most of the research in this area is retrospective in nature, but, importantly, prospective studies have supported a relationship between PTSD (Breslau et al., 1991) or physical assault (Cisler et al., 2011) and later smoking or nicotine dependence. A variety of other factors may influence these relationships, including sex. For instance, PTSD symptoms were strongly associated with nicotine dependence in male, but not female, subjects from a study on the relationship between depression vulnerability and smoking (Thorndike et al., 2006).
It should be noted that a history of smoking and the development of nicotine dependence might influence the subsequent course of psychiatric illnesses. Major depression and alcoholism are associated with longer periods of nicotine withdrawal in comorbid individuals, and with smoking relapse to alleviate nicotine withdrawal symptoms (Weinberger et al., 2009). Prospective studies are required to support the conflation of nicotine use and psychiatric illness, particularly with regard to specific symptoms (domains of function) that are similarly affected in psychiatric disorders and nicotine dependence. Indeed, even though there may be overall relationships between smoking and psychiatric disorders, smoking may more closely correlate with particular symptoms associated with psychiatric illness. For instance, smoking in patients with major depressive disorder is associated with melancholic features, in particular psychomotor agitation and decreased appetite (Leventhal et al., 2008). As another example, allelic variation in the 15q25 gene cluster, that includes several nicotinic receptor subunit genes, is associated not only with nicotine dependence, schizophrenia, and bipolar disorder, but is specifically associated with the negative symptoms of schizophrenia (Jackson et al., 2013). In PTSD patients, the recall of stressful or traumatic events increases craving, negative affect, and PTSD symptoms (Beckham et al., 2007). In that study, the craving and affective symptoms were greater in PTSD patients who smoked and were reduced by smoking either nicotinized or denicotinized cigarettes, although another study found greater effects of nicotine compared to placebo (Buckley et al., 2007). Thus, smoking and nicotine dependence may be more closely associated with particular symptoms of psychiatric disorders than with psychiatric diagnoses per se.
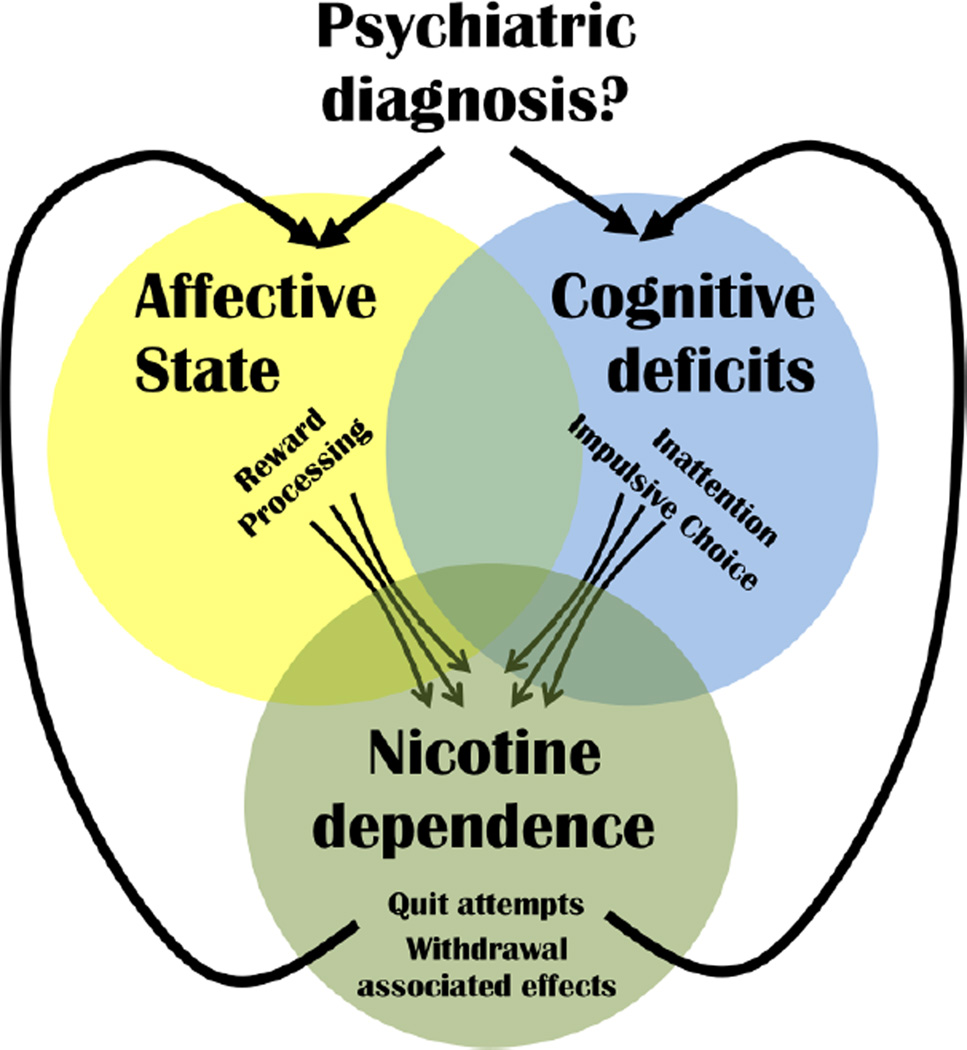
The ability to control the history of nicotine exposure in animal models might help unravel the relationship between nicotine dependence and other co-morbid psychiatric conditions. Specifically, such models could examine whether similar underlying mechanisms predispose individuals to both dependence and affected domains of function, or whether premorbid phenotypes or withdrawal-associated phenotypes lead to self-treatment with nicotine. The potential relationships between these factors are depicted in Figure 1.
Figure 1. Possible contribution to nicotine dependence and continued use.
Although a psychiatric diagnosis is not a pre-requisite for nicotine dependence, such a diagnosis greatly increases the likelihood of dependence. Natural tendencies (sub-clinical behaviors) tending toward a) negative affective states, e.g., lower reward processing capabilities, or b) cognitive deficits, e.g., inattention or impulsivity, could contribute toward initial nicotine use given nicotine-induced improvements in these behaviors in non-smokers. Once dependent however, the more pronounced negative reinforcement resulting from alleviation of nicotine withdrawal (exacerbating natural affective or cognitive impairments) likely strongly contributes to failed quite attempts (relapse). The mechanism(s) underlying the acquisition of nicotine dependence based upon positive reinforcement of nicotine intake likely differ from those underlying withdrawal-induced impact on affect or cognition (negative reinforcement of nicotine intake), suggesting that treatments to prevent relapse should be developed to counter negative reinforcement mechanisms. Withdrawal-induced effects on specific affective or cognitive domains often vary by individual and treatments developed to enhance quit attempts could be tailored to those domains.
Consequences of nicotine withdrawal that may negatively reinforce nicotine dependence
Nicotine withdrawal produces a number of undesirable effects, including changes in mood, cognition, and weight gain. Human self-reports and correlational studies commonly suggest that relapse to smoking is often brought about by these effects (Patterson et al., 2010) (Fig. 2). One of the unanswered questions in smoking cessation research is the extent to which these deficits exist prior to smoking, as is presumably the case for nicotine dependent individuals with comorbid psychiatric conditions, or are purely the result of withdrawal. Of course, it is quite likely that there is substantial individual heterogeneity in the causative factors of nicotine dependence and that in some individuals the states may be pre-morbid in origin, while in others, they result from nicotine dependence and occur during periods of nicotine withdrawal and attempted abstinence.
Figure 2. Theoretical model of the opponent-process theory of smoking and withdrawal-induced relapse.
Initiation of smoking likely produces cognitive benefits which dissipate over time with chronic use. Upon withdrawal, cognitive performance drops. For some, performance may not drop too far and eventually their performance normalizes again before a relapse incident (blue). In some individuals, cognitive performance may drop to intolerable levels and so they relapse and start smoking again (relapse, shown in green). Developing a treatment that could predate nicotine withdrawal however, to minimize withdrawal-induced deficits in cognition, would greatly enhance the likelihood of successfully quitting (red).
Nicotine withdrawal has been shown to produce a variety of cognitive impairments in humans that might lead to smoking as self-medication. Nicotine withdrawal produces deficits in attention in the Stroop task (Gross et al., 1993), attention and reaction time in a go-no go task (Hughes et al., 1989; Keenan et al., 1989), verbal working memory (Sweet et al., 2010) and spatial working memory (Carlson et al., 2009). There is a substantial literature that suggests that smoking alleviates such withdrawal-induced cognitive deficits (see (Heishman et al., 1994) for review). Smoking, or nicotine administration (transdermally), improves performance on a variety of attentional, mnemonic and cognitive tasks in which smokers show deficits (Atzori et al., 2008; Kollins et al., 2009; Myers et al., 2008; Snyder and Henningfield, 1989). Withdrawal-induced increases in response inhibition errors are positively correlated with baseline plasma cotinine and boredom susceptibility in smokers (Pettiford et al., 2007). Although nicotine improves cognition in both smokers and nonsmokers (Froeliger et al., 2009), greater impairments in abstinent smokers, combined with experience with these effects, may lead to self-medication. Indeed, working memory deficits predict smoking resumption after short-term abstinence (Patterson et al., 2010).
Sensitivity to stress and anxiety may also influence smoking, as is obvious from the relationship of nicotine dependence with PTSD and anxiety disorders, but smoking may be driven by acute stress and anxiety as well. Individuals may learn to self-treat for acute stress and anxiety, and individuals that experience more stress and anxiety may be more likely to form these associations. Certainly, subjective self-reports of “stress-reduction” from smoking, and exacerbation of stress during nicotine withdrawal, are quite common. Indeed, stress is often given as a reason for failure of abstinence. Stress increases motivation for nicotine in abstinent smokers (Colamussi et al., 2007) and even nicotine-associated stimuli can alleviate stress (Levin et al., 1991). Withdrawal-induced changes in mood, including dysphoria and irritability, are commonly reported. Even in individuals without a prior history of depression, which could indicate self-medication in the development of nicotine dependence, withdrawal-induced dysphoria can be ameliorated by nicotine (Myers et al., 2008). This finding might indicate that such withdrawal-induced dysphoria may be important at later stages of the addictive process in maintaining nicotine dependence.
Nicotine withdrawal produces a number of effects that may negatively reinforce subsequent nicotine self-administration, or nicotine withdrawal may exacerbate pre-existing impairments, which are then ameliorated by nicotine self-administration resulting in negative reinforcement. Such impairments include several aspects of attention and cognition (George et al., 2002; Hatsukami et al., 1989), which can be ameliorated by nicotine or nicotinic receptor agonists (Loughead et al., 2010; Sacco et al., 2005). Attenuating these deficits is an important consideration for nicotine cessation strategies as smokers that experience greater cognitive impairments during withdrawal have an increased likelihood of relapse during smoking cessation trials (Patterson et al., 2010).
It may be interesting to consider the phenomenon of “incubation” with regard to potential negative-reinforcement mechanisms that might underlie drug-seeking behavior in abstinent smokers (relapse). Incubation refers to the phenomenon whereby the sensitivity to stimuli that lead to reinstatement of drug-seeking behavior during this period of forced abstinence becomes progressively more potent over longer periods of abstinence. The level and persistence of this responding suggests that it is not simply spontaneous reinstatement. Incubation is a phenomenon that has recently been described in animal models of reinstatement of drug self-administration (for review see (Pickens et al., 2011)), and this phenomenon has been observed for nicotine (Abdolahi et al., 2010). A similar phenomenon was originally described in humans (Gawin and Kleber, 1986), and cue-induced craving in abstinent smokers has been suggested to show “incubation”-like properties (Bedi et al., 2011). Reinstatement of drug-seeking behavior after a forced period of abstinence (and withdrawal) has been used for some time to model relapse in humans. The fundamental psychological nature of the phenomenon remains uncertain, although one might be tempted to speculate that rumination may play a part in incubation, as it has been suggested to do so for PTSD (Heron-Delaney et al., 2013). Reconsolidation of memories of drug-associated cues has been suggested to be an important part of the process (Lee et al., 2006). Incubation occurs for conditioned aversive responses as well (Pickens et al., 2010), which may model similar processes that occur in PTSD. Consequently, incubation may have importance for nicotine cessation treatments as relapse can occur after prolonged periods of abstinence, even after initial success of nicotine cessation treatments. Rates of relapse in nicotine cessation trials are notoriously high (for review, see (Velicer et al., 1992)).
