Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inheritable heart muscle disease that predominantly affects the right ventricle (RV) and predisposes to ventricular arrhythmias and sudden cardiac death (SCD).1–17
In the last three decades, there have been a significant number of studies defining the pathogenesis, genetic aspects, and clinical manifestations of the disease (See ‘Etiology, pathogenesis, diagnosis and natural history’ as Supplementary material online). In 1994 and 2010, an International Task Force (ITF) document proposed guidelines for the standardized diagnosis of ARVC/D based on electrocardiographic (ECG), arrhythmic, morphological, histopathological, and clinico-genetic factors.18,19
The growing knowledge regarding arrhythmic outcome, risk factors, and life-saving therapeutic interventions, make it particularly timely to critically address and place into perspective the issues relevant to the clinical management of ARVC/D patients. The present ITF consensus statement is a comprehensive overview of currently used risk stratification algorithms and approaches to therapy, either pharmacological or non-pharmacological, which often poses a clinical challenge to cardiovascular specialists and other practitioners, particularly those infrequently engaged in the management of ARVC/D. This document should be regarded as a guide to clinical practice where rigorous evidence is still lacking, because of the relatively low disease prevalence and the absence of controlled studies. Recommendations are based on available data derived from non-randomized and observational studies and consensus within the conference panellists. When development of prognostic-therapeutic algorithms was controversial, management decisions were recommended to be individualized.
Recommendation and level of evidence of specific management options were classified according to predefined scales, as outlined in Tables 1 and 2 (http://www.escardio.org/guidelines-surveys/esc-guidelines/about/Pages/rules-writing.aspx). Because randomized studies are not available, most consensus recommendations on treatment of ARVC/D are based on data derived from follow-up registries and/or experts opinions (i.e. level of evidence B or C).
Table 1.
Classes of recommendations
| Classes of recommendations | Definition | Suggested wording to use |
|---|---|---|
| Class I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective. | Is recommended/is indicated |
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment of procedure. | |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy. | Should be considered |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion | May be considered |
| Class III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful | Is not recommended |
Table 2.
Levels of evidence
| Level of evidence A | Data derived from multiple randomized clinical trials or meta-analysis |
| Level of evidence B | Data derived from a single randomized clinical trial or large non-randomized studies |
| Level of evidence C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries. |
All members of the writing group of this consensus document provided disclosure statements of all relationships that might present conflicts of interest.
Risk stratification
The natural history of ARVC/D is predominantly related to ventricular electrical instability which may lead to arrhythmic SCD, mostly in young people and athletes.2,8,10 In advanced disease, progression of RV muscle disease and left-ventricular involvement may result in right or biventricular heart failure.3,4 The available outcome studies are based on small patients cohorts followed for a relatively short follow-up period (Table 3).22–36 The estimated overall mortality rate varies among different studies, ranging from 0.08% per year during a mean follow-up of 8.5 years in the series by Nava et al.20 to 3.6% per year during a mean follow-up of 4.6 years in the series by Lemola et al.21
Table 3.
Clinical variables associated with an increased risk of major arrhythmic events in arrhythmogenic right-ventricular cardiomyopathy/dysplasia*
| Risk factor | Definition | Patients, n | Study endpoint | HR/OR | 95% CI | P-value | References |
|---|---|---|---|---|---|---|---|
| Cardiac arrest | Aborted SCD due to VF | 132 | ICD interventions on rapid VT/VF | 79 | 6.8–90.6 | <0.001 | Corrado et al. Circulation 200322 |
| Unstable sustained VT | Sustained (>30 s) VT causing syncope or haemodynamic collapse | ICD interventions on rapid VT/VF | 14 | 1.7–21.1 | 0.015 | ||
| Sustained VT or VF | VT lasting >30 s or VF | 108 | Any appropriate ICD intervention | N/A | N/A | 0.003 | Link et al. JACC 201423 |
| VT lasting >30 s or VF | 50 | Cardiac death (SCD in 67% and heart failure in 33%) | 22.97 | 2.33–2.66 | 0.007 | Watkins et al. Heart Rhythm 200924 | |
| Syncope | Syncopal episodes unrelated to extracardiac causes and occurring in the absence of documented ventricular arrhythmias and/or circumstances clearly leading to reflex-mediated changes in vascular tone or heart rate | 132 | ICD interventions on rapid VT/VF | 7.5 | 0.84–1.81 | 0.07a | Corrado et al. Circulation 200322 |
