Abstract
Lactobacillus plantarum FH185 was isolated from the feces of healthy adults. In our previous study, L. plantarum FH185 was demonstrated that it has anti-obesity effect in the in vitro and in vivo test. In order to determine its potential for use as a probiotic, we investigated the physiological characteristics of L. plantarum FH185. The optimum growth temperature of L. plantarum FH185 was 40℃. L. plantarum FH185 showed higher sensitivity to novobiocin in a comparison of fifteen different antibiotics and showed higher resistance to polymyxin B and vancomycin. It also showed higher β-galactosidase and N-acetyl-β-glucosaminidase activities. Moreover, it was comparatively tolerant to bile juice and acid, and inhibited the growths of Salmonella Typhimurium and Staphylococcus aureus with rates of 44.76% and 53.88%, respectively. It also showed high adhesion activity to HT-29 cells compared to L. rhamnosus GG.
Keywords: Lactobacillus plantarum, probiotic characteristic, human feces
Introduction
The word ‘probiotics,’ which is derived from the Greek and means for life, was first used by Lilley and Stillwell (1965) to describe the substances secreted by one microorganism to stimulate the growth of another, as an antonym of ‘antibiotic’. In 1974, Parker defined probiotic “as organisms and substances which contribute to intestinal microbial balance”. Fuller (1989) redefined probiotics as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance”.
Lactic acid bacteria (LAB) have complex nutritional requirements and are frequently used as probiotics or in the fermentation of food products. Probiotics consisting of one or more species of live bacteria, such as Lactobacillus and Bifidobacteria, not only affect the intestinal flora directly, but also affect other organs by modulating immunological parameters and intestinal permeability and producing bioactive or regulatory metabolites (de Vrese and Schrezenmeir, 2008; Delzenne et al., 2011; Gerritsen et al., 2011). Probiotics produce a health benefit when administered to animals, including humans. Several studies have reported the health-promoting effects of probiotics, including the maintenance of intestinal mucosal resistance to pathogenic microorganisms (Mennigen and Bruewer, 2009), prevention of diarrhea (Guandalini, 2008), stabilization of gut microflora (Gibson et al., 1997), alleviation of lactose intolerance (de Vrese et al., 2001), immunomodulation (Perdigón et al., 2001), reduced serum cholesterol levels (Nguyen et al., 2007), reduction of bodyweight and metabolic disorders (Lee et al., 2007), and the prevention of allergic diseases and cancers (Isolauri and Salminen, 2008; Kumar et al., 2010).
Probiotics must be safe for their intended use. The 2002 FAO/WHO guidelines recommend that, though bacteria may be generally recognized as safe (GRAS), the safety of a potential probiotic should be assessed by the minimum required testing, i.e., determination of antibiotic resistance patterns, assessment of certain metabolic activities, acid and bile salt tolerance, ability to adhere to the intestinal epithelium of the hosts, antagonistic activity against pathogenic bacteria, and assessment of the ability to maintain their viability during processing and storage (Lin et al., 2006; Lonkar et al., 2005; Rial, 2000; Schlundt, 2002).
In our previous study, L. plantarum FH185 was observed to exhibit lipase inhibitory activity of 70.09±2.04% and to inhibit the adipocyte differentiation of 3T3-L1 cells (18.63±0.98%) at a concentration of 100 μg/mL. It was also demonstrated that the strain has an effect on the reduction of adipocyte size and gut microbial changes in diet-induced obese mice. Thus, this study was performed to investigate the physiological characteristics of L. plantarum FH185 in order to determine its potential as a starter for functional food products.
Materials and Methods
Bacterial strains
A LAB strain having an anti-obesity effect, namely, L. plantarum FH185, was isolated from the feces of healthy adults. In our previous study, L. plantarum FH185 was found to have lipase inhibitory activity of 70.09±2.04% and to inhibit the adipocyte differentiation of 3T3-L1 cells (18.63±0.98%) at a concentration of 100 μg/mL. It was also demonstrated that the strain has an effect on the reduction of adipocyte size and gut microbial changes in diet-induced obese mice (Park et al., 2015). The strain was incubated in a Lactobacilli MRS broth (Difco, USA) as the growth medium at 37℃ for 18 h.