Premorbid conditions that may predispose individuals to nicotine dependence: Animal models
Although the preceding discussion has emphasized pathological states in comorbid conditions that may increase the likelihood of nicotine dependence, it is likely that variation in a number of traits, whether associated with psychiatric diagnoses or not, may contribute to smoking. These traits include elevated levels of anxiety and stress sensitivity, reduced hedonic tone, impulsivity, attentional impairment, and baseline impairments in learning and memory functions. These dysfunctions may represent the ends of the normal distribution of these phenotypes, situational responses to circumstances or sub-clinical conditions. In any case, nicotine can improve all of these functions, which is likely to lead to self-medication with nicotine.
Some smokers consistently indicate that nicotine is “stress-reducing” and that they smoke in order to benefit from these effects. Although these reports are quite common, there is not very much evidence that nicotine induces stress-relief or has anxiolytic effects in animal models. Perhaps the absence of such data is because the anxiolytic effects of nicotine in rodents, like many of its other effects, occur over very narrow dose ranges, so that such effects are only observed at quite low doses (Adriani et al., 2004; Balerio et al., 2005, 2006), while higher doses produce anxiogenic effects (Balerio et al., 2005, 2006). Nicotinic agonists with more selective nicotinic subunit profiles can also exert anxiolytic effects (Decker et al., 1994; Feuerbach et al., 2009). As the anxiolytic and anxiogenic effects of nicotine appear to be mediated by different receptor subtypes, it seems likely that agonists that are more specific might have anxiolytic effects over broader dose ranges than nicotine. It may also be the case, as it is for other effects of nicotine, that anxiolytic or stress-alleviating effects of nicotine are only observed in certain individuals that have higher levels of anxiety or stress-sensitivity, or individuals that are predisposed to more robust effects of nicotine. Additionally, the anxiolytic effects of nicotine may only be apparent under certain conditions. For example, although nicotine by itself did not have anxiolytic effects in one study, it did reverse the anxiogenic effects of caffeine (Kayir and Uzbay, 2006). Such effects might explain the common combinations of smoking and consumption of caffeinated beverages. Both genetic differences and sex may also contribute to the interaction of stress and nicotine consumption. Mice that over-express the R isoform of acetylcholinesterase have increased anxiety that is normalized by chronic forced nicotine consumption (Salas and De Biasi, 2008). Female, but not male, mice were more anxious in the elevated plus maze after chronic nicotine consumption (Caldarone et al., 2008).
Chronic nicotine treatments also reverse cognitive and affective deficits produced by chronic mild stress (Andreasen et al., 2011). Chronic stress increases the expression of α7 nicotinic receptor mRNA in the hippocampus (Hunter et al., 2010). As discussed below, this nicotinic receptor subunit has been linked to some of the cognitive effects of nicotine. Premorbid sensitivity to stress might be hypothesized to affect several different aspects of the addictive process. However, in mice selected for differential stress sensitivity, initial nicotine self-administration did not differ, but reinstatement of nicotine self-administration induced by a stressor was observed in high-stress mice but not low-stress mice (Bilkei-Gorzo et al., 2008). Such individual differences in animals may model the sort of individual differences observed in humans, highlighting the contribution of a particular domain to stress-induced 'relapse', reflecting the heterogeneity of mechanisms involved in quit-attempt failures. Individually tailoring treatment approaches to these underlying causes of quit-attempt failures should improve nicotine cessation treatment.
There is evidence that the anxiogenic and anxiolytic effects of nicotine depend on different nicotine receptor subtypes. Anxiogenic effects of nicotine are partially mediated by β3 and β4 nicotinic-containing receptors (Booker et al., 2007; Salas et al., 2003). Interestingly, genetic deletion of the α7 nicotinic receptor subunit does not affect anxiety (Salas et al., 2007), nor does it have effects on punishment-related/contextual learning (Davis and Gould, 2006, 2007b; Davis et al., 2007; Wehner et al., 2004; Young et al., 2011). The α7 subunit containing nicotinic receptors may mediate other cognitive effects of nicotine; e.g., there is evidence that they may affect working memory (Levin et al., 2009) and they are required for normal positive reward associative learning (Young et al, 2011) and attention (Young et al, 2004; Hoyle et al, 2006). The effects of nicotine may also be more stress relieving than anxiolytic, as nicotine reduces stress-induced hyperthermia (Vinkers et al., 2009).
Nicotine improves memory and cognition, although these effects are highly task-dependent and, in some cases, are more likely to be observed when animals have pre-existing (or treatment-induced) impairments. Nicotine administration during acquisition of contextual fear conditioning enhances learning, while nicotine administration only during expression of contextual fear conditioning does not enhance performance (Davis and Gould, 2006; Davis et al., 2005; Davis et al., 2007; Davis et al., 2006; Gould, 2003; Gould and Higgins, 2003; Gould and Lommock, 2003; Gould and Wehner, 1999; Gulick and Gould, 2008a; Raybuck and Gould, 2007; Wehner et al., 2004). These effects of nicotine are not observed for delay cued fear conditioning (Davis and Gould, 2006; Davis et al., 2005; Davis et al., 2007; Davis et al., 2006; Gould and Wehner, 1999; Gulick and Gould, 2008a; Raybuck and Gould, 2007; Wehner et al., 2004). Interestingly, nicotine does enhance trace cued fear conditioning (Gould et al., 2004; Raybuck and Gould, 2009), which, like contextual conditioning, but not delay cued fear conditioning, is hippocampal dependent (McEchron et al., 1998). In many of these studies nicotine was given during acquisition and retrieval, but the discriminative stimulus properties of nicotine, or other state-dependent effects, are not thought to be important in producing these learning effects (Gould, 2003). Indeed, when assessed at a later time-point, and without nicotine administration, contextual conditioning was still enhanced (Gould and Higgins, 2003). Furthermore, direct dorsal hippocampus infusion at training alone was sufficient to enhance learning (Kenney et al., 2012). That these effects are hippocampal in origin has been demonstrated by direct injections of nicotine into the dorsal hippocampus (Davis et al., 2007; Gulick and Gould, 2009), although other structures, such as the medial prefrontal cortex, are also involved (Raybuck and Gould, 2010). The dorsal hippocampus is also involved in the reversal of ethanol-induced learning impairments by nicotine (Rezayof et al., 2010). Another study found that direct nicotine infusion into the cingulate cortex, but not the hippocampus, reversed ethanol-induced learning deficits (Gulick and Gould, 2009). Contextual learning in these paradigms actually involves multiple processes, including learning about the context and associating the context with a particular internal state. These processes may be separated in the context pre-exposure facilitation effect procedure, which indicates that nicotine enhances contextual learning, but not associative learning (Kenney and Gould, 2008b).
Nicotine improves learning in a variety of other learning tasks as well, including aversively motivated discrimination learning (Bovet-Nitti, 1969; Castellano, 1976), passive avoidance (Bovet et al., 1966; Ciamei et al., 2001), inhibitory avoidance (Brioni and Arneric, 1993), social recognition (Feuerbach et al., 2009), transfer of aversive conditioning (Oliverio, 1968) and open arm avoidance learning in the plus maze (Biala and Kruk, 2008). Nicotine also improves learning deficits induced by certain drugs or aging (Bontempi et al., 2003; Gulick and Gould, 2010; Meguro et al., 1994; Mizoguchi et al., 2011). Subtype-specific nicotinic agonists improve aversive conditioning, inhibitory avoidance, social recognition memory, object recognition and working memory, either under baseline conditions or by reversing impairments induced by other conditions or treatments (Andre et al., 2011; Azizbeigi et al., 2011; Boess et al., 2007; Bontempi et al., 2003; Decker et al., 1994; Feuerbach et al., 2009; Gatto et al., 2004; Gulick and Gould, 2008b; Obinu et al., 2002; Rueter et al., 2004; Rushforth et al., 2010; Rushforth et al., 2011). Given the variety of learning and memory tasks in which nicotine has been shown to have beneficial effects, it is not surprising that many effects of nicotine are not hippocampally mediated. Nicotine injected into the anterior cingulate cortex reverses ethanol-induced learning impairments for several tasks (Gulick and Gould, 2009, 2011). Given the ability of ethanol to impair learning, it is perhaps not surprising that ethanol and nicotine are often taken together, although there are certainly other effects of both drugs, and characteristics of alcohol and nicotine dependent individuals, that might contribute to co-administration of these drugs.
One of the fundamental questions about the effects of nicotine in these studies is whether nicotine affects a specific part of the mnemonic process, or perhaps some other aspect of cognitive function that influences memory acquisition, retention or retrieval. It is also possible that nicotine exerts effects on multiple aspects of cognition that may vary by cognitive process, and there is evidence to suggest that this is the case. Many of the studies discussed above suggest that nicotine improves memory acquisition. Nicotine has a relatively short duration of action, so it might be thought unlikely that injections prior to learning experiences influence memory consolidation. However, nicotine does improve memory consolidation under some circumstances (Castellano, 1976; Oliverio, 1968), but not others (Gould and Higgins, 2003). Nicotine also has effects on memory retention (Valzelli et al., 1986), albeit only in mice that were poor learners to begin with, as well as retrieval under some conditions (Zarrindast et al., 1996). The effects on memory retrieval may relate to some of the effects of nicotine on acquisition; improved retrieval of memories of previous trials might accelerate the rate of learning during acquisition. However, this may vary across learning paradigms that engage different neural substrates because nicotine can enhance one trial learning (Gould et al., 2004).
There is a specific pharmacology to the effects of nicotine on learning, which has influenced efforts to develop nootropic drugs. Evidence suggests the involvement of α7 and β2 nicotinic receptor subunits in the effects of nicotine on spatial and contextual learning ((Davis and Gould, 2009; Levin et al., 2009; Raybuck and Gould, 2009; Ren et al., 2007; Wehner et al., 2004) but see also (Paylor et al., 1998)), as well as some other types of learning (Picciotto et al., 1995). The involvement of the nicotinic acetylcholine receptor subunits may vary with cognitive task as β2 but not α7 subunits appear to be involved in the effects of nicotine on contextual learning, while both subunits appear to be involved in the effects of nicotine on spatial working memory.
In most of the studies discussed above, behavioral changes are observed in the absence of any initial impairment. Observations in dopamine transporter (DAT) knockout mice are perhaps of most relevance to the question of whether premorbid impairments in learning lead to self-medication with nicotine. In the Morris Water Maze, learning impairments were ameliorated by chronic nicotine treatment (Weiss et al., 2007a). This observation is of special interest as DAT knockout mice have been suggested to model aspects of attentional impairments in schizophrenia and ADHD (Arime et al., 2012; Yamashita et al., 2006), that can be reversed by acute nicotine administration (Uchiumi et al., 2013). DAT knockout mice are also more sensitive to the hypolocomotor effects of nicotine and less sensitive to the anxiogenic effects of nicotine (Weiss et al., 2007a). These behavioral changes are accompanied by regional and sub-type specific changes in nicotine receptor density (Weiss et al., 2007b).
