Abstract
Pathologic alterations in podocytes lead to failure of an essential component of the glomerular filtration barrier and proteinuria in chronic kidney diseases. Elevated levels of saturated free fatty acid (FFA) are harmful to various tissues, implemented in the progression of diabetes and its complications such as proteinuria in diabetic nephropathy. Here, we investigated the molecular mechanism of palmitate cytotoxicity in cultured mouse podocytes. Incubation with palmitate dose-dependently increased cytosolic and mitochondrial reactive oxygen species, depolarized the mitochondrial membrane potential, impaired ATP synthesis and elicited apoptotic cell death. Palmitate not only evoked mitochondrial fragmentation but also caused marked dilation of the endoplasmic reticulum (ER). Consistently, palmitate upregulated ER stress proteins, oligomerized stromal interaction molecule 1 (STIM1) in the subplasmalemmal ER membrane, abolished the cyclopiazonic acid-induced cytosolic Ca2+ increase due to depletion of luminal ER Ca2+. Palmitate-induced ER Ca2+ depletion and cytotoxicity were blocked by a selective inhibitor of the fatty-acid transporter FAT/CD36. Loss of the ER Ca2+ pool induced by palmitate was reverted by the phospholipase C (PLC) inhibitor edelfosine. Palmitate-dependent activation of PLC was further demonstrated by following cytosolic translocation of the pleckstrin homology domain of PLC in palmitate-treated podocytes. An inhibitor of diacylglycerol (DAG) kinase, which elevates cytosolic DAG, strongly promoted ER Ca2+ depletion by low-dose palmitate. GF109203X, a PKC inhibitor, partially prevented palmitate-induced ER Ca2+ loss. Remarkably, the mitochondrial antioxidant mitoTEMPO inhibited palmitate-induced PLC activation, ER Ca2+ depletion and cytotoxicity. Palmitate elicited cytoskeletal changes in podocytes and increased albumin permeability, which was also blocked by mitoTEMPO. These data suggest that oxidative stress caused by saturated FFA leads to mitochondrial dysfunction and ER Ca2+ depletion through FAT/CD36 and PLC signaling, possibly contributing to podocyte injury.
Podocytes are the terminally differentiated visceral epithelial cells in the glomerular filtration barrier that have a critical role in conserving macromolecules in the plasma. Injury, cell death and detachment of podocytes lead to proteinuria, an early prognostic symptom of chronic kidney disease (CKD).1 Epidemiologic studies have shown that the majority of CDKs in patients are caused by glomerular disorders with diabetic nephropathy (DN).2, 3 One of the major pathogenic mediators in type 2 diabetes and its complications is dyslipidemia, resulting in high saturated free fatty-acid (FFA) concentrations. In normal adults, the total plasma FFA concentration is reported to be 200–600 μM, and this value increases up to fourfold in type 2 diabetes.4 Palmitate is the most abundant saturated FFA in the plasma of humans and rodents accounting for ~25% of total fatty acids.5, 6
Saturated FFAs in the cytosol induce reactive oxygen species (ROS) production as observed in pancreatic β-cells, hepatic cells and skeletal muscle cells.7, 8, 9 In muscle cells, palmitate stimulates superoxide generation through the mitochondrial electron transport chain and NADPH oxidase activities.10 This oxidative stress leads to mitochondrial dysfunction, mitochondrial permeability transition (PT), cytochrome c release and apoptosis.8, 11, 12 The role of unsaturated FFA in this process has been controversial, but accumulating evidence suggests a protective function counteracting the cytotoxic effect of saturated FFA.8, 13, 14
Furthermore, palmitate elicits the unfolded protein response and endoplasmic reticulum (ER) stress-induced cell death in various cell types.15, 16 Particularly in pancreatic β-cells, ER stress by palmitate leads to defective insulin secretion contributing to the progression of type 2 diabetes.17 An explanation for the induction of ER stress may be that saturated FFA disrupts ER Ca2+ homeostasis in β-cells.15, 18 Depletion of ER Ca2+ impairs the function of ER-resident chaperones, resulting in the recruitment of the immunoglobulin heavy chain binding protein (BIP) into the lumen of the organelle.19 A series of ER stress responses are initiated by the release of BIP from sensors in the ER membrane including inositol-requiring protein 1, activating transcription factor 6 and PKR-like ER kinase (PERK). Downstream signaling cascades of these activators lead to translation attenuation, upregulation of ER chaperone expression, ER-associated protein degradation and apoptosis.20
The ER is highly sensitive to the redox status.21 In the ER lumen, protein folding by disulfide isomerase requires oxidation, which depends on hydrogen peroxide generated by ER oxidoreductase (ERO1).22 Accumulation of misfolded proteins in the ER lumen produces excessive ROS through ERO1-mediated oxidation.23 Moreover, the induction of C/EBP homology protein (CHOP) caused by PERK activation during ER stress also increases ERO1 expression, which further accelerates ROS generation.24, 25 Intriguingly, Ca2+ release from the ER is increased during ER stress, which is blocked by silencing of ERO1 or type 1 inositol (1,4,5)-trisphosphate (IP3) receptor.23 These findings imply that oxidative stress aggravates ER stress in a vicious cycle by reducing the Ca2+ level in the ER lumen.
Several reports have investigated the pathologic changes induced by palmitate in podocytes, pertinent to the development of proteinuria and the progression of glomerular disease. Palmitate inhibits insulin signaling and insulin-stimulated glucose uptake in human podocytes.26 Palmitate-induced apoptosis associated with ER stress was partially prevented by silencing of CHOP in mouse podocytes.14 Furthermore, palmitate-dependent cytotoxicity was attenuated by the co-treatment with mono-unsaturated FFA or the activation of stearoyl-CoA desaturase, which converts saturated to unsaturated FFA.27
In this study, we aimed to identify the molecular mechanisms linking FFA-induced superoxide production associated with mitochondrial dysfunction and ER stress in mouse podocytes. We clearly show that palmitate disrupts Ca2+ homeostasis in the cytosol and intracellular organelles, crucial for the development of ER stress and cytotoxicity in podocytes. We further demonstrate that mitochondrial oxidative stress acts upstream of palmitate-induced ER Ca2+ depletion and ER stress through phospholipase C (PLC) activation. These perturbations of cellular homeostasis in podocytes with altered cytoskeletal arrangement likely contribute to the deterioration of the filtration barrier function in DN.
Results
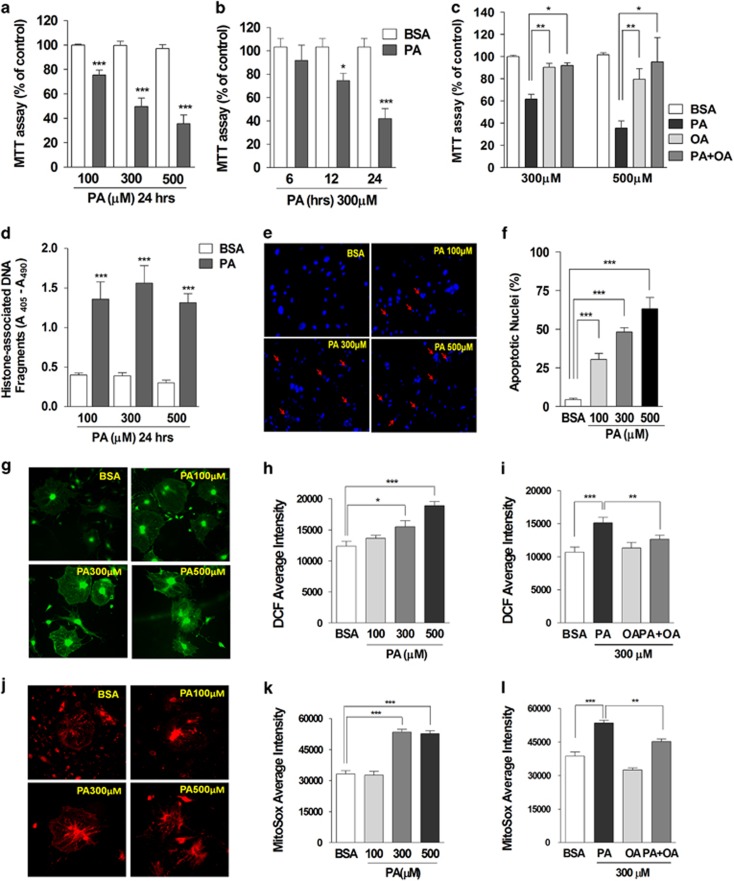
We previously demonstrated full-podocyte differentiation after 14 days in culture at 37 °C without interferon-γ.28 To investigate the cytotoxic effects of FFA, we administered bovine serum albumin (BSA)-conjugated palmitate or oleate to differentiated podocytes. Using a 3-(4,5-dimethylhioazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, we observed dose-dependent reduction of cell viability by 100–500 μM palmitate (Figure 1a). Palmitate (300 μM)-induced cytotoxicity was marked between 12 and 24 h of incubation (Figure 1b). Oleate did not affect cell survival and even protected from palmitate-induced cytotoxicity (Figure 1c). Incubation with palmitate for 24 h prominently raised apoptotic DNA fragments (Figure 1d). Palmitate dose-dependently increased the percentage of fragmented and condensed apoptotic nuclei (Figures 1e and f).
