Abstract
Chicken breast dipped with citric acid (CA) was treated by sous vide processing and stored in a refrigerated state for 0, 3, 6, 9, and 14 d. A non-dipped control group (CON) and three groups dipped in different concentrations of citric acid concentration were analyzed (0.5%, 0.5CIT; 2.0%, 2CIT and 5.0%, 5CIT; w/v). Cooking yield and moisture content increased due to the citric acid. While the redness of the juice and meat in all groups showed significant increase during storage, the redness of the citric acid groups was reduced compared to the control group (p<0.05). The percentage of myoglobin denaturation (PMD) of the CA groups was also increased according to the level of CA during storage. Total aerobic counts, Enterobacteriaceae counts, volatile basic nitrogen and thiobarbituric acid reactive substances (TBARS) were generally lower in the citric acid-treated samples than in untreated ones, indicating extended shelf life of the cooked chicken breast dipped in citric acid solution. The shear force of the 2CIT and 5CIT groups was significantly lower (p<0.05). The findings indicated positive effects in the physicochemical properties and storage ability of sous vide chicken breast at 2% and 5% citric acid concentrations.
Keywords: citric acid, pink color, sous-vide, chicken breast
Introduction
Sous vide technology incorporates the control of temperature, cooking time and stability of the core temperature during the cooking of ingredients that are vacuum packaged later. After the process, the ingredients undergo a chilling process (Schellekens, 1996). Sous vide technology is well known for its minimal denaturation of protein and lipids via heat processing, as well as for the preservation of sensitive nutrients relative to cooked products. A variety of characteristics of several products treated with the sous vide process have been researched. In particular, meat products treated in such a way have shown improvement in tenderness and juiciness (Buck et al., 1979; Diaz et al., 2008; Sebastia et al., 2010) because heat denaturation of collagen and actin does not occur below 60℃ (Offer et al., 1984). The texture of meat products highly influences consumer sensory evaluation, and moreover, the tenderness of sous vide meat products can obtain more positive evaluations by minimizing the loss of water during cooking (Buck et al., 1979; Liu et al., 2004; Schilling et al., 2003). Because of the cooking temperature below 100℃, sous vide poultry meat was reported to have pink color defect, which ultimately induces a negative consumer perception (Heaton et al., 2000; Holownia et al., 2004). Therefore, many researchers have attempted to reduce the pink color defect of meat (Sammel and Claus, 2003; Sammel et al., 2007). Further, many have also tried to prevent appearance of the pink color by treating the ingredients with substances such as diethylenetriamine pentaacetic acid, trans 1,2-diaminocuclohexane-N,N,N’,N’ tetraacetic acid disodium salt, etc. However, such ingredients cannot be legally utilized as additives (Schwarz et al., 1997).
Due to consumer interest in healthy foods and protein intake, the consumption of broiler breast has slightly increased throughout the world (USDA, 2009). Researchers have reported improvement of the texture of roasted chicken breast with certain marinades, cooking temperatures, storage conditions, and control of the pH (Barbanti and Pasquini, 2005; Yang and Chen, 1993). Some researchers indicated that a marinade of citric acid improved the quality of meat products while reducing the pink color of sous vide products (Ke, 2006; Sammel and Claus, 2003). According to the Code of Federal Regulations (1998), the level of citric acid solution acceptable for additives allows marination of meat products at below 10%. However, citric acid marinade can reduce the pH of meat products at a certain point, as well as generate sour flavors, thereby leading to consumer repulsion. During refrigerated storage, the redness of the drip from sous vide chicken breasts tends to increase.
The objective of the present study was to investigate the effects of the level of citric acid on reduction of the pink color of chicken breasts treated with the sous vide process. Changes in other characteristics, including physicochemical, microbiological and sensory properties of the chicken breast, were also examined according to different concentrations of citric acid.
Materials and Methods
Preparation of sous vide products
In each of three independent replicate trials, freshly skinned and deboned chicken breast fillets (n=100, weight=100±5 g) were obtained from a local meat processing plant. The chicken breasts fillets were divided into four groups: 100 chicken breasts were randomly assigned to 4 treatments, each with 25 chicken breasts. CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution. The solutions containing citric acid at 0.5, 2 and 5% were prepared in distilled water. Each treatment was divided into 5 chicken breasts (about 100 g each) and allocated to the five periods. Chicken breasts were dipped in the citric acid solutions at a ratio of 10: 1 (chicken breast: citric acid solution, w/v) in polyamide-poly-propylene pouches, and the pouches were heat sealed using a vacuum sealing machine (FJ500XL, Fujee, Korea). The pouches were stored at 4℃ for 12h. After dipping process, the citric acid solutions were removed and the chicken breasts were then packaged in polyamide-poly-propylene pouches. The pouches were heat sealed using the vacuum machine and then cooked at 61℃ for 100 min with a water cascading retort (Diamond M, Julabo, Germany). The internal temperature at 61℃ for 30 min was measured with a thermocouple. After heating, the samples were immediately put through a chilling process for 10 min. The samples were then stored at 4℃ for 0, 4, 7, 10 and 14 d in a refrigerator.
Moisture content and cooking yield
The moisture content of the samples was determined using an official method (AOAC, 1995).
Cooking yield (%) = [Wt after cooking / Wt before cooking] × 100
Expressible drip
The measurement of expressible drip was carried out according to the method of Chantachum, Benjakul and Sriwirat (2000) with a slight modification. A weight of samples was placed between Whatman No. 1 filter papers on top and below. A 0.5 g weight was then placed on top within 30 s and held for 2 min. The refrigerated samples were placed at room temperature (25℃) for 20 min and the samples were pressed at 100 tons/m2 using a hydraulic press (Ilshintech. Co., LTD, Korea). The samples were then removed and weighed (B). The drip under pressure was determined as [(A − B)/A] × 100 (Hasegawa, 1987).
pH and warner bratzler shear force
The pH of the samples was measured with a pH meter (pH 900, Precisa Co, Dietikon, Switzerland). For measurement, 2 g samples were homogenized with 18 mL of distilled water for 90 s using a Bag mixer 400 (Interscience Co, St Nom la Bretêche, France).
For measurement of the shear value was carried out following as the method of Juneja et al. (2006). 4 cm strips were cut from the chicken breast samples (Height 1.5 cm). The shear value was then measured on a texture analyzer (TA-XT2, Stable micro system, Scarsdale, NY) equipped with a Warner Bratzler shear force cell under a cross head speed of 2 mm/sec. The height of the blade was set 60 mm above the base. The unit used for the shear force was kg/cm2.