Improvements in learning produced by nicotine may involve aspects of cognitive function other than memory processes, such as attentional function (see (Levin et al., 2006) for review). Visuospatial attention has been studied in rodents using the 5-choice serial reaction time task (5CSRTT; (Carli et al., 1983; Humby et al., 1999)). Attentional load may be altered in this task several ways, such as decreasing the stimulus intensity or duration, adding a distracting stimulus or decreasing the predictability of the stimulus (de Bruin et al., 2006). Nicotine improves attentional performance in the 5CSRTT, as judged by increases in accuracy and decreases in omission errors (Hahn et al., 2002; Stolerman et al., 2000; Young et al., 2004), although this is not always observed (Hoyle et al., 2006), and chronic nicotine treatment may be necessary to produce attentional improvements under some circumstances (Pattij et al., 2007). Furthermore, nicotine improves accuracy and reduces omissions in the presence of a distracting stimulus (Hahn et al., 2002) or under other conditions that degrade performance (Stolerman et al., 2000). These effects involve nicotine actions in the prelimbic region of the prefrontal cortex, but not the dorsal hippocampus, based on experiments using localized intracerebral nicotine injections (Hahn et al., 2003b). Other nicotinic receptor agonists also improve performance in the 5CSRTT, although notably, not a selective α7 nicotinic receptor agonist (Grottick et al., 2003; Hahn et al., 2003a). It must be noted, however, that the effects of nicotine on omissions in this task are reversed by a selective α7 nicotinic receptor antagonist (Hahn et al., 2011). Impairments of choice accuracy in the 5CSRTT are observed in α5 nicotinic receptor subunit knockout mice (Bailey et al., 2010) and α7 nicotinic receptor subunit knockout mice (Hoyle et al., 2006). The effects of nicotine were assessed in both studies. Bailey et al. (2010) assessed only one dose of nicotine, which did not improve performance in the knockout mice, and produced slight impairments in wild-type mice. Hoyle et al. (2006) assessed a wide range of nicotine doses, but did so under conditions that equalized performance, at high levels, between knockout and wild-type mice. The differences between these studies may result in part from differences in baseline performance; that is, the effects of nicotine may only be apparent when performance is poor. Supporting this view, nicotine improves attentional performance in the 5CSRTT at intermediate doses under conditions that produce suboptimal performance (de Bruin et al., 2006).
Classical attention/vigilance tests in humans are based on signal detection theory. These tests involve separating attention to signal from noise, and as such require that on some trials there are non-target signals. This requirement is one shortcoming of the 5CSRTT, which led to subsequent modifications of the task to incorporate non-target trials, resulting in the 5-choice continuous performance test (5CCPT) (Young et al., 2009). Nicotine and the selective α4β2 nicotinic receptor agonist ABT-418, but not the α7 nicotinic receptor agonist PNU 282987, improved performance under baseline conditions in the 5CCPT, and ameliorated impairments induced by scopolamine (Young et al., 2013). Importantly, because of the presence of non-target trials, it became clear that nicotine improved attentional performance by increasing attention to target signals without simply increasing overall responding as no effect was observed on non-target signals. Although there has been some emphasis on selective α7 nicotinic receptor agonists in the development of pro-cognitive therapeutics (Wallace and Bertrand, 2013; Wallace and Porter, 2011), these effects appear to be restricted to limited cognitive domains. Other pro-cognitive effects of nicotine appear to be mediated by other receptor subtypes. Thus, in an assessment of a range of cognitive functions, α7 nicotinic receptor subunit knockout mice were found to have impaired procedural learning, but no deficits in attentional set-shifting, reversal learning, span capacity, aversive-motivated learning, short-term memory or motivation (Young et al., 2011). Although no deficits were observed in attentional set shifting in α7 nicotinic receptor subunit knockout mice, nicotine has been shown to improve attentional set shifting, in terms of both intradimensional and extradimensional shifts in the Attentional Set Shifting Task (Allison and Shoaib, 2013). This task is analogous to the Wisconsin Card Sorting Task. Impairments in this task are found in schizophrenia (Dolan et al., 2004; Yip et al., 2009), and these impairments are associated with failure in nicotine cessation (Dolan et al., 2004).
Deficits in sensorimotor integration are known to exist in schizophrenia, as well as other frontostriatal disorders (Braff et al., 2001; Kohl et al., 2013). Differences in hippocampal mediated gating of novel auditory stimuli across mouse strains appear to be mediated by α7 nicotinic receptors (Mexal et al., 2007; Stevens et al., 1996), and this function in poorer performing strains is improved by nicotine (Stevens et al., 1996). A selective α7 nicotinic receptor agonist also improves this function in DBA/2 mice that show reduced auditory gating (Feuerbach et al., 2009; Stevens et al., 1998). Nicotine also enhances prepulse inhibition in mice (Gould et al., 2005), but does so over a rather narrow dose range (Rollema et al., 2009), as did varenicline in that study. As noted above, deficits in prepulse inhibition are observed in DAT knockout mice (Ralph et al., 2001; Yamashita et al., 2006), and these deficits can be improved by acute nicotine treatment (Uchiumi et al., 2013). Although α7 nicotinic receptor knockout mice do not have deficits in prepulse inhibition (Paylor et al., 1998; Young et al., 2011), the effects of nicotine in DAT KO mice can be reversed by the α7 nicotinic receptor antagonist methyllycaconitine (Uchiumi et al., 2013), suggesting that this nicotinic receptor subunit may mediate these effects of nicotine.
Nicotine self-administration in the context of premorbid conditions that may motivate self-medication
The research discussed above identifies a variety of effects of nicotine that might lead to self-medication. However, little research has been done that might demonstrate self-medication in animal models. Indeed, research into factors that may contribute to the development of nicotine dependence has focused primarily upon factors that might influence the positively reinforcing effects of nicotine. This work has primarily examined home-cage two-bottle oral consumption or intravenous operant self-administration procedures. Both methods have been used in rats and mice to examine traits that may contribute to increased consumption/self-administration of nicotine, or the change in consumption/self-administration over time (for review see (Caille et al., 2012; Hall et al., 2012)). This work has examined both genetic and environmental contributions to nicotine dependence, but little work has focused on any circumstance in which subjects might self-medicate – or at least in which it can be demonstrated that their nicotine intake is for that purpose and has those effects.
Potential genetic contributions to nicotine dependence have been examined primarily in genetically modified mice, genetic techniques being only recently available in rats. Nicotine has a particularly short half-life in mice (Petersen et al., 1984), so that higher nicotine doses, and more frequent administration, is required to reach plasma levels that are typically observed in rats (Marks et al., 2004). The short half-life of nicotine, and the notoriously narrow dose ranges for the effects of nicotine in CPP and operant self-administration procedures make such studies quite difficult, with effective dose ranges varying substantially (Blokhina et al., 2005; Contet et al., 2010; Martellotta et al., 1995; Paterson et al., 2003; Rasmussen and Swedberg, 1998). Key parameters that facilitate nicotine self-administration in mice include prior training for food reward, initial exposure to low i.v. nicotine doses, a slower rate of drug delivery, priming infusions, testing in darkness, and testing at consistent times each day (Fowler and Kenny, 2011). Under these conditions mice self-administer nicotine on more demanding schedules, over a broader range of doses, more robustly (comparing the active and inactive levers), and switch responding between levers if the contingencies were changed (see also discussion in (Galeote et al., 2009)).
Based on findings in a number of genetically modified mice, it has been suggested that an important determinant of addictive potential are the negative effects of drugs of abuse associated with the descending limb of dose-response curves (Uhl et al., 2002). This determinant may be particularly relevant to nicotine, so that tolerance or innate tolerance to these effects may have large effects on the net reinforcing effects of nicotine and the range of doses that will produce positive reinforcement. For instance, in the case of α5 nicotinic receptor subunit knockout mice, there is a substantial widening of the effective dose range for nicotine self-administration because of reduced aversive effects of high nicotine (Fowler et al., 2011). Medial habenular α5 nicotinic receptors were shown to mediate these effects based on viral-mediated gene rescue of the wild-type phenotype by medial habenular expression of α5 nicotinic receptors in knockout mice.
Although self-administration procedures model drug-seeking behavior in a somewhat obvious manner, these procedures do not necessarily model drug dependence very well, including several key features of diagnostic criteria for nicotine dependence. In recent years, focus in some studies has turned to such features. While high reactivity to novelty predicts initiation of cocaine intake in rats (Belin et al., 2008), impulsivity predicts escalated rates of cocaine intake (Belin et al., 2008; Dalley et al., 2007). Importantly, impulsivity predicts features of drug taking in self-administration procedures that model dependence that include: (1) persistence of drug taking in the face of negative consequences, (2) increased motivation for drug-seeking, and (3) inability to refrain from drug-seeking behavior (Belin et al., 2008). These features were operationally defined as (1) operant responding for cocaine during a concurrent punishment schedule, (2) a progressive ratio breakpoint, and (3) responses during an extinction session, respectively. Negative reinforcement may influence all of these factors by providing a very strong incentive for drug seeking behavior that may overcome negative consequences and may actually be exacerbated during extinction (e.g., abstinence or withdrawal).
Given the context of the present review, it will be important to incorporate examination of affective and cognitive phenotypes prior to, during, and after extended periods of nicotine self-administration. The question to be answered by such studies is whether premorbid impairments predict subsequent nicotine self-administration, or whether effects that emerge after long periods of nicotine self-administration, the development of tolerance and experience with withdrawal lead to greater nicotine self-administration. Several instances of this type have been discussed in this review, but experiments of this type remain quite rare.
Animal models of nicotine dependence: nicotine withdrawal
The findings discussed above address the effects of nicotine in non-dependent animals. Many effects of nicotine are observable in naïve animals and, as is apparent in at least some cases, the effects of nicotine may be greater in subjects that have baseline deficits in affective or cognitive function. This circumstance is certainly less well-studied than deficits in affective or cognitive function that result from nicotine withdrawal.
Animal models of nicotine dependence: affective effects of nicotine withdrawal
Several approaches have been taken to examine the basis of negative affective states produced by nicotine withdrawal (see Table 2 for a summary of the studies discussed here, including the approaches used to induce nicotine tolerance/withdrawal). Animal models of withdrawal-induced affective effects have focused primarily upon aversive components of withdrawal. For example, mice will avoid a place that has been paired with mecamylamine-induced withdrawal (Jackson et al., 2009a). It is not possible to specify the nature of the affective state involved using the conditioned place aversion paradigm, but several likely candidates exist, as discussed below. Another study found that although mecamylamine-induced aversions in rats after chronic treatments with saline or nicotine, dihydro-β-erythroidine induced selective place aversions after chronic nicotine treatment (Watkins et al., 2000b). In any case, the negative affective states underlying conditioned place aversions could include withdrawal-induced anxiety and/or dysphoria, both of which have been demonstrated in other paradigms (see below).
Table 2.
