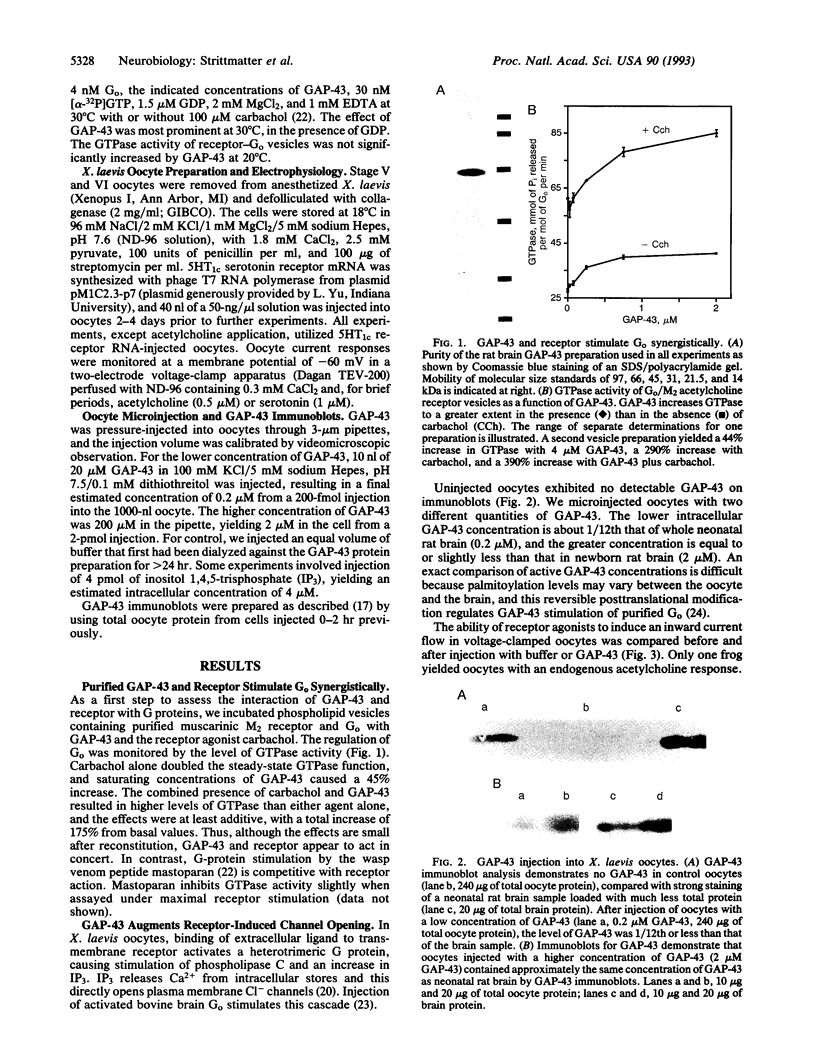
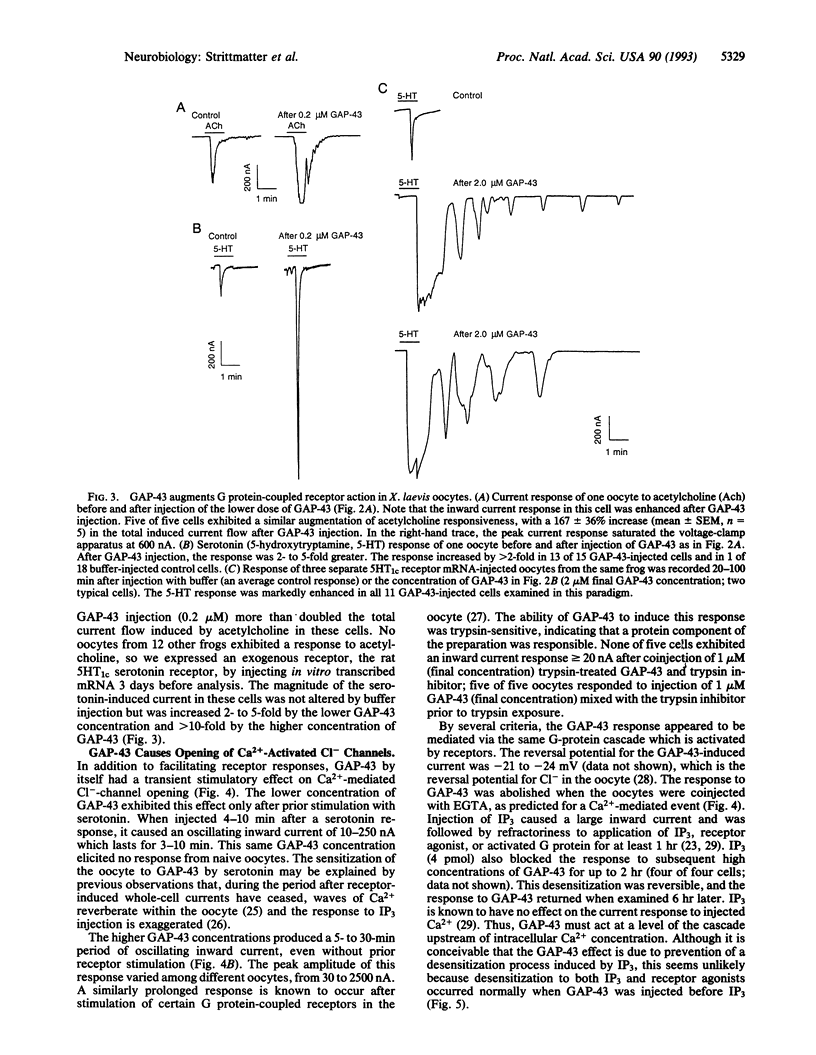
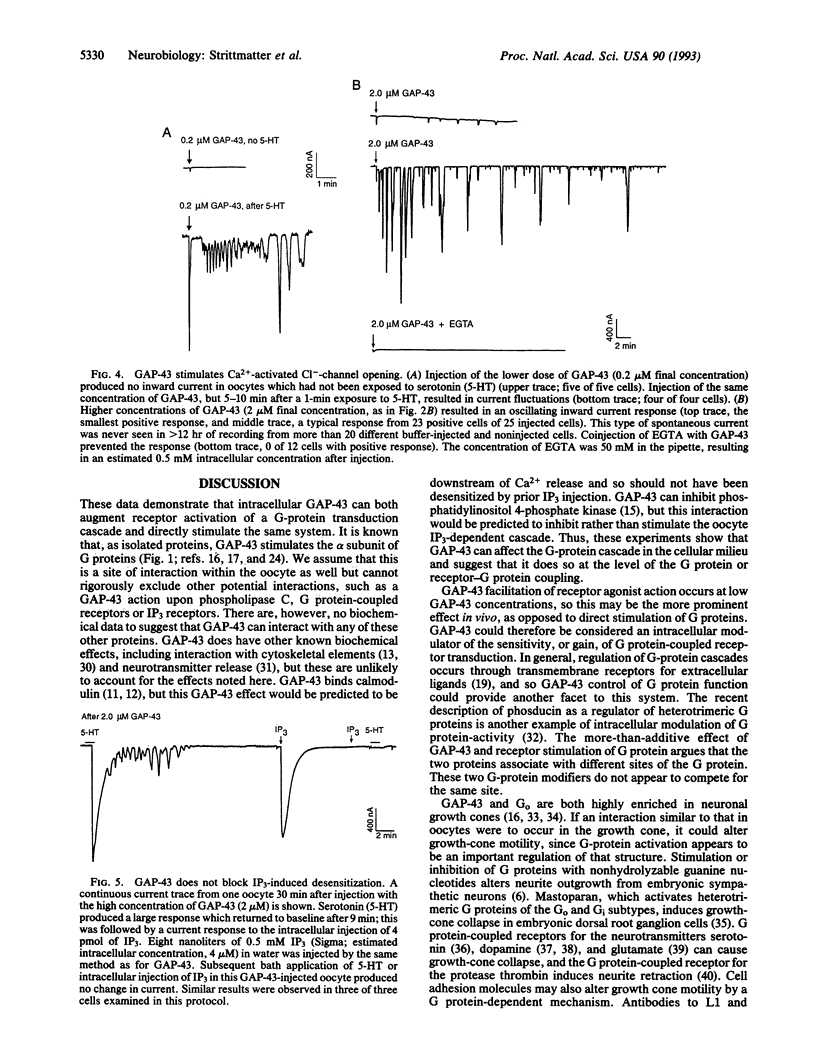
Abstract
The neuronal protein GAP-43 is thought to play a role in determining growth-cone motility, perhaps as an intracellular regulator of signal transduction, but its molecular mechanism of action has remained unclear. We find that GAP-43, when microinjected into Xenopus laevis oocytes, increases the oocyte response to G protein-coupled receptor agonists by 10- to 100-fold. Higher levels of GAP-43 cause a transient current flow, even without receptor stimulation. The GAP-43-induced current, like receptor-stimulated currents, is mediated by a calcium-activated chloride channel and can be desensitized by injection of inositol 1,4,5-trisphosphate. This suggests that neuronal GAP-43 may serve as an intracellular signal to greatly enhance the sensitivity of G protein-coupled receptor transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K. A., Wakim B. T., Doyle G. S., Walsh K. A., Storm D. R. Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein. J Biol Chem. 1988 Jun 5;263(16):7544–7549. [PubMed] [Google Scholar]
- Baetge E. E., Hammang J. P. Neurite outgrowth in PC12 cells deficient in GAP-43. Neuron. 1991 Jan;6(1):21–30. doi: 10.1016/0896-6273(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Bauer P. H., Müller S., Puzicha M., Pippig S., Obermaier B., Helmreich E. J., Lohse M. J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992 Jul 2;358(6381):73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- Benowitz L. I., Apostolides P. J., Perrone-Bizzozero N., Finklestein S. P., Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988 Jan;8(1):339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. R., Au D., Alexander K. A., Nicolson T. A., Storm D. R. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J Biol Chem. 1991 Jan 5;266(1):207–213. [PubMed] [Google Scholar]
- Coggins P. J., Zwiers H. Evidence for a single protein kinase C-mediated phosphorylation site in rat brain protein B-50. J Neurochem. 1989 Dec;53(6):1895–1901. doi: 10.1111/j.1471-4159.1989.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Cox E. C., Müller B., Bonhoeffer F. Axonal guidance in the chick visual system: posterior tectal membranes induce collapse of growth cones from the temporal retina. Neuron. 1990 Jan;4(1):31–37. doi: 10.1016/0896-6273(90)90441-h. [DOI] [PubMed] [Google Scholar]