Visible spectrum analysis
The redness of meat juices was analyzed by using slightly modified Liu and Chen (2001) and Milar, Moss and Stevenson (1996) methods. The redness of the samples was measured by determination of the absorbance of the juices from each sample. The juice of the sous vide products was collected in a tube, and the tubes were centrifuged at 4000 rpm for 15 min. They were then filtered through Whatman No. 1 filter paper, and the absorbance at 550 nm was measured using a spectrophotometer (Optizen 2120UV, Mecasys, Korea).
Determination of percentage of myoglobin denaturation (PMD) in sous vide products
The myoglobin extraction was carried out according to the method of Warris (1979). Briefly, 4 g samples were minced with 20 mL phosphate buffer solution (pH 6.8) using a homogenizer (AM-7, Nihonseiki Kaisha, Japan) at 13,000 rpm for 10 s. The minced samples were stored at 4℃ for 1 h, and centrifuged at 5,000 g for 30 min at 5℃. The supernatant was filtered through Whatman No.1 paper and the absorbance at 525 and 700 nm was measured by spectrophotometer (Optizen 2120 UV, Mecasys, Korea).
Myoglobin (mg/mL) = (A525 − A700) × 2.303 × dilution factor
Percentage of myoglobin denaturation (PMD %) = [1 − (myoglobin concentration after cooking / myoglobin concentration before cooking)] × 100
Measurement of color
The instrumental color of the samples, internal and external, was measured using a colorimeter (NR-300, Nippon Denshoku, Japan). The samples were placed at room temperature, and then sliced through the center parallel to the surface. The machine was calibrated with a white plate (CIE L* = +94.48, a* = −0.67, b*= +3.31). Values for CIE L* (lightness), CIE a* (redness), and CIE b*(yellowness) were expressed.
Determination of thiobarbituric acid reactive substances (TBARS)
TBA values of the sous vide products stored for different time periods were determined according to a method modified from Witte et al. (1970). The absorbance of the supernatant was measured at 532 nm using a spectrophotometer (Optizen 2120UV, Mecasys, Korea), and the results were expressed as mg malonaldehyde (MDA)/kg sample.
Determination of volatile basic nitrogen (VBN)
VBN of the sous vide products stored for different periods was determined according to the method of Conway (1950). Briefly, 5 g of each sample was blended with 15 mL of distilled water and then homogenized at 12,000 rpm for 1 min, after which distilled water was added up to 50 mL. Filtration was then carried out with Whatman No. 1 filter paper. The Conway cover was greased with Vaseline for sealing, and then 1 mL of mixed liquid was added to the central well and 1 mL of 0.01 N H3BO3 was added the outer well. Following in this step, 100 uL of Conway reagent was added to the central well and 1 mL of 50% K2CO3 was added to the outer well. After the cell was shaken horizontally, it was incubated at 37℃ for 2 h. The mixture was then titrated with 0.02 N H2SO4. Finally, the VBN was calculated as follows:
- a, b:
titration of the sample and blank, respectively (amounts (mL) of 0.02 N H2SO4);
- d:
1/15 (dilution ratio of 1mL used to 15 mL);
- S:
the amount of sample (g);
- F:
1.0 (factor of 0.02 N H2SO4 used).
The results were expressed as VBN values (mg/kg meat).
Microbiological analysis
Sampling was performed during storage (0, 4, 7, 10 and 14 d). For each time period, 2 g samples were homogenized in 18 mL of sterile 0.85% NaCl solution for 90 s with a bag mixer. Subsequent 101 serial dilutions were carried out for analysis.
Total plate counts were enumerated on standard plate count agar (Difco, USA) after 24-48 h of incubation at 36-37℃. Enterobacteriaceae count was carried out on Petri film Enterobacteriaceae count plates (3M) after incubation for 24 at 35-37℃, according to the Food and Drug Administration (FDA) method.
Statistical analysis
A 4-treatment and 5-storage-period factorial design was carried out, and data were analyzed by analysis of variance (ANOVA). On each storage days, 5 replications per treatment were analyzed, for a total of 25 replications. All statistical data were analyzed using the General Linear Model (GLM) procedure of SPSS 19.0 (SPSS, Inc., USA). The means were further compared for significance (p<0.05) using the Tukey test between groups and periods. Pearson correlation coefficients were calculated among the measured variables at a 95% confidence level.
Results and Discussion
The effect of citric acid on cooking yield, expressible drip and moisture content of chicken breast treated with sous vide process
Moisture content varied among treatments and ranged from 67.11 to 70.73%. Moisture content of 0.5CIT, 2CIT and 5CIT was significantly higher than that of CON (p<0.05, Table 1). The sample marinated with citric acid solution resulted in higher moisture content and cooking yield. The similar result was reported by Serdaroglu, Abdraimov and Onenc (2006). Due to immersion in acidic treatments, which influences the uptake of water compared to non-treated groups has been reported (Burke and Monahan, 2003; Ke et al., 2009). Other studies also reported that the acid concentration demonstrated a positive correlation with the moisture content of meat products marinated in weak acid (Aktas and Kaya, 2001; Aktas et al., 2003).
Table 1. Effects of citric acid on cooking yield, expressible drip, and moisture content of sous vide cooked broiler breast.
| Contents (%) | Treatments |
|||||
|---|---|---|---|---|---|---|
| CON | 0.5CIT | 2CIT | 5CIT | SEM | p-value | |
| Cooking yield | 79.43b | 80.47b | 81.51b | 85.32a | 0.78 | <0.001 |
| Expressible drip | 39.30ns | 38.02 | 37.03 | 35.08 | 1.17 | 0.15 |
| Moisture | 67.11b | 69.60a | 69.10ab | 70.73a | 0.79 | 0.07 |
All values are the means of three replicates (n=15); -, not detected.
1)CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution.
2)NS, not significant.
3)Different superscript letters in the same row indicate significant differences, p<0.05.
Change in pH during storage
The pH value of the chicken breast in all groups increased slightly during refrigerated storage (Table 2). The citric acid level affected the pH value during storage, appearing at the pH of CON > 0.5CIT > 2CIT > 5CIT. The pH of 5CIT was significantly lower than all other groups (p<0.05). Rio et al. (2007) stated that immersion of chicken meat in citric acid significantly decreased the pH after marination. The low pH value of meat products is known to influence several factors during storage, such as loss of redness, prolongation of storage, stability of water binding capacity, and texture (Sammel and Claus, 2003). It is well known that myosin plays an important role in water-binding, and the isoelectric point of this protein is 5.2- 5.3 in chicken meat (Alvarado and McKee, 2007; Castellini et al., 2002). Similar results are also found in the present study, in that the tenderness and shearforce of the meat products were affected by the change in pH.