Growth of strain
The number of viable L. plantarum FH185 was determined by serial ten-fold dilution in 0.1% peptone water. Ten microliter of L. plantarum FH185 was inoculated into 150 mL of 10% reconstituted skimmed milk (105 CFU/mL), and then the culture was incubated at 3 h intervals for 24 h at 34℃, 37℃ and 40℃. All of the pour plates were incubated aerobically at 37℃ for 48 h using a BCP plate count agar (Eiken, Japan).
Antibiotic tolerance
L. plantarum FH185 was grown at 37℃ for 18 h in MRS broth and inoculated (1%, v/v) into a MRS broth supplemented with antibiotics (amikacin, gentamicin, kanamycin, neomycin, streptomycin, penicillin-G, methicillin, oxacillin, ampicillin, bacitracin, rifampicin, novobiocin, lincomycin, polymyxin B and chloramphenicol; Sigma) at various concentrations in a two-fold dilution step. The minimal inhibitory concentration (MIC) was determined by checking the moment at which the strain stopped growing after incubation at 37℃ for 48 h.
Enzyme activity
An API ZYM kit (Apibio-Mérieux) was used to study enzyme activity. L. plantarum FH185 was grown at 37℃ for 18 h in MRS broth. Sediment from the centrifuged broth culture was used to prepare the suspension at 105-106 CFU/mL. After inoculation, the cultures were incubated for 5 h at 37℃. The addition of a surface active agent (ZYM A reagent) to the cupules facilitated the solubilization of the ZYM B reagent in the medium. Color was allowed to develop for at least 5 min, and values ranging from 0-5 (corresponding to the colors developed) were assigned. The approximate number for the free nmol hydrolyzed substrate was determined based on the color strength: 0, negative reaction; 1, 5 nmol; 2, 10 nmol; 3, 20 nmol; 4, 30 nmol; 5, 40 nmol or higher.
Bile tolerance
Bile tolerance was tested as described by Gilliland and Walker (1990). L. plantarum FH185 was grown at 37℃ for 18 h in MRS broth. Each 1% of the L. plantarum FH185 strain culture was inoculated into sterilized MRS broth containing 0.05% L-cysteine (Sigma) with or without 0.3% oxgall (Sigma), and then the growth potential was compared in the presence of the bile. Then, the cultures were incubated anaerobically at 1 h intervals for 7 h at 37℃. All of the pour plates were incubated anaerobically at 37℃ for 48 h using a BCP plate count agar.
Acid tolerance
Acid tolerance was tested as described by Clark et al (1993). Solutions of 37% HCl in double-distilled water were adjusted to pH levels of 2.0, 3.0, and 4.0. Sterile double-distilled water (pH 6.4) served as the control. 10 mL of each pH solution was transferred into sterile test tubes. One milliliter of stock culture containing approximately 109 CFU/mL of L. plantarum FH185 using MRS agar containing 0.05% cysteine was then transferred into each of the four pH solutions. The pH solutions containing L. plantarum FH185 were then incubated anaerobically at 37℃, followed by intermittent plating after 1, 2, and 3 h to stimulate the survival of L. plantarum FH185 under pH conditions common to the human stomach. Samples from the pH solution were re-suspended and subjected to serial dilutions. All of the pour plates were then incubated anaerobically at 37℃ for 48 h using a BCP plate count agar.
Antimicrobial activity
Antimicrobial activity was tested as described by Gilliland and Speck (1977). Escherichia coli KFRI 174, Salmonella Typhimurium KFRI 250, and Staphylococcus aureus KFRI 219 (obtained from the culture collection of the Korea Food Research Institute [Korea]) were enumerated on an EMB agar (Difco), on a Bismuth sulfite agar (Difco), and on a Baird Parker agar (Difco), respectively. All of the plates were incubated for 48 h at 37℃. Both the control culture and the associative culture were incubated for 6 h at 37℃. At the end of the incubation period, the samples were removed and placed in an ice bath until analysis. The number of CFU of pathogens per mL was determined using the appropriate selective medium. The percentages of inhibition were determined using the following formula:
Adherence assay
The adhesion of L. plantarum FH185 was studied using the HT-29 intestinal epithelial cell line (Kim et al., 2008). HT-29 cells were obtained from the Korea Cell Line Bank (Korea). The cells were cultured at 37℃ in a 5% CO2-95% air atmosphere in RPMI 1640 (GIBCO) supplemented with 10% FBS. The sub-cultured (3 times) L. plantarum FH185 was harvested by centrifugation at 12,000 rpm for 3 min, and then washed three times with PBS to remove any remaining MRS broth. The washed bacteria were then re-suspended in an RPMI 1640 medium to an optical density at 600 nm (OD 600) of 0.5 (approximately 107 CFU/mL). The re-suspended bacteria were appropriately diluted and plated on a BCP plate count agar. To investigate the adhesion activity, post-confluent HT-29 cells were washed twice with PBS. After washing, 1 mL of the bacteria in the RPMI 1640 medium was added to each well of the tissue-culture plate (12 wells), which was then incubated for 2 h. After incubation, the cells were washed five times with sterile PBS and harvested with a trypsin-EDTA (0.25% trypsin and 0.02% EDTA; GIBCO). It was appropriately diluted and plated on a BCP plate count agar to determine the number of viable cell-associated bacteria.
