Abstract
Background. Serum omentin-1 level was low in the most types of ischemic heart disease compared to normal subjects; it also dependently correlated with coronary heart disease; thus, omentin-1 is regarded as a novel biomarker in IHD. Objective. The aim of the present study was to establish the links between omentin-1 and acute myocardial infarction in metformin patients. Subjects and Methods. A cross-sectional study was performed on eighty-five patients with type II DM and acute MI. They are divided as follows: Group I, 62 patients with type II DM who received metformin prior to onset of acute MI; Group II, 23 patients with type II DM who did not receive metformin prior to onset of acute MI; and Group III, 30 normal healthy controls. Venous blood was drawn from each participant for determination of lipid profile, plasma omentin-1, cardiac troponin-I (cTn-I) and other routine tests. Results. Patients that presented with acute MI that received metformin show a significant difference in all biochemical parameters (p < 0.001); metformin increases serum omentin-1 level and decreases serum cardiac troponin-I level compared with control subjects and nonmetformin treated patients. Conclusion. Metformin pharmacotherapy increases omentin-1 serum levels and may be regarded as a potential agent in the prevention of the occurrences of acute MI in diabetic patients.
1. Introduction
Acute myocardial infarction is one of the main diseases leading to death worldwide and is caused by atherosclerosis and other metabolic syndromes that induce inflammatory and immunological reactions that are caused by adipokines like Visfatin [1]. Omentin-1 is secreted from omentum and visceral fat also, it is found in the heart, lung, placenta, and ovary [2], it produced protective roles in regulating inflammatory and immunological response to induction of insulin sensitivity and downregulation of tumor necrosis factor (TNF) [3], and it inhibits free radicals and superoxide formations that play an important role in the vascular inflammation and smooth muscle remodeling; so it is regarded as protective adipokine in IHD [4]. Moreover, omentin-1 levels are inversely correlated with obesity, body mass index, hemoglobin, cholesterol, and type 2 diabetes mellitus [5]. Serum omentin-1 level was low in the most types of ischemic heart disease compared to normal subjects [6], but serum omentin-1 is positively correlated with high density lipoprotein (HDL) that is independently correlated with IHD, and many studies showed a significant correlation between low omentin-1 serum level and progressions of IHD [7].
Omentin-1 produced significant vasodilatations through induction of endothelial nitric oxide (eNO) phosphorylation; thus, NO inhibitor will abolish the vasodilating effect of omentin-1 [8]; additionally, it inhibits C-reactive protein and nuclear factor kappa-light chain of immune cells; so, it prevents the associations for inflammation, angiogenesis, and pathogenesis of coronary artery disease [9].
Additionally, omentin-1 triggers endothelial cells adenosine monophosphate protein kinase (AMPK) leading to potent anti-inflammatory action which modulates visceral fat macrophage activity [10].
Metformin is one of the most prescribed agents as a first line drug for treatment of type II diabetes mellitus worldwide; it acts through inhibition of hepatic glucose production and intestinal glucose absorption; also, it accelerates glucose uptake and consumption [11]. Long term uses of metformin lead to cardiovascular protection for prevention of microvascular and macrovascular complications which mediate diabetic harmful effect on the cardiovascular system [12].
Therefore, the aims of the present study are to establish the links between omentin-1 and acute myocardial infarction in patients treated with metformin prior to the onset of acute MI.
2. Subjects and Methods
This cross-sectional study was performed on eighty-five patients with type II DM with the age of 49–66 years that were screened for acute myocardial infarction at the coronary care unit (CCU) in Al-Yarmook Teaching Hospital in cooperation with Clinical Pharmacology, College of Medicine, Al-Mustansiriyia University, from January to July, 2015; this study was approved by the Scientific Committee Review Board in the first of January 2015 (ethical committee permission number 159). All enrolled patients offered oral and written informed permission for this study and acquiescence to use their samples for prospect study. The patients were selected according to grading of New York Heart Association (NYHA) [13].
2.1. Study Design
Eighty-five patients with type II DM (51 males + 34 females) and acute MI were selected. They are divided as follows: Group I, 62 patients with type II DM (42 males + 20 females) who received metformin prior to onset of acute MI; Group II, 23 patients with type II DM (9 males + 14 females) who did not receive metformin prior to onset of acute MI; and Group III, 30 normal healthy controls (20 males + 10 females) who have not received any drugs.
2.2. Exclusion Criteria
Patients with any other systemic diseases other than type II DM and acute MI are excluded.
2.3. Sample Processing
10 mL of venous blood was drawn after overnight fasting for lipid profile and other routine tests, and 3 mL was transferred into EDTA anticoagulant tubes centrifuged at 2000 r/min; then, supernatant was stored in the refrigerator to be used for estimation of plasma omentin-1, cardiac troponin-I (cTn-I), and other routine investigations by ELISA Kit, while atherogenic index (AI) = log(TG/HDL), low risk was <0.11, intermediate risk was 0.11–0.21, and high risk was >0.21.