Figure 1.
Palmitate, but not oleate, increased cytosolic and mitochondrial ROS production and induced cytotoxicity in mouse podocytes. Palmitate (PA) or bovine serum albumin (BSA) was applied to differentiated mouse podocytes with different concentrations ((a), N=10) and durations ((b), N=7). The effect of oleate (OA) on PA-induced cytotoxicity was also analyzed ((c), N=3). Cytotoxicity by palmitate treatment (24 h) was evaluated by MTT assay (a–c) and quantitative determination of apoptotic DNA fragments ((d), N=5). Condensed and fragmented apoptotic nuclei induced by palmitate were detected by DAPI staining (e) and are expressed as the percent of apoptotic nuclei ((f), N=8). Podocytes treated with BSA or palmitate (PA) were loaded with DCF-DA (g–i) or mitoSox (j–l) and the fluorescence intensity was measured using a fluorescence microscope imaging system. Average florescence intensities of DCF (19–29 images from ≥5 independent experiments; (b) and (c)) or mitoSox (7–10 images from ≥3 independent experiments; e and f) were analyzed. Data are presented as mean±S.E.M., and *, **, *** denote P<0.05, P<0.01 and P<0.001, respectively
Cytosolic ROS generation was followed using the 2′,7′-Dichlorofluorescein diacetate (DCF-DA) fluorescence dye (Figures 1g–i). Treatment with palmitate for 24 h increased DCF fluorescence intensity. In contrast, oleate did not elevate cytosolic ROS. Moreover, palmitate-induced ROS generation was prevented by oleate co-incubation.
Mitochondrial superoxide was measured using mitoSox (Figures 1j–l). Palmitate treatment for 24 h increased the fluorescence intensity of mitoSox-loaded podocytes. Oleate did not affect mitochondrial ROS production, and attenuated the palmitate-induced increase of mitochondrial superoxide. These data demonstrate that the mono-unsaturated fatty-acid oleate protects from oxidative stress and cell death induced by saturated FFA in mouse podocytes.
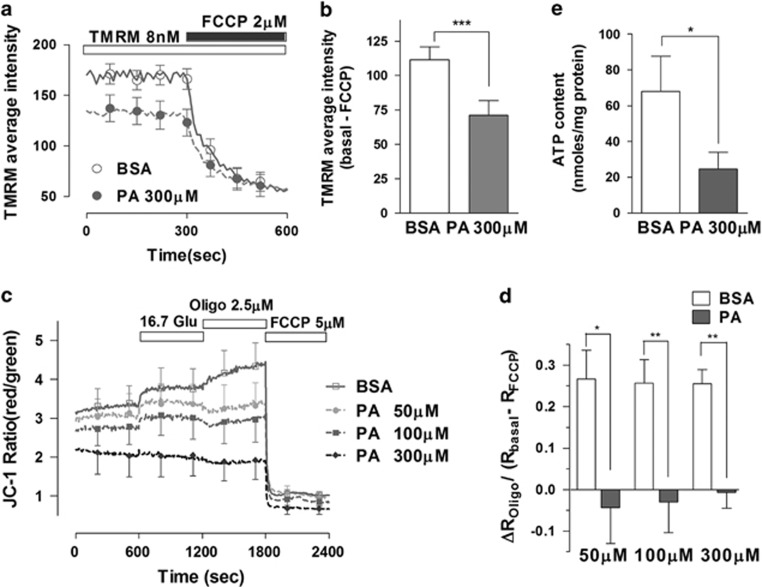
We investigated whether palmitate incubation could alter the mitochondrial membrane potential (ΔΨm) and ATP generation as a consequence of mitochondrial oxidative stress. We used two potential-sensitive fluorescence dyes, tetramethylrhodamine methylester (TMRM) and JC-1 to measure the ΔΨm. TMRM (8 nM) was used in a non-quenching mode, distributing according to the Nernstian equation across the inner mitochondrial membrane. We observed that treatment with 300 μM palmitate for 24 h lowered the average fluorescence intensity of TMRM in podocytes compared with control BSA-treated cells (Figures 2a and b).
Figure 2.
Palmitate elicited mitochondrial depolarization and ATP depletion in mouse podocytes. (a, b) Differentiated podocytes were loaded with the potential-sensitive probe TMRM (8 nM for 30 min) and changes in fluorescence intensity in the mitochondrial area were measured using a confocal microscope imaging system. Average TMRM fluorescence intensities were compared between BSA- and PA-treated podocytes (N=11). (c, d) Podocytes were loaded with JC-1 (350 nM for 30 min) and the fluorescence intensity was measured using a fluorescence multi-plate reader. The JC-1 fluorescence ratio of red over green, which reflects mitochondrial membrane potential, was recorded from BSA- or palmitate (PA)-treated cells (N=6–8). Oligomycin-induced changes in the JC-1 ratio (ΔROligo) were normalized to the resting mitochondrial membrane potential (Rbasal−RFCCP). (e) Podocytes were incubated with BSA or PA for 24 h and the cellular ATP content in podocytes was measured using a bioluminescence assay (N=13). Data are presented as mean±S.E.M., and *, ** and *** denote P<0.05, P<0.01 and P<0.001, respectively
As an alternative method to study ΔΨm, we measured the JC-1 fluorescence ratio (red/green) from BSA- or palmitate-treated podocytes (Figures 2c and d).29 The resting ΔΨm and glucose (16.7 mM)-stimulated hyperpolarization were lower in palmitate-treated cells. Oligomycin, a selective blocker of the F1F0-ATPase, causes hyperpolarization in normally functioning mitochondria, but depolarization in dysfunctional mitochondria.30 In control cells, oligomycin caused hyperpolarization but induced partial depolarization of the Ψm in podocytes treated with low concentration of palmitate. This finding suggests that palmitate treatment increases the fraction of impaired mitochondria consuming ATP through F1F0-ATPase to maintain the ΔΨm.
The lowered driving force across the inner mitochondrial membrane may also inhibit ATP synthesis. Incubation of podocytes with palmitate (300 μM) for 24 h reduced the cellular ATP content compared with that of control cells (Figure 2e). Our results demonstrate palmitate-induced impairment of mitochondrial energy metabolism.
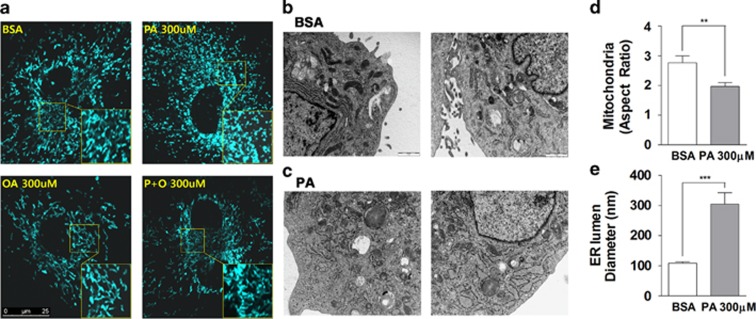
Oxidative stress and depolarization of the Ψm has a negative impact on mitochondrial fusion resulting in shortened and fragmented mitochondria.31, 32 We observed that control podocytes had interconnected and rod-shaped mitochondria (Figure 3a). On the contrary, palmitate (300 μM)-treated podocytes had dot-like round and fragmented mitochondria, suggesting either reduced mitochondrial fusion or accelerated fission. Oleate alone or added in combination with palmitate did not affect mitochondrial morphology.
Figure 3.