Statistical analysis
The results are expressed as the mean±standard deviation (SD). Statistical analysis was performed with a statistical analysis system (SAS, SAS Institute Inc., USA). The significance of the differences was analyzed by conducting a one-way analysis of variance (ANOVA) with Duncan’s multiple range tests. Values of p<0.05 were considered statistically significant.
Results and Discussion
Growth of strain
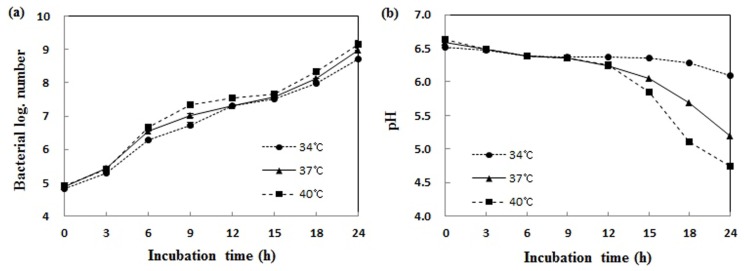
As the result of incubation of L. plantarum FH185 in 10% reconstituted skimmed milk at 34℃, 37℃ and 40℃ for 24 h, the highest growth rate identified at 40℃. The pH value was also the lowest at 40℃. The optimum growth temperature of L. plantarum FH185 was found to be 40℃ (Fig. 1).
Fig. 1. Growth curve (a), and pH changes (b) of Lactobacillus plantarum FH185 in MRS broth at various temperatures. (a) All values are within the mean±standard deviation of the three replicates.
Antibiotic tolerance
Several studies on the antibiotic sensitivity and resistance of dairy starter bacteria were conducted over a period of many years (Salminen et al., 1998). Although some resistance appeared to be a strain, a specific pattern for classification has not emerged (Reinbold and Reddy, 1974).
Table 1 shows the tolerance of the L. plantarum FH185 strain to sixteen kinds of antibiotics. The results showed that L. plantarum FH185 showed itself to be more sensitive to novobiocin and penicillin-G in a comparison of fourteen different antibiotics, and exhibited the greatest resistance to polymyxin B and vancomycin.
Table 1. Antibiotics susceptibility of Lactobacillus plantarum FH185.
| Antimicrobal agents | Minimal inhibitory concentrations (μg/mL) |
|---|---|
| Aminoglycosides | |
| Amikacin | 20±0 |
| Gentamycin | 80±0 |
| Kanamycin | 100±0 |
| Neomycin* | 12.5±0 |
| Streptomycin | 400±0 |
| β-lactams | |
| Penicillin-G* | 10±0 |
| Methicillin | 160±0 |
| Oxacillin | 30±0 |
| Ampicillin | 320±0 |
| Gram-positive spectrum | |
| Bacitracin* | 15±0 |
| Rifampicin | 240±0 |
| Novobiocin | 7.5±0 |
| Lincomycin* | 12.5±0 |
| Gram-negative spectrum | |
| Polymyxin B* | 1200±0 |
| Broad spectrum | |
| Chloramphenicol | 80±0 |
| Vancomycin | 1600±0 |
*units/mL
All values are the mean±standard deviation of three replicates.