Table 2. Effect of citric acid on pH of sous-vide cooked broiler breast.
| Treatments | Storage (d) |
SEM |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 14 | Treatment | Time | Treatment×Time | ||
| CON | 6.55a C | 6.56a C | 6.55a C | 6.60a B | 6.71a A | 0.04 | <0.001 | <0.001 | 0.103 |
| 0.5CIT | 6.33ab C | 6.32b C | 6.38b B | 6.41b B | 6.48b A | ||||
| 2CIT | 6.12b A | 6.27b B | 6.26c B | 6.36b A | 6.36c A | ||||
| 5CIT | 5.63c E | 5.71c D | 5.85d C | 5.89c B | 5.97d A | ||||
| SEM | 0.10 | 0.07 | 0.02 | 0.06 | 0.03 | ||||
All values are the means of three replicates (n=15); -, not detected.
1)CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution.
2)NS, not significant.
3)a-dDifferent superscript letters in the same column indicate significant differences among all groups, p<0.05.
4)A-EDifferent superscript letters in the same row indicate significant differences among periods.
The effect of citric acid on volatile basic nitrogen of chicken breast processed by the sous vide method
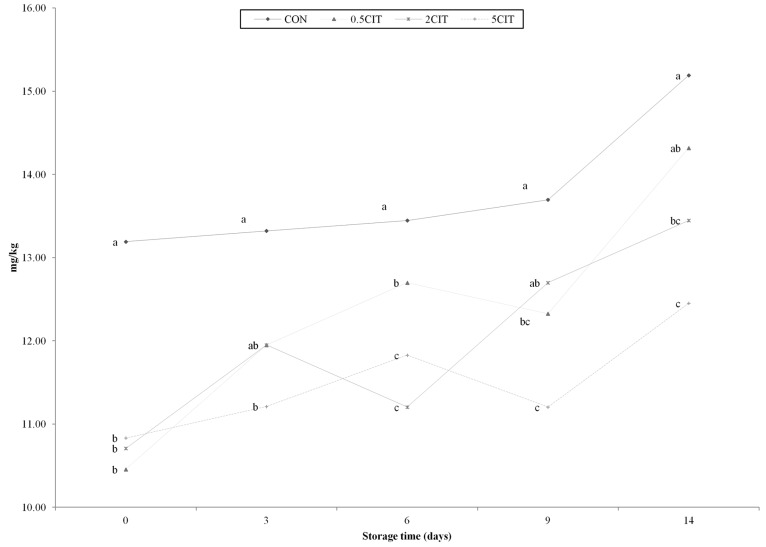
The volatile basic nitrogen of all groups increased during refrigerated storage (Fig. 1). According to previous studies, such an increasing tendency may be the result of the deamination of amnino acids and the production of ammonia during storage (Byun et al., 2003). In general, VBN is used for the determination of the major parameters of fresh poultry meat. The VBN values of all citric acid groups were significantly lower than that of CON during storage (p<0.05), decreasing according to the level of citric acid. Several studies also reported VBN to be an indicator of the quantity of biogenic amines, which are produced in the microbiological contamination of foods (Min et al., 2004; Min et al., 2007; Vinci and Antonelli, 2002). Therefore, the antimicrobial effects of citric acid may have caused the relative decrease in VBN during storage.
Fig. 1. Effect of citric acid on volatile basic nitrogen of packaged sous vide broiler breast. CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution. Different letters at a time point indicate significant differences, p<0.05.
The effect of citric acid on lipid oxidation in chicken breast processed by the sous vide method
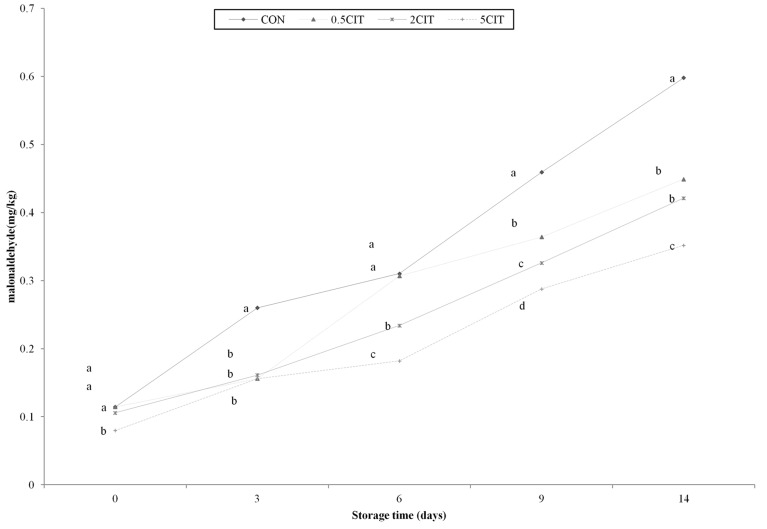
Acid treatment is well known to increase the water-holding capacity and texture properties of meat products. However, several reports concluded that there are negative effects on lipid oxidation stability in muscle foods above certain acid concentrations (Morrissey et al., 1998; Ogden et al., 1995). A significant effect of the treatments and storage time on lipid oxidation was observed in all samples (p<0.05). At the initial phase, the treatment containing 5% CA had the lowest TBA values among the groups (p<0.05), while significant differences were not observed among the control group, 0.5CIT and 2CIT. The TBA value of the sous vide chicken breast tended to increase during storage (Fig. 2). The lowest value of TBA was found in 5CIT during storage (p<0.05). The TBA values of all groups decreased with increasing CA concetration, in the following order: CON > 0.5CIT > 2CIT > 5CIT. In addition, Sommers et al. (2003) suggested that antioxidnat effect of citric acid was proved in meat product treated with citric acid. Ke et al. (2009) suggested that the inhibition of lipid oxidation was proven because of elimination of heme proteins. The inhibition of lipid oxidation in sous-vide products treated with citric acid could be influenced the formation of denatured heme proteins by decreasing pH of meat. Overall, citric acid may act as a food acidulant, antioxidant and a metal chelator (Contini et al., 2014).
Fig. 2. Effect of TBA value of packaged sous vide broiler breast during storage. CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution. Different letters at a time point indicate significant differences, p<0.05.
