The anti-CD20 monoclonal antibody rituximab has revolutionized the management of B-cell non-Hodgkin's lymphoma and chronic lymphocytic leukemia (CLL). Addition of rituximab to the frontline standard chemotherapy (fludarabine–cyclophosphamide, FC) within the rituximab fludarabine cyclophosphamide regimen has recently shown to statistically increase both progression-free survival (PFS) and overall survival (OS) in the randomized CLL8 phase III trial of the German CLL Group.1 This was the first example of improved OS in first-line CLL, although restricted to those patients fit enough to receive FC chemotherapy. However, the translation of these results into the clinical setting is hampered by the fact that ca. one half of CLL patients are 72 years at the time of first therapy, 40% being aged more than 75 years2 with increased burden of comorbidities (for example, kidney function impairment) and thus not eligible for FC chemotherapy.
Based on the results of the CALGB studies3 and the German CLL5 trial,4 chlorambucil remains a treatment option of choice in those CLL patients in Europe. Despite its limited efficacy as a single agent, the favorable safety profile of chlorambucil led to its application as a backbone for reduced intensity immuno-chemotherapy in combination with rituximab and obinutuzumab (GA101). Obinutuzumab is a novel glycoengineered type II CD20 antibody that has enhanced FcgRIII affinity resulting in superior antibody-dependent cell cytotoxicity (ADCC) and antibody dependent cell phagocytosis induction as compared with rituximab; and mediates strong direct cell-death induction with a concomitant reduction in complement dependent cytotoxicity.5 As a type II CD20 antibody, it also shows reduced CD20 internalization in CLL samples; a mechanism that may further enhance its capability to mediate ADCC.6 In a series of ex vivo-treated CLL whole-blood samples, Patz et al.7 elucidated obinutuzumab mechanism of action against CLL cells, and demonstrated that the superior B-cell depletion of obinutuzumab observed is to a large extent due to enhanced ADCC through recruitment of (CD16)-bearing immune effector cells, as compared with rituximab at a saturating dose of 10 μg/ml. Similarily, Rafiq et al.8 confirmed the role of ADCC for B-cell depletion by obinutuzumab. The German CLL group has designed the CLL11 that randomized chlorambucil alone, rituximab–chlorambucil (R-Clb) and obinutuzumab–chlorambucil (G-Clb) in patients with CLL and coexisting conditions.9 Clinical data from the CLL11 trial demonstrated an acceptable safety profile with a higher rate of first infusion related reactions, but rapid and complete drop in peripheral CLL cells counts for the G-Clb arm, and improved PFS over Clb alone, as well as R-Clb (median PFS 26.7 for G-Clb vs 15.2 months for R-Clb; hazards ratio (HR), 0.39; 95% confidence interval (CI), 0.31–0.49, P<0.001). Most notably, the G-Clb combination induced a higher rate of complete responses and minimal residual disease negativity in a significant proportion of CLL patients, whereas minimal residual disease-negative patients in the R-Clb arm were rarely observed.9 These data lead to the approval of obinutuzumab in combination with chlorambucil for first-line treatment of CLL in the United States10 and Europe. Updated results from the CLL11 trial confirmed the previously observed OS benefit of G-Clb over Clb monotherapy (HR, 0.47; 95% CI, 0.29–0.76, P=0.0014).11 Interestingly, response to G-Clb improved outcome in all analyzed subgroups, except in patients with del(17p) where it was not significant.9
Here, we aimed to investigate whether rituximab and obinutuzumab B-cell depletion in ex vivo CLL samples is modulated by CLL-related prognostic markers, such as interphase fluorescent in situ hybridization (FISH) and conventional cytogenetics, immunoglobulin heavy-chain variable region mutational status (IGHV), β2-microglobulin level, recurrent somatic mutations (for example, TP53, NOTCH1 and SF3B1). All these parameters have been linked to reduce efficacy of chlorambucil monotherapy in the UK LRF CLL4 trial.12, 13 To date, no study has evaluated the frequency of these molecular markers across patient's age groups, but it is likely that they can decrease the efficacy of chlorambucil and other chemotherapies, making it desirable to assess the efficacy of rituximab and obinutuzumab as a single agent in a large series of ex vivo-treated CLL samples. For this purpose, we collected samples from 96 patients after signed, informed consent to correlate anti-CD20 B-cell depletion with modern and classical prognostic parameters (for example, FISH, immunoglobulin gene heavy variable mutational status, age, gender, Binet stage, β2-microglobulin and bulky adenopathies >5 cm) (Table 1). Fresh peripheral blood mononuclear cells were isolated from blood samples by Ficoll gradient centrifugation and subsequently cultured in high density cultures (10.10E6 cells/ml) allowing to work with viable cultures for more than 7 days.14 Antibody-mediated B-cell depletion was determined by enumerating trypan blue-negative, flow cytometrically CD5/CD19-positive B lymphocytes after treatment at a fixed concentration of 10 μg/ml of anti-CD20 antibodies, in RPMI+10% complement-free FCS, for 7 days.