2.4. Estimation of Serum Omentin-1 Concentration
Omentin-1 concentration was determined by ELISA Kit (Wellbiotechnology, Changsha) pg/mL.
2.5. Estimation of Plasma Troponin-I Concentration
Troponin-I concentration was determined by ELISA Kit (Cardiac Troponin-I ELISA Kit Catalog number E-EL-R1253 pg./mL, Elabscience, China).
2.6. Statistical Analysis
SPSS 22.0 software was used for statistical analysis, when p < 0.01 was regarded as significant. The variables were presented as mean ± SD; differences in mean values among groups were compared by ANOVA. Correlations between serum concentrations of omentin-1 and other variables were done via Pearson correlation analysis.
3. Results
Patients with acute MI at a coronary care unit (CCU) presented with miscellaneous clinical and biochemical variables with complete preceding history (Table 1).
Table 1.
Clinical and metabolic characteristics of the patients with type II DM at coronary care unit.
| Characteristics | Number (%), mean ± SD |
|---|---|
| Age (years) | 57.5 ± 12.02 |
| Male : female ratio | 60% male, 40% female |
| Race, white : black ratio | 100 : 0 |
| Onset of chest pain (hrs) | 4.42 ± 2.69 (1–7) |
| Positive history for IHD | 70 (82.35) |
| Hypertension | 69 (81.17) |
| Hyperlipidemia | 79 (92.94) |
| Duration of hospitalization | 6 ± 2.27 (4–10) |
| Troponin I positive | 42 (95.45) |
| Troponin negative | 2 (4.54) |
| Types of MI | |
| Anterior | 39 (45.88) |
| Anteroseptal | 12 (14.11) |
| Anterolateral | 13 (15.29) |
| Posterior | 14 (16.47) |
| Inferior | 7 (6.81) |
| Complications | 12 (14.11) |
| Death | 2 (2.35) |
| Arrhythmia | 7 (8.23) |
| Shock | 1 (1.17) |
| Acute heart failure | 2 (2.35) |
| Pharmacotherapy | |
| Antiplatelets | 85 (100) |
| Anticoagulant | 55 (64.70) |
| Anti-ischemic | 78 (91.76) |
| Antidiabetic agents | 85 (100) |
| Metformin | 62 (72.94) |
| Others | 23 (27.05) |
| ACEI | 80 (94.11) |
| Hypolipidemic agents | 78 (91.76) |
| Analgesics | 22 (50) |
| Direct current shock (DC) | 6 (70.58) |
ACEI: angiotensin converting enzyme inhibitor.
The patients that presented with acute MI that received metformin show a significant difference in all biochemical parameters (p < 0.001). Serum omentin-1 was 27.13 ± 1.55 pg/mL in control subjects and 25.43 ± 1.2 pg/mL in patients with acute MI that were previously treated with metformin prior to the occurrence of MI, while serum cardiac troponin l increased from 21.202 ± 0.636 pg/mL in normal healthy controls to 75.453 ± 5.62 pg/mL in the patients with acute MI that were previously treated with metformin prior to the occurrence of MI. Other biochemical parameters also showed the same difference (Table 2).
Table 2.
Biochemical changes and serum omentin-1 level in acute MI in the patients treated with metformin prior to acute MI in comparison with normal healthy control subjects.
| Biochemical parameters | Group III (n = 30) Mean ± SD |
Group I (n = 62) Mean ± SD |
t | 95% CI Upper–lower limits |
p |
|---|---|---|---|---|---|
| S. omentin-1 pg/mL | 27.13 ± 1.55 | 25.43 ± 1.2∗ | 6.0073 | 27.7088–26.5512 | <0.0001 |
| S. troponin-I pg/mL | 21.202 ± 3.483 | 75.453 ± 5.62∗ | 36.4115 | 21.4395–20.9645 | <0.0001 |
| FBG mg/dL | 118.96 ± 9.704 | 137.75 ± 13.139∗ | 10.6056 | 122.5835–15.3365 | <0.0001 |
| Total cholesterol mg/dL | 140.96 ± 30.091 | 152.33 ± 16.877∗∗ | −2.0696 | 152.1962–129.7238 | 0.0475 |
| TG mg/dL | 138.66 ± 10.366 | 149.34 ± 23.31∗ | −5.6431 | 142.5307–134.7893 | <0.0001 |
| HDL mg/dL | 51.23 ± 5.882 | 54.43 ± 8.355∗ | −2.9798 | 53.4264–49.0336 | 0.0058 |
| VLDL mg/dL | 27.64 ± 2.082 | 29.86 ± 4.662∗ | 6.0073 | 27.7088–26.5512 | <0.0001 |
| LDL mg/dL | 62.08 ± 3.834 | 68.13 ± 3.937∗ | −66.274 | 62.2667–61.8933 | <0.0001 |
| AI | 0.072 ± 0.008 | 0.078 ± 0.009∗ | −4.1079 | 0.075–0.069 | 0.0003 |
| SPB mmHg | 123.16 ± 9.042 | 140.33 ± 8.773∗ | 10.4008 | 126.5363–119.7837 | <0.0001 |
| DPB mmHg | 75.50 ± 1.643 | 82.16 ± 8.773∗ | −22.202 | 76.1135–74.8865 | <0.0001 |
Group I: patients received metformin prior to acute MI. Group III: normal healthy controls. ∗ p < 0.001;∗∗ p < 0.05.