Methods used to induce dependence/withdrawal in rodent models of nicotine dependence
| Article | Species | Method | Type of Withdrawal (or Tolerance) | Dose/Treatment/Regimen |
|---|---|---|---|---|
| Marks and Collins (1985) | Mouse | Continuous Intravenous Infusion | Tolerance | 1 increasing to 8 mg/kg/hr NB over 7–10 days, continued for 10 more days |
| Marks et al. (1986a) | Mouse | Continuous Intravenous Infusion | Tolerance | 1 increasing to 3 mg/kg/hr NB over 7–10 days, continued for 10 more days |
| Marks et al. (1986b) | Mouse | Continuous Intravenous Infusion | Tolerance | 1 increasing to 2/4/6 mg/kg/hr NB over 7–10 days, continued for 10 more days |
| Epping-Jordan et al. (1998) | Rat | Osmotic Minipump | Spontaneous Precipitated: DHβE | 1 wk, 3.16 mg/kg/day NB |
| Watkins et al. (2000b) | Rat | Osmotic Minipump | Precipitated: DHβE, MEC, CHLOR | 6–24 days, 3.16 mg/kg/day NB |
| Castane et al. (2002) | Mouse | Osmotic Minipump | Precipitated: MEC | 6 days, 10 mg/kg/day NT |
| Damaj et al. (2003) | Mouse | Osmotic Minipump | Spontaneous Precipitated: DHβE, MEC, MLA, HEX | 2/4/8 wk, 6–48 mg/kg/day NT |
| Semenova and Markou (2003) | Rat | Osmotic Minipump | Tolerance Spontaneous | 1 wk, 3.16 mg/kg/day NB |
| Biala and Weglinska (2005) | Mouse | Repeated Injection | Precipitated: MEC | 1 wk, 4× daily, 2.5 mg/kg SC NT |
| Berrendero et al. (2005) | Mouse | Osmotic Minipump | Precipitated: MEC | 6 days, 25 mg/kg/day NT |
| Davis et al. (2005) | Mouse | Osmotic Minipump | Spontaneous | 12/14 days, 6.3 mg/kg/day NB |
| Kenny and Markou (2005) | Rat | Osmotic Minipump | Precipitated: DHβE | 7–11 days, 3.16 mg/kg/day NB |
| Naylor et al. (2005) | Mouse | Subcutaneous | Tolerance | 4/8 wk, 1× daily, 1.2 mg/kg NT |
| Shoaib and Bizarro (2005) | Rat | Osmotic Minipump | Spontaneous Precipitated: DHβE, MLA | 1 wk, 3.16 mg/kg/day NB |
| LeSage et al. (2006) | Rat | Osmotic Minipump | Spontaneous | 9 days, 3.2/8 mg/kg/day NB |
| McCallum et al. (2006) | Mouse | Continuous Intravenous Infusion | Tolerance | 1/2/4 mg/kg/hr NB over 10–13 days |
| Davis and Gould (2007a) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3 mg/kg/day NB |
| George et al. (2007) | Rat | Osmotic Minipump Intravenous Self-administration | Precipitated: MEC Spontaneous | 2 wk, 3.16 mg/kg/day NB After acquisition of nicotine self-administration and 2–6 wks of maintenance (0.03 mg/kg/injection) rats subjected to repeated periods of forced abstinence |
| Portugal and Gould (2007) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3 mg/kg/day NB |
| Salas et al. (2007) | Mouse | Intraperitoneal Osmotic Minipump | Tolerance Precipitated: MEC, MLA | 3 days, 3× daily, 0.5 mg/kg NB 13 days, 24 mg/kg/day NB |
| Semenova et al. (2007) | Rat | Osmotic Minipump | Spontaneous Precipitated: MEC | 2 wk, 6.31 mg/kg/day NB |
| Andre et al. (2008) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3/12.6 mg/kg/day NB |
| Johnson et al. (2008) | Mouse | Repeated Injection Osmotic Minipump | Spontaneous Spontaneous Precipitated: MEC | 1 wk, 4× daily, 2 mg/kg SC NB 13 days, 24 mg/kg/day NB 8–10 days, 24 mg/kg/day NB |
| Jonkman et al. (2008) | Rat | Osmotic Minipump | Spontaneous Precipitated: DHβE, MEC | 4 wk, 3.16 mg/kg/day NB |
| Manhaes et al. (2008) | Mouse | Oral, Forced Oral, Voluntary | Spontaneous | 50 µg/mL NB |
| Portugal et al. (2008) | Mouse | Osmotic Minipump | Spontaneous Precipitated: DHβE | 12 days, 6.3 mg/kg/day NB |
| Raybuck et al. (2008) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3 mg/kg/day NB |
| Stoker et al. (2008) | Mouse | Osmotic Minipump | Spontaneous Precipitated: DHβE, MEC | 2/4 wk, 10–40 mg/kg/day NB |
| Davis and Gould (2009) | Mouse | Osmotic Minipump IC via Osmotic Minipump | Precipitated: IC DHβE Spontaneous | 12 days, 6.3 mg/kg/day NB 12–14 days, 0.175 µg/side/hr NB |
| Jackson et al. (2009a) | Mouse | Osmotic Minipump | Precipitated: MEC, DHβE, MLA | 2/4 wk, 36 mg/kg/day NB |
| Jackson et al. (2009b) | Mouse | Osmotic Minipump | Spontaneous Precipitated: α-conotoxin H9A;L15A | 2/4 wk, 36 mg/kg/day NB |
| Portugal and Gould (2009) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3 mg/kg/day NB |
| Raybuck and Gould (2009) | Mouse | Osmotic Minipump | Spontaneous Precipitated: DHβE, MLA | 12 days, 6.3 mg/kg/day NB |
| Jackson et al. (2010) | Mouse | Osmotic Minipump | Tolerance Spontaneous Precipitated: MEC | 1/2/4 wk, 36 mg/kg/day NB |
| Grieder et al. (2010) | Mouse Rat | Osmotic Minipump Osmotic Minipump | Spontaneous | 13 days, 1.4/7 mg/kg/day NB 7 days, 1/3.15 mg/kg/day NB |
| Scott and Hiroi (2010) | Mouse | Oral, Forced | Precipitated: MEC | 12 days, 200 μg/mL NB |
| Der-Avakian and Markou (2010) | Rat | Osmotic Minipump | Spontaneous | 4 wk, 9 mg/kg/day NT |
| Hayase (2011) | Mouse | Subcutaneous | Spontaneous | 4 days, 4× daily, 0.3 mg/kg |
| Scott and Hiroi (2011) | Mouse | Oral, Forced | Precipitated: MEC | 14 days, 200 μg/mL NB |
| Stoker et al. (2011) | Mouse | Osmotic Minipump | Spontaneous Precipitated: MEC | 9–28 days, 40 mg/kg/day NB |
| Wilkinson and Gould (2011) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3 mg/kg/day NB |
| Young et al. (2012) | Mice | Osmotic Minipump | Spontaneous | 4 wk, 40 mg/kg/day, NB |
| Cohen et al. (2013) | Rat | Intravenous Self-administration | Spontaneous Precipitated: MEC | After acquisition of nicotine self-administration and 6–14 wks of maintenance (0.03 mg/kg/injection) rats subjected to repeated periods of forced abstinence |
| Poole et al. (2014) | Mouse | Osmotic Minipump | Spontaneous | 12 days, 6.3 mg/kg/day NB |
| Pergadia et al. (2014) | Rat | Osmotic Minipump | Spontaneous | 4 wk, 6.3 mg/kg/day NB |
Abbreviations: NB, nicotine base; NT nicotine tartrate salt; CHLOR, chlorisondamine; DHβE, Dihydro-β-erythroidine; HEX, hexamethonium; MEC, mecamylamine; MLA, methyllycaconitine.
“Physiological” (or somatic) dependence and withdrawal has been demonstrated by repeated or continuous nicotine administration over a period of 1 to 4 weeks using several different approaches. These approaches include repeated daily intraperitoneal or subcutaneous injections, continuous subcutaneous administration via osmotic minipumps and oral self-administration. Since osmotic minipumps first became available in 1977, this method of nicotine administration has been the most commonly used approach by far. The use of osmotic minipumps has a number of implications for nicotine dependence/withdrawal studies since there are substantial differences between the fluctuating levels produced by repeated daily injections or oral consumption, and the continuous levels produced by osmotic minipumps. Ultimately, the primary interest is in studying the effects of withdrawal from chronic nicotine on behaviors and both techniques can prove useful in experimental control of nicotine exposure levels. Hence, both of these approaches have been shown to produce “physical” dependence and withdrawal on a variety of outcomes, such as changes in locomotion, rearing, motor coordination, startle, temperature, heart rate, and operant responding for food reinforcement (Marks and Collins, 1985; Marks et al., 1986a; Marks et al., 1986b; McCallum et al., 2006; Naylor et al., 2005). The rate of the development of dependence and the magnitude of dependence varies across outcomes and differs among rodent strains (Marks et al., 1986a; Marks et al., 1986b). That these effects have somewhat different bases can be seen in α7 nicotinic receptor subunit knockout mice, in which somatic withdrawal symptoms are largely eliminated, but tolerance to the locomotor-decreasing effects of nicotine are unaffected (Salas et al., 2007; Stoker et al., 2011). Distinct somatic withdrawal signs, such as piloerection, ptosis, wet dog shakes, teeth chattering, paw tremor, body tremor and scratches, are among the most common ways to measure withdrawal (Berrendero et al., 2005; Castane et al., 2002). At least part of the basis of somatic withdrawal may also be peripheral, rather than central, in nature (Watkins et al., 2000b). Since the different behavioral effects of nicotine show different rates of tolerance development, and consequent withdrawal, it will be important to measure other outcomes that may be more relevant to the symptoms that humans report during initial abstinence and withdrawal.
To induce a nicotine withdrawal state in animals, several techniques are available as mentioned above, each with advantages and disadvantages. One advantage of using osmotic minipumps is that when removed, they produce a relatively long-lasting withdrawal state, so that it is possible to assess several different types of withdrawal effects in the same animals. For instance, Damaj and colleagues (Jackson et al., 2009b) evaluated mice 18 – 24 h after several weeks of nicotine administration via osmotic minipumps, examining anxiety-related behavior in the elevated plus maze, somatic signs of withdrawal, and nociception. These effects were all shown to be dependent on κ opioid receptors in a subsequent study (Jackson et al., 2010), as well as highly dependent on the strain studied (Jackson et al., 2009c). There may also be a change in μ opioid systems, as naloxone-precipitated withdrawal produces a place aversion after chronic nicotine treatment (Watkins et al., 2000b). Another benefit as alluded to above is that minipumps produce a known constant level of circulating nicotine in the animal, which contrasts with self-administration where the animal may simply have a lower intake that is required for experimental conditions. The use of minipumps also benefits from its implantation and removal being minor procedures which overall are likely to be less stressful to the animal than long-term injections every day. Although these constant levels may allow for more experimenter control, they do not reflect the fluctuating levels (e.g. (Russell et al., 1976)), and constant adaptations to these fluctuating levels, that are characteristic of the behavior of smokers. Long-term administration by multiple daily experimenter administered injections, or long-term oral or intravenous self-administration may model these fluctuations more closely, but these techniques each have their own drawbacks, including stress. The handling associated with multiple daily injections is stressful, as is the surgery necessary for intravenous self-administration and the long-term maintenance of catheters, and, in most cases, it is necessary to use forced oral nicotine consumption (using nicotine solutions as the only fluid source) to induce high enough levels of nicotine consumption to induce withdrawal, which is also stressful. Self-administration techniques more closely mimic the bolus dosing that occurs in humans if the animal is given access throughout the day and inter-individual differences in choice of intake can be linked with the degree of withdrawal etc., but as mentioned the experimental conditions are more difficult to control.
Spontaneous nicotine withdrawal, occurring after removal of an osmotic minipumps delivering s.c. nicotine, produced a conditioned place aversion, but these effects were short lasting (Grieder et al., 2010). One could speculate, however, that nicotine self-administration at this time would produce negative reinforcement. Experiments by Scott and Hiroi suggest such a possibility. Mecamylamine-precipitated withdrawal induced a place aversion after a period of chronic forced consumption of nicotine (Scott and Hiroi, 2011). A tone paired with the mecamylamine-induced withdrawal state was then used to reinstate nicotine conditioned place preference (CPP) that had been extinguished by a long delay between nicotine and place preference testing (Scott and Hiroi, 2010). The anticipation of nicotine withdrawal produced by the stimulus associated with nicotine withdrawal elicited drug seeking behavior (i.e., approach to the compartment previously paired with nicotine). These experiments used oral nicotine administration, which is more likely to induce fluctuating levels of nicotine than osmotic minipumps, and spontaneous acute withdrawal, although the experiments still used drug-induced withdrawal. Neither approach completely models the sort of fluctuating levels that are likely to occur on a daily basis in smokers that provide many more opportunities to experience withdrawal, albeit brief and perhaps not as great in magnitude, but which may have more relevance to negative reinforcement in smokers. A major challenge in this field will be to develop animal models that have more validity for this aspect of nicotine dependence.
Animal models of nicotine dependence: effects of nicotine withdrawal on anxiety
Self-reports of increased anxiety during nicotine withdrawal in humans are quite common, as has been reported in the literature (Hughes et al., 1991). The effects of nicotine on stress or anxiety that are most relevant to negative reinforcement may develop in individuals with premorbid stress and anxiety, but may emerge even in individuals without such susceptibility after experiencing nicotine withdrawal. Nicotine withdrawal increases sensitivity to stressors in the light-enhanced startle paradigm (Jonkman et al., 2008). In that study, startle responses were increased in the more stressful bright environment during nicotine withdrawal. This increase in startle reactivity may represent a general increase in response to anxiogenic stimuli as acute withdrawal produced by nicotine receptor antagonist administration increases anxiety as measured in the elevated plus maze (Biala and Weglinska, 2005; Damaj et al., 2003; Manhaes et al., 2008) and the light-dark box (Stoker et al., 2008).