Palmitate-induced mitochondrial fragmentation and ER lumen dilation in mouse podocytes. (a) Differentiated podocytes were infected with adenovirus encoding mitochondrial-targeted cyan fluorescence protein (mitoCFP) and treated with BSA or palmitate (PA) for 24 h. Mitochondrial morphology was observed using a confocal microscope imaging system. (b, c) Structural alterations in intracellular organelles were analyzed from electron microscopy (EM) images. The aspect ratio of mitochondria (longer/shorter diameter, (d)) and the diameter of the ER lumen (e) were obtained from EM images and compared between BSA (N=13)- and PA (N=16)-treated podocytes. Data are presented as mean±S.E.M. ** and *** denote P<0.01 and P<0.001, respectively
Using electron microscopy (EM), we analyzed micro-structural alterations in intracellular organelles focusing on mitochondria and the ER (Figures 3b–e). BSA-treated cells had elongated mitochondria and regular width of ER lumen. On the other hand, shortened mitochondria and severely dilated ER was observed in palmitate-treated podocytes. We compared the mitochondrial aspect ratio (AR), which is calculated from the ratio of the longer diameter divided by the shorter one. Palmitate-treated podocytes had a lower mitochondrial AR value than BSA-treated cells. The mean ER diameter in palmitate-treated podocytes was conspicuously wider than the diameter in control cells, suggesting an ER stress response in palmitate-treated cells.33
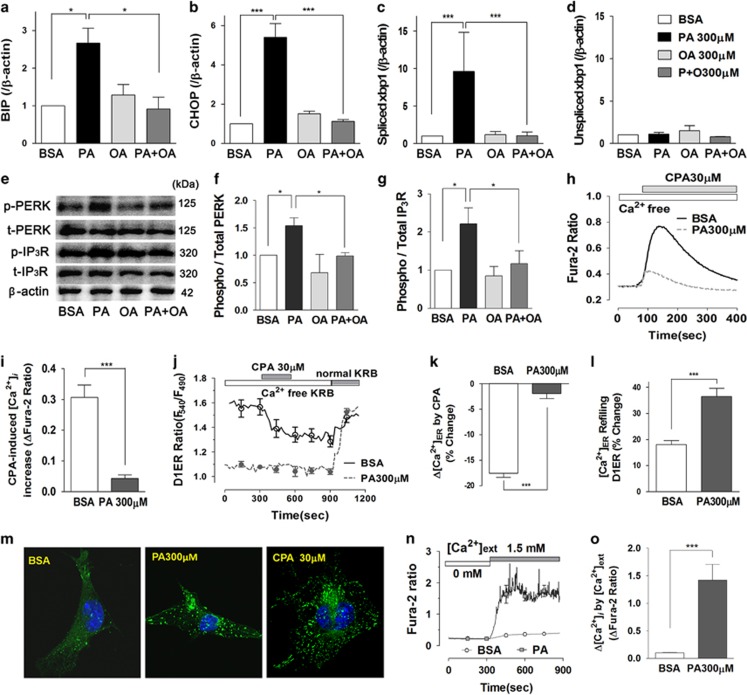
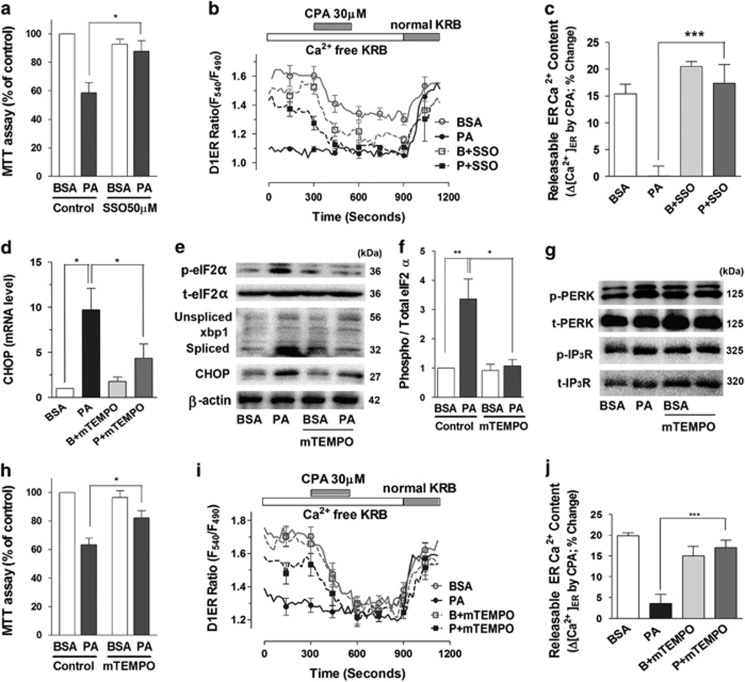
To investigate whether ER stress occurs in palmitate-treated podocytes, we checked transcriptional regulation of ER stress response genes (Figures 4a–d).15 Palmitate (300 μM) increased mRNA levels of BIP and CHOP. Unspliced xbp1 (X-box BIP 1) was unaltered, whereas spliced xbp1 was highly upregulated by palmitate treatment. Oleate alone did not affect and in combination with palmitate prevented upregulation of ER stress markers. Palmitate increased phosphorylation of PERK and type 1 IP3 receptor (IP3R). These activations were prevented by oleate co-incubation (Figures 4e–g).
Figure 4.
Palmitate depleted the ER Ca2+ pool and elicited ER stress in mouse podocytes. (a–d) Transcript levels of BIP (a), CHOP (b) and spliced (c) and unspliced xbp1 (d) were analyzed using quantitative RT-PCR from differentiated podocytes treated with BSA-, palmitate (PA)-, oleate (OA) and PA with OA for 24 h (N=3). (e–g) Protein levels of phosphorylated and total PKR-like ER kinase (PERK) and type 1 inositol (1,4,5)-trisphosphate receptor (IP3R) were analyzed using western blot. (h, i) Changes in cytosolic Ca2+ ([Ca2+]i) induced by cyclopiazonic acid (CPA) were recorded by using a fluorescence microscope imaging system, and reflect the amount of ER Ca2+ release. Before Fura-2 loading, podocytes were incubated with BSA or PA in Ca2+-free KRB solution for 90 min (N=8–9). (j–l) Luminal Ca2+ content in ER ([Ca2+]ER) was measured in podocytes transfected with plasmid encoding ER Ca2+-sensing fluorescence protein D1ER using a confocal microscope imaging system. CPA-induced reduction of [Ca2+]ER and ER Ca2+ refilling by 1.8 mM extracellular Ca2+ were compared between podocytes treated with BSA (N=37) or PA (N=34) in Ca2+-free KRB solution for 90 min. (m) To visualize the localization of STIM1, podocytes were transfected with a plasmid encoding STIM1-yellow fluorescence protein (YFP). Confocal YFP fluorescence images were obtained from BSA-, PA- (for 24 h) or CPA- (for 10 min) treated podocytes. (n, o) Changes in [Ca2+]i by extracellular Ca2+ addition were compared between BSA- or PA-treated podocytes in Ca2+-free KRB solution for 90 min (N=5). Data are presented as mean±S.E.M. * and *** denote P<0.05 and P<0.001, respectively
Because of the strong Ca2+ dependency of ER chaperones, ER Ca2+ depletion can cause misfolding of luminal proteins and as a consequence ER stress.15, 18 To estimate ER Ca2+ levels, we measured cytosolic Ca2+ ([Ca2+]i) changes in response to the application of cyclopiazonic acid (CPA), an inhibitor of the ER Ca2+ ATPase (SERCA) (Figures 4h and i). Podocytes were incubated with BSA or palmitate in Ca2+-free Krebs-Ringer bicarbonate (KRB) solution for 90 min. CPA caused a pronounced rise of [Ca2+]i in control cells. This calcium rise was almost completely prevented when podocytes were treated with palmitate. The results reveal palmitate-induced loss of the ER Ca2+ store.
Next, we directly measured luminal Ca2+ level in the ER with D1ER (Figures 4j–l).18, 34, 35 In BSA-treated cells perfused with Ca2+-free KRB solution, the CPA-induced inhibition of the ER Ca2+ pump decreased the D1ER ratio. Palmitate-treated podocytes had a very low D1ER ratio at resting state, and did not significantly respond to CPA treatment. After wash out of CPA, the ER Ca2+ levels returned to basal level when exposed to 1.8 mM Ca2+. Following depletion of ER Ca2+ stores palmitate-treated podocytes showed more rapid and pronounced increases in ER Ca2+ refilling. These results demonstrate that store-operated Ca2+ entry (SOCE) and refilling of ER Ca2+ via the SERCA pump is not impaired following palmitate incubation.
SOCE is initiated by oligomerization of stromal interaction molecule 1 (STIM1) and Orai at the plasma membrane-ER junction area.36 Oligomerization in podocytes was followed in cells expressing yellow fluorescence protein (YFP)-tagged STIM1. Palmitate treatment-elicited redistribution of YFP into subplasmalemmal bright fluorescent spots suggestive of STIM1 oligomerization. A concentration of STIM1-YFP fluorescence was also observed in cells depleted for ER Ca2+ using CPA (Figure 4m). STIM1 oligomerization is consistent with palmitate-mediated ER Ca2+ depletion. To demonstrate the activation of SOCE by palmitate, we applied extracellular Ca2+ to podocytes incubated with palmitate in Ca2+-free KRB solution for 90 min. Palmitate-treated cells had pronounced [Ca2+]i increase upon Ca2+ addition via SOCE compared to BSA-treated cells (Figures 4n and o).