FBG: fasting blood glucose, TG: triglyceride, HDL: high density lipoprotein, VLDL: very low density lipoprotein, LDL: low density lipoprotein, AI: atherogenic index, SPB: systolic blood pressure, and DPB: diastolic blood pressure.
In the patients who presented with acute MI in the coronary care unit (CCU) that were not previously treated with metformin, there is a significant difference in all biochemical parameters (p < 0.0001). Regarding serum omentin-1 level, it decreased from 27.13 ± 1.55 pg/mL in healthy controls to 23.71 ± 1.161 pg/mL in patients with MI not previously treated with metformin, while serum cardiac troponin-I increased from 21.202 ± 0.636 pg/mL in healthy controls to 79.53 ± 3.60 pg/mL in patients with MI not previously treated with metformin (Table 3).
Table 3.
Biochemical changes and serum omentin-1 levels in acute MI in the patients that were not previously treated with metformin prior to acute MI in comparison with normal healthy control subjects.
| Biochemical parameters | Group III (n = 30) Mean ± SD |
Group II (n = 23) Mean ± SD |
t | 95% CI Upper-lower limits |
p |
|---|---|---|---|---|---|
| S. omentin-1 pg/mL | 27.13 ± 1.55 | 23.71 ± 1.161∗ | 12.0852 | 27.7088–26.5512 | <0.0001 |
| S. troponin-I pg/mL | 21.202 ± 2.636 | 79.53 ± 3.60∗ | −121.197 | 22.1863–20.2177 | <0.0001 |
| FBG mg/dL | 118.96 ± 9.704 | 138.77 ± 17.467∗ | −11.181 | 122.5835–115.3365 | <0.0001 |
| Total cholesterol mg/dL | 140.96 ± 30.091 | 242.44 ± 12.552∗ | −18.4716 | 152.1962–129.7238 | <0.0001 |
| TG mg/dL | 138.66 ± 10.366 | 246.55 ± 57.607∗ | −57.007 | 142.530–134.7893 | <0.0001 |
| HDL-C mg/dL | 51.23 ± 5.882 | 38.77 ± 4.570∗ | 11.6026 | 53.4264–49.0336 | <0.0001 |
| VLDL-C mg/dL | 27.64 ± 2.082 | 49.30 ± 11.524∗ | −56.9821 | 28.4174–26.8626 | <0.0001 |
| LDL-C mg/dL | 62.08 ± 3.834 | 154.37 ± 4.316∗ | −722.133 | 62.3414–61.8186 | <0.0001 |
| AI | 0.072 ± 0.008 | 0.443 ± 0.0084∗ | −254.006 | 0.075–0.069 | <0.0001 |
| SPB mmHg | 123.16 ± 9.042 | 162.33 ± 10.61∗ | −23.727 | 126.5363–119.7837 | <0.0001 |
| DPB mmHg | 75.50 ± 11.643 | 105.16 ± 21.784∗ | −13.953 | 79.8476–71.1524 | <0.0001 |
Group II: patients have not received metformin prior to acute MI. Group III: normal healthy controls have not received any drugs.
∗ p < 0.0001. FBG: fasting blood glucose, TG: triglyceride, HDL: high density lipoprotein, VLDL: very low density lipoprotein, LDL: low density lipoprotein, AI: atherogenic index, SPB: systolic blood pressure, and DPB: diastolic blood pressure.
Furthermore, there is a significant difference in most of biochemical parameters regarding serum omentin-1 and cardiac troponin-I between the patients that received metformin and the patients that did not receive metformin prior to occurrences of acute MI (p < 0.01). Thus, metformin elevates omentin-1 from 23.71 ± 1.161 pg/mL to 25.43 ± 1.2 pg/mL and lower serum cardiac troponin-I from 79.53 ± 3.60 pg/mL to 75.453 ± 5.62 pg/mL significantly in patients who presented with acute MI (Table 4).
Table 4.
Biochemical changes and serum omentin-1 level in acute MI in the patients that were not previously treated with metformin prior to acute MI in comparison with the patients previously treated with metformin.