Regarding the relative importance of premorbid anxiety and the development of such effects, it is interesting to note that under at least some circumstances, premorbid anxiety levels in naïve mice do not predict subsequent oral nicotine consumption (Abreu-Villaca et al., 2006). In contrast, however, anxiety levels during withdrawal are associated with subsequent voluntary oral nicotine consumption (Manhaes et al., 2008). It must also be noted that differences in these mice were dependent on earlier (adolescent) experience with nicotine. High-anxiety animals that were exposed to nicotine in adolescence consumed less nicotine than low-anxiety animals. It is difficult to judge what factors determined these outcomes, and whether different effects might result from other experimental conditions (e.g. nicotine doses, duration of exposures, ages, etc.) but the combination of factors did influence nicotine consumption. It would appear therefore that the ability to self-medicate requires a history of drug taking, is influenced by previous experience, and perhaps experience early in life may be more important than exposure later in life.
Animal models of nicotine dependence: reward deficits induced by nicotine withdrawal
Several approaches have been used to examine nicotine withdrawal-induced reward deficits. Both spontaneous and precipitated (mecamylamine) withdrawal elevated thresholds for rewarding brain stimulation (Epping-Jordan et al., 1998; Johnson et al., 2008; Stoker et al., 2008; Watkins et al., 2000b), although a peripherally acting nicotine receptor antagonist also produces effects in this model (Watkins et al., 2000b). This latter effect may result from nicotine-associated cues. Indeed, stimuli associated with nicotine withdrawal elevate intracranial self-stimulation (ICSS) thresholds in rats (Kenny and Markou, 2005), an effect that could be described as conditioned withdrawal-induced dysphoria. Spontaneous withdrawal also reduced motivated behavior for a monetary reward using a progressive ratio procedure in humans (Kollins et al., 2013). Similarly, in rats, the motivation to obtain a sucrose reward using the progressive ratio procedure was blunted during spontaneous nicotine withdrawal, although this effect appears to be specific to rats that were chronically exposed to higher, but not lower, nicotine doses (Der-Avakian and Markou, 2010; LeSage et al., 2006). These data suggest that motivated behavior is one aspect of reward processing that is impaired during nicotine withdrawal, yet it remains to be determined whether resumption of smoking or nicotine administration restores these motivational deficits.
Reward responsiveness (i.e., the propensity to modulate future behavior as a function of prior positive reinforcement experiences) has also been shown to be impaired during nicotine withdrawal in both humans and rats. Reward responsiveness is assessed using the Response Bias Probabilistic Reward Task that measures the degree of response bias when choosing between two positively reinforced stimuli that differ in reinforcement frequency (Der-Avakian et al., 2013; Pizzagalli et al., 2005). Increased response bias for the more frequently reinforced stimulus reflects elevated reward responsiveness. Using an analogous task developed for use in both species, spontaneous withdrawal from nicotine blunted reward responsiveness similarly in humans and rats (Pergadia et al., 2014). Interestingly, in rats previously exposed to chronic nicotine via minipumps, acute nicotine re-exposure potentiated response bias during protracted withdrawal, suggesting that the enhancement of reward responsiveness during resumption of smoking may contribute to relapse during protracted withdrawal (Pergadia et al., 2014).
The ability of nicotine to alleviate these reward deficits induced by withdrawal, and consequent negative reinforcement, could be an important mediator of drug seeking behavior in later stages of dependence. As suggested by Koob and colleagues (Koob, 2013; Koob and Le Moal, 2008), later stages of drug dependence may involve compulsive drug taking mediated more by the alleviation of negative affective states, and negative reinforcement, than by positive affective consequences of nicotine and positive reinforcement.
Nicotine withdrawal itself also engages stress systems (George et al., 2007), which have been associated with several aspects of nicotine withdrawal, including increases in anxiety and hyperalgesia (Cohen et al., 2013). Furthermore, these systems have been associated with elevations in nicotine self-administration after extended access followed by nicotine withdrawal (George et al., 2007). Increases in nicotine self-administration in this study were associated with activation of CRF systems and withdrawal-induced anxiety. Indeed, the presence of withdrawal symptoms in rats predicts subsequent nicotine intake (Cohen et al., 2013). At early stages of withdrawal, only 2 hours after a chronic nicotine treatment regimen, mice exhibit a depression-like profile similar to a chronic stress regimen (Hayase, 2011). In the same study, chronic nicotine treatment reversed anhedonia induced by chronic stress. This finding suggests that cross-sensitization between stress and nicotine (or perhaps nicotine withdrawal experiences) may influence nicotine seeking in animals that have had both experiences. Overall, these studies support the idea of self-medication, resulting either from nicotine withdrawal or from other experiences that induce symptoms that can be alleviated by nicotine. Furthermore, in both cases, subsequent drug-seeking behavior may be driven by negative reinforcement mechanisms that anticipate such affective states.
Animal models of nicotine dependence: effects of nicotine withdrawal on pain
Nicotine induces analgesia, although this generally occurs at rather high nicotine doses; 3 mg/kg s.c. or more (Berrendero et al., 2005). As for many other nicotine effects, nicotine receptor antagonist precipitated withdrawal produces the opposite effect, hyperalgesia (Cohen et al., 2013; Damaj et al., 2003). The importance of this effect of nicotine, compared to other effects, is less well known and has been examined to a limited extent. However, one may speculate that given the incidence of neuropathic pain, these mechanisms might certainly be important in some individuals. A variety of analgesic compounds have been found to produce a place preference for a location associated with relief from pain (for review see (Navratilova et al., 2013)).
Animal models of nicotine dependence: withdrawal effects on learning
Nicotine affects a variety of cognitive processes, quite notably hippocampal dependent learning (for review, see (Kenney and Gould, 2008a)) and the discussion above regarding effects of nicotine in non-dependent animals). It is not surprising that nicotine would have such effects given the location of specific nicotinic receptor subtypes within portions of hippocampal circuitry involved in learning and memory processes (Tang and Dani, 2009; Zhang et al., 2010). Furthermore, these effects of nicotine are highly task-dependent. Even in quite similar procedures, nicotine dose, the chronicity and timing of treatments and characteristics of the subjects (e.g., strain, sex, age, etc.), affect the outcomes. Administration of nicotine during acquisition of the Morris water maze can produce impairments (Moragrega et al., 2003) or improvements (Bernal et al., 1999), which may depend on the duration of previous nicotine exposure. Whether this difference reflects the development of an initial tolerance to certain effects of nicotine or sensitization of others remains to be seen.
Chronic administration of nicotine, nicotine withdrawal, and nicotine administration to nicotine-dependent animals is of most relevance to nicotine dependence in humans. Not surprisingly, studies of such circumstances reveal a variety of adaptations to chronic nicotine exposure that are typically in the opposite direction to the generally beneficial effects of acute nicotine on cognition. For instance, spontaneous nicotine withdrawal impairs contextual fear conditioning, although it does not seem to affect delay cued fear conditioning (Andre et al., 2011; Davis et al., 2005; Portugal and Gould, 2009; Portugal et al., 2008; Raybuck and Gould, 2009). This pattern of results may suggest that the adaptations occurring in response to chronic nicotine exposure differ across the brain areas impacted by acute nicotine injections.
Acute nicotine treatment reverses the impairment produced by nicotine withdrawal on contextual fear conditioning (Davis et al., 2005), as do other putative or current nicotine cessation treatments, including bupropion (Portugal and Gould, 2007), varenicline (Raybuck et al., 2008), donepezil (Poole et al., 2014) and galantamine (Wilkinson and Gould, 2011). The deficits in contextual fear conditioning in these studies has been compared to cognitive deficits in ADHD and are reversed by atomoxetine (Davis and Gould, 2007a). As seen with spontaneous withdrawal, precipitated nicotine withdrawal with the high-affinity nicotine receptor antagonist dihydro-β-erythroidine impairs acquisition of trace (Raybuck and Gould, 2009) and contextual (Davis and Gould, 2009) fear conditioning, which suggests that α4β2 nicotinic acetylcholine receptor subunits may be mediating these effects. Chronic nicotine administration ameliorates stress-induced impairments in spontaneous alteration (Andreasen et al., 2011) and spatial learning deficits in the Morris water maze produced by prenatal barbiturate exposure (Beer et al., 2005). Hence, chronic nicotine treatment can attenuate deficient learning in rodents, while withdrawal from nicotine impairs certain aspects of learning, notably contextual but not cued fear conditioning.
Animal models of nicotine dependence: withdrawal effects on attention
As noted above, nicotine has been shown to improve attention upon acute treatment in normal subjects and in subjects with attentional impairments. In a manner that appears to reflect a type of opponent-process to the effects of acute nicotine in the 5CSRTT, spontaneous or precipitated nicotine withdrawal impairs choice accuracy and increases omission errors (Semenova et al., 2007; Shoaib and Bizarro, 2005). These effects did not seem to involve α7 nicotinic receptors as the selective antagonist methyllycaconitine did not induce attentional deficits, but the relatively non-selective antagonist dihydro-β-erythroidine did induce deficits. Withdrawal from chronic nicotine (minipump) also impaired vigilance of mice as measured by the 5CCPT 4, 28, and 52 hours later (Young et al., 2012). Interestingly, although acute nicotine treatment improves attention by increasing target responding, withdrawal from nicotine impairs performance by increasing response disinhibition, e.g. responding to non-target signals, and reducing accuracy as seen in the 5CSRTT. Hence, differing mechanisms may contribute to the initial beneficial effects of nicotine on cognition compared with nicotine withdrawal.
Nicotine and nicotine withdrawal may also influence attentional mechanisms by influencing preattentional mechanisms, or sensory-motor gating, such as prepulse inhibition. Nicotine withdrawal impairs prepulse inhibition of acoustic startle in DBA2J mice (Semenova et al., 2003), and in human subjects (Kumari and Gray, 1999). However, effects of nicotine withdrawal were not observed in another study that used C57BL6/J mice (Andre et al., 2008). It would appear that genetic background, and some predisposition to such deficits is an important factor, as appears to be the case for other cognitive effects of nicotine.
Pharmacological treatment of nicotine dependence
Several medications are now available for the treatment of nicotine dependence, but they may work in quite different ways, under quite different circumstances. Additionally, a number of other drugs are being considered for the treatment of nicotine dependence, or explored in clinical or preclinical studies with that aim in mind (see Table 1 in (Elrashidi and Ebbert, 2014) who provide an excellent summary of the current state of drug development in this area). As described in that review, existing treatments for nicotine dependence include various types of nicotine replacement therapies, bupropion, and varenicline, as well as off-label use of nortriptyline and clonidine. Various other previously approved compounds are also being investigated as potential smoking cessation treatments, including atomoxetine, baclofen, carvedilol, labetalol, lobeline, mecamylamine, naltrexone, reboxetine, rimonabant, surinabant, selegiline, EVT 302, tiagabine, topiramate, vigabatrin, cytosine, buspirone, and d-cycloserine. Given the different mechanisms of action of drugs used (or suggested) to treat nicotine dependence, it might be thought that they may work under certain specific circumstances and that their effects may be highly heterogeneous, reflecting the underlying mechanisms that may drive smoking behavior in different individuals. The first treatment developed for smoking cessation was nicotine replacement, via gum or patch (Silagy et al., 2004), which certainly alleviates withdrawal symptoms and in a similar manner alleviates premorbid conditions associated with smoking. However, nicotine does nothing to reverse the dependent state or to address learning associated with negative reinforcement. Nicotine taken in this manner has led to only limited success in nicotine cessation trials, prompting the development of other approaches. The partial α4β2 nicotinic acetylcholine receptor agonist varenicline is also approved for smoking cessation, with some success in clinical trials (Gonzales et al., 2006; Nides et al., 2006; Oncken et al., 2006). However, the effects of varenicline in animal models appear to be primarily upon positive reinforcement, even under conditions that produce dependence and withdrawal (George et al., 2011).