Table 1. Clinical characteristics of CLL patients.
| Characteristics | n | |
|---|---|---|
| Binet stage | ||
| A | 96 | 65.6% |
| B/C | 96 | 34.3% |
| Median age (years) | 96 | 67 (41–83) |
| Age>70 | 96 | 30 |
| Gender (M/F) | 96 | 69/27 |
| Median leukocytosis per μl | 96 | 53 800 |
| Bulky>5 cm | 84 | 22.6% |
| FISH | ||
| Del 13q alone/normal | 85 | 24.7% |
| Del 11q | 62 | 19.4% |
| Del 17p | 85 | 5.9% |
| Tri12 | 62 | 22.6% |
| Mutations | ||
| IGHV (MUT/UNMUT) | 70 | 35/35 |
| TP53 | 63 | 11.1% |
| NOTCH1 | 63 | 12.7% |
| SF3B1 | 62 | 4.7% |
| CD20 ABC low/high | 21 | 10/11 |
Abbreviations: CD20 ABC, CD20 antibody bound per cell; del 11, 11q deletion; del 13, 13q deletion; del 17, 17p deletion; M, male; F, female; FISH, fluorescent in situ hybridization; tri12, trisomy 12. IGHV status: M, mutated; UM, unmutated.
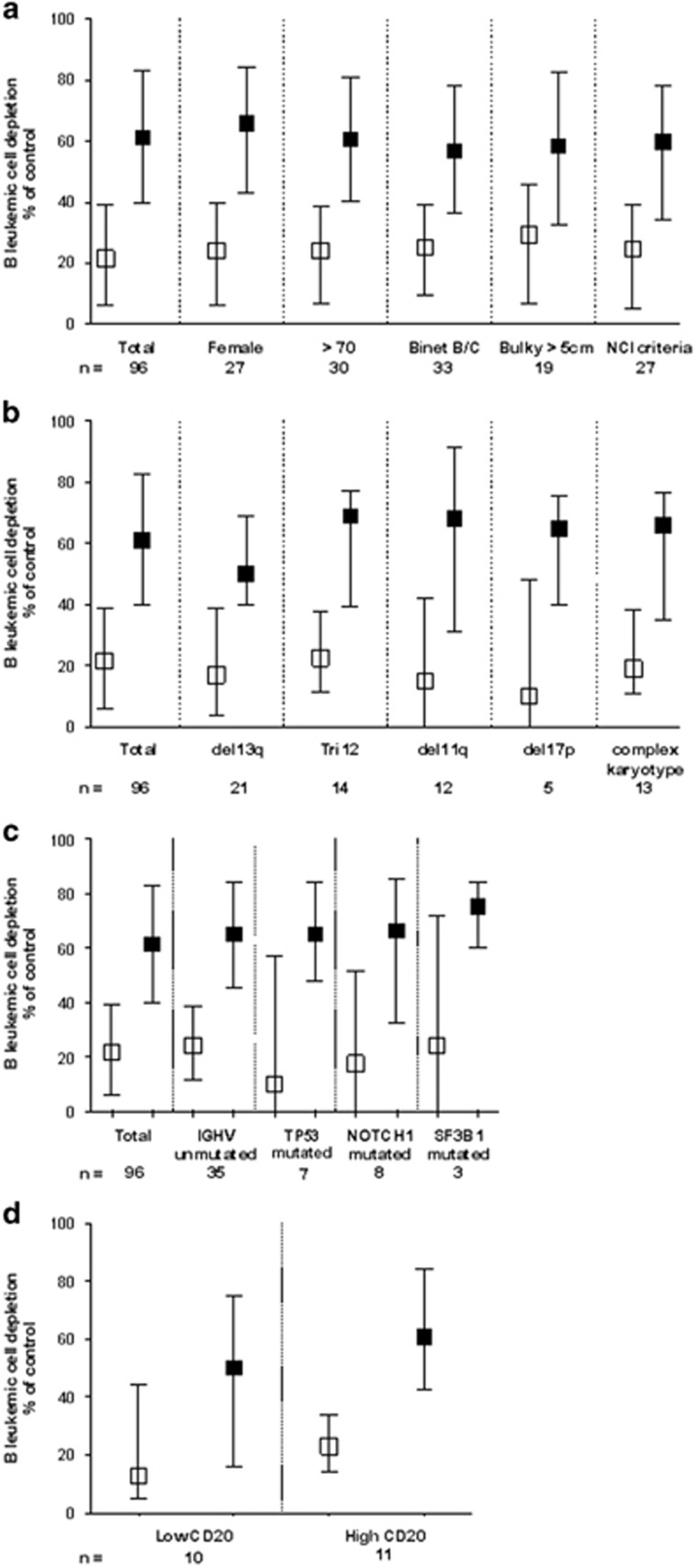
We chose the whole-blood B-cell depletion assay as most relevant assay to compare the efficacy of obinutuzumab and rituximab as it integrates all described mechanisms of action of CD20 antibodies such as direct cell killing, ADCC, antibody dependent cell phagocytosis and complement dependent cytotoxicity. The median percentages of B-cell depletion in 96 patients were 22% with rituximab and 62.8% with obinutuzumab (P<0.001). Obinutuzumab had higher activity than rituximab in 83% of patients, resulting in B-cell depletion >50% in 64.6% of cases versus 16.8% for rituximab. Deletion of 11q locus (by FISH), and disruption of the p53 pathway, either by deletion of the 17p13 locus (by FISH) or TP53 gene mutation, were parameters associated with decreased rituximab efficacy, as previously proposed elsewhere.14 We extended these preliminary data and demonstrate here that obinutuzumab-induced depletion is not affected in these high-risk CLL subsets (Figures 1a–c). Mutational