Vancomycin resistance is a matter of great importance in that vancomycin is one of the last antibiotics to remain widely efficacious against clinical infections caused by multidrug-resistant pathogens (Zhou et al., 2005). A few gram-positive bacteria, including Lactobacillus species, are essentially resistant to vancomycin (Swenson et al., 1990; Hamilton-Miller and Shah, 1998). Irreversible loss of antibiotic resistance from a strain as a result of a treatment known to eliminate plasmids is an indication that the resistance is plasmid-linked. However, in the case of the Lactobacillus strain, there has been no indication so far that vancomycin resistance would represent an inducible, transmissible genetic system (Salminen et al., 1998). For confirmation of the safety, cloning and expression of the gene related to anti-obesity effect from L. plantarum FH185 could be an alternative way.
Enzyme activity
There are many causes of cancer. The formation of carcinogens might be due to an association of bacterial enzymes like β-glucuronidase and nitroreductase, which are involved in the transformation of pro-carcinogens into carcinogen (Goldin, 1990). L. plantarum FH185 did not produce β-glucuronidase; rather, it produced such enzymes as leucine arylamidase, acid phosphatase, naphtol-AS-BI-phosphohydrolase, α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, and N-acetyl-β-glucosaminidase. Especially, L. plantarum FH185 produced more β-galactosidase and N-acetyl-β-glucosaminidase than other enzymes (Table 2). According to Zielinska et al. (2015), β-glucuronidase was not produced by any Lactobacillus isolated from traditional fermented cabbage and cucumber. However esterase, leucine arylamidase, valine arylamidase, cystine arylamidase, naphthol-AS-BI-phosphohydrolase, β-galactosidase, α and β-glucosidase, and N-acetyl-β-glucosaminidase were detected. This enzyme profile is similar to that of the L. plantarum FH185 strain.
Table 2. Enzyme patterns of Lactobacillus plantarum FH185.
| Enzyme | L. plantarum FH185 |
|---|---|
| Alkaline phosphatase | 1 |
| Esterase (C4) | 1 |
| Esterase Lipase (C8) | 1 |
| Lipase (C14) | 1 |
| Leucine arylamidase | 4 |
| Valine arylamidase | 1 |
| Cystinearylamidase | 2 |
| Trypsin | 0 |
| α-chymotrypsin | 1 |
| Acid phosphatase | 3 |
| Naphtol-AS-BI-phosphohydrolase | 3 |
| α-galactosidase | 3 |
| β-galactosidase | 5 |
| β-glucuronidase | 0 |
| α-glucosidase | 3 |
| β-glucosidase | 4 |
| N-acetyl-β-glucosaminidase | 5 |
| α-mannosidase | 0 |
| α-fucosidase | 1 |
*A value ranging from 0 to 5 is assigned to the standard color: zero represents a negative; 5 represents a reaction of maximum intensity. Values 1 through 4 represent intermediate reactions depending on the level of intensity. The approximate activity may be estimated from the color strength: 1 corresponds to the liberation of 5 nanomoles, 2 to 10 nanomoles, 3 to 20 nanomoles, 4 to 30 nanomoles, and 5 to 40 nanomoles or more.
Bile and acid tolerance
Tolerance to gastric juice and bile salts is a crucial factor in the selection of probiotic strains (Caggia et al., 2015). In order to ensure their beneficial effects after consumption, probiotics must be viable in the food and survive the gastrointestinal ecosystem with a pH ranging from 1.0 to 3.0 in the stomach and approximate bile salt concentrations of 0.3 in the small intestine (Mainville et al., 2005; Shah, 2007).