The dopamine/norepinephrine reuptake inhibitor bupropion has also been used in the treatment of nicotine dependence for some time (Goldstein, 1998), but the beneficial effects may be limited to certain individuals. These limitations of the beneficial effects of bupropion are borne out by research using animal models. For instance, bupropion did not attenuate self-administration or the discriminative stimulus effects of nicotine in rats (Shoaib et al., 2003). It has instead been suggested that bupropion acts to alleviate nicotine withdrawal (Cryan et al., 2003; Malin et al., 2006; Warner and Shoaib, 2005; Wing and Shoaib, 2007), which is not surprising, considering that the original indication for this medication was depression. Indeed, bupropion reversed anhedonia induced by nicotine withdrawal (Cryan et al., 2003; Paterson et al., 2007), as well as withdrawal-associated conditioned place aversion (Malin et al., 2006). In a clinical study, bupropion reduced affective symptoms during withdrawal but not craving (Shiffman et al., 2000).
For similar reasons other antidepressant compounds have been examined in both preclinical and clinical studies. For example, nortriptyline, like bupropion, reduces somatic signs of nicotine withdrawal in rats (Wing and Shoaib, 2007) and humans (Prochazka et al., 1998). Acute administration of fluoxetine, a selective serotonin reuptake inhibitor commonly administered chronically for depression, reversed nicotine withdrawal-induced ICSS threshold elevations (i.e., anhedonia) when co-administered with p-MPPI [4-(2'-methoxy-phenyl)-1-[2'-(n-(2"-pyridinyl)-p-iodobenzamido]-ethylpiperazine], a 5-HT1A receptor antagonist (Harrison et al., 2001). Similarly, acute administration of paroxetine, an antidepressant that blocks the reuptake of serotonin and norepinephrine, reversed amphetamine withdrawal-induced anhedonia when co-administered with p-MPPI, suggesting a common mechanism for the treatment of withdrawal from different classes of psychostimulant drugs (Markou et al., 2005). These drug combinations act to enhance serotonergic transmission rapidly, suggesting that nicotine withdrawal-induced anhedonia may be mediated by decreased serotonergic function, as is hypothesized for depression. Interestingly, fluoxetine and p-MPPI did not alleviate somatic signs of nicotine withdrawal (Harrison et al., 2001), indicating a clear dissociation between affective and somatic signs, demonstrating that it is not somatic discomfort that induces anhedonia.
Based on the high rates of smoking in individuals with schizophrenia, treatments for schizophrenia may also alleviate some aspects of nicotine dependence. Consistent with this view, clozapine - an atypical antipsychotic that exhibits high affinity for several subtypes of serotonin and dopamine receptors - reversed nicotine withdrawal-induced ICSS threshold elevations after chronic administration (Semenova and Markou, 2003). Besides its known actions at serotonin and dopamine receptors, clozapine acts as an α2 adrenergic antagonist, which is thought to contribute to its antipsychotic effects (Litman et al., 1996). Consequently, blockade of α2 adrenergic receptors with idazoxan also reversed nicotine withdrawal-induced ICSS threshold elevations (Semenova and Markou, 2010), suggesting a common mechanism between the reward deficits observed during nicotine withdrawal and schizophrenia.
Several other classes of drugs are being considered for the treatment of nicotine dependence, in part because they may affect mechanisms involved in withdrawal. The aversive state associated with mecamylamine-precipitated nicotine withdrawal is κ opioid receptor dependent, based upon conditioned place aversion studies (Jackson et al., 2010). This mechanism is in contrast to the positive reinforcing effects of nicotine, which are μ opioid receptor dependent (Berrendero et al., 2002). This fundamental difference in mechanisms of positive and negative reinforcement may suggest that treatments for nicotine dependence that target positive reinforcement may be misplaced, depending upon the relative contribution of each mechanism to drug-seeking behavior. Somatic withdrawal is also attenuated by the L-type calcium channel antagonists nimodipine, verapamil, flunarizine and diltiazem (Biala and Weglinska, 2005), which may suggest a potential role for this class of drugs in treating nicotine dependence.
Conclusions
The research reviewed here clearly indicates that there are both premorbid and postmorbid (e.g. withdrawal-induced) intra-individual differences that may drive smoking behavior. Premorbid affective and cognitive impairments may be improved by smoking, resulting in negative reinforcement that drives drug-seeking behavior. The substantial comorbidity between nicotine dependence and diverse psychiatric disorders supports this idea, although such effects may also be present in individuals with more moderate impairments that do not have diagnosable disorders. These relationships, supported by numerous findings in animal models discussed in this review, further suggest that the underlying reasons for smoking may be quite different across individuals. The diverse behavioral effects of nicotine on several different aspects of affect and cognition, that have different neurobiological and genetic bases, also suggest that there is substantial heterogeneity in the underlying causes of smoking. Postmorbid effects of nicotine withdrawal may overlap with the behaviors relevant to premorbid drug seeking, but there are also distinct differences. These differences likely arise from differing neural mechanisms and contribute to relapse via negative reinforcement. In other words, as the diverse behavioral effects of nicotine withdrawal are felt, people will relapse to alleviate these symptoms. Considering the number of people that already smoke, it is just as important to model the behavioral effects of nicotine withdrawal in order to develop treatments to minimize relapse during quit attempts. The diverse neurobiological and genetic mechanisms underlying smoking liability and relapse susceptibility will necessitate animal models that separately address these diverse causes of smoking and nicotine withdrawal. The identification of these mechanisms should lead to diverse treatments that will specifically target the behaviors affected in individuals for personalized treatment.
Highlights.
Substantial psychiatric co-morbidities exist in nicotine dependent individuals
Underlying symptoms in nicotine dependent individuals are heterogeneous
Symptoms include both affective and cognitive impairments
Nicotine dependent individuals self-treat with nicotine
Self-treatment needs to be reflected in animal models of nicotine dependence
Acknowledgments
This work was supported in part by NIDA grants DA011946 (AM), DA017949 (TJG), MH104344 (JWY) and in part by intramural funding from the National Institute on Drug Abuse (FSH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhaes AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2006;167:175–182. doi: 10.1016/j.bbr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacol. 2004;29:869–878. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Allison C, Shoaib M. Nicotine improves performance in an attentional set shifting task in rats. Neuropharmacology. 2013;64:314–320. doi: 10.1016/j.neuropharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- Andre JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behav Brain Res. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre JM, Leach PT, Gould TJ. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology. 2011;60:617–625. doi: 10.1016/j.neuropharm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen JT, Henningsen K, Bate S, Christiansen S, Wiborg O. Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: comparison with sertraline. J Psychopharmacol. 2011;25:1134–1141. doi: 10.1177/0269881110391831. [DOI] [PubMed] [Google Scholar]
- Arime Y, Kasahara Y, Hall FS, Uhl GR, Sora I. Cortico-Subcortical Neuromodulation Involved in the Amelioration of Prepulse Inhibition Deficits in Dopamine Transporter Knockout Mice. Neuropsychopharmacol. 2012;37:2522–2530. doi: 10.1038/npp.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori G, Lemmonds CA, Kotler ML, Durcan MJ, Boyle J. Efficacy of a nicotine (4 mg)-containing lozenge on the cognitive impairment of nicotine withdrawal. J Clin Psychopharmacol. 2008;28:667–674. doi: 10.1097/JCP.0b013e31818c9bb8. [DOI] [PubMed] [Google Scholar]
- Azizbeigi R, Ahmadi S, Babapour V, Rezayof A, Zarrindast MR. Nicotine restores morphine-induced memory deficit through the D1 and D2 dopamine receptor mechanisms in the nucleus accumbens. J Psychopharmacol. 2011;25:1126–1133. doi: 10.1177/0269881111405354. [DOI] [PubMed] [Google Scholar]
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl) 2005;181:260–269. doi: 10.1007/s00213-005-2238-y. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl) 2006;184:504–513. doi: 10.1007/s00213-005-0251-9. [DOI] [PubMed] [Google Scholar]
- Batel P. Addiction and schizophrenia. Eur Psychiat. 2000;15:115–122. doi: 10.1016/s0924-9338(00)00203-0. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addict Behav. 2007;32:2900–2915. doi: 10.1016/j.addbeh.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, Levin ED, Rose JE, Fairbank JA. Smoking in Vietnam Combat Veterans with Posttraumatic-Stress-Disorder. J Trauma Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer A, Slotkin TA, Seidler FJ, Aldridge JE, Yanai J. Nicotine therapy in adulthood reverses the synaptic and behavioral deficits elicited by prenatal exposure to phenobarbital. Neuropsychopharmacol. 2005;30:156–165. doi: 10.1038/sj.npp.1300582. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal MC, Vicens P, Carrasco MC, Redolat R. Effects of nicotine on spatial learning in C57BL mice. Behav Pharmacol. 1999;10:333–336. doi: 10.1097/00008877-199905000-00010. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, Maldonado R. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Kruk M. Cannabinoid receptor ligands suppress memory-related effects of nicotine in the elevated plus maze test in mice. Behav Brain Res. 2008;192:198–202. doi: 10.1016/j.bbr.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Biala G, Weglinska B. Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res. 2005;51:483–488. doi: 10.1016/j.phrs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Darvas M, Maldonado R, Zimmer A. A common genetic predisposition to stress sensitivity and stress-induced nicotine craving. Biol Psychiatry. 2008;63:164–171. doi: 10.1016/j.biopsych.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G. The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2- carboxamide improves working and recognition memory in rodents. J Pharmacol Exp Ther. 2007;321:716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Whelan KT, Risbrough VB, Lloyd GK, Menzaghi F. Cognitive enhancing properties and tolerability of cholinergic agents in mice: a comparative study of nicotine, donepezil, and SIB-1553A, a subtype-selective ligand for nicotinic acetylcholine receptors. Neuropsychopharmacol. 2003;28:1235–1246. doi: 10.1038/sj.npp.1300150. [DOI] [PubMed] [Google Scholar]
- Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007;87:146–157. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Bovet-Nitti F. Facilitation of simultaneous visual discrimination by nicotine in four "inbred" strains of mice. Psychopharmacologia. 1969;14:193–199. doi: 10.1007/BF00404217. [DOI] [PubMed] [Google Scholar]
- Bovet D, Bovet-Nitti F, Oliverio A. Effects of nicotine on avoidance conditioning of inbred strains of mice. Psychopharmacologia. 1966;10:1–5. doi: 10.1007/BF00401895. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Arneric SP. Nicotinic receptor agonists facilitate retention of avoidance training: participation of dopaminergic mechanisms. Behav Neural Biol. 1993;59:57–62. doi: 10.1016/0163-1047(93)91159-k. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Holohan DR, Mozley SL, Walsh K, Kassel J. The effect of nicotine and attention allocation on physiological and self-report measures of induced anxiety in PTSD: A double-blind placebo-controlled trial. Exp Clin Psychopharm. 2007;15:154–164. doi: 10.1037/1064-1297.15.2.154. [DOI] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods Mol Biol. 2012;829:243–256. doi: 10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;439:187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Gilbert DG, Riise H, Rabinovich NE, Sugai C, Froeliger B. Serotonin transporter genotype and depressive symptoms moderate effects of nicotine on spatial working memory. Exp Clin Psychopharmacol. 2009;17:173–180. doi: 10.1037/a0016384. [DOI] [PubMed] [Google Scholar]
- Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Castellano C. Effects of nicotine on discrimination learning, consolidation and learned behaviour in two inbred strains of mice. Psychopharmacology (Berl) 1976;48:37–43. doi: 10.1007/BF00423304. [DOI] [PubMed] [Google Scholar]
- Chen LS, Xian H, Grucza RA, Saccone NL, Wang JC, Johnson EO, Breslau N, Hatsukami D, Bierut LJ. Nicotine dependence and comorbid psychiatric disorders: Examination of specific genetic variants in the CHRNA5-A3-B4 nicotinic receptor genes. Drug Alcohol Depen. 2012;123:S42–S51. doi: 10.1016/j.drugalcdep.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciamei A, Aversano M, Cestari V, Castellano C. Effects of MK-801 and nicotine combinations on memory consolidation in CD1 mice. Psychopharmacology (Berl) 2001;154:126–130. doi: 10.1007/s002130000584. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. A prospective examination of the relationships between PTSD, exposure to assaultive violence, and cigarette smoking among a national sample of adolescents. Addict Behav. 2011;36:994–1000. doi: 10.1016/j.addbeh.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol. 2013 doi: 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;88:251–258. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Whisler KN, Jarrell H, Kenny PJ, Markou A. Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacology (Berl) 2010;212:283–299. doi: 10.1007/s00213-010-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine Tob Res. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F, Becker J. Effect of History of Alcoholism or Major Depression on Smoking Cessation. Am J Psychiat. 1993;150:1546–1547. doi: 10.1176/ajp.150.10.1546. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacol. 2007a;32:2011–2019. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta 2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology. 2007b;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur Neuropsychopharmacol. 2009;19:551–561. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin NM, Fransen F, Duytschaever H, Grantham C, Megens AA. Attentional performance of (C57BL/6Jx129Sv)F2 mice in the five-choice serial reaction time task. Physiol Behav. 2006;89:692–703. doi: 10.1016/j.physbeh.2006.08.009. [DOI] [PubMed] [Google Scholar]