| Biochemical parameters | Group I (n = 62) Mean ± SD |
Group II (n = 23) Mean ± SD |
t | 95% CI Upper-lower limits |
p |
|---|---|---|---|---|---|
| S. omentin-1 pg/mL | 25.43 ± 1.2∗ | 23.71 ± 1.161 | 11.2861 | 25.7347–25.1253 | <0.0001 |
| S. troponin-I pg/mL | 75.453 ± 5.62∗ | 79.53 ± 3.60 | −5.7122 | 76.8802–74.0258 | <0.0001 |
| FBG mg/dL | 137.75 ± 13.139 | 138.77 ± 17.467 | −0.6113 | 141.0867–134.4133 | 0.5433 |
| Total cholesterol mg/dL | 152.63 ± 16.877∗ | 242.44 ± 12.552 | −41.9011 | 156.916–148.344 | <0.0001 |
| TG mg/dL | 149.34 ± 23.31∗ | 246.55 ± 57.607 | −32.8371 | 155.2596–143.4204 | <0.0001 |
| HDL-C mg/dL | 54.24 ± 8.355∗ | 38.77 ± 4.570 | 14.5794 | 56.3618–52.1182 | <0.0001 |
| VLDL-C mg/dL | 29.86 ± 4.662∗ | 49.30 ± 11.524 | −32.8337 | 31.0439–28.6761 | <0.0001 |
| LDL-C mg/dL | 68.13 ± 3.937∗ | 154.37 ± 4.316 | −13581.08 | 68.1427–68.1173 | <0.0001 |
| AI | 0.082 ± 0.09∗ | 0.443 ± 0.084 | −31.5835 | 0.1049–0.0591 | <0.0001 |
| SPB mmHg | 140.33 ± 8.773∗ | 162.33 ± 10.61 | −19.745 | 142.5579–138.1021 | <0.0001 |
| DPB mmHg | 82.16 ± 8.773∗ | 105.16 ± 21.784 | −20.6431 | 84.3879–79.9321 | <0.0001 |
Group I: patients received metformin prior to acute MI. Group II: patients have not received metformin prior to acute MI.
∗ p < 0.001. FBG: fasting blood glucose, TG: triglyceride, HDL: high density lipoprotein, VLDL: very low density lipoprotein, LDL: low density lipoprotein, AI: atherogenic index, SPB: systolic blood pressure, and DPB: diastolic blood pressure.
The results of the present study highlight the cardiovascular protective influence of pretreatment with metformin, manifested by ameliorating the acute MI associated biochemical changes, in patients with type 2 DM (Figure 1).
Figure 1.
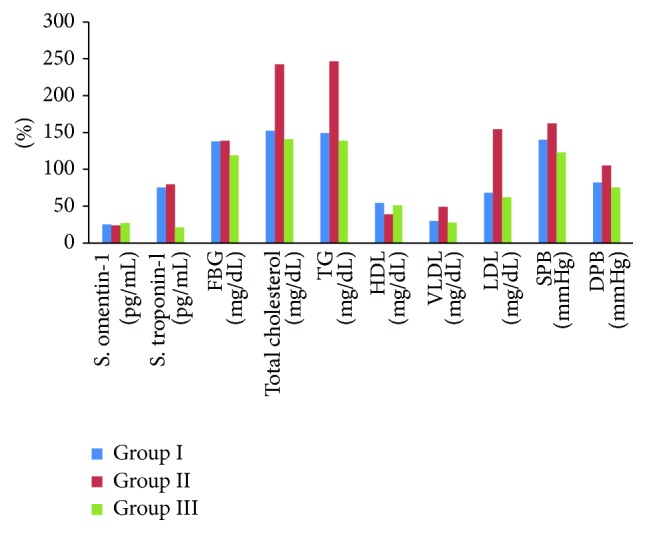
Biochemical parameter changes in acute MI regarding the previous metformin therapy in comparison with nonmetformin treated patients and healthy control subjects.
Regarding mean differences and 95% Confidence Interval of the difference (lower and upper limits) between control normal, healthy subjects and the patients with acute MI that either were previously treated with metformin or not, serum omentin-1 concentrations showed a high significant difference between those patients and normal controls (Table 5).
Table 5.
Serum omentin-1 concentration changes between control normal, healthy subjects and the patients with acute MI that either were previously treated with metformin or not.
| S. omentin 1 | Mean difference | t | df | Sig. (two-tailed) | 95% CI |
|---|---|---|---|---|---|
| Group I | 75.453 | 75.83 | 61 | 0.000301 | 73.423–77.482 |
| Group II | 79.530 | 93.71 | 22 | 0.000586 | 77.739–81.320 |
| Group III | 21.202 | 182.51 | 29 | 0.000545 | 20.964–21.439 |
Group I: who received metformin prior to acute MI. Group II: who did not receive metformin prior to acute MI. Group III: normal healthy controls.
Serum omentin-1 was not correlated with serum cardiac troponin-I in normal healthy subjects (R = 0.0) (Figure 2).
Figure 2.
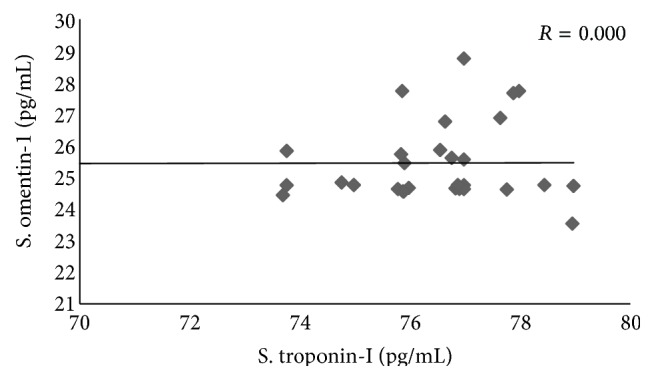
Correlation between serum omentin-1 and cardiac troponin-I in normal healthy control subjects.