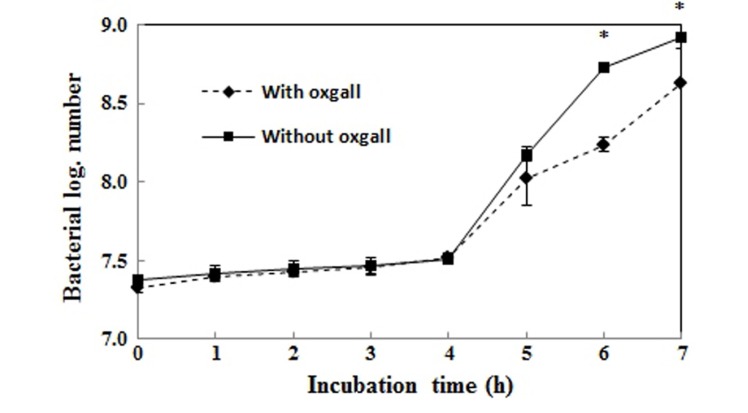
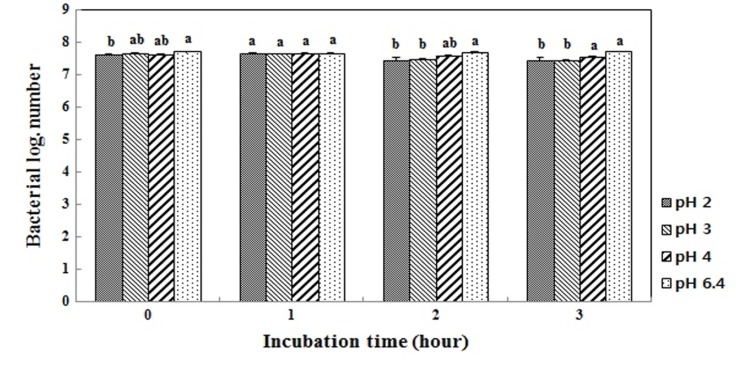
Fig. 2 shows the growth curves in MRS broth or MRS broth containing 0.3% bile. The log value of the population after incubation for 7 h without 0.3% oxgall was 8.9, but it was 8.6 with the addition of 0.3% bile. Therefore, the survival rate of L. plantarum FH185 in MRS broth containing 0.3% bile was 96.6%. Also, Fig. 3 shows the pH tolerance of L. plantarum FH185. It showed a 97.4% survival rate after incubation for 3 h in highly acidic conditions (pH 2.0).
Fig. 2. Growth of Lactobacillus plantarum FH185 in MRS broth containing 0.05% L-cysteine with/without 0.3% ox gall. *p<0.05 between ox gall and without ox gall (t-test).
Fig. 3. Survival of Lactobacillus plantarum FH185 after three hours in HCl solution (pH 2.0, 3.0, 4.0 and 6.4). a,bMeans values with different superscript within same time are significantly different (p<0.05).
According to Guo et al. (2015), thirty kinds of Lactobacillus strains isolated from the suan-tsai and koumiss sample were tested with regard to their acid and bile tolerance. The acid resistance values of the lactobacilli ranged from 44.1 to 85.2%, while their bile tolerance values ranged from 4.6 to 34.2%. L. plantarum FH185 has probiotic potential because a comparatively high percentage of the strain survived in MRS broth containing 0.3% bile salt, under a highly acidic condition.
Antimicrobial activity
Foodborne diseases arising from the consumption of food contaminated with pathogenic bacteria such as Salmonella sp., Listeria monocytogenes, Staphylococcus sp., and E.coli is of vital concern to public health (Oussalah et al., 2007). As the number of multidrug-resistant pathogens expands, and recognition of the role that human microbiota play in health and disease increases, it is becoming increasingly interesting to use probiotics as a therapeutic agent (Britton and Versalovic, 2008). For instance, many researchers have demonstrated the anti-pathogenic effects of LAB (Casey et al., 2007). The antagonistic activities demonstrated by lactic acid bacteria may be due to the production of substances with antibacterial properties in particular: hydrogen peroxide, organic acid and bacteriocins (Tejero-Sarinena et al., 2012).
Table 3 shows the antimicrobial activity of L. plantarum FH185 against various pathogenic strains. L. plantarum FH185 did not show any inhibition against E. coli, but it showed inhibition against S. Typhimurium and S. aureus at rates of 44.4% and 53.9%, respectively. The pH value of pathogens after incubation for 7 h was 6.4, but the pH value of a mixed culture with L. plantarum FH185 and pathogens was around 5.5-5.6. This means that even lactic acid produced during incubation affected the antimicrobial activity, it was not a large effect. Although L. plantarum FH185 did not show resistance against E. coli, the strain showed comparatively excellent inhibition against S. Typhimurium and S. aureus.
Table 3. Inhibition of pathogens by Lactobacillus plantarum FH185 in MRS broth.
| Indicators | Indicatorsa | L. plantarum FH185a + Indicators | Inhibition (%) | ||
|---|---|---|---|---|---|
| CFU/mL | pH | CFU/mL | pH | ||
| Escherichia coli | 2.0±0.2×107 | 6.4 | 3.2±0.3×107 | 5.6 | - |
| Salmonella Typhimurium | 7.2±0.4×106 | 6.4 | 4.0±0.2×106 | 5.5 | 44.4 |
| Staphyloccous aureus | 3.3±0.1×108 | 6.4 | 1.7±0.3×108 | 5.6 | 53.9 |
*Initial count of L. plantrum FH185: 4.4±0.1×106 CFU/mL
aDetermined after 6 h of incubation at 37℃
All values are the mean±standard deviation of three replicates.