- de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM. Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry. 1995;152:453–455. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Sullivan JP, Buckley MJ, Radek RJ, Raszkiewicz JL, Kang CH, Kim DJ, Giardina WJ, Wasicak JT, et al. (S)-3-methyl-5-(1-methyl-2-pyrrolidinyl)isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: II. In vivo characterization. J Pharmacol Exp Ther. 1994;270:319–328. [PubMed] [Google Scholar]
- Der-Avakian A, D'Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiat. 2013;3 doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DL, O'Malley SS, Canive J, Thuras P, Westermeyer J. Nicotine dependence and psychiatric and substance use comorbidities in a sample of American Indian male veterans. Drug Alcohol Depen. 2009;99:169–175. doi: 10.1016/j.drugalcdep.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Sacco KA, Termine A, Seyal AA, Dudas MM, Vessicchio JC, Wexler BE, George TP. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophr Res. 2004;70:261–275. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology (Berl) 2007;194:279–288. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Elrashidi MY, Ebbert JO. Emerging drugs for the treatment of tobacco dependence: 2014 update. Expert Opin Emerg Dr. 2014;19:243–260. doi: 10.1517/14728214.2014.899580. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clin Psychol Rev. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Field M, Rush M, Cole J, Goudie A. The smoking Stroop and delay discounting in smokers: effects of environmental smoking cues. J Psychopharmacol. 2007;21:603–610. doi: 10.1177/0269881106070995. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Gilbert DG, McClernon FJ. Effects of nicotine on novelty detection and memory recognition performance: double-blind, placebo-controlled studies of smokers and nonsmokers. Psychopharmacology (Berl) 2009;205:625–633. doi: 10.1007/s00213-009-1571-y. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina VM, Obinu MC, Laville M, Reibaud M, Pradier L, Dunbar G, Bencherif M. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects. CNS Drug Rev. 2004;10:147–166. doi: 10.1111/j.1527-3458.2004.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl) 2011;213:715–722. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, Duncan EJ. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: involvement of nicotinic receptor mechanisms. Schizophr Res. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacol. 2002;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette-Smoking - Implications for Psychiatric-Illness. Am J Psychiat. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Psychiatry and cigarettes. Arch Gen Psychiat. 1998;55:692–693. doi: 10.1001/archpsyc.55.8.692. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss J, Cooper TB. Smoking Cessation, Clonidine, and Vulnerability to Nicotine among Dependent Smokers. Clin Pharmacol Ther. 1993;54:670–679. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- Glautier S. Measures and models of nicotine dependence: positive reinforcement. Addiction. 2004;99(Suppl 1):30–50. doi: 10.1111/j.1360-0443.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Goldstein MG. Bupropion sustained release and smoking cessation. J Clin Psychiatry. 1998;59(Suppl 4):66–72. [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci. 2003;38:124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rukstalis M, Lewis MC. Atomoxetine and nicotine enhance prepulse inhibition of acoustic startle in C57BL/6 mice. Neurosci Lett. 2005;377:85–90. doi: 10.1016/j.neulet.2004.11.073. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Meyer C, Hapke U, Rumpf HJ, Freyberger HJ, Dilling H, John U. Lifetime-comorbidity of obsessive-compulsive disorder and subclinical obsessive-compulsive disorder in northern Germany. Eur Arch Psy Clin N. 2001;251:130–135. doi: 10.1007/s004060170047. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Sellings LH, Vargas-Perez H, Ting AKR, Siu EC, Tyndale RF, van der Kooy D. Dopaminergic signaling mediates the motivational response underlying the opponent process to chronic but not acute nicotine. Neuropsychopharmacol. 2010;35:943–954. doi: 10.1038/npp.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology (Berl) 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Haman M, Wyler R, Higgins GA. Reversal of a vigilance decrement in the aged rat by subtype-selective nicotinic ligands. Neuropsychopharmacol. 2003;28:880–887. doi: 10.1038/sj.npp.1300102. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2008a;196:483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Varenicline ameliorates ethanol-induced deficits in learning in C57BL/6 mice. Neurobiol Learn Mem. 2008b;90:230–236. doi: 10.1016/j.nlm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The hippocampus and cingulate cortex differentially mediate the effects of nicotine on learning versus on ethanol-induced learning deficits through different effects at nicotinic receptors. Neuropsychopharmacol. 2009;34:2167–2179. doi: 10.1038/npp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Nicotine acts in the anterior cingulate, but not dorsal or ventral hippocampus, to reverse ethanol-induced learning impairments in the plus-maze discriminative avoidance task. Addict Biol. 2010;16:176–188. doi: 10.1111/j.1369-1600.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Nicotine acts in the anterior cingulate, but not dorsal or ventral hippocampus, to reverse ethanol-induced learning impairments in the plus-maze discriminative avoidance task. Addiction Biology. 2011;16:176–188. doi: 10.1111/j.1369-1600.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Sharples CGV, Wonnacott S, Shoaib M, Stolerman P. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003a;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology. 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Involvement of the prefrontal cortex but not the dorsal hippocampus in the attention-enhancing effects of nicotine in rats. Psychopharmacology. 2003b;168:271–279. doi: 10.1007/s00213-003-1438-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psychopharmacology. 2011;217:75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Hall FS, Markou A, Levin ED, Uhl GR. Mouse models for studying genetic influences on factors determining smoking cessation success in humans. Ann N Y Acad Sci. 2012;1248:39–70. doi: 10.1111/j.1749-6632.2011.06415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiat. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YTB, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacol. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Hartsough CS, Lambert NM. Pattern and progression of drug use among hyperactives and controls: a prospective short-term longitudinal study. J Child Psychol Psychiatry. 1987;28:543–553. doi: 10.1111/j.1469-7610.1987.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Fletcher L, Morgan S, Keenan R, Amble P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. J Subst Abuse. 1989;1:407–416. [PubMed] [Google Scholar]
- Hayase T. Depression-related anhedonic behaviors caused by immobilization stress: a comparison with nicotine-induced depression-like behavioral alterations and effects of nicotine and/or "lantidepressant" drugs. J Toxicol Sci. 2011;36:31–41. doi: 10.2131/jts.36.31. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and Smoking: A review of effects on nicotine performance. Exp Clin Psychopharm. 1994;2:345–395. [Google Scholar]
- Heron-Delaney M, Kenardy J, Charlton E, Matsuoka Y. A systematic review of predictors of posttraumatic stress disorder (PTSD) for adult road traffic crash survivors. Injury. 2013;44:1413–1422. doi: 10.1016/j.injury.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of Tobacco Withdrawal - a Replication and Extension. Arch Gen Psychiat. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keenan RM, Yellin A. Effect of tobacco withdrawal on sustained attention. Addict Behav. 1989;14:577–580. doi: 10.1016/0306-4603(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Bloss EB, McCarthy KJ, McEwen BS. Regulation of the nicotinic receptor alpha7 subunit by chronic stress and corticosteroids. Brain Res. 2010;1325:141–146. doi: 10.1016/j.brainres.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Sachs DPL, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. New Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Fanous AH, Chen JC, Kendler KS, Chen XN. Variants in the 15q25 gene cluster are associated with risk for schizophrenia and bipolar disorder. Psychiat Genet. 2013;23:20–28. doi: 10.1097/YPG.0b013e32835bd5f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology. 2009a;56:970–974. doi: 10.1016/j.neuropharm.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009b;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Walters CL, Miles MF, Martin BR, Damaj MI. Characterization of pharmacological and behavioral differences to nicotine in C57Bl/6 and DBA/2 mice. Neuropharmacology. 2009c;57:347–355. doi: 10.1016/j.neuropharm.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav. 2008;90:409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacol. 2008;33:2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayir H, Uzbay IT. Nicotine antagonizes caffeine- but not pentylenetetrazole-induced anxiogenic effect in mice. Psychopharmacology (Berl) 2006;184:464–469. doi: 10.1007/s00213-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Keenan RM, Hatsukami DK, Anton DJ. The effects of short-term smokeless tobacco deprivation on performance. Psychopharmacology (Berl) 1989;98:126–130. doi: 10.1007/BF00442018. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008a;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008b;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuthen NJ, Niaura RS, Borrelli B, Goldstein M, DePue J, Murphy C, Gastfriend D, Reiter SR, Abrams D. Comorbidity, smoking behavior and treatment outcome. Psychother Psychosom. 2000;69:244–250. doi: 10.1159/000012403. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiat. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders - Apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kollins SH, English JS, Roley ME, O'Brien B, Blair J, Lane SD, McClernon FJ. Effects of smoking abstinence on smoking-reinforced responding, withdrawal, and cognition in adults with and without attention deficit hyperactivity disorder. Psychopharmacology. 2013;227:19–30. doi: 10.1007/s00213-012-2937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Epstein JN. Effects of smoking abstinence on reaction time variability in smokers with and without ADHD: an ex-Gaussian analysis. Drug Alcohol Depend. 2009;100:169–172. doi: 10.1016/j.drugalcdep.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology (Berl) 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Hum Psychopharmacol. 2001;16:321–326. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. Bmc Public Health. 2009;9 doi: 10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Pentel PR. Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Be. 2006;83:585–591. doi: 10.1016/j.pbb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Witt CF, Zimmerman M. Associations between depression subtypes and substance use disorders. Psychiat Res. 2008;161:43–50. doi: 10.1016/j.psychres.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rose JE, Behm F, Caskey NH. The effects of smoking-related sensory cues on psychological stress. Pharmacol Biochem Behav. 1991;39:265–268. doi: 10.1016/0091-3057(91)90177-4. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB, Conners CK. Nicotine effects and attention deficit disorder. In: Newhouse P, Iasecki M, editors. Nicotine in psychiatry: psychopathology and emerging therapeutics. New York: Wiley; 2000. pp. 203–214. [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacol. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Litman RE, Su TP, Potter WZ, Hong WW, Pickar D. Idazoxan and response to typical neuroleptics in treatment-resistant schizophrenia - Comparison with the atypical neuroleptic, clozapine. Brit J Psychiat. 1996;168:571–579. doi: 10.1192/bjp.168.5.571. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, Jennings RE, Erwin DS, Presley SE, Perales BA. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 2006;184:494–503. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- Manhaes AC, Guthierrez MC, Filgueiras CC, Abreu-Villaca Y. Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2008;193:216–224. doi: 10.1016/j.bbr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res. 2004;67:157–166. doi: 10.1016/S0920-9964(02)00523-6. [DOI] [PubMed] [Google Scholar]
- Markou A, Harrison AA, Chevrette J, Hoyer D. Paroxetine combined with a 5-HT1A receptor antagonist reversed reward deficits observed during amphetamine withdrawal in rats. Psychopharmacology. 2005;178:133–142. doi: 10.1007/s00213-004-2008-2. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacol. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Collins AC. Tolerance, cross-tolerance, and receptors after chronic nicotine or oxotremorine. Pharmacol Biochem Behav. 1985;22:283–291. doi: 10.1016/0091-3057(85)90392-2. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Romm E, Gaffney DK, Collins AC. Nicotine-induced tolerance and receptor changes in four mouse strains. J Pharmacol Exp Ther. 1986a;237:809–819. [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. J Pharmacol Exp Ther. 1986b;239:358–364. [PubMed] [Google Scholar]