In previously metformin treated patients with acute MI, serum omentin-1 is significantly correlated with serum cardiac troponin-I (R = 1.56) (Figure 3).
Figure 3.
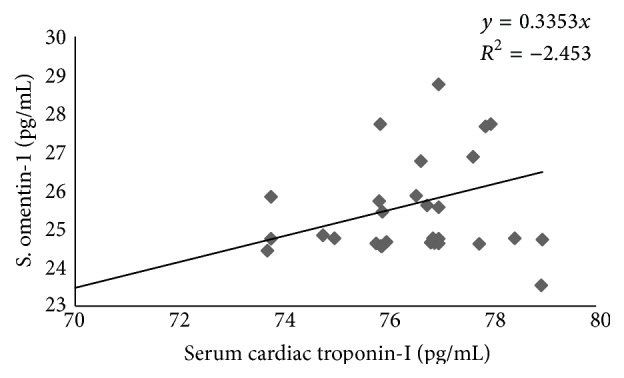
Correlation between serum omentin-1 and cardiac troponin-I in previously metformin treated patients with acute MI.
In the patients with acute MI that were not previously treated with metformin, serum omentin-1 is highly correlated with serum cardiac troponin-I (R = 1.76) (Figure 4).
Figure 4.
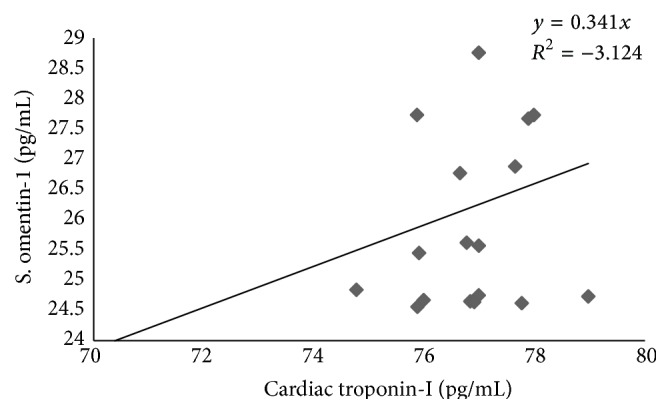
Correlation between serum omentin-1 and cardiac troponin-I in patients with acute MI not previously treated with metformin.
Atherogenic index, which is mainly reflecting lipid profile effects, was high in nonmetformin treated patients (5.252) but it was low in metformin treated patients (1.814) and control healthy subjects (1.748) (Figure 5).
Figure 5.
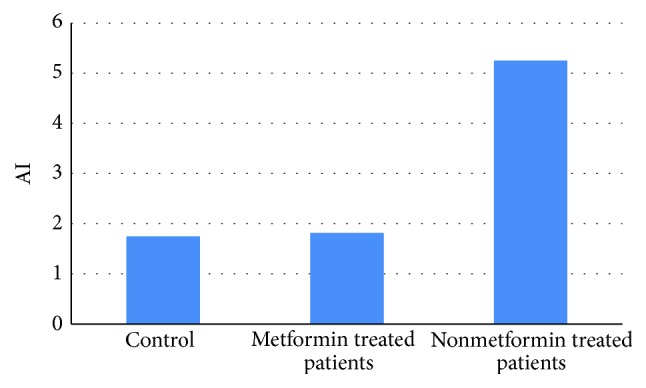
Differential atherogenic index levels in acute MI regarding metformin treatment.
The interesting finding in the present study is the gender effects on the amplitude of changing in serum omentin-1 levels between healthy and diseased groups (Figure 6).
Figure 6.
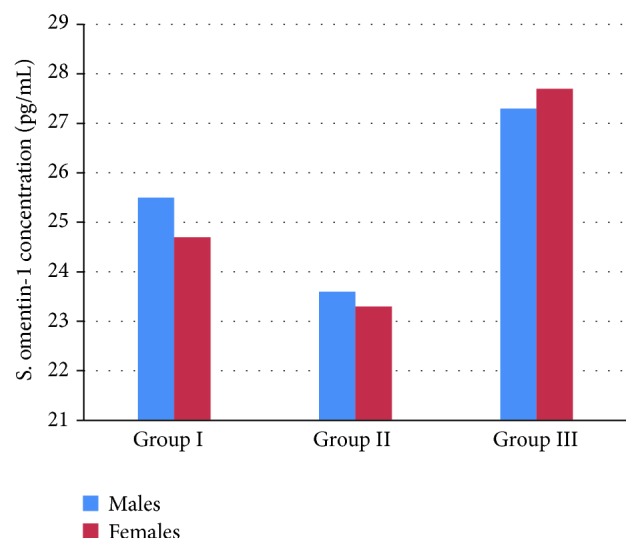
Gender differences in serum omentin-1 level in acute MI as compared with normal healthy group.