FAT/CD36 is a fatty-acid transporter also known as scavenger receptor, which mediates adverse effects of saturated FFA.37 To test whether FAT/CD36 was required for palmitate-induced [Ca2+]ER loss and cytotoxicity, we pretreated podocytes with the inhibitor, sulfosuccinimidyl oleate (SSO), 30 min before palmitate incubation (Figures 5a–c). SSO (50 μM) abrogated palmitate-induced cytotoxicity compared with palmitate alone. Consistently, SSO restored the D1ER ratio and recovered the CPA-induced ER Ca2+ release. These results show that inhibition of FAT/CD36 prevents palmitate-induced depletion of ER Ca2+.
Figure 5.
Palmitate-induced ER Ca2+ depletion and cytotoxicity were attenuated by a FAT/CD36 inhibitor and a mitochondrial antioxidant. (a) Sulfosuccinimidyl oleate (SSO), a FAT/CD36 inhibitor, was used to pretreat podocytes for 30 min before BSA or palmitate (PA, 300 μM) incubation (for 24 h), and cytotoxicity was estimated by using an MTT assay (N=5). (b, c) Luminal Ca2+ content in ER ([Ca2+]ER) was measured in D1ER-transfected podocytes incubated with BSA or PA in Ca2+-free KRB solution for 90 min (N=5). (d–j) A mitochondrial antioxidant, mitoTEMPO (mTEMPO; 100 nM), was pretreated to podocytes for 30 min before BSA or PA incubation. The mRNA (d) and protein (e–g) levels of ER stress-related proteins were analyzed, and cytotoxicity induced by palmitate ((h), N=11) were estimated. Effects of mTEMPO pretreatment on [Ca2+]ER levels were analyzed in D1ER-transfected podocytes incubated with BSA or PA upon cyclopiazonic acid (CPA) application ((i, j), N=13–15). Data are presented as mean ± S.E.M. *, ** and *** denote P<0.05, P<0.01 and P<0.001, respectively
To investigate the role of palmitate-induced ROS on ER Ca2+ depletion and ER stress, we preincubated podocytes with the mitochondrial antioxidant, mitoTEMPO (100 nM). Palmitate (300 μM)-induced cytotoxicity was significantly attenuated by preincubation with mitoTEMPO (Figure 5g). Transcriptional upregulation of CHOP by palmitate (300 μM) was also inhibited by mitoTEMPO (Figure 5d). Palmitate increased the phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), PERK and IP3R as well as the protein levels of CHOP and spliced but not unspliced xbp1. All these palmitate-induced changes were prevented by mitoTEMPO-mediated ROS scavenging (Figures 5e–g). The reduction of releasable ER Ca2+ and refilling of ER Ca2+ stores by palmitate were prevented by pretreatment with mitoTEMPO (Figures 5i and j). These data support our hypothesis that the oxidative stress originating from mitochondria participates in ER Ca2+ depletion and ER stress, which is instrumental in palmitate-elicited cytotoxicity.
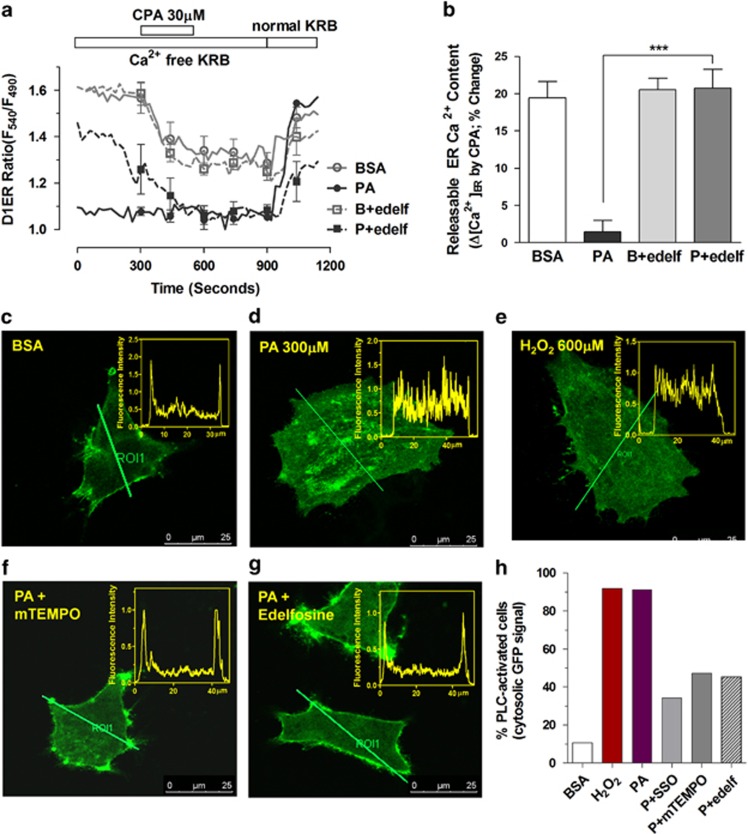
We applied the PLC inhibitor edelfosine to test whether PLC activation is involved in palmitate-induced ER Ca2+ release. Pretreatment with edelfosine completely restored the CPA-releasable ER Ca2+ pool in podocytes treated with palmitate (Figures 6a and b).
Figure 6.
Inhibition of phospholipase C prevented intracellular IP3 generation and loss of the ER Ca2+ pool in palmitate-treated podocytes. (a, b) Changes in luminal Ca2+ content in ER ([Ca2+]ER) upon cyclopiazonic acid (CPA) application were measured in D1ER-transfected podocytes. Edelfosine (edelf; 10 μM), a phospholipase C (PLC) inhibitor, was used to pretreat cells for 30 min before BSA or palmitate (PA, 300 μM) incubation in Ca2+-free KRB solution for 90 min (N=4). (c–h) To observe IP3 generation from PIP2, a plasmid encoding the pleckstrin homology domain of phospholipase Cδ (PLCδ-PH)-green fluorescence protein (GFP) was transfected into podocytes. (c–g) Podocytes were incubated with BSA, PA or H2O2 in Ca2+-free KRB solution for 90 min and GFP distribution was observed using a confocal microscope. Edelfosine (f) or a mitochondrial antioxidant, mitoTEMPO (mTEMPO; 100 nM) (g) was used to pretreat cells for 30 min before BSA or PA incubation. Line analyses of the fluorescence intensity for each picture are shown on the right. The percentage of cells showing PLCδ-PH-GFP translocation from the plasma membrane to the cytosol reflects PLC activation and IP3 synthesis. (h) Data are presented as mean±S.E.M. *** denote P<0.001
To directly visualize PLC activation in palmitate-treated podocytes, we overexpressed the pleckstrin homology (PH) domain of PLC-δ fused to green fluorescent protein (PH-GFP), which can bind phosphatidylinositol 4,5-bisphosphate (PIP2) in plasmalemmal lipid vesicles and also IP3 in the cytosol (Figures 6c–h).38 The activation of PLC elicits marked redistribution of the PH-GFP signal from the plasma membrane to the cytosol. PH-GFP fluorescence is concentrated in subplasmalemmal areas of control podocytes. This is conspicuously different from that of palmitate-treated cells, most of which have uniformly distributed GFP signal indicating re-localization with IP3 to the cytosol. The redistribution of the PH-GFP by palmitate was completely reproduced by H2O2 treatment, confirming oxidative stress-mediated PLC activation. Pretreatment with SSO to inhibit FAT/CD36 markedly augmented the proportion of palmitate-treated cells with subplasmalemmal GFP signal. PLC activation was also blocked by mitoTEMPO and the PLC inhibitor edelfosine in palmitate-treated cells. Based on the above results, we suggest that activation of PLC triggered by mitochondrial ROS is a critical step resulting in palmitate-induced ER Ca2+ depletion in podocytes.
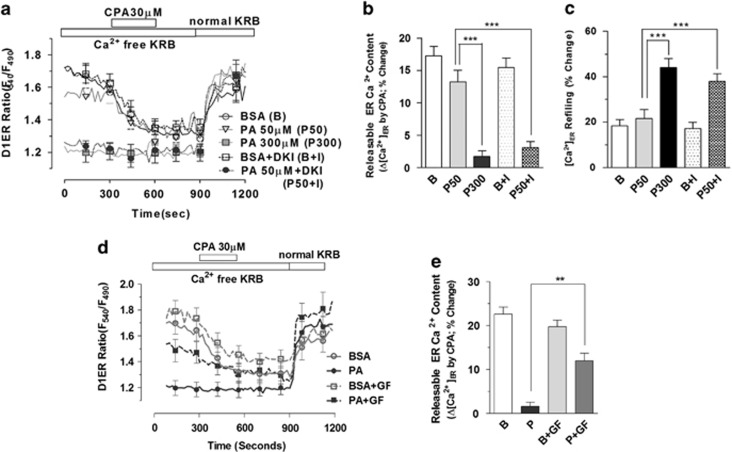
Using PIP2 as a substrate, PLC also generates diacylglycerol (DAG), which activates protein kinase C (PKC).39 DAG is further converted into phosphatidic acid by DAG kinase. Consequently, inhibition of DAG kinase increases DAG content and PKC activity.40 To evaluate the role of DAG in ER Ca2+ depletion, we co-incubated podocytes with R59022, a DAG kinase inhibitor (DKI), and palmitate (Figures 7a–c). ER Ca2+ homeostasis was not affected by low concentrations of palmitate (50 μM) alone. However, the DKI (10 μM) enabled palmitate (50 μM) to deplete the ER Ca2+ store similar to the effect of high palmitate concentration (300 μM). ER Ca2+ refilling was also strongly stimulated by the DKI in the presence of 50 μM palmitate when compared with the same concentration of palmitate alone.