Changes in meat, juice color and denatured myoglobin contents during refrigerated storage
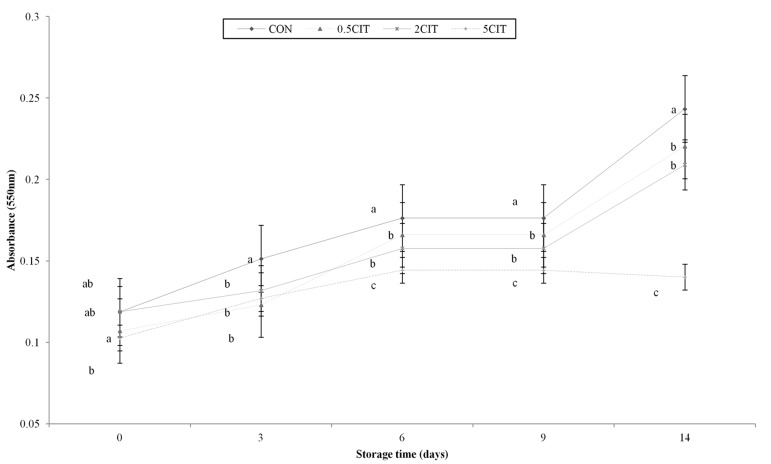
The pink color, related to the visual appearance analysis, indicates the degree of redness of the meat juice, as shown in Fig. 3. The changes in meat color during storage were shown in Table 3. The absorbance of pink color in the juice of all groups significantly increased during refrigerated storage (p<0.05). Likewise, the external and internal redness (CIE a*) in all groups increased significantly during storage (p<0.05). A significant treatment × time interaction was not detected in internal redness (p>0.05), while there was a significant treatment × time interaction in external redness (p<0.05). The increment of external redness during the storage might be expected that water-soluble heme protein appeared from the internal chicken breast of these samples (Berry et al., 2003). Citric acid treated groups reduced redness when compared to both internal and external redness of CON (p<0.05). The redness of meat color was positively correlated with the absorbance of pink color (r=0.431, p=0.001). The increase in redness of vacuum packaged meat products during storage was reported by Nam and Ahn (2002) and the pink color of the juice may be influenced by pink pigments leaking from the meat product. However, a decrease in the intensity of pink color relative to the control was observed during storage, according to the increasing the level of CA. As the increase in the level of CA also induced the denaturation of myoglobin due to reduced thermal stability, the PMD of all groups were significantly increased during refrigerated storage (p<0.05, Table 4). Because of myoglobin with lower thermal stability, increasing PMD during refrigerated storage was also reported by Chen (2003). The pink color is associated with the formation of globinhemochrome by the denaturation of myoglobin in vacuum packed cooked meat products during storage (Belitz et al, 2004; King and Whyte, 2006; Suman and Joseph, 2013). The pink color of meat was also shown to increase due to the higher thermal stability of the pink color-conferring form of myoglobin in meat at high pH (Hunt et al., 1999). Herein, the increase in the level of CA showed a tendency to reduce the redness of the juice during storage. Although the pink color of 0.5CIT and 2CIT were significantly lower than the non-treated group (p<0.05), no significant difference was found between 0.5 CIT and 2 CIT during storage. Some researchers reported the reduction of the pink color defect in cooked meat marinated with citric acid, as marinade with fruit juice plentiful in citrus acid reduced the redness of meat products during storage (Lynch and Faustman, 2000; Sammel and Claus, 2003). The decrease in the red color of meat products during storage was deduced to occur due to the correlation between lipid and pigment oxidation in meats. This result could be explained by the binding of the heme iron of myoglobin with citric acid or the prevention of the formation of pink pigments by acidification (Kieffer et al., 2000). The external lightness (CIE L*) of all groups was significantly decreased, while the external yellowness (CIE b*) of all groups was increased during storage (p<0.05). Nevertheless, the increase in the level of CA enhanced the lightness of the meat (p<0.05). This result may be induced by the higher moisture content and lower expressible drip due to samples treated with citric acid (Bojarska et al., 2003). Moreover, the ratio of the increased external yellowness in all groups was higher compared to the internal yellowness during storage.
Fig. 3. Changes in pink color of packaged sous vide broiler breast during storage. CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution. Different letters at a time point indicate significant differences, p<0.05.
Table 3. Effect of citric acid on color (lightness, redness and yellowness) of sous-vide cooked broiler breast.
| Place | Contents | Treatments | Storage (d) |
SEM |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 14 | Treatment | Time | Time×Treatment | ||||
| Internal | CIE L* | CON | 82.81bC | 83.85nsB | 83.81bB | 85.01nsA | 85.12bA | 0.30 | 0.001 | 0.395 | 0.770 |
| 0.5CIT | 84.18aC | 84.48C | 83.97abC | 84.24C | 83.40abC | ||||||
| 2CIT | 83.47aC | 84.25C | 84.34aC | 83.70B | 85.44abA | ||||||
| 5CIT | 83.37aC | 84.20BC | 84.26aB | 85.21AB | 86.27aA | ||||||
| SEM | 0.31 | 0.28 | 0.28 | 0.31 | 0.47 | ||||||
| CIE a* | CON | 4.41aC | 4.47nsB | 5.83aA | 4.42aB | 4.68aAB | 0.21 | <0.001 | 0.005 | 0.055 | |
| 0.5CIT | 2.41bC | 3.57B | 4.01abA | 4.26aA | 4.58aA | ||||||
| 2CIT | 3.78bC | 4.39A | 3.75bAB | 3.95abB | 2.73bD | ||||||
| 5CIT | 3.05bC | 3.96A | 3.96bA | 3.82bAB | 3.61abB | ||||||
| SEM | 0.22 | 0.20 | 0.20 | 0.22 | 0.28 | ||||||
| CIE b* | CON | 10.69nsNS | 10.08b | 10.23ns | 9.84b | 11.93a | 0.26 | 0.276 | 0.027 | 0.362 | |
| 0.5CIT | 11.41A | 11.73aA | 9.90B | 10.30aAB | 10.02bAB | ||||||
| 2CIT | 10.17NS | 10.33ab | 9.60 | 10.22a | 10.77ab | ||||||
| 5CIT | 10.38AB | 10.89ab A | 9.50B | 9.85bAB | 9.40bB | ||||||
| SEM | 0.28 | 0.25 | 0.25 | 0.28 | 0.34 | ||||||
| External | CIE L* | CON | 84.51aA | 80.23aC | 81.71nsBC | 79.27bC | 82.89nsB | 0.37 | 0.012 | 0.821 | 0.015 |
| 0.5CIT | 81.81bB | 83.54bA | 82.13B | 82.20aB | 82.04B | ||||||
| 2CIT | 82.89bNS | 82.89ab | 81.51 | 81.60ab | 82.37 | ||||||
| 5CIT | 83.13ab A | 81.93abB | 81.42B | 81.48abB | 81.64B | ||||||
| SEM | 1.01 | 0.65 | 1.01 | 0.95 | 0.52 | ||||||
| CIE a* | CON | 3.07nsC | 4.68aB | 4.03nsB | 5.16aA | 4.3aB | 0.16 | 0.018 | <0.001 | 0.003 | |
| 0.5CIT | 3.45A | 2.72bB | 3.39A | 3.67abA | 3.89abA | ||||||
| 2CIT | 2.81C | 3.41bA | 3.99B | 3.89abB | 3.19abC | ||||||
| 5CIT | 3.33A | 3.27bB | 3.35A | 3.03bB | 2.86bC | ||||||
| SEM | 0.45 | 0.30 | 0.35 | 0.53 | 0.30 | ||||||
| CIE b* | CON | 10.15bB | 12.33A | 12.61nsA | 12.35nsA | 11.97bAB | 0.33 | 0.001 | 0.250 | 0.682 | |
| 0.5CIT | 11.72aB | 12.45nABs | 12.95AB | 13.06A | 11.94bB | ||||||
| 2CIT | 11.22abB | 13.66A | 11.75B | 11.85B | 13.66aA | ||||||
| 5CIT | 11.08abB | 13.12A | 12.92AB | 13.08A | 13.35abA | ||||||
| SEM | 1.52 | 0.37 | 0.65 | 0.73 | 0.38 | ||||||
All values are the means of three replicates (n=15); -, not detected.