4. Discussion
The current study shows a significant change in the serum omentin-1 levels in patients who presented with acute MI at the CCU as compared with normal control subjects, since omentin-1 was expressed in visceral fat more than subcutaneous fat; thus, it decreased in the obesity and shows a negative correlation with BMI [14]. Low omentin-1 serum level was concerned with ischemic heart diseases [15]; also, omentin-1 induced NO production through activation of AMPK eNOS phosphorylation which suppressed p38-mediated e-selectin induction and so it improve vascular endothelial functions [16]. Therefore, omentin-1 effects on isolated rat blood vessels inhibit noradrenaline induced vasoconstriction and cause cardiac vasodilatations; also, omentin-1 reduced vascular endothelial dysfunction via amelioration of inflammatory process through inhibition of CRP, vascular endothelial growth factor (VEGF), NF-kB, and TNF [3]; these studies correspond with findings of present study which showed a significant reduction of omentin-1 serum concentrations in patients with acute MI in relation to BMI.
Thus, omentin-1 is regarded as protective adipokine in IHD and metabolic syndrome via inhibition of superoxide and free radical formations [17].
Moreover, findings of present study showed a considerable elevation in lipid profile and diminution in serum HDL level in relation to reduction of serum omentin-1 concentration.
Omentin-1 plays a potential role in lipid metabolism and insulin sensitivity thus, when omentin-1 decreased leading to insulin resistance and hyperinsulinemia which per se encourage hepatic triglyceride (TG) synthesis which blocks conversion of very low density lipoprotein (VLDL) into high density lipoprotein (HDL), this leads to high VLDL and low HDL which are concerned with progression of atherosclerosis and IHD; thus, omentin-1 is a protective factor in IHD [18, 19].
Regarding gender differences in omentin-1 serum level, female fat mass is higher than male fat mass, and because omentin-1 is negatively correlated with adiposity, since omentin-1 serum level is higher in male than female due to negative correlation between omentin-1 and ß-estradiol [20], this corresponds with our findings that showed a higher serum omentin-1 levels in males than females in patients with acute MI regardless of previous pharmacotherapy.
Moreover, a study showed that serum omentin-1 level did not differ significantly between males and females, and males with metabolic syndrome had 20% reduction in plasma omentin-1 as compared with females having the metabolic syndrome [21, 22].
The present study also showed that serum omentin-1 might predict cardiac measures in patients with acute MI.
Several adipokines and omentin-1 have beneficial roles, but the precise mechanism of these adipokines is still indistinct; also, low omentin-1 serum concentration may predict the occurrence of IHD [23]; in addition, the present study showed that serum omentin-1 levels positively correlated with cardiac troponin-I and this correlation may be of significance concerning peripheral biochemical disorders with cardiac ischemia.
Serum omentin-1 level was not correlated with BNP in heart failure, suggesting a different path-physiological progression of heart failure [24], but in IHD serum omentin-1 level is negatively correlated with cardiac troponin-I and IL-6, since both omentin-1 and IL-6 are produced by stromal vascular cells of fatty tissue with paracrine effect; so IL-6 may inhibit omentin-1 production, but cardiac troponin-I reflects cardiomyocyte damage that increased coincidentally with IL-6 [25].
Therefore, omentin-1 represents an optimistic biomarker for a prognosis of ischemic myocardial damage, when serum omentin-1 was low and high cardiac troponin-I indicates a poor prognosis, while normal or elevated omentin-1 serum level in IHD regardless of cardiac troponin-I indicates a good prognosis [26].
Metformin accounted for improving lipid profiles via decreasing plasma, serum levels of total cholesterol, triglyceride, LDL, VLDL, and increase in the HDL level [27]. Additionally, metformin was shown to lessen coagulation and accelerate fibrinolysis through inhibition of plasminogen activator factor inhibitor [28]; also, it reduced platelet activation and aggregation in the atherogenic vascular site [29].
Moreover, metformin was reported to prevent hyperglycemia induced cardiovascular damage through oxidative stress pathway since chronic hyperglycemia in DM activates protein kinase C (PKC) which leads to increases in the endothelial permeability, neutrophil activation, and cytokine stimulation, with all of these leading to vascular and cardiac complications of DM, therefore, metformin, leading to significant cardiovascular protection in diabetic patients through modulation of PKC [30, 31]. Long term effects of metformin lead to fatty acid oxidation stimulation and inhibition of PKC-NAD pathway which per se prevent vascular oxidative damage and inhibition of de novo diacylglycerol biosynthesis [32].
The pleiotropic effects of metformin are anti-ischemic activity, attenuation in ventricular postischemic dysfunction, raising tolerance to ischemia, and improving coronary blood flows leading to significant reduction in the incidence of MI [33].
So the present study confirmed the cardioprotective actions of metformin which might mediated, at least in part, via rising of omentin-1 serum level.