- Martellotta MC, Kuzmin A, Zvartau E, Cossu G, Gessa GL, Fratta W. Isradipine inhibits nicotine intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1995;52:271–274. doi: 10.1016/0091-3057(95)00096-f. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology (Berl) 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Meguro K, Yamaguchi S, Arai H, Nakagawa T, Doi C, Yamada M, Ikarashi Y, Maruyama Y, Sasaki H. Nicotine improves cognitive disturbance in senescence-accelerated mice. Pharmacol Biochem Behav. 1994;49:769–772. doi: 10.1016/0091-3057(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Mexal S, Jenkins PM, Lautner MA, Iacob E, Crouch EL, Stitzel JA. alpha7 nicotinic receptor gene promoter polymorphisms in inbred mice affect expression in a cell type-specific fashion. J Biol Chem. 2007;282:13220–13227. doi: 10.1074/jbc.M610694200. [DOI] [PubMed] [Google Scholar]
- Miller EN, Fujioka TA, Chapman LJ, Chapman JP. Hemispheric asymmetries of function in patients with major affective disorders. J Psychiatr Res. 1995;29:173–183. doi: 10.1016/0022-3956(95)00011-s. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Ibi D, Takase F, Nagai T, Kamei H, Toth E, Sato J, Takuma K, Yamada K. Nicotine ameliorates impairment of working memory in methamphetamine-treated rats. Behav Brain Res. 2011;220:159–163. doi: 10.1016/j.bbr.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Moragrega I, Carrasco MC, Vicens P, Redolat R. Spatial learning in male mice with different levels of aggressiveness: effects of housing conditions and nicotine administration. Behav Brain Res. 2003;147:1–8. doi: 10.1016/s0166-4328(03)00112-8. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacol. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Addiction Reviews, Vol 1282. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor C, Quarta D, Fernandes C, Stolerman IP. Tolerance to nicotine in mice lacking alpha7 nicotinic receptors. Psychopharmacology (Berl) 2005;180:558–563. doi: 10.1007/s00213-005-2187-5. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Obinu MC, Reibaud M, Miquet JM, Pasquet M, Rooney T. Brain-selective stimulation of nicotinic receptors by TC-1734 enhances ACh transmission from frontoparietal cortex and memory in rodents. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:913–918. doi: 10.1016/s0278-5846(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Oliverio A. Effects of nicotine and strychnine on transfer of avoidance learning in the mouse. Life Sci. 1968;7:1163–1167. doi: 10.1016/0024-3205(68)90225-7. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. European Journal of Neuroscience. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–587. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, A, D-A, MS, DS, PAF, M, AC, H, S, S, A, M, DA, P Withdrawal of nicotine blunts reward responsiveness in humans and rats. The Journal of the American Medical Association. 2014 doi: 10.1001/jamapsychiatry.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Pettiford J, Kozink RV, Lutz AM, Kollins SH, Rose JE, McClernon FJ. Increases in impulsivity following smoking abstinence are related to baseline nicotine intake and boredom susceptibility. Addict Behav. 2007;32:2351–2357. doi: 10.1016/j.addbeh.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Navarre BM, Nair SG. Incubation of conditioned fear in the conditioned suppression model in rats: role of food-restriction conditions, length of conditioned stimulus, and generality to conditioned freezing. Neuroscience. 2010;169:1501–1510. doi: 10.1016/j.neuroscience.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal detection approach. Biol Psychiat. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Poole RL, Connor DA, Gould TJ. Donepezil Reverses Nicotine Withdrawal-Induced Deficits in Contextual Fear Conditioning in C57BL/6J Mice. Behav Neurosci. 2014 doi: 10.1037/bne0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol Biochem Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacol Biochem Behav. 2009;92:117–123. doi: 10.1016/j.pbb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med. 1998;158:2035–2039. doi: 10.1001/archinte.158.18.2035. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: Differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Swedberg MD. Reinforcing effects of nicotinic compounds: intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1998;60:567–573. doi: 10.1016/s0091-3057(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behav Neurosci. 2007;121:1119–1124. doi: 10.1037/0735-7044.121.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice--a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. Eur J Neurosci. 2009;29:377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Thinschmidt J, Liu J, Ai L, Papke RL, King MA, Hughes JA, Meyer EM. alpha7 Nicotinic receptor gene delivery into mouse hippocampal neurons leads to functional receptor expression, improved spatial memory-related performance, and tau hyperphosphorylation. Neuroscience. 2007;145:314–322. doi: 10.1016/j.neuroscience.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Shirazi-Zand Z, Zarrindast MR, Nayer-Nouri T. Nicotine improves ethanol-induced memory impairment: the role of dorsal hippocampal NMDA receptors. Life Sci. 2010;86:260–266. doi: 10.1016/j.lfs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Fuemmeler BF, McClernon FJ, Beckham JC. Association between trauma exposure and smoking in a population-based sample of young adults. J Adolesc Health. 2008;42:266–274. doi: 10.1016/j.jadohealth.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, Lin NH, Osinski MA, Reinhart GA, Buckley MJ, Martin RL, McDermott JS, Preusser LC, Seifert TR, Su Z, Cox BF, Decker MW, Sullivan JP. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev. 2004;10:167–182. doi: 10.1111/j.1527-3458.2004.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 2010;471:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine Improves Working Memory Span Capacity in Rats Following Sub-Chronic Ketamine Exposure. Neuropsychopharmacol. 2011;36:2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MAH, Feyerabend C, Cole PV. Plasma Nicotine Levels after Cigarette-Smoking and Chewing Nicotine Gum. Brit Med J. 1976;1:1043–1046. doi: 10.1136/bmj.1.6017.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Salas R, De Biasi M. Opposing actions of chronic stress and chronic nicotine on striatal function in mice. Neurosci Lett. 2008;440:32–34. doi: 10.1016/j.neulet.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Hiroi N. Emergence of dormant conditioned incentive approach by conditioned withdrawal in nicotine addiction. Biol Psychiatry. 2010;68:726–732. doi: 10.1016/j.biopsych.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Hiroi N. Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry. 2011;69:1052–1059. doi: 10.1016/j.biopsych.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Bespalov A, Markou A. Decreased prepulse inhibition during nicotine withdrawal in DBA/2J mice is reversed by nicotine self-administration. Eur J Pharmacol. 2003;472:99–110. doi: 10.1016/s0014-2999(03)01904-6. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiat. 2003;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The alpha 2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. Eur Neuropsychopharm. 2010;20:731–746. doi: 10.1016/j.euroneuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Be. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology (Berl) 2003;165:405–412. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Barch DM, Wolf TJ, Mamah D, Csernansky JG. Elevated rates of substance use disorders in non-psychotic siblings of individuals with schizophrenia. Schizophr Res. 2008;106:294–299. doi: 10.1016/j.schres.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder FR, Henningfield JE. Effects of nicotine administration following 12 h of tobacco deprivation: assessment on computerized performance tasks. Psychopharmacology (Berl) 1989;97:17–22. doi: 10.1007/BF00443406. [DOI] [PubMed] [Google Scholar]
- Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. A prospective study of smoking in young women and risk of later psychiatric hospitalization. Nord J Psychiat. 2011;65:3–8. doi: 10.3109/08039481003786386. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacol. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Role of alpha7- and beta4-Containing Nicotinic Acetylcholine Receptors in the Affective and Somatic Aspects of Nicotine Withdrawal: Studies in Knockout Mice. Behav Genet. 2011 doi: 10.1007/s10519-011-9511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Mulligan RC, Finnerty CE, Jerskey BA, David SP, Cohen RA, Niaura RS. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Res. 2010;183:69–74. doi: 10.1016/j.pscychresns.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63:673–682. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike FP, Wernicke R, Pearlman MY, Haaga DAF. Nicotine dependence, PTSD symptoms, and depression proneness among male and female smokers. Addict Behav. 2006;31:223–231. doi: 10.1016/j.addbeh.2005.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depen. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Uchiumi O, Kasahara Y, Fukui A, Hall FS, Uhl GR, Sora I. Serotonergic involvement in the amelioration of behavioral abnormalities in dopamine transporter knockout mice by nicotine. Neuropharmacology. 2013;64:348–356. doi: 10.1016/j.neuropharm.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatr. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Valzelli L, Baiguerra G, Giraud O. Difference in learning and retention by Albino Swiss mice. Part III. Effect of some brain stimulants. Methods Find Exp Clin Pharmacol. 1986;8:337–341. [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111:23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, de Jong NM, Kalkman CJ, Westphal KG, van Oorschot R, Olivier B, Korte SM, Groenink L. Stress-induced hyperthermia is reduced by rapid-acting anxiolytic drugs independent of injection stress in rats. Pharmacol Biochem Behav. 2009;93:413–418. doi: 10.1016/j.pbb.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin Ther Tar. 2013;17:139–155. doi: 10.1517/14728222.2013.736498. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Porter RHP. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem Pharmacol. 2011;82:891–903. doi: 10.1016/j.bcp.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10:219–231. doi: 10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000a;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000b;292:1053–1064. [PubMed] [Google Scholar]
- Waxmonsky JA, Thomas MR, Miklowitz DJ, Allen MH, Wisniewski SR, Zhang HW, Ostacher MJ, Fossey MD. Prevalence and correlates of tobacco use in bipolar disorder: data from the first 2000 participants in the Systematic Treatment Enhancement Program. Gen Hosp Psychiat. 2005;27:321–328. doi: 10.1016/j.genhosppsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Maciejewski PK, Mckee SA, Reutenauer EL, Mazure CM. Gender Differences in Associations between Lifetime Alcohol, Depression, Panic Disorder, and Posttraumatic Stress Disorder and Tobacco Withdrawal. Am J Addiction. 2009;18:140–147. doi: 10.1080/10550490802544888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Nosten-Bertrand M, McIntosh JM, Giros B, Martres MP. Nicotine improves cognitive deficits of dopamine transporter knockout mice without long-term tolerance. Neuropsychopharmacol. 2007a;32:2465–2478. doi: 10.1038/sj.npp.1301385. [DOI] [PubMed] [Google Scholar]
- Weiss S, Tzavara ET, Davis RJ, Nomikos GG, Michael McIntosh J, Giros B, Martres MP. Functional alterations of nicotinic neurotransmission in dopamine transporter knock-out mice. Neuropharmacology. 2007b;52:1496–1508. doi: 10.1016/j.neuropharm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Wilkinson DS, Gould TJ. The effects of galantamine on nicotine withdrawal-induced deficits in contextual fear conditioning in C57BL/6 mice. Behav Brain Res. 2011;223:53–57. doi: 10.1016/j.bbr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Examining the clinical efficacy of bupropion and nortriptyline as smoking cessation agents in a rodent model of nicotine withdrawal. Psychopharmacology (Berl) 2007;195:303–313. doi: 10.1007/s00213-007-0902-0. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y, Kobayashi H, Sora I. Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacol. 2006;31:2132–2139. doi: 10.1038/sj.npp.1301009. [DOI] [PubMed] [Google Scholar]
- Yip SW, Sacco KA, George TP, Potenza MN. Risk/reward decision-making in schizophrenia: A preliminary examination of the influence of tobacco smoking and relationship to Wisconsin Card Sorting Task performance. Schizophr Res. 2009;110:156–164. doi: 10.1016/j.schres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacol. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86:1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-Choice Continuous Performance Test: Evidence for a Translational Test of Vigilance for Mice. Plos One. 2009;4 doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behavioural Brain Research. 2013;240:119–133. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in alpha 7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Markou A, Eyler LT, Geyer MA. Withdrawal from chronic nicotine impairs attention in mice and humans as measured by the 5-choice continuous performance test: A model for identifying treatments. Society for Neuroscience. 2012:696.604. [Google Scholar]
- Zarrindast MR, Sadegh M, Shafaghi B. Effects of nicotine on memory retrieval in mice. Eur J Pharmacol. 1996;295:1–6. doi: 10.1016/0014-2999(95)00628-1. [DOI] [PubMed] [Google Scholar]
- Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci. 2010;30:6443–6453. doi: 10.1523/JNEUROSCI.0458-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]