Adhesion ability
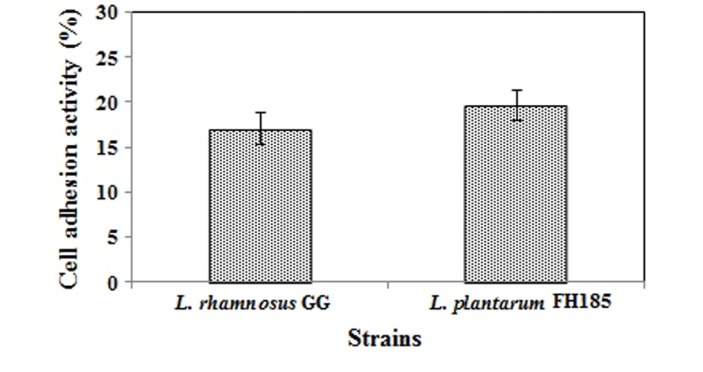
Adherence ability to the intestinal epithelium of the hosts is one of the main criteria for selecting probiotic strains. Attachment to mucosa prolongs the time probiotics can influence the gastrointestinal immune system and microbiota of the host. Thus, the ability to adhere to intestinal surfaces is thought to correspond to the efficacy of the probiotic strain (O’Halloran et al., 1997). As shown in Fig. 4, 19.62% of L. plantarum FH185 adhered to HT-29 cell and 17.02% of the L. rhamnosus GG strain adhered to the cell. L. rhamnosus GG was used as the positive control. In many studies, it was demonstrated that L. rhamnosus GG has a great ability to adhere to the epithelial cell line (Gopal et al., 2001; Tuomola and Salminen, 1998). Thus, we could define that L. plantarum FH185 exhibits great adherence to the epithelial surface.
Fig. 4. Adhesive ability of Lactobacillus plantarum FH185 to HT-29 cell. All values are the mean±SD of three replicates. There are no significantly different (p<0.05).
Conclusion
In our previous study, Lactobacillus plantarum FH185 was isolated from the feces of healthy adults and demonstrated to have anti-obesity effects. We investigated the physiological characteristics of L. plantarum FH185 for potential use as probiotics. The essential and fundamental properties of probiotics - such as growth pattern, antibiotic tolerance, enzyme activity, bile tolerance, acid tolerance, antimicrobial activity and adhesion ability - were tested. The optimum growth temperature of L. plantarum FH185 was 40℃. L. plantarum FH185 was able to survive in antibiotic conditions at a low concentration and did not produce carcinogenic enzymes such as β-glucuronidase. Moreover, it was found to be comparatively tolerant to bile juice and acid, and displayed inhibition against two kinds of pathogenic strains. It also showed high adhesion activity to HT-29 cells compared to L. rhamnosus GG. These results demonstrate that L. plantarum FH185 could be used as a probiotic.
Acknowledgments
This study was supported by a grant from the Korea Food Research Institute (project no. E0143023839).