- Davies J. A., Cook G. M., Stern C. D., Keynes R. J. Isolation from chick somites of a glycoprotein fraction that causes collapse of dorsal root ganglion growth cones. Neuron. 1990 Jan;4(1):11–20. doi: 10.1016/0896-6273(90)90439-m. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., De Graan P. N., Oestreicher A. B., Versteeg D. H., Gispen W. H. Inhibition of noradrenaline release by antibodies to B-50 (GAP-43). Nature. 1989 Nov 2;342(6245):74–76. doi: 10.1038/342074a0. [DOI] [PubMed] [Google Scholar]
- Doherty P., Ashton S. V., Moore S. E., Walsh F. S. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991 Oct 4;67(1):21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Gianotti C., Nunzi M. G., Gispen W. H., Corradetti R. Phosphorylation of the presynaptic protein B-50 (GAP-43) is increased during electrically induced long-term potentiation. Neuron. 1992 May;8(5):843–848. doi: 10.1016/0896-6273(92)90198-m. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science. 1989 May 26;244(4907):980–983. doi: 10.1126/science.2543072. [DOI] [PubMed] [Google Scholar]
- Haydon P. G., Man-Son-Hing H., Doyle R. T., Zoran M. FMRFamide modulation of secretory machinery underlying presynaptic inhibition of synaptic transmission requires a pertussis toxin-sensitive G-protein. J Neurosci. 1991 Dec;11(12):3851–3860. doi: 10.1523/JNEUROSCI.11-12-03851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon P. G., McCobb D. P., Kater S. B. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984 Nov 2;226(4674):561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Burnier J., Ross E. M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990 Aug 25;265(24):14176–14186. [PubMed] [Google Scholar]
- Igarashi M., Strittmatter S. M., Vartanian T., Fishman M. C. Mediation by G proteins of signals that cause collapse of growth cones. Science. 1993 Jan 1;259(5091):77–79. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- Kater S. B., Mills L. R. Regulation of growth cone behavior by calcium. J Neurosci. 1991 Apr;11(4):891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford K. L., DeMello F. G., Klein W. L. D1-type dopamine receptors inhibit growth cone motility in cultured retina neurons: evidence that neurotransmitters act as morphogenic growth regulators in the developing central nervous system. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2839–2843. doi: 10.1073/pnas.85.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Hellmiss R., Duerson K., Ennulat D., David N., Clapham D., Peralta E. Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J. 1990 Dec;9(13):4381–4390. doi: 10.1002/j.1460-2075.1990.tb07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockerbie R. O. The neuronal growth cone: a review of its locomotory, navigational and target recognition capabilities. Neuroscience. 