Figure 7.
Diacylglycerol and protein kinase C are involved in ER Ca2+ depletion in palmitate-treated podocytes. Changes in luminal Ca2+ content in ER ([Ca2+]ER) upon cyclopiazonic acid (CPA) application were measured in D1ER-transfected podocytes incubated with BSA or PA (300 μM) in Ca2+-free KRB solution for 90 min. (a–c) A diacylglycerol (DAG) kinase inhibitor, R59022 (DKI) was used to pretreat cells for 30 min before 50 μM PA incubation, and CPA-induced reduction of [Ca2+]ER (b) and ER Ca2+ refilling by 1.8 mM extracellular Ca2+ (c) were compared among different groups (N=13–24). (d) An inhibitor of protein kinase C, GF109203X was used to pretreat cells for 30 min before BSA or PA incubation, and CPA-induced reduction of [Ca2+]ER was analyzed (N=19). Data are presented as mean±S.E.M. ** and *** denote P<0.01 and P<0.001, respectively
The role of PKC, which is a downstream effector of DAG, on palmitate-induced ER Ca2+ depletion was investigated using GF109203X, a nonspecific PKC inhibitor. GF109203X (5 μM) partially restored the palmitate (300 μM)-induced loss of releasable ER Ca2+ content (Figures 7d and e). These results show that activation of the PLC-DAG-PKC pathway is, at least in part, involved in ER Ca2+ depletion induced by palmitate.
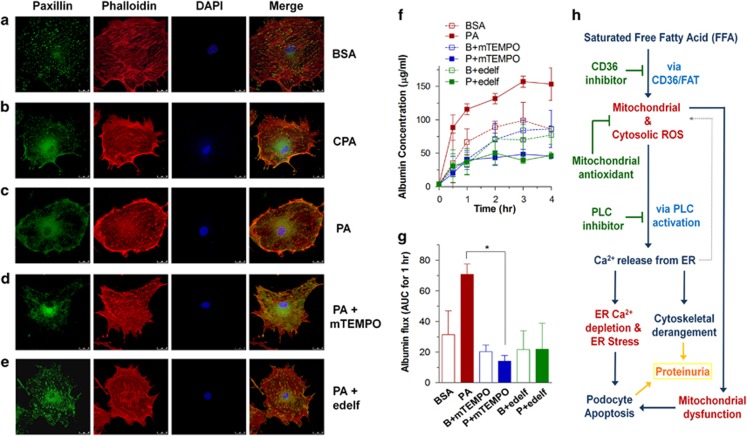
Alteration of the cytoskeletal structure in podocytes impairs barrier function and protein filtration. To observe cytoskeletal rearrangements, fluorescence-labeled phalloidin and immunostaining of paxillin were used for visualizing actin fibers and focal adhesions, respectively (Figures 8a–e). BSA-treated differentiated podocytes have well-organized stress fiber bundles in the cytosol extended into the cell periphery. Paxillin was found at focal adhesions at the tip of actin fibers. Similar to the phenotype of CPA-treated podocytes, palmitate elicits cortical rearrangement of actin fibers with reduced focal adhesion points. Palmitate-induced changes were prevented by pretreatement with mitoTEMPO and edelfosine.
Figure 8.
Alterations in the actin cytoskeleton and albumin permeability in palmitate-treated podocytes. Differentiated podocytes cultured on coverslips were incubated in Ca2+-free KRB solution containing BSA (a) or palmitate (PA; 300 μM) (c) for 90 min, or cyclopiazonic acid (CPA; 15 μM) for 15 min (b). A mitochondrial antioxidant, mitoTEMPO (mTEMPO; 100 nM) (d) or a phospholipase C (PLC) inhibitor, edelfosine (edelf; 10 μM) (e), were used to pretreat podocytes for 15 min before PA incubation. Actin filaments were stained with phalloidin (red) and focal adhesions were detected by paxillin (green) staining. (f, g) The effects of BSA or PA on albumin permeability were estimated using transwell chambers with FITC-labeled BSA. (h) Proposed hypothetical model for palmitate-induced toxicity
As a read-out of podocyte function, we measured the permeability of confluent podocyte layers to FITC-labeled BSA using transwell chambers. Palmitate treatment markedly increased albumin permeation due to defective barrier function of the podocytes (Figure 8f). Consistent with the restoration of cytoskeletal structure, high albumin permeability caused by palmitate was reduced by either mitoTEMPO or edelfosine (Figure 8g). These findings suggest that the pathogenic impact of palmitate on filtration barrier function in podocytes is closely related to ER Ca2+ release mediated by oxidative stress and PLC activation.
Discussion
High serum levels of saturated FFA in diabetic patients have a detrimental effect on insulin secreting β-cells and target cells of the hormone. Previous studies have documented palmitate-induced pathologic changes in human and mouse cultured podocytes, including insulin resistance, ER stress and apoptosis.14, 26 We report in this study on mouse podocytes that palmitate, but not oleate, (1) induced mitochondrial and cytosolic ROS production and apoptotic cell death, (2) impaired mitochondrial energy metabolism (depolarization of the ΔΨm leading to defective ATP synthesis), (3) caused alterations of mitochondrial and ER morphology, (4) decreased the ER Ca2+ concentration, (5) elicited ER stress responses and (6) caused cytoskeletal rearrangement and albumin permeability in podocyte monolayers. Most of these palmitate-induced changes were prevented by co-incubation with oleate, inhibition of CD36/FAT, inhibition of PLC or scavenging of mitochondrial ROS.
Oxidative stress affects protein-folding capacity of the ER and activates the unfolded protein response.21 Persistent exposure to ROS initiates the pro-apoptotic pathway of ER stress and caspase activation.25 Both oxidative stress and ER stress may increase Ca2+ release from the ER, which further aggravates the ER function and cell fate.23 A key finding of this study was the pronounced palmitate-induced depletion of the ER Ca2+ pool. This was monitored directly by measuring luminal ER Ca2+ levels.34 The loss of ER Ca2+ stores by palmitate was complete, as shown by the absence of a response upon inhibition of the ER Ca2+ ATPase (SERCA). In addition, cytosolic Ca2+ rises upon stimulation of ER Ca2+ release were abolished in palmitate-treated podocytes. As a consequence of the pronounced depletion of ER Ca2+, we also observed STIM1 oligomerization following palmitate treatment. STIM1 oligomerization is part of the mechanism leading to store-operated calcium entry (SOCE), an attempt of palmitate-treated cells to replenish their ER calcium content.36 Downstream consequences of this impairment of ER Ca2+ homeostasis were dilatation of the ER lumen and ER stress responses. The reduced ER Ca2+ levels are causally related to the upregulation of ER stress-associated proteins, including CHOP and the initiation of apoptosis.
The present study furthers our understanding of the molecular mechanisms linking mitochondrial ROS production to ER stress. Activation of PLC has a central role in this process. This was demonstrated using a PH domain containing fluorescent protein, which allows visualization of PLC activation and PIP2 hydrolysis.38 Palmitate-induced PIP2 hydrolysis was prevented by a mitochondrial antioxidant similar to the effect of a PLC inhibitor. ER Ca2+ depletion was also abolished by the mitochondrial antioxidant as well as by PLC inhibition in the podocytes. Relevant in this context, a recent study proposed that ROS generated from NADPH oxidase 2 directly induces activation of PLC-γ via inhibition of DAG kinase in a lung ischemia–reperfusion model.41 Based on our result, we suggest that parallel activations of IP3 and DAG-PKC by PLC may contribute to palmitate-induced ER Ca2+ depletion and ER stress. Further clarification of the underlying molecular mechanisms is necessary.