The cardioprotective mechanism of metformin was through activation of AMPK (which increases tolerance to ischemia), stimulation of endothelial NO synthase (which protects against ischemic-reperfusion), and anti-inflammatory effects via inhibition of IL-8, IL-6, IL-1, and TNF [34, 35].
Therefore, metformin increases serum omentin-1 levels which reflect improvement in insulin sensitivity and BMI; also, metformin is regarded as anti-inflammatory agent through modulation of certain cytokines that prevents the deleterious effects of inflammatory burdens on omentin-1 gene expression; so an elevation of omentin-1 serum level after metformin pharmacotherapy may be regarded as potential factor in prevention of diabetic cardiovascular complications [36, 37]; these facts explained the lower atherogenic index (AI) in metformin treated patients that presented with acute MI in the present study.
Additionally, metformin improves omentin-1 serum level even in normal healthy subjects [38]; from herein, metformin treatment even in nondiabetic patients produced a significant cardioprotection directly or indirectly through elevation of protective omentin-1 adipokine [39].
5. Conclusion
Metformin pharmacotherapy increases omentin-1 serum level and may be regarded as a potential agent in the prevention of the occurrences of acute MI in diabetic patients.
Acknowledgments
The authors would like to express thanks to the research committees and Professor Sadiq M. Alhamash, Dean of College of Medicine, Al-Mustansiriya University, Iraq, for his enormous maintaining in the march of scientific advancement and scientific reality in the Faculty of Medicine.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Lago F., Dieguez C., Gómez-Reino J., Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine & Growth Factor Reviews. 2007;18(3-4):313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 2.De Souza Batista C. M., Yang R.-Z., Lee M.-J., et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 3.Kazama K., Usui T., Okada M., Hara Y., Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. European Journal of Pharmacology. 2012;686(1–3):116–123. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Cherian E., Sudheesh N. P., Janardhanan K. K., Patani G. Free-radical scavenging and mitochondrial antioxidant activities of Reishi-Ganoderma lucidum (curt: Fr) P. Karst and arogyapacha-Trichopus zeylanicus gaertn extracts. Journal of Basic and Clinical Physiology and Pharmacology. 2009;20(4):289–307. doi: 10.1515/jbcpp.2009.20.4.289. [DOI] [PubMed] [Google Scholar]
- 5.Yan P., Li L., Yang M., et al. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on plasma omentin-1 levels in patients with type 2 diabetes mellitus. Diabetes Research and Clinical Practice. 2011;92(3):368–374. doi: 10.1016/j.diabres.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Shang F.-J., Wang J.-P., Liu X.-T., et al. Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers. 2011;16(8):657–662. doi: 10.3109/1354750x.2011.622789. [DOI] [PubMed] [Google Scholar]
- 7.Dilip C., Cholamugath S., Baby M., Pattani D. Prevalence of cardiovascular risk factors and management practices of acute coronary syndrome in a tertiary care hospital. Journal of Basic & Clinical Pharmacology. 2015;26(6):547–554. doi: 10.1515/jbcpp-2014-0055. [DOI] [PubMed] [Google Scholar]
- 8.Ouwens D. M., Sell H., Greulich S., Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. Journal of Cellular and Molecular Medicine. 2010;14(9):2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupinski J., Font A., Luque A., Turu M., Slevin M. Angiogenesis and inflammation in carotid atherosclerosis. Frontiers in Bioscience. 2008;13(17):6472–6482. doi: 10.2741/3167. [DOI] [PubMed] [Google Scholar]
- 10.Šenolt L., Polanská M., Filková M., et al. Vaspin and omentin: new adipokines differentially regulated at the site of inflammation in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2010;69(7):1410–1411. doi: 10.1136/ard.2009.119735. [DOI] [PubMed] [Google Scholar]
- 11.Mathew E. M., Rajiah K. Assessment of medication adherence in type-2 diabetes patients on poly pharmacy and the effect of patient counseling given to them in a multispecialty hospital. Journal of Basic and Clinical Pharmacy. 2014;5(1):15–18. doi: 10.4103/0976-0105.128251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acharya K. G., Shah K. N., Solanki N. D., Rana D. A. Evaluation of antidiabetic prescriptions, cost and adherence to treatment guidelines: a prospective, cross-sectional study at a tertiary care teaching hospital. Journal of Basic and Clinical Pharmacy. 2013;4(4):82–87. doi: 10.4103/0976-0105.121653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th. Boston, Mass, USA: Little, Brown and Company; 1994. [Google Scholar]
- 14.Yang R.-Z., Lee M.-J., Hu H., et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. American Journal of Physiology—Endocrinology and Metabolism. 2006;290(6):E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 15.Yamawaki H., Kameshima S., Usui T., Okada M., Hara Y. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochemical and Biophysical Research Communications. 2012;423(1):152–157. doi: 10.1016/j.bbrc.2012.05.103. [DOI] [PubMed] [Google Scholar]