1)CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution.
2)NS, not significant.
3)a-dDifferent superscript letters in the same column indicate significant differences among all groups, p<0.05.
4)A-EDifferent superscript letters in the same row indicate significant differences among periods.
Table 4. Effect of citric acid on percentage of myoglobin denaturation (PMD) of sous-vide cooked broiler breast (%).
| Contents | Treatments | Storage (d) |
SEM |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 14 | Time | Treatment | Time×Treatment | |||
| PMD (%) | CON | 63.23cC | 55.29cD | 69.31B | 85.32bA | 85.66nsA | 0.75 | <0.001 | <0.001 | <0.001 |
| 0.5CIT | 72.49bcC | 65.87bEc | 71.96bC | 80.95bB | 86.72A | |||||
| 2CIT | 73.28bC | 71.96bC | 74.87bB | 82.41bA | 86.98A | |||||
| 5CIT | 82.01aB | 78.30aC | 82.54aB | 90.34aA | 89.10A | |||||
| SEM | 0.94 | 1.23 | 2.55 | 0.76 | 0.79 | |||||
All values are the means of three replicates (n=15); -, not detected.
1)CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution.
2)NS, not significant.
3)a-dDifferent superscript letters in the same column indicate significant differences among all groups, p<0.05.
4)A-EDifferent superscript letters in the same row indicate significant differences among periods.
Shear force
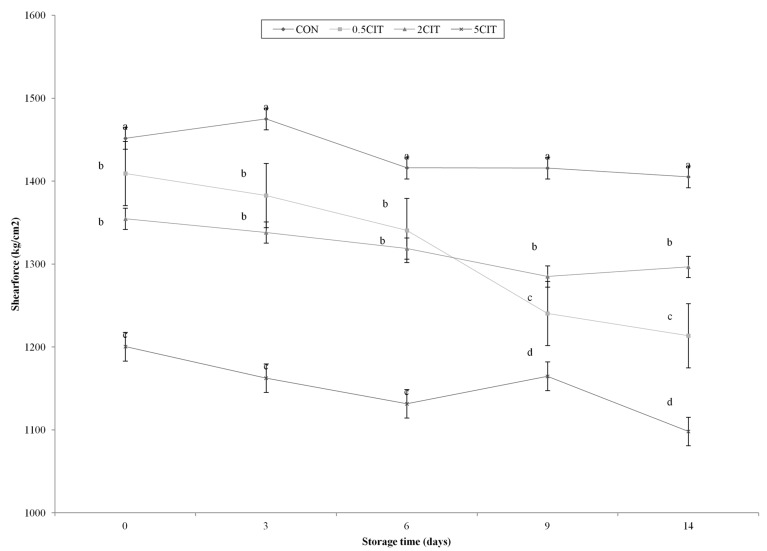
All groups showed a decline of the shear force during storage. Lipid oxidation affects the decrease in tenderness of meat products, which can be related to the interaction of lipid oxidation and cross-linking of proteins (Lund et al., 2007; Xiong et al., 2009). The shear forces of the CA groups were significantly lower than that of CON during storage (p<0.05) (Fig. 4). The decrease in shear force of the meat products with increasing citric acid concentration was due to the reduction of pH, which influences the water binding capacity (Burke and Monahan, 2003). Okeudo and Moss (2005) was demonstrated that shear force has negative correlation with cooking yield and moisture content. Especially, the shear force of 5CIT was significantly lower than that of the other groups (p<0.05). The denaturation of muscle protein due to treatment in acidic solutions is known to induce tissue breakdown and decrease the tenderness of meat (Aktas and Kaya, 2001; Ke et al., 2009). Therefore, denaturation of protein and moisture content could influence the reduction of shear force due to samples treated with citric acid.
Fig. 4. Changes in shear force of packaged sous vide broiler breast duirng storage. CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution. Different letters at a time point indicate significant differences, p<0.05.