In addition to ER Ca2+ release, SOCE through plasmalemmal orai channels could increase cytosolic as well as mitochondrial matrix Ca2+ concentrations. Ca2+ overload in the matrix facilitates mitochondrial superoxide generation.42 This mitochondrial ROS may further accelerate ER Ca2+ release.23 Such a positive feedback loop established from oxidative stress and ER-mitochondrial Ca2+ transfer compromises mitochondrial function and elicits PT. The observed reduction of the electrical gradient across the inner mitochondrial membrane, defective ATP synthesis and podocyte apoptosis could be the result of this type of mitochondrial oxidative stress. The importance of ER Ca2+ release in the promotion of cell death has already been demonstrated. The overexpression of SERCA promotes oxidative stress-mediated mitochondrial PT pore opening and apoptosis.43
The here shown tight interaction between mitochondrial and ER ROS and calcium-handling occurs in the regions of close proximity between mitochondria and the ER.44 These contact sites, mitochondria-associated ER membranes (MAMs), are a signaling platform and essential for normal ER and mitochondrial function.45 Loss of functional MAMs has been associated with hepatic insulin resistance in type 2 diabetes.46 In contrast to these findings, higher IP3 receptor expression and enlarged MAMs were reported in diabetic mice or control mice on a high-fat diet.42 The reason for these conflicting results remains to be elucidated.
To maintain proper glomerular filtration barrier function, the structure of the actin cytoskeleton in podocytes should adapt to environmental changes to prevent permeability to large molecules. Particularly, Ca2+-dependent remodeling of the actin cytoskeleton is essential for the maintenance of the glomerular slit diaphragm structure.47 Sustained Ca2+ elevation leads to the loss of stress fibers and foot effacement in podocytes, which causes glomerulosclerosis in mice.48 Palmitate-induced cytoskeleton rearrangement built cortical actin bundles (Figure 8). Blocking of either mitochondrial ROS or PLC signaling prevented actin cytoskeleton remodeling as well as albumin permeability of confluent podocyte monolayers. Our study provides evidence that Ca2+ release from the ER via oxidative stress and PLC activation could be responsible for pathologic actin remodeling and albumin permeability. In addition to podocyte apoptosis, alterations in the cytoskeleton may actively participate in the proteinuric phenotype induced by saturated FFA in DN.
Based on these results, we suggest several targets to achieve protection from glomerular injury and proteinuria as well as cellular damage in other tissues caused by high plasma levels of saturated FFA. First, scavenging the mitochondrial ROS could be an effective strategy to prevent the vicious cycle of oxidative stress and disturbance of organellar Ca2+. Mitochondrial antioxidants counteracted palmitate-mediated ER stress and cell death. Second, palmitate-induced pathogenic alterations were consistently alleviated by a mono-unsaturated FFA. Third, reducing PLC signaling, lowering downstream signaling pathways linked to IP3, DAG and PKC, could attenuate ER Ca2+ depletion and ER stress, as shown here using a PLC inhibitor. Last, but not least, pharmacological inhibition of FAT/CD36 efficiently reduces entry of FFA and its subsequent toxicity. In conclusion, we propose a model for the mechanism of saturated FFA-induced cytotoxicity in podocytes, which provides evidence for the close interaction between oxidative stress and Ca2+ homeostasis in mitochondria and the ER (Figure 8h). Our work contributes to the elucidation of high-fat-induced glomerular pathophysiology and paves the way for therapeutic approaches to various disease conditions with inherent ER stress and mitochondrial dysfunction.
Materials and Methods
Cell culture and drugs
Immortalized mouse podocyte cell line was a kind gift from Professor Peter Mundel (Harvard Medical School, Charlestown, MA, USA). These cells were grown on collagen I (Catalog # A10483-01, Life Technologies Corporation, Grand Island, NY, USA) coated dishes with low glucose (5.5 mM) Dulbecco's modified Eagles medium supplemented with 5% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at two different temperatures.49, 50 At 33 °C, cells are allowed to proliferate (permissive condition) in the presence of 20 U/ml mouse recombinant IFN-γ (R & D Systems, Minneapolis, MN, USA). For the induction of differentiation (non-permissive condition), podocytes were thermo-shifted to 37 °C in the absence of IFN-γ for 14 days. Expression of synaptopodin, a specific marker for podocyte differentiation, was gradually increased during culture in non-permissive conditions (37 °C), whereas it was not detected in podocytes maintained under permissive conditions (33 °C with interferon-γ).
Palmitate and oleate were prepared by conjugation with fatty-acid-free BSA. First, sodium palmitate or sodium oleate (100 mM) was dissolved in autoclaved distilled water (DW) at 70 °C for 30 min. BSA (10%) was also dissolved in autoclaved DW at 55 °C for 30 min and filtered. Dissolved palmitate or oleate solution was added dropwise into the BSA solution and stored as a stock (10 mM) at −80 °C until use. KRB solution contained (in mM) 135 NaCl, 3.6 KCl, 2 NaHCO3, 0.5 NaH2PO4, 0.5 MgSO4, 1.5 CaCl2, 10 HEPES, 5.5 glucose (pH 7.4, 318 mOsm/kg H2O). 2′,7′-Dichlorofluorescein diacetate DCF-DA, mitoSox, JC-1 and TMRM were purchased from Molecular Probes (Invitrogen, Grand Island, NY, USA). SSO was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and mitoTEMPO was purchased from Enzo Life Sciences (Enzo Life Sciences, Inc., Farmingdale, NY, USA). Sodium palmitate, sodium oleate, fatty-acid-free BSA, MTT, 4′,6′-diamidino-2-phenylindole (DAPI), edelfosine, DKI R59022, GF109203X and other drugs were purchased from Sigma (St. Louis, MO, USA).
Assays for apoptotic cell death
Cytotoxicity was estimated by MTT colorimetric assay. Differentiated podocytes seeded onto a 96-well plate were incubated with MTT (50 μg/well) for 2 h. The supernatant was removed and 100 μl dimethylsulfoxide was subsequently added to each well. After shaking the plate, the absorbances of each well at 570 and 630 nm were measured, and subtracted by a microplate ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Apoptosis of podocyte was detected by nuclear staining using DAPI. Podocytes were grown on collagen-coated coverslips and maintained for 14 days in non-permissive conditions. Before fixation, the medium was discarded and cells were carefully washed three times with phosphate-buffered saline (PBS). The cells were fixed using 4% paraformaldehyde in PBS for 15 min at 37 °C, followed by washing with PBS for three times. DAPI (1 μg/ml, 5 min) was used for nuclear staining. Cells fixed on coverslips were washed out three times with PBS and mounted on a glass slide using Vectashield mounting solution (Vector Laboratories, Inc., Burlingame, CA, USA). Apoptotic cells displayed characteristic features including chromatin condensation and nuclear fragmentation resulting from activation of endogenous nucleases, and were counted under an epifluorescence microscope (excitation at 358 nm and emission at 461 nm).
Apoptotic internucleosomal DNA fragmentation was quantitatively assayed by antibody-mediated capture of cytoplasmic oligonucleosome-associated histone-DNA complexes (Cell Death Detection ELISA Plus kit; Roche Molecular Biochemicals, Mannheim, Germany). Differentiated podocytes cultured in 24-well plates (5 × 104 cells/well) were resuspended in 200 μl lysis buffer supplied by the manufacturer, and incubated for 30 min at room temperature. After pelleting nuclei by centrifugation (200 × g, 10 min), 20 μl of supernatant (cytoplasmic fraction) was used in the enzyme-linked immunosorbent assay (ELISA), following the manufacturer's standard protocol. Finally, the absorbances at 405 and 490 nm (reference wavelength), upon incubating with a peroxidase substrate for 5 min, were determined with a microplate ELISA reader (Molecular Devices).
Measurement of cytosolic and mitochondrial ROS generation
Cytosolic ROS generation was measured using CM-H2DCF-DA (2′-7′ dichlorofluorescin diacetate) (Molecular Probes, Eugene, OR, USA). Differentiated podocytes on 12-mm coverslips were loaded with 5 μM CM-H2DCF-DA for 20 min at 37 °C, and excess dye was then washed out using KRB solution. Fluorescence intensity was measured using an inverted microscope (IX81, Olympus, Tokyo, Japan) equipped with an array laser Nipkow spinning disk (CSU10, Yokogawa Electric Corporation, Tokyo, Japan). The wavelengths for the measurement of DCF fluorescence were 490 nm for excitation and 535 nm for emission and were analyzed using Metamorph 6.1 software (Molecular Devices).
Mitochondrial superoxide generation was detected using mitoSox (Molecular Probes), a red fluorescent dye localized to mitochondria. Once it enters the mitochondria, mitoSox is specifically oxidized by superoxide and exhibits red fluorescence. MitoSox (5 μM) was used to load the differentiated podocytes cultured on 12 mm coverslips for 14 days at 37 °C. Cells were washed twice with KRB solution and the red fluorescent intensity from podocytes was measured by using the Nipkow spinning disk confocal microscopic system with wavelengths of 514 and 560 nm for excitation and emission, respectively.