- 16.Yamawaki H., Kuramoto J., Kameshima S., Usui T., Okada M., Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochemical and Biophysical Research Communications. 2011;408(2):339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Zhong X., Zhang H.-Y., Tan H., et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacologica Sinica. 2011;32(7):873–878. doi: 10.1038/aps.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z., Lee J. Omentin a novel adipokine in human adipose tissue. American Journal of Physiology: Endocrinology and Metabolism. 2008;290(6):1253–1281. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 19.Xie H., Xie P.-L., Wu X.-P., et al. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovascular Research. 2011;92(2):296–306. doi: 10.1093/cvr/cvr200. [DOI] [PubMed] [Google Scholar]
- 20.Tan B. K., Adya R., Farhatullah S., et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome. Ex vivo in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57(4):801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 21.Vu A., Sidhom M. S., Bredbeck B. C., Kosmiski L. A., Aquilante C. L. Evaluation of the relationship between circulating omentin-1 concentrations and components of the metabolic syndrome in adults without type 2 diabetes or cardiovascular disease. Diabetology & Metabolic Syndrome. 2014;6, article 4 doi: 10.1186/1758-5996-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo H. J., Hwang S. Y., Hong H. C., et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovascular Diabetology. 2011;10, article 103 doi: 10.1186/1475-2840-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsutamoto T., Tanaka T., Sakai H., et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. European Heart Journal. 2007;28(14):1723–1730. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- 24.Narumi T., Watanabe T., Kadowaki S., et al. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovascular Diabetology. 2014;13, article 84 doi: 10.1186/1475-2840-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunetti L., Leone S., Orlando G., et al. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacological Reports. 2014;66(6):991–995. doi: 10.1016/j.pharep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Wal P., Wal A., Nair V. R., Rai A. K., Pandey U. Management of coronary artery disease in a Tertiary Care Hospital. Journal of Basic and Clinical Pharmacy. 2013;4(2):31–35. doi: 10.4103/0976-0105.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michael U. A., David B. U., Theophine C. O., Philip F. U., Ogochukwu A. M., Benson V. A. Antidiabetic effect of combined aqueous leaf extract of vernonia amygdalina and metformin in rats. Journal of Basic and Clinical Pharmacy. 2010;1(3):197–202. [PMC free article] [PubMed] [Google Scholar]
- 28.Krysiak R., Gdula-Dymek A., Okopień B. Effect of metformin on selected parameters of hemostasis in fenofibrate-treated patients with impaired glucose tolerance. Pharmacological Reports. 2013;65(1):208–213. doi: 10.1016/s1734-1140(13)70980-0. [DOI] [PubMed] [Google Scholar]
- 29.Kabil Kucur S., Gozukara I., Aksoy A., et al. How medical treatment affects mean platelet volume as a cardiovascular risk marker in polycystic ovary syndrome? Blood Coagulation and Fibrinolysis. 2014 doi: 10.1097/mbc.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 30.Batchuluun B., Inoguchi T., Sonoda N., et al. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232(1):156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A. A., Jadhav P. R., Deshmukh Y. A. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. Journal of Basic and Clinical Pharmacy. 2014;5(3):p. 79. doi: 10.4103/0976-0105.139731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen T. M. D., Seigneurin F., Froment P., Combarnous Y., Blesbois E. The 5′-AMP-activated protein kinase (AMPK) is involved in the augmentation of antioxidant defenses in cryopreserved chicken sperm. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0134420.e0134420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farshad F., Gloria S., Nikolay P., Shiqin X. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. Journal of the American Heart Association. 2014;3(6) doi: 10.1161/JAHA.114.001202.e001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng S., Cao J., He Q., et al. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. The Journal of Biological Chemistry. 2015;290(6):3393–3802. doi: 10.1074/jbc.m114.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baus D., Heermeier K., De Hoop M., et al. Identification of a novel AS160 splice variant that regulates GLUT4 translocation and glucose-uptake in rat muscle cells. Cellular Signalling. 2008;20(12):2237–2246. doi: 10.1016/j.cellsig.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Feng W.-H., Yuan X.-W., Tong G.-Y., et al. Correlated increase of omentin-1 and adiponectin by exenatide, avandamet and dietary change in diet-induced obese rats. Folia Biologica. 2013;59(6):217–224. doi: 10.14712/fb2013059060217. [DOI] [PubMed] [Google Scholar]
- 37.Li S.-N., Wang X., Zeng Q.-T., et al. Metformin inhibits nuclear factor κB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart and Vessels. 2009;24(6):446–453. doi: 10.1007/s00380-008-1137-7. [DOI] [PubMed] [Google Scholar]
- 38.De Leo V., La Marca A., Orvieto R., Morgante G. Effect of metformin on insulin-like growth factor (IGF) I and IGF-binding protein I in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2000;85(4):1598–1600. doi: 10.1210/jc.85.4.1598. [DOI] [PubMed] [Google Scholar]
- 39.Preiss D., Lloyd S. M., Ford I., et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. The Lancet Diabetes and Endocrinology. 2014;2(2):116–124. doi: 10.1016/s2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]