status of IGHV genes and NOTCH1 gene did not impact on either rituximab or obinutuzumab efficacy. Occurence of SF3B1 mutation was rare in our cohort, despite a trend toward a slightly decreased efficacy for rituximab, no effect for obinutuzumab was observed. This observation may be linked to the higher reported frequency of this mutation in del(11q) patients. As NOTCH1 mutations have been reported in up to one-third of trisomy 12 (tri12) patients, and tri12 being a cytogenetic abnormality with the highest CD20 expression,15 our results of conserved rituximab efficacy in these subgroups are not surprising. However, direct comparison between in vitro and in vivo data have to be taken with caution as NOTCH1 mutations may alter the results of rituximab fludarabine cyclophosphamide regimen in a subgroup analysis of the CLL8 trial.
Figure 1.
Anti-leukemic activity of a fixed saturating dose of anti-CD20 antibodies. (a–c) B-cell depletion was assessed using flow cytometry among PBMC from CLL patients, after 7 days (7d) of incubation with either rituximab (□) or GA101 (obinutuzumab) (▪). A saturating dose of 10 μg/ml was used in a complement-free medium, so results observed are due to both direct cell-death induction and antibody-dependent cell cytotoxicity. Boxes indicate means and whiskers confidence intervals. Comparisons were made using paired Student's t-test, all with P<0.05. (d) Depletion of leukemic cells according to CD20 surface antigen quantitative assessment. Cutoff value used (high vs low) was the median CD20 expression in a cohort of 21 patients (8100 antibodies bound per cell, using Quantibrite kits), and B-cell depletion was measured flow cytometrically after 7d in culture with 10 μg/ml of the indicated antibodies (rituximab, □ or GA101, ▪). Comparisons were made using paired Student's t-test, all with P<0.05.