Assays for ΔΨm and ATP generation
Mitochondrial membrane potential of podocytes was measured using the lipophilic cationic dye 5,5′,6,6′-tetrachloro-1,19,3,39-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) (Molecular Probes). JC-1 monomers enter into mitochondria based on ΔΨm and form J-aggregates inside the mitochondria transmitting red fluorescence (excitation/emission wavelength: 540/590 nm), whereas the rest of the monomers transmit green fluorescence (excitation/emission wavelength: 490/540 nm). The ratio of red/green fluorescence is used as an indicator of ΔΨm. For this experiment, differentiated podocytes were grown on 96-well flat, clear bottom, black-walled polystyrene TC-treated microplates (Corning Incorporated, Corning, NY, USA). Cells were washed twice with KRB solution after loading with JC-1 (300 nM) for 40 min and the fluorescence was recorded by a fluorescence microplate reader (Flexstation II, Molecular Devices), as described previously.29
As an alternative method to measure the ΔΨm, podocytes seeded on collagen I-coated coverslips were loaded with 8 nM TMRM for 20 min. Cells were placed on the inverted microscope and perfused with KRB solution containing 8 nM TMRM. Fluorescence images with 514 nm excitation and 560 nm emission were recorded with the Nipkow spinning disk confocal microscopic system and analyzed using Metamorph 6.1 software.
The ATP content in cell lysates was measured using a bioluminescence assay, as described previously.29 Differentiated podocytes seeded onto 24-well plates (105 cells/well) were preincubated with glucose-free KRB solution for 2 h prior to incubation with 5.5 mM normal KRB solution for 30 min. Then, supernatants were removed and 100 μl lysis buffer supplied by the ATP measurement kit (Roche HS-II Biolumniscence kit, Mannheim, Germany) was added. After 5 min, cells from each well were scraped with pipette tips and transferred to a tube for centrifugation. The supernatant was put into a 96-well plate and the luminescence was measured using a luminometer (Synergy, BioTek Instruments, Winooski, VT, USA) after addition of the luciferase reagent (50 μl) supplied with the kit. Measurement of the protein concentration of cell lysates was performed using the Bradford assay (BCA protein assay kit, Thermo Scientific, Rockford, IL, USA).
Morphological analysis of mitochondria and ER
Adenovirus encoding mitochondrial-targeted cyan fluorescence protein (mitoCFP) were applied together with adenovirus expressing the reverse tetracyclin transactivator for 90–120 min at 37 °C, as described previously.29 After 48 h incubation, cells were fixed with 4% paraformaldehyde in PBS for 15 min. Fluorescence images for mitoCFP (excitation/emission: 440/490 nm) were obtained using a laser-scanning mode confocal microscopic system (TCS SPE, Leica Microsystems GmbH, Wetzlar, Germany).
To observe STIM1 localization in the ER, differentiated podocytes were transiently transfected with a plasmid encoding YFP-tagged STIM1 using the FuGENE HD transfection reagent (Promega, Madison, WI, USA) according to the manufacturer's instructions. Fluorescence images for YFP-STIM1 (excitation/emission: 488/535 nm) were obtained 48 h after transfection using the laser-scanning mode confocal microscope. DAPI (1 μg/ml, 5 min) was used for nuclear staining.
To analyze changes in the electron microscopic ultrastructures of mitochondria and ER, differentiated podocytes were collected by centrifugation and treated with 2.5% glutaraldehyde fixative at 4 °C for 24 h. These samples were then rinsed with 0.1 M sodium cacodylate buffer and post-fixed with 1% osmium tetroxide in the same buffer for 2 h. After rinsing with 0.1 M cacodylate buffer, they were dehydrated for 15-min periods in increasing concentrations of ethanol (70, 80, 90, 95 and 100% v/v), exchanged through propylene oxide, and embedded in a mixture of epoxy resin. Sections were cut with a diamond knife on an ultramicrotome (ULTRACUT E, Reichert-Jung, Vienna, Austria) and were stained with 1% uranyl acetate for 14 min, followed by a lead staining reagent for 3 min. The sections were examined with a transmission electron microscope JEM 1200 EXII (JEOL, Tokyo, Japan).
Quantitative real-time PCR and western blot anlaysis
Total RNA was isolated from cultured podocytes using the RNeasy kit (Qiagen GmbH, Hilden, Germany). The quantity and quality of RNA were assessed using a spectrophotometer set at 260 nm. First-strand cDNA was synthesized from 1 μg of total RNA with a reverse transcription kit (Applied Bioscience, Foster City, CA, USA) using oligo-dT in a reaction volume of 20 μl, according to the manufacturer's protocol. Parallel reactions without reverse transcriptase were performed to confirm the absence of genomic DNA amplification. Quantitative real-time PCR was performed using sequence-specific primers to measure the mRNA levels of BIP (GRP78; (+) ACT TGG GGA CCA CCT ATT CC, (−) AGG AGT GAA GGC CAC ATA CG), CHOP (C/EBP homologous protein; (+) CAC CAC ACC TGA AAG CAG AA, (−) ATC CTC ATA CCA GGC TTC CA), and the spliced ((+) TGA GTC CGC AGC AGG TG, (−) GCA GAC TCT GGG GAA GGA C) and unspliced ((+) AAG AAC ACG CTT GGG AAT GG, (−) CAT AGT CTG AGT GCT GCG GA) forms of xbp1 (X-box BIP 1). β-actin was used as reference control. For the analysis of the expression of each gene, experiments were conducted in triplicate in a real-time PCR system (7900HT, ABI prism, Foster city, CA, USA) using SYBR Green PCR Master Mix (Qiagen), as described previously.34
For total protein extraction, cells seeded on six-well plates were washed with ice-cold PBS and lysed with cold RIPA buffer (Thermo Fisher Scientific Inc.) containing protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). The supernatants from lysates were electrophoresed on SDS-PAGE gels and then transferred to a polyvinylidene difluoride membrane (Merck Millipore, Billerica, MA, USA). The membrane was blocked in 5% BSA or 6% skim milk for 1h at room temperature, followed by incubation with primary antibody at 4 °C overnight. To estimate the ER stress response, the membrane was incubated with primary antibodies against phosphorylated and total eIF2α (1 : 1000, Catalog # sc-101670 and sc-133132, Santa Cruz Biotechnology), phosphorylated and total PERK (1 : 2000, Catalog # sc-32579 and sc-13073), xbp1 (1 : 1000, Catalog # sc-7160, Santa Cruz Biotechnology) and CHOP (1:500, Catalog # 612202, BioLegend, San Diego, CA, USA). Primary antibodies against phosphorylated and total IP3R (1 : 2000, Catalog # 8548 and # 3763, Cell signaling Technology, Danvers, MA, USA) and as a loading control, β-actin (1 : 5000, Catalog # ab6276, Abcam, Cambrige, UK) were used. Membranes were incubated for 1 h at room temperature in horseradish peroxidase-conjugated secondary antibody against either mouse or rabbit IgG (Catalog # 31450 and 31460, Thermo Scientific). The bands were visualized with an UVP Biospectrum-600 imaging system using enhanced chemiluminescence solution (Luminata Forte, Millipore Corporation, Billerica, MA, USA).
Measurement of cytosolic and ER luminal Ca2+ concentrations
To measure cytosolic Ca2+ concentrations ([Ca2+]i), podocytes cultured on coverslips were loaded with fura-2/AM (5 μM) in a dark room for 40–60 min at room temperature. Fura-2-loaded podocytes were then washed and transferred to a perfusion chamber on an inverted microscope (IX-70, Olympus). The cells were alternately excited at 340 and 380 nm by a monochromatic light source (LAMDA DG-4; Sutter, Novato, CA, USA), and fluorescence images were captured at 510 nm with an intensified CCD camera (Cascade; Roper, Duluth, GA, USA). The fluorescence intensity ratio from the two excitation wavelengths (F340/F380) reflecting [Ca2+]i was estimated by using MetaFluor 6.1 software.
Luminal Ca2+ content in the ER ([Ca2+]ER) was measured with FRET-based cameleon protein probe D1ER, which allows ratiometric recording of emitted fluorescence from YFP (540 nm) and CFP (490 nm), as described previously.34, 35 The D1ER plasmid was transiently transfected into podocytes using FuGENE HD. Forty-eight hours after transfection, cells on coverslips were excited at 440 nm by using the Nipkow spinning disk confocal microscopic system, and the D1ER fluorescence intensity ratio, which reflects the [Ca2+]ER, derived from measuring two emission wavelengths (F540/F490), was determined using MetaFluor 6.1 software.
Assay for PIP2 hydrolysis
Differentiated podocytes were transfected with plasmid encoding the PH domain of the PLC-δ-GFP fusion construct using FuGENE HD.38 The PH domain of PLC-δ can selectively bind PIP2 in plasmalemmal lipid vesicles and also IP3 in the cytosol, but only weakly bind other inositol phosphates and phosphatidylinositol 4-phosphate. Therefore, hydrolysis of PIP2 by endogenous PLCs and propagation of generated IP3 should shift the distribution of GFP fluorescence from the plasma membrane to the cytosol.38 After 48 h incubation, fluorescence images for GFP (excitation/emission: 488/535 nm) were obtained using a confocal microscope.