1987 Mar;20(3):719–729. doi: 10.1016/0306-4522(87)90235-1. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Dou P., Kater S. B. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988 Jun;8(6):2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri K. F., Gordon-Weeks P. R. GAP-43 in growth cones is associated with areas of membrane that are tightly bound to substrate and is a component of a membrane skeleton subcellular fraction. J Neurosci. 1990 Jan;10(1):256–266. doi: 10.1523/JNEUROSCI.10-01-00256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri K. F., Pfenninger K. H., Willard M. B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986 May;83(10):3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Padrell E., Carty D. J., Omri G., Landau E. M., Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990 Jan 4;343(6253):79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Oestreicher A. B., Van Dongen C. J., Zwiers H., Gispen W. H. Affinity-purified anti-B-50 protein antibody: interference with the function of the phosphoprotein B-50 in synaptic plasma membranes. J Neurochem. 1983 Aug;41(2):331–340. doi: 10.1111/j.1471-4159.1983.tb04747.x. [DOI] [PubMed] [Google Scholar]
- Parker E. M., Kameyama K., Higashijima T., Ross E. M. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J Biol Chem. 1991 Jan 5;266(1):519–527. [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. On the importance of being inhibited, or saying no to growth cones. Neuron. 1988 Jun;1(4):263–267. doi: 10.1016/0896-6273(88)90074-8. [DOI] [PubMed] [Google Scholar]
- Raper J. A., Kapfhammer J. P. The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron. 1990 Jan;4(1):21–29. doi: 10.1016/0896-6273(90)90440-q. [DOI] [PubMed] [Google Scholar]
- Rodrigues P. dos S., Dowling J. E. Dopamine induces neurite retraction in retinal horizontal cells via diacylglycerol and protein kinase C. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9693–9697. doi: 10.1073/pnas.87.24.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch U., Lohse M. J., Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989 Jul;3(1):13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Perrone-Bizzozero N. I., Beermann M. L., Benowitz L. I. Phospholipid-mediated delivery of anti-GAP-43 antibodies into neuroblastoma cells prevents neuritogenesis. J Neurosci. 1991 Jun;11(6):1685–1690. doi: 10.1523/JNEUROSCI.11-06-01685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D., Boton R., Moran O., Dascal N. Short- and long-term desensitization of serotonergic response in Xenopus oocytes injected with brain RNA: roles for inositol 1,4,5-trisphosphate and protein kinase C. Pflugers Arch. 1990 Apr;416(1-2):7–16. doi: 10.1007/BF00370215. [DOI] [PubMed] [Google Scholar]