Anti-CD20 MoAb activity was also reported to depend on the absolute CD20 expression level on the surface of CLL cells. Recently, CD20 internalization or CD20 shaving (by monocytic cells) after rituximab treatment, have been proposed as evasion mechanisms to antibody therapy.6, 16 We used the Quantibrite CD20 kit (BD QuantiBRITE fluorescent assay, BD Biosciences, Le Pont de Claix, France) to quantitatively assess CD20 expression at the surface of CLL cells from 24 patients. A cutoff value of a median CD20 expression of 8100 antibodies bound per cell was applied to categorize high versus low CD20-expressing samples (Figure 1d). CD20 expression levels was not linked to classical clinical prognostic factors, but did affect both rituximab-induced (12 vs 25%, P=0.37) and obinutuzumab-induced (42.5 vs 67.5%, P<0.05) median B-cell depletion. In both instances, obinutuzumab retained superior activity than rituximab (P<0.01), suggesting target modulation at the tumor surface had stronger impact on type I rather than type II antibody, as previously reported.16
The challenge in developing novel anti-CD20 antibodies is to demonstrate superior clinical efficacy over that achieved with rituximab. Our data suggest that based on the in vitro data across all prognostic subgroups of CLL patients ex vivo, obinutuzumab appears to work independent of (genetic) risk groups and may further qualify as a therapeutic regimen with the activity in bad prognosis CLL patients where current therapies have only limited efficacy. The data from the CLL11 study9 provide support for these preclinical findings supports further studies of obinutuzumab in different CLL risk groups in combination with chemotherapy- and novel-targeted reagents to ultimately improve the clinical management of CLL patients.
Acknowledgments
This study was supported in part by research funding from La Ligue contre le Cancer (AQM). We thank Drs S Struski, N Dastugue, E Delabesse and N Prade who performed karyotypes and genetic profiling of CLL patients, and CLL patients who gave consent to participate in this study.
Footnotes
CK is an employee of Hoffmann La Roche. The remaining authors declare no conflict of interest.
References
- 1Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174. [DOI] [PubMed] [Google Scholar]
- 2Diehl LF, Karnell LH, Menck HR. The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer 1999; 86: 2684–2692. [PubMed] [Google Scholar]
- 3Woyach JA, Ruppert AS, Rai K, Lin TS, Geyer S, Kolitz J et al. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential cancer and leukemia group B studies. J Clin Oncol 2013; 31: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009; 114: 3382–3391. [DOI] [PubMed] [Google Scholar]
- 5Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115: 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011; 118: 2530–2540. [DOI] [PubMed] [Google Scholar]
- 7Patz M, Isaeva P, Forcob N, Muller B, Frenzel LP, Wendtner CM et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol 2011; 152: 295–306. [DOI] [PubMed] [Google Scholar]
- 8Rafiq S, Butchar JP, Cheney C, Mo X, Trotta R, Caligiuri M et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol 2013; 190: 2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–1110. [DOI] [PubMed] [Google Scholar]
- 10Lee HZ, Miller BW, Kwitkowski VE, Ricci S, DelValle P, Saber H et al. US Food and drug administration approval: obinutuzumab in combination with chlorambucil for the treatment of previously untreated chronic lymphocytic leukemia. Clin Cancer Res 2014; 20: 3902–3907. [DOI] [PubMed] [Google Scholar]
- 11Goede V, Fischer K, Engelke A, Schlag R, Lepretre S, Montero LF et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia 2015; 29: 1602–1604. [DOI] [PubMed] [Google Scholar]
- 12Oscier D, Wade R, Davis Z, Morilla A, Best G, Richards S et al. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica 2010; 95: 1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Oscier DG, Rose-Zerilli MJ, Winkelmann N, Gonzalez de Castro D, Gomez B, Forster J et al. The clinical significance of NOTCH1 and SF3B1 mutations in the UK LRF CLL4 trial. Blood 2013; 121: 468–475. [DOI] [PubMed] [Google Scholar]
- 14Laprevotte E, Ysebaert L, Klein C, Valleron W, Blanc A, Gross E et al. Endogenous IL-8 acts as a CD16 co-activator for natural killer-mediated anti-CD20 B cell depletion in chronic lymphocytic leukemia. Leuk Res 2013; 37: 440–446. [DOI] [PubMed] [Google Scholar]
- 15Tam CS, Otero-Palacios J, Abruzzo LV, Jorgensen JL, Ferrajoli A, Wierda WG et al. Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent in-situ hybridization in 510 patients. Br J Haematol 2008; 141: 36–40. [DOI] [PubMed] [Google Scholar]
- 16Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood 2010; 115: 5191–5201. [DOI] [PubMed] [Google Scholar]