References
- 1.Britton R. A., Versalovic J. Probiotics and gastrointestinal infections. Interdiscip. Perspect. Infect. Dis. (2008) doi: 10.1155/2008/290769. 290769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caggia C., de Angelis M., Pitino I., Pino A., Randazzo C. L. Probiotic features of Lactobacillus strains isolated from Ragusano and Pecorino Siciliano cheeses. Food Microbiol. (2015);50:109–117. doi: 10.1016/j.fm.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Casey P. G., Gardiner G. E., Casey G., Bradshaw B., Lawlor P. G., Lynch P. B., Leonard F. C., Stanton C., Ross R. P., Fitzgerald G. F., Hill C. A five-strain probiotic combination reduced pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. (2007);73:1858–1863. doi: 10.1128/AEM.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark P. A., Cotton L. N., Martin J. H. Selection of bifidobacteria for use as dietary adjuncts in cultured dairy foods: II-tolerance to simulated pH of human stomachs. Cul. Dairy Prod. J. (1993);28:11–14. [Google Scholar]
- 5.de Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng.. Biotechnol. (2008);111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 6.de Vrese M., Steglman A., Richter B., Fenselau S., Laue C., Scherezenmeir J. Probiotics-compensation for lactase insufficiency. Am. I. Clin. Nutr. (2001);73:421–429. doi: 10.1093/ajcn/73.2.421s. [DOI] [PubMed] [Google Scholar]
- 7.Delzenne N. M., Neyrinck A. M., Backhed F., Cani P. D. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat. Rev. Endocrinol. (2011);7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 8.Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. (1989);66:365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 9.Gerritsen J., Smidt H., Rijkers G. T., de Vos W. M. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. (2011);6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson G. R., Saveedra J. M., MacFarlane S., MacFarlane G. T. Probiotics and intestinal infections In: Fuller R., editor. Probiotic. 2: Applications and practical Aspects. Chapman & Hall; New York: pp. 10–39. [Google Scholar]
- 11.Gilliland S. E., Speck M. L. Antagonistic action of Lactobacillus acidophilus toward intestinal and foodborne pathogens in associative cultures. J. Food Prot. (1977);40:820–823. doi: 10.4315/0362-028X-40.12.820. [DOI] [PubMed] [Google Scholar]
- 12.Gilliland S. E., Walker D. K. Factors to consider when selecting a culture of Lactobacillus acidophilus as a dietary adjunct to produce a hypocholesterolemic effect in humans. J. Dairy Sci. (1990);73:905–911. doi: 10.3168/jds.S0022-0302(90)78747-4. [DOI] [PubMed] [Google Scholar]
- 13.Goldin B. R. Intestinal microflora: Metabolism of drugs and carcinogens. Ann. Med. (1990);22:43–48.. doi: 10.3109/07853899009147240. [DOI] [PubMed] [Google Scholar]
- 14.Gopal P. K., Prasad J., Smart J., Gill H. S. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Esherichia coli. Int. J. Food Microbiol. (2001);47:207–216. doi: 10.1016/s0168-1605(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 15.Guandalini S. Probiotics for children with diarrhea: an update. J. Clin. Gastroentrerol. (2008);42:S53–S57. doi: 10.1097/MCG.0b013e3181674087. [DOI] [PubMed] [Google Scholar]
- 16.Guo C. F, Zhang S., Yuan Y. H., Yue T. L., Li J. Y. Comparison of lactobacilli isolated from Chinese suan-tsai and Koumiss for their probiotic and functional properties. J. Funct. Foods. (2015);12:294–302. doi: 10.1016/j.jff.2014.11.029. [DOI] [Google Scholar]
- 17.Hamilton-Miller J. M., Shah S. Vancomycin susceptibility as an aid to the identification of Lactobacilli. Lett. Appl. Microbiol. (1998);27:121–123. doi: 10.1046/j.1472-765X.1998.t01-2-00400.x. [DOI] [PubMed] [Google Scholar]
- 18.Isolauri E., Salminen S. Probiotics: use in allergic disorders: a Nutrition, Allergy, Mucosal Immunology, and Intestinal Microbiota (NAMI) research group report. J. Clin. Gastroenterol. (2008);42:S91–S96. doi: 10.1097/MCG.0b013e3181639a98. [DOI] [PubMed] [Google Scholar]
- 19.Kim S. J., Cho S. Y., Kim S. H., Song O. J., Shin I. S., Cha D. S., Park H. J. Effect of microencapsulation on viability and other characteristics in Lactobacillus acidophilus ATCC 43121. LWT-Food Sci. Technol. (2008);41:493–500. doi: 10.1016/j.lwt.2007.03.025. [DOI] [Google Scholar]
- 20.Kumar M., Kumar A., Nagpal R., Mohania D., Behare P., Verma V., Kumar P., Paddar D., Aggarwal P. K., Henry C. J., Jain S., Yadav H. Cancer-preventing attributes of probiotics: and update. Int. J. Food. Sci. Nutr. (2010);61:473–496. doi: 10.3109/09637480903455971. [DOI] [PubMed] [Google Scholar]