- Skene J. H. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Jacobson R. D., Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986 Aug 15;233(4765):783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Fishman M. C. The neuronal growth cone as a specialized transduction system. Bioessays. 1991 Mar;13(3):127–134. doi: 10.1002/bies.950130306. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M. GAP-43 as a modulator of G protein transduction in the growth cone. Perspect Dev Neurobiol. 1992;1(1):13–19. [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Sudo Y., Linder M. E., Fishman M. C. An intracellular guanine nucleotide release protein for G0. GAP-43 stimulates isolated alpha subunits by a novel mechanism. J Biol Chem. 1991 Nov 25;266(33):22465–22471. [PubMed] [Google Scholar]
- Strittmatter S. M., Vartanian T., Fishman M. C. GAP-43 as a plasticity protein in neuronal form and repair. J Neurobiol. 1992 Jul;23(5):507–520. doi: 10.1002/neu.480230506. [DOI] [PubMed] [Google Scholar]
- Sudo Y., Valenzuela D., Beck-Sickinger A. G., Fishman M. C., Strittmatter S. M. Palmitoylation alters protein activity: blockade of G(o) stimulation by GAP-43. EMBO J. 1992 Jun;11(6):2095–2102. doi: 10.1002/j.1460-2075.1992.tb05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan H. S., Stone S. R., Hemmings B. A., Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992 Feb;8(2):363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Van Hooff C. O., Holthuis J. C., Oestreicher A. B., Boonstra J., De Graan P. N., Gispen W. H. Nerve growth factor-induced changes in the intracellular localization of the protein kinase C substrate B-50 in pheochromocytoma PC12 cells. J Cell Biol. 1989 Mar;108(3):1115–1125. doi: 10.1083/jcb.108.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Benowitz L. I., Villa-Komaroff L., Neve R. L. Transfection of PC12 cells with the human GAP-43 gene: effects on neurite outgrowth and regeneration. Brain Res Mol Brain Res. 1990 Jan;7(1):39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Zuber M. X., Goodman D. W., Karns L. R., Fishman M. C. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989 Jun 9;244(4909):1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]