Total plate count and enterobacteriaceae counts
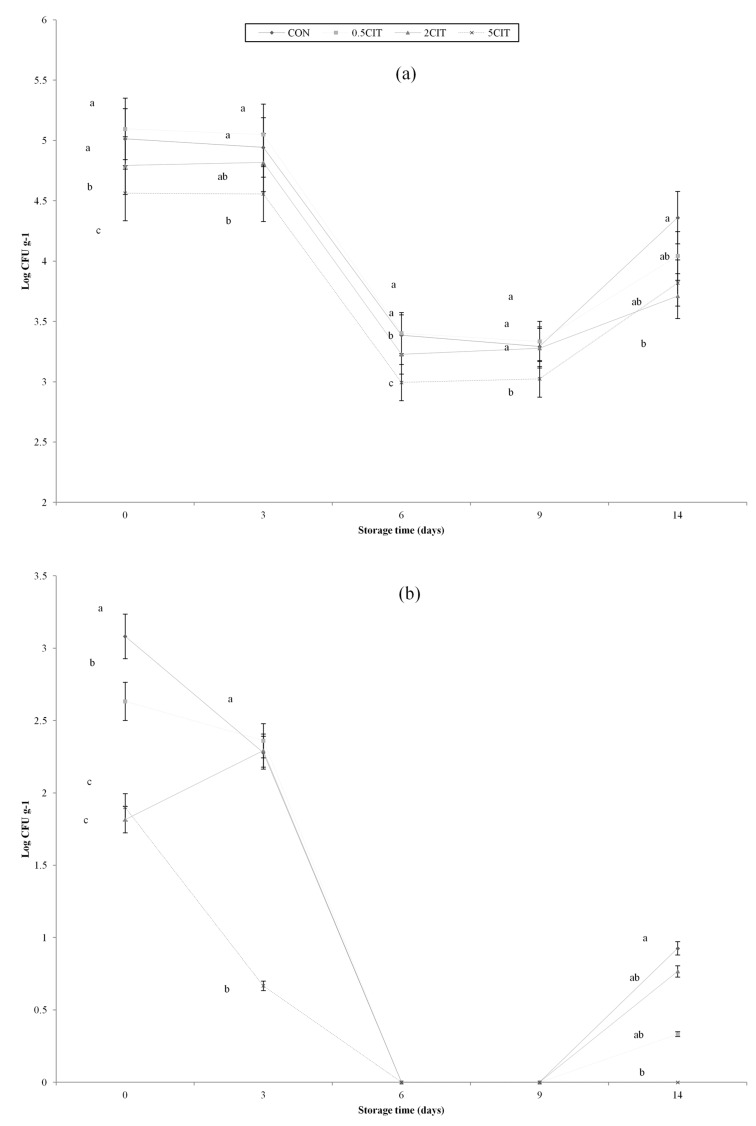
Total plate counts of all groups were between 4.5-5.0 Log CFU g−1 at on average at the start (day 0), and were significantly decreased during the first half of storage (p<0.05) (Fig. 5). At the initial phase, the number of Enterobacteriaceae in CON was higher than those of the other groups; further, the Enterobacteriaceae of all groups significantly decreased during the first half of storage (p<0.05). A similar result of the growth of Enterobacteriaceae in sous-vide products was reported by Garcia-Linares et al. (2004). Other reports showed that sous vide processed meat products showed excellent stability of microbial growth during refrigerated storage (Hansen et al., 1995; Wang et al., 2004). Cold shock of the microorganisms through the cooking and chilling processes contributed to the decrease in the number of total viable counts (Mcdonald, 2000). The microbial growth of CON and 0.5CIT rapidly increased again to about 4.0 Log CFU g-1 by day 14. These results were similar to the study of Garcia-Linares et al. (2004), that the reduction in microbial growth could be explained by heat processing, which induces the pasteurization of heat resistant bacteria. Through 2CIT and 5CIT showed a slight increasing tendency at day 14, the rate of increase was significantly lower than CON (p<0.05). This result might imply that both control of the pH and the chelating properties of citric acid proved the antimicrobial effects (Alakomi et al., 2007; No et al., 2002).
Fig. 5. Changes in microbiological counts of packaged sous vide broiler breast during storage (Log CFU/g). (a) Total plate count. (b) Enterobacteriaceae Count CON, breast muscle treated without citric acid solution; 0.5CIT, dipped in 0.5% citric acid solution; 2CIT, dipped in 2% citric acid solution; 5CIT, dipped in 5% citric acid solution. Different letters at a time point indicate significant differences, p<0.05.
Conclusion
Dipping in citric acid solution was proven to prevent the redness in meat products conferred by the sous-vide process. The increase in CA concentration had a positive influence on reduction of the pink color by inducing the thermal denaturation of myoglobin during refrigerated storage. Increasing CA concentration also had an antibacterial effect on the growth of microorganisms. Moreover, the 5CIT group showed excellent prevention of lipid oxidation compared to the other groups. Consequently, 2CIT and 5CIT showed great reduction of the pink color in juice and lipid oxidation stability. For these reasons, 2% and 5% citric acid solutions were found to be suitable for sous vide processed chicken breasts.
Acknowledgments
This research was supported by the Technology Commercialization Support Program, iPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
References
- 1.AOAC. Official methods of analysis of AOAC. 17th Ed. Association of Official Analytical Chemists; Washington D.C.: (1995). [Google Scholar]
- 2.Aktas N., Aksu M., Kaya M. The effect of organic acid marinade on tenderness, cooking loss and bound water content of beef. J. Muscle Foods. (2003);14:181–194. doi: 10.1111/j.1745-4573.2003.tb00699.x. [DOI] [Google Scholar]
- 3.Aktas N., Kaya M. The influence of marinating with weak organic acids and salts on the intramuscular connective tissue and sensory properties of beef. Eur. Food Res. Techn.ol. (2001);213:88–94. doi: 10.1007/s002170100329. [DOI] [Google Scholar]
- 4.Alakomi H. L., Puupponen-pimia R., Aura A. M., Helader I. M., Nohynek L., Oksman-Caldentey K. M. Weakening of Salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J. Agr. Food Chem. (2007);55:3905–3912. doi: 10.1021/jf070190y. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado C., McKee S. Marination to improve functional properties and safety of poultry meat. J. Appl. Poult. Res. (2007);16:113–120. doi: 10.1093/japr/16.1.113. [DOI] [Google Scholar]
- 6.Barbanti D., Pasquini M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT-Food Sci. Technol. (2005);38:895–901. doi: 10.1016/j.lwt.2004.08.017. [DOI] [Google Scholar]
- 7.Belitz H. D., Grosch W., Schieberle P. Food Chemistry. 4th ed. Springer; Berlin: (2004). Fruits and fruit products [Google Scholar]
- 8.Holzer Z., Berry B. W., Campbell A. M., Spanier A. M., Solomon M. B. Effect of koshering and hydrodynamic pressure on beef color, odor, and microbial loads. J. Muscle Foods. (2003);15:69–82. [Google Scholar]
- 9.Bojarska U., Batura J., Cierach M. The effect of measurement site on the evaluation of tom breast muscle color. Polish J. Food Nutr. Sci. (2003);53:45–49. [Google Scholar]
- 10.Burke R. M., Monahan F. J. The tenderization of shin beef using a citrus juice marinade. Meat Sci. (2003);63:161–168. doi: 10.1016/S0309-1740(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 11.Buck E. M., Hickey A. M., Rosenau J. Low temperature air oven vs a water bath for the preparation of rare beef. J. Food Sci. (1979);44:1602–1605. doi: 10.1111/j.1365-2621.1979.tb09099.x. [DOI] [Google Scholar]
- 12.Byun J. S., Min J. S., Kim I. S., Kim J. W., Chung M. S., Lee M. Comparison of indicators of microbial quality of meat during aerobic cold storage. J. Food Prot. (2003);66:1733–1737. doi: 10.4315/0362-028x-66.9.1733. [DOI] [PubMed] [Google Scholar]
- 13.Castellini C., Mugani C., Boso A. D. Effect of organic production system on broiler carcass and meat quality. Meat Sci. (2002);60:219–225. doi: 10.1016/S0309-1740(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 14.Chantachum S., Benjakul S., Sriwirat N. Separation and quality of fish oil from precooked and non-precooked tuna heads. Food Chem. (2000);69:289–294. doi: 10.1016/S0308-8146(99)00266-6. [DOI] [Google Scholar]