- 21.Lee K., Paek K., Lee H. Y., Park J. H., Lee Y. Anti-obesity effect of trans-10, cis-12 conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J. Appl. Microbiol. (2007);103:1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 22.Lilley D. M., Stillwell R. H. Probiotics: growth promoting factors produced by microorganisms. Science. (1965);147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 23.Lin W. H., Hwang C. F., Chen L. W., Tsen H. Y. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiol. (2006);23:74–81. doi: 10.1016/j.fm.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Lonkar P., Harne S. D., Kalorey D. R., Kurkure N. V. Isolation, in vitro antibacteria activity, bacteria sensitivity and plasmid profile of Lactobacilli. Asian-Aust. J. Anim. Sci. (2005);18:1336–1342. doi: 10.5713/ajas.2005.1336. [DOI] [Google Scholar]
- 25.Mainville I., Arcand Y., Farnworth E. R. A dynamic model that simulates the human upper gastro-intestinal tract for the study of probiotics. Int. J. Food Microbiol. (2005);99:287–296. doi: 10.1016/j.ijfoodmicro.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Mennigen R., Bruewer M. Effect of probiotics on intestinal barrier function. Ann. NY. Acad. Sci. (2009);1165:183–189. doi: 10.1111/j.1749-6632.2009.04059.x. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T. D., Kand J. H., Lee M. S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. (2007);113:358–361. doi: 10.1016/j.ijfoodmicro.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 28.O’Halloran S., Feeney M., Morrissey D., Murphy L., Thornton G., Shanahan F., O’Sullivan G. C., Collins J. K. Adhesion of potential probiotic bacteria to human epithelial cell lines; Poster in conference: Functional Foods: Designer Foods for the Future; Cork, Ireland. (1997). [Google Scholar]
- 29.Oussalah M., Caillet S., Saucier L., Lacroix M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhymurium, Staphylococcus aureus and Listeria monocytogenes. Food Control. (2007);18:414–420. doi: 10.1016/j.foodcont.2005.11.009. [DOI] [Google Scholar]
- 30.Park S. Y., Cho S. A., Lee M. K., Lim S. D. Effect of Lactobacillus plantarum FH185 on the reduction of adipocyte size and gut microbial changes in mice with diet-induced obesity. Korean J. Food Sci. An. (2015);35:171–178. doi: 10.5851/kosfa.2015.35.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker R. B. Probiotics, the other half of the antibiotic story. Animal Nutr. Hlth. (1974);29:4–8. [Google Scholar]
- 32.Perdigón G., Fuller R., Raya R. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. (2001);2:27–42. [PubMed] [Google Scholar]
- 33.Reinbold G. W., Reddy M. S. Sensitivity or resistance of dairy starter and associated microorganisms to selected antibiotics. J. Milk Technol. (1974);37:517–521. [Google Scholar]
- 34.Rial R. D. The role of probiotic cultures in the control of gastro-intestinal health. J. Nutr. (2000);130:396–402. [Google Scholar]
- 35.Salminen S., von Wright A., Morelli L., Marteau P., Brassart D., de Vos W. M., Fonden R., Saxelin M., Collins K., Mogensen G., Birkeland S. E., Mattila-Sandholm T. Demonstration on safety of probiotics - A review. Int. J. Food Microbiol. (1998);44:93–106. doi: 10.1016/S0168-1605(98)00128-7. [DOI] [PubMed] [Google Scholar]
- 36.Schlundt J. New directions in foodborne disease prevention. Int. J. Food Microbiol. (2002);78:3–17. doi: 10.1016/S0168-1605(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 37.Shah N. P. Functional cultures and health benefits. Int. Dairy J. (2007);17:1262–1277. doi: 10.1016/j.idairyj.2007.01.014. [DOI] [Google Scholar]
- 38.Swenson J. M., Fracklam R. R., Thornsberry C. Antimicrobial susceptibility of vancomycin-resistant Leuconostoc, Pediococcus, and Lactobacillus species. Antimicrobiol. Agents Chemother. (1990);34:543–549. doi: 10.1128/AAC.34.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tejero-Sarinena S., Barlow J., Costabile A., Gibson G. R., Rowland J. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acid. Anaerobe. (2012);18:530–538. doi: 10.1016/j.anaerobe.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Tuomola E. M., Salminen S. J. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food. Microbiol. (1998);41:45–51. doi: 10.1016/S0168-1605(98)00033-6. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J. S., Pillidge C. J., Gopal P. K., Gill H. S. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int. J. Food Microbiol. (2005);98:211–217. doi: 10.1016/j.ijfoodmicro.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Zielinska D., Rzepkowska A., Radawska A., Zielinski K. In vitro screening of selected probiotic properties of Lactobacillus strains isolated from traditional fermented cabbage and cucumber. Curr. Microbiol. (2015);70:183–194. doi: 10.1007/s00284-014-0699-0. [DOI] [PubMed] [Google Scholar]