Staining for actin cytoskeleton
Differentiated podocytes cultured on coverslips were washed twice with PBS and fixed with 4% paraformaldehyde in PBS for 15 min at 37 °C. Cells were incubated with Alexa549-phalloidin (Invitrogen) for 20 min at room temperature. Then, cells were permeabilized with 0.25% Triton X-100 and blocked with 1% BSA for 30 min. Incubation with primary antibody against paxillin (Abcam) was performed overnight at 4 °C, followed by incubation with secondary antibody (Life Technology). The cells were washed and stained with DAPI for 5 min. Fluorescence images were obtained using a confocal microscope.
Albumin permeability assay
Differentiated podocytes were seeded on transwell bicameral chambers (0.4 μm pore; Corning, Cambridge, MA, USA) and transepithelial passage of FITC-labeled BSA (Sigma) was measured. Confluent differentiated podocytes were exposed to palmitate for 90 min in Ca2+-free KRB solution. In the upper compartment of transwell chambers, culture medium was replaced with 2 ml of FITC-BSA (250 μg/ml). From the lower compartment 100 μl samples were drawn after 0.5, 1, 2, 3 and 4 h of diffusion. The chamber was replenished with an equal volume fresh medium each time a sample was withdrawn. The fluorescence intensity of collected samples was measured using a fluorescence microplate reader (Flexstation II) at 450 nm, and the albumin concentrations in the samples were calculated using linear regression.
Data analysis
Statistical comparisons between two groups of data were performed using two-tailed unpaired Student's t-tests. Multiple comparisons were determined using one-way analysis of variance followed by a post hoc test (Tukey's multiple comparison test). P-values <0.05 were considered significant.
Acknowledgments
We are grateful to Professor Hoon Young Choi (Gangnam Severance Hospital, Yonsei University, Seoul, Korea), Professor Jochen Reiser (Rush University, Chicago, IL, USA), Professor Nicolas Demaurex (University of Geneva, Geneva, Switzerland), Professor Dong Min Shin (School of Dentistry, Yonsei University, Seoul, Korea) and Professor Yup Kang (School of Medicine, Ajou University, Suwon, Korea) for providing podocytes and plasmids. This work was supported by the grant from the National Research Foundation (NRF-2013R1A1A4A01010780) and Yonsei University Future-leading Research Initiative of 2014 (2014-22-0127).
Glossary
- FFA
free fatty acid
- ER
endoplasmic reticulum
- STIM1
stromal interaction molecule 1
- PLC
phospholipase C
- DAG
diacylglycerol
- CKD
chronic kidney disease
- DN
diabetic nephropathy
- ROS
reactive oxygen species
- PT
permeability transition
- BIP
immunoglobulin heavy chain binding protein
- IRE1
inositol-requiring protein 1
- ATF6
activating transcription factor 6
- PERK
PKR-like ER kinase
- PDI
protein disulfide isomerase
- ERO1
ER oxidoreductase
- CHOP
C/EBP homology protein
- IP3
inositol (1,4,5)-trisphosphate
- SCD
stearoyl-CoA desaturase
- ΔΨm
mitochondrial membrane potential
- AR
aspect ratio
- xbp1
X-box-binding protein 1
- CPA
cyclopiazonic acid
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SSO
sulfosuccinimidyl oleate
- eIF2α
eukaryotic translation initiation factor 2α
- PH
pleckstrin homology
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- DKI
DAG kinase inhibitor
- SOC
store-operated calcium
- MAMs
mitochondria-associated ER membranes
The authors declare no conflict of interest.
Footnotes
Edited by C Munoz-Pinedo
References
- 1Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat Rev Nephrol 2013; 9: 328–336. [DOI] [PubMed] [Google Scholar]
- 2Abboud H, Henrich WL. Clinical practice. Stage IV chronic kidney disease. N Engl J Med 2010; 362: 56–65. [DOI] [PubMed] [Google Scholar]
- 3Ising C, Koehler S, Brahler S, Merkwirth C, Hohne M, Baris OR et al. Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Mol Med 2015; 7: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Van der Vusse GJ, Roemen TH. Gradient of fatty acids from blood plasma to skeletal muscle in dogs. J Appl Physiol 1995; 78: 1839–1843. [DOI] [PubMed] [Google Scholar]
- 5Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res 1995; 36: 229–240. [PubMed] [Google Scholar]
- 6Miyasaka CK, Mendonca JR, Nishiyama A, de Souza JA, Pires de Melo M, Pithon-Curi TC et al. Comparative effects of fish oil given by gavage and fish oil-enriched diet on leukocytes. Life Sci 2001; 69: 1739–1751. [DOI] [PubMed] [Google Scholar]
- 7Yuan H, Zhang X, Huang X, Lu Y, Tang W, Man Y et al. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of beta-cells via JNK, p38 MAPK and p53 pathways. PloS One 2010; 5: e15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 2010; 299: E1096–E1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Srivastava S, Chan C. Hydrogen peroxide and hydroxyl radicals mediate palmitate-induced cytotoxicity to hepatoma cells: relation to mitochondrial permeability transition. Free Rad Res 2007; 41: 38–49. [DOI] [PubMed] [Google Scholar]
- 10Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol 2008; 216: 796–804. [DOI] [PubMed] [Google Scholar]
- 11Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 2000; 279: H2124–H2132. [DOI] [PubMed] [Google Scholar]
- 12Koshkin V, Dai FF, Robson-Doucette CA, Chan CB, Wheeler MB. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic beta-cells. J Biol Chem 2008; 283: 7936–7948. [DOI] [PubMed] [Google Scholar]
- 13Miller TA, LeBrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y et al. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem Biophys Res Commun 2005; 336: 309–315. [DOI] [PubMed] [Google Scholar]
- 14Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 2010; 299: F821–F829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 2008; 121((Pt 14)): 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006; 147: 943–951. [DOI] [PubMed] [Google Scholar]
- 17Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008; 29: 42–61. [DOI] [PubMed] [Google Scholar]
- 18Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab 2009; 296: E690–E701. [DOI] [PubMed] [Google Scholar]
- 19Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat 2004; 28: 51–65. [DOI] [PubMed] [Google Scholar]
- 20Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic beta-cell dysfunction. Diabetes Obes Metab 2010; 12((Suppl 2)): 76–82. [DOI] [PubMed] [Google Scholar]
- 21Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 2007; 9: 2277–2293. [DOI] [PubMed] [Google Scholar]
- 22Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 2000; 290: 1571–1574. [DOI] [PubMed] [Google Scholar]
- 23Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 2009; 186: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 2008; 105: 18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 2012; 81: 767–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dialysis Transplant 2009; 24: 3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Sieber J, Weins A, Kampe K, Gruber S, Lindenmeyer MT, Cohen CD et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol 2013; 183: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Das R, Xu S, Quan X, Nguyen TT, Kong ID, Chung CH et al. Upregulation of mitochondrial Nox4 mediates TGF-beta-induced apoptosis in cultured mouse podocytes. Am J Physiol Renal Physiol 2014; 306: F155–F167. [DOI] [PubMed] [Google Scholar]
- 29Park KS, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou JC, Marchetti P et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 2008; 283: 33347–33356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet 2003; 35: 367–371. [DOI] [PubMed] [Google Scholar]
- 31Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 2006; 22: 79–99. [DOI] [PubMed] [Google Scholar]
- 32Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012; 337: 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 2006; 47: 2726–2737. [DOI] [PubMed] [Google Scholar]
- 34Park KS, Poburko D, Wollheim CB, Demaurex N. Amiloride derivatives induce apoptosis by depleting ER Ca(2+) stores in vascular endothelial cells. Br J Pharmacol 2009; 156: 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA 2004; 101: 17404–17409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005; 437: 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol 2007; 39: 2012–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol 1998; 143: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 1984; 312: 315–321. [DOI] [PubMed] [Google Scholar]
- 40de Chaffoy de Courcelles DC, Roevens P, Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem 1985; 260: 15762–15770. [PubMed] [Google Scholar]
- 41Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun 2012; 3: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Arruda AP, Pers BM, Parlakgul G. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 2014; 20: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 2003; 300: 135–139. [DOI] [PubMed] [Google Scholar]
- 44Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998; 280: 1763–1766. [DOI] [PubMed] [Google Scholar]
- 45Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci 2011; 124((Pt 13)): 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Tubbs E, Theurey P, Vial G, Bendridi N, Bravard A, Chauvin MA et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 2014; 63: 3279–3294. [DOI] [PubMed] [Google Scholar]
- 47Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 2010; 3: ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Greka A, Mundel P. Calcium regulates podocyte actin dynamics. Semin Nephrol 2012; 32: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997; 236: 248–258. [DOI] [PubMed] [Google Scholar]
- 50Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 2009; 58: 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]