- 15.Chen H. H. Effect of cold storage on the stability of chub and horse mackerel myoglobins. J. Food Sci. (2003);68:1416–1419. doi: 10.1111/j.1365-2621.2003.tb09659.x. [DOI] [Google Scholar]
- 16.Contini C., Alavarez R., O’Sullivan M., Dowling D. P., Gargan S. O., Monahan F. J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. (2014);96:1171–1176. doi: 10.1016/j.meatsci.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Conway E. J. Micro diffusion analysis and volumetric error. Crosby, Lockwood and Son Ltd.; London: (1950). [Google Scholar]
- 18.Code of Federal Regulations. Approval of substances for use in the preparation of food products. Code of Federal Regulations, Title 9. Chapter 3. Part 318. (9CFR318.7) US Government Printing Office; Washington, DC: (1998). pp. 241–256. [Google Scholar]
- 19.Diaz P., Nieto G., Garrido M. D., Banon S. Microbial, physical-chemical and sensory spoilage during the refrigerated storage of cooked pork loin processed by the sous vide method. Meat Sci. (2008);80:287–292. doi: 10.1016/j.meatsci.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Rio D. E., Muriente R., Prieto M., Alonso-calleja C., Capite R. Effectiveness of trisodiumphosphate, acidified sodium chlorite, citric acid and peroxy acids against pathogenic bacteria on poultry during refrigerated storage. J. Food Protect. (2007);70:2063–2071. doi: 10.4315/0362-028x-70.9.2063. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Linares M. C., Gonzalez-Fofandos E., Garcia-Fernandez M. C., Garcia-Arias M. T. Microbiological and nutritional quality of sous vide or traditionally processed fish: Influence of fat content. J. Food Quality. (2004);27:371–387. doi: 10.1111/j.1745-4557.2004.00676.x. [DOI] [Google Scholar]
- 22.Gault N. F. S. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. (1985);15:15–30. doi: 10.1016/0309-1740(85)90071-3. [DOI] [PubMed] [Google Scholar]
- 23.Hansen T. B., Knochel S., Juncher D., Bertelsen G. Storage characteristics of sous vide cooked roast beef. Int. J. Food Sci. Technol. (1995);30:365–378. [Google Scholar]
- 24.Hasegawa H. Laboratory manual on analytical methods and procedures for fish and fish products. Marine Fishiers Research Department, Southeast Asian Fisheries Development Center; Singapore: (1987). [Google Scholar]
- 25.Heaton K. M., Cornforth D. P., Moiseeve I. V., Egbert W. R., Carpenter C. E. Minimum sodium nitrate levels for pinking of various cooked meats as related to use of direct or indirect- dried soy isolates in poultry roll. Meat Sci. (2000);55:321–329. doi: 10.1016/S0309-1740(99)00160-6. [DOI] [PubMed] [Google Scholar]
- 26.Holownia K., Chinnan M. S., Reynolds A. E., Davis J. W. Relating induced in situ conditions of raw chicken breast meat to pinking. Poult.. Sci. (2004);83:109–118. doi: 10.1093/ps/83.1.109. [DOI] [PubMed] [Google Scholar]
- 27.Hunt M. C., Sørheim O., Slinde E. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. (1999);64:847–851. doi: 10.1111/j.1365-2621.1999.tb15925.x. [DOI] [Google Scholar]
- 28.Juneja V. K., Fan X., P-Ramos A., D-Cinco M., P-Aguilar R. The effect of grapefruit extract and temperature abuse on growth of Clostridium perfrigens from spore inocula in marinated, sous-vide chicken products. Innov. Food Sci. Emerg. (2006);7:100–106. doi: 10.1016/j.ifset.2005.09.004. [DOI] [Google Scholar]
- 29.Mcdonald K., Sun D. W., Kenny T. Comparison of the quality of cooked beef products cooled by vacuum cooling and by conventional cooling. Lebensm-Wiss. u.-Technol. (2000);33:21–29. doi: 10.1006/fstl.1999.0603. [DOI] [Google Scholar]
- 30.Ke S. Effect of pH and salts on tenderness and water-holding capacity of muscle foods. Ph.D thesis. Massachisetts Univ. Amherst, US; (2006). [Google Scholar]
- 31.Ke S., Hung Y., Decker E. A., Hultin H. O. Impact of citric acid on the tenderness, microstructure and oxidative stability of beef muscle. Meat Sci. (2009);82:113–118. doi: 10.1016/j.meatsci.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Kieffer K. J., Claus J. R., Wang H. Inhibition of pink color development in cooked uncured ground turkey by the addition of citric acid. J. Muscle Foods. (2000);11:235–243. [Google Scholar]
- 33.King N. J., Whyte R. Does it look cooked? A review of factors that influence cooked meat color. J. Food Sci. (2006);71:31–40. [Google Scholar]
- 34.Liu Y., Lyon B. G., Windham W. R., Lyon C. E., Savage E. M. Principal component analysis of physical, color, and sensory characteristics of chicken breast deboned at two, four, six, and twenty-four hours postmortem. Poult. Sci. (2004);83:101–108. doi: 10.1093/ps/83.1.101. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Chen Y. R. Analysis of visible reflectance spectra of stored, cooked and diseased chicken meats. Meat Sci. (2001);58:395–401. doi: 10.1016/s0309-1740(01)00041-9. [DOI] [PubMed] [Google Scholar]
- 36.Lund M. N., Lametsch R., Hviid M. S., Jensen O. N., Skibsted L. H. High oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. (2007);77:295–303. doi: 10.1016/j.meatsci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Effect of aldehyde lipid oxidation products on myoglobin. J. Agr. Food Chem. (2000);48:600–604. doi: 10.1021/jf990732e. [DOI] [PubMed] [Google Scholar]
- 38.Milar S. J., Moss B. W., Stevenson M. H. Some observation on the absorbance spectra of various myoglobin derivatives found in meat. Meat Sci. (1996);42:277–288. doi: 10.1016/0309-1740(94)00045-x. [DOI] [PubMed] [Google Scholar]
- 39.Min J. S., Lee S. O., Jang A., Lee M., Kim Y. Quantitative analysis of biogenic amines in raw and processed foods of animal origin on Korean domestic market. Asian-Aust. J. Anim. Sci. (2004);17:1764–1768. [Google Scholar]
- 40.Min J. S., Lee S. O., Jang A., Jo C., Park C. S., Lee M. Relationship between the concentration of biogenic amines and volatile basic nitrogen in fresh beef, pork and chicken meat. Asian-Aust. J. Anim. Sci. (2007);20:1278–1284. [Google Scholar]
- 41.Morrissey P. A., Sheehy P. J. A., Galvin K., Kerry J. P., Buckley D. J. Lipid stability in meat and meat products. Meat Sci. (1998);49:73–86. [PubMed] [Google Scholar]
- 42.Mur M. M., Yuste J. High pressure processing applied to cooked sausage manufacture: physical properties and sensory analysis. Meat Sci. (2003);65:1187–1191. doi: 10.1016/S0309-1740(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 43.Nam K. C., Ahn D. U. Carbon monoxide-heme pigment is responsible for the pink color in irradiated raw turkey breast meat. Meat Sci. (2002);60:25–33. doi: 10.1016/s0309-1740(01)00101-2. [DOI] [PubMed] [Google Scholar]
- 44.No H. K., Park N. Y., Lee S. H., Meyers S. P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. (2002);74:65–72. doi: 10.1016/s0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 45.Ogden S. K., Guerrero I., Tayor A. J., Buendia H. E., Gallardo F. Changes in odour, colour and texture during the storage of acid preserved meat. Lebensm.-Wiss u.-Technol. (1995);28:521–527. [Google Scholar]
- 46.Offer G., Restall D., Trinick J. Water-holding in meat In: Bailey A. J., editor. Recent Advances in Chemistry of Meaty. The Royal Society of Chemistry; London: (1984). pp. 71–83. [Google Scholar]
- 47.Okeudo N. J., Moss B. W. Interrealtionships amongst carcass and meat quality characteristics of sheep. Meat Sci. (2005);69:1–8. doi: 10.1016/j.meatsci.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Onenc A., Serdarpglu M., Abdraimov K. Effect of various additives to marinating baths on some properties of cattle meat. Eur. Food Res. Technol. (2004);218:114–117. [Google Scholar]
- 49.Sammel L. M., Claus J. R. Citric acid and sodium citrate effects on reducing pink color defect of cooked intact turkey breast and ground turkey rolls. J. Food Sci. (2003);68:874–878. [Google Scholar]
- 50.Sammel L. M., Claus J. R. Calcium chloride and tricalcium phosphate effects on the pink color defect in cooked ground and intact turkey breast. Meat Sci. (2007);77:492–498. doi: 10.1016/j.meatsci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Sammel L. M., Claus J. R., Greaser M. L., Lucey J. A. Identifying constituents of whey protein concentrates that reduce the pink color defect in cooked ground turkey. Meat Sci. (2007);77:529–539. doi: 10.1016/j.meatsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Saunders A. B. The effect of acidification on myofibrillar proteins. Meat Sci. (1994);37:271–280. doi: 10.1016/0309-1740(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 53.Schellekens M. New research issues in sous-vide cooking. Trends Food Sci. Technol. (1996);7:256–262. [Google Scholar]
- 54.Schilling M. W., Schiling J. K., Claus J. R., Marrott N. G., Duncan S. E., Wang H. Instrumental texture assessment and consumer acceptability of cooked broiler breast evaluated using a geometrically uniform-shaped sample. J. Muscle Foods. (2003);14:11–23. [Google Scholar]
- 55.Schwarz S. J., Claus J. R., Wang H., Marritt N. G., Gragam P. P., Fernandes C. F. Inhibition of pink color development in cooked, uncured ground turkey through the binding of non-pink generating ligands to muscle pigments. Poult. Sci. (1997);76:1450–1456. doi: 10.1093/ps/76.10.1450. [DOI] [PubMed] [Google Scholar]
- 56.Sebastia C., Soriano J. M., Iranzo M., Rico H. Microbiological quality of sous vide cook-chill preserved food at different shelf life. J. Food Process Preserv. (2010);34:964–974. [Google Scholar]
- 57.Serdaroglu M., Abdraimov K., Onenc A. The effects of marinating with citric acid solutions and grapefruit juice on cooking and eating quality of turkey breast. J. Muscle Foods. (2006);18:162–172. [Google Scholar]
- 58.Sommers C. H., Fan X., Handel A. P., Sorakai K. B. Effect of citric acid on the radiation resistance of Listeria monocytogenes and frankfurter quality factors. Meat Sci. (2003);63:407–415. doi: 10.1016/s0309-1740(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 59.Suman S. P., Joseph P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. T. (2013);4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- 60.USDA. [Accessed Feb.26, 2010];USDA long-term projections. Economic Research Service. (2009) Available from: http:www.ers.usda.gov/publications/oce091/oce091d.pdf .
- 61.Vinci G., Antonelli M. L. Biogenic amines: quality index of freshness in red and white meat. Food Control. (2002);13:519–524. [Google Scholar]
- 62.Warriss P. D. The extraction of haem pigments from fresh meat. J. Food Technol. (1979);14:75–80. [Google Scholar]
- 63.Wang S. H., Chang M. H., Chen T. C. Shelf-life and Microbiological profiler of chicken wing products following sous vide treatment. Int. J. Poultry Sci. (2004);3:326–332. [Google Scholar]
- 64.Witte V. C., Krause G. F., Bailey M. E. A new extraction method for determining 2-thiobarbituric acid values of pork. J. Food Sci. (1970);35:582–585. [Google Scholar]
- 65.Xiong Y. L., Park D., Ooizumi T. Variation in the cross-linking pattern of porcine myofibrillar protein exposed to three oxidative environments. J. Agr. Food Chem. (2009);57:153–159. doi: 10.1021/jf8024453. [DOI] [PubMed] [Google Scholar]
- 66.Yang C. C., Chen T. C. Effects of refrigerated storage, pH, adjustment, and marinade on color of raw and microwave cooked chicken meat. Poult. Sci. (1993);72:355–362. [Google Scholar]