| Idem | 106 | Any appropriate ICD intervention | 2.94 | 1.83–4.67 | 0.013 | Corrado et al. Circulation 201025 | |
| ICD interventions on rapid VT/VF | 3.16 | 1.39–5.63 | 0.005 | ||||
| N/A | 50 | Cardiac death (SCD in 67% and heart failure in 33%) | 10.73 | 1.88–61.8 | 0.008 | Watkins et al. Heart Rhythm 200924 | |
| Non-sustained VT | ≥3 consecutive ventricular beats with a rate >100 beats/min, lasting <30 s, documented during exercise testing or 24-h Holter | 84 | Any appropriate ICD intervention | 10.5 | 2.4–46.2 | 0.003 | Bhonsale et al. JACC 201126 |
| Idem | 106 | Any appropriate ICD intervention | 1.62 | 0.96–4.62 | 0.068a | Corrado et al. Circulation 201025 | |
| LV dysfunction | Angiographic LV EF <55% | 132 | ICD interventions on rapid VT/VF | 0.94 | 0.89–0.95 | 0.037 | Corrado et al. Circulation 200322 |
| Angiographic LV EF <40% | 130 | Cardiac death (SCD in 33% and heart failure in 67%) | 10.9 | 2.8–41.7 | <0.001 | Hulot et al. Circulation 200427 | |
| Angiographic LV EF <55% | 60 | Any appropriate ICD intervention | 1.94 | 0.93–4.05 | 0.078a | Wichter et al. Circulation 200428 | |
| Echocardiographic LV EF <50% | 61 | Cardiac death and heart transplantation (SCD in 53%, heart failure death in 13%, heart transplantation in 34%) | N/A | N/A | <0.05 | Lemola et al. Heart 200521 | |
| Angiographic LV EF <55% | 313 | Sudden cardiac death | 14.8 | 2.37–53.5 | <0.001 | Peters, J Cardiovasc Med 200739 | |
| RV dysfunction | Angiographic RV EF <45% | 60 | Any appropriate ICD intervention | 2.09 | 1.03–4.23 | 0.041 | Wichter et al. Circulation 200428 |
| FAC % per unit decrease | 70 | Composite (death in 0%, heart transplantation in 7%, ventricular fibrillation in 10%, sustained ventricular tachycardia in 36%, arrhythmic syncope in 4%). | 1.08 | 1.04–1.12 | <0.001 | Saguner, Circ Cardiovasc Imaging 201429 | |
| RV dilation | RV end-diastolic area, cm2, per unit increase | 70 | As above | 1.05 | 1.01–1.08 | 0.004 | Saguner, Circ Cardiovasc Imaging 201429 |
| Right-atrial dilation | Right atrium, short axis, mm, per unit increase | 70 | As above | 1.03 | 1.00–1.06 | 0.037 | Saguner, Circ Cardiovasc Imaging 201429 |
| Biventricular dysfunction | Echocardiographic RV and LV dysfunction (EF <50%) | 96 | Cardiac death and heart transplantation (SCD in 30%, heart failure death in 30%, death of unknown cause in 5%, heart transplantation in 35%) | 6.3 | 2.17–17.5 | <0.001 | Pinamonti, Eur Heart J 201130 |
| Heart failure | Clinical signs of RV heart failure | 130 | Cardiac death (SCD in 33% and heart failure in 67%) | 13.7 | 2.58–71.4 | 0.002 | Hulot et al. Circulation 200427 |
| Clinical signs or symptoms of congestive heart failure | 61 | Cardiac death and heart transplantation (SCD in 53%, heart failure death in 13%, heart transplantation in 34%) | N/A | N/A | <0.05 | Lemola et al. Heart 200521 | |
| Young age | Per 5 years increment | 132 | ICD interventions on rapid VT/VF | 0.77 | 0.57–0.96 | 0.007 | Corrado et al. Circulation 200322 |
| Per 1 year increment | 108 | ICD interventions on rapid VT/VF | N/A | N/A | 0.03 | Link et al. JACC 201423 | |
| Male gender | 215 | Composite (cardiac arrest in 9%, ICD intervention in 22%, sustained VT in 69%) | 1.8 | 1.2–2.8 | 0.004 | Bhonsale et al. Circ AE 201331 | |
| 134 | Composite (SCD in 5%, cardiac arrest 27%, sustained VT 64%, ICD shock 5%) | 2.76 | 1.19–6.41 | 0.02 | Rigato et al. Circ Gen 201332 | ||
| Complex genotype | Compound or digenic heterozygosisity | 134 | Composite (SCD in 5%, cardiac arrest 27%, sustained VT 64%, ICD shock 5%) | 3.71 | 1.54–8.92 | 0.003 | Rigato et al. Circ Gen 201332 |
| Proband status | First family member affected by the genetic defect who seeks medical attention because of the occurrence of clinical manifestations | 215 | Composite (cardiac arrest in 9%, ICD intervention in 22%, sustained VT in 69%) | 7.7 | 2.8–22.5 | <0.001 | Bhonsale et al. Circ AE 201331 |
| Inducible VT/VF | VT or VF that lasted >30 s or required termination because of haemodynamic compromise | 84 | Any appropriate ICD intervention | 4.5 | 1.4–15.0 | 0.013 | Bhonsale et al. JACC 201126 |
| N/A | 60 | Any appropriate ICD intervention | 2.16 | 0.94–5.0 | 0.069a | Wichter et al. Circulation 200428 | |
| N/A | ICD intervention on fast VT/VF | N/A | N/A | N/A | |||
| VT that lasted >30 s or required termination because of haemodynamic compromise. Induction of VF not considered | 62 | Composite (cardiac death in 13%, heart transplantation in 10%, unstable VT/VF in 70%, syncope in 7%). | 2.5 | 1.0–6.2 | 0.04 | Saguner, Am J Cardiol 201333 | |
| Extent of electroanatomic scar on RV endocardial voltage mapping | low-voltage (<0.5 mV) areas on bipolar electroanatomic voltage mapping. Per 5% increment. | 69 | Composite arrhythmic (SCD in 5%, ICD intervention in 37%, sustained VT in 58%) | 1.6 | 1.2–1.9 | <0.001 | Migliore et al. Circ AE 201334 |
| Fragmented electrograms on RV endocardial voltage mapping | Multiple (>3) discrete deflections, amplitude <1.5 mV, and duration >100 ms | 95 | Any appropriate ICD intervention | 21.2 | 1.8–251.8 | 0.015 | Santangeli et al. Heart Rhythm 201235 |
| T-wave inversion in inferior leads | Negative T-waves in leads II, III, aVF | 108 | Any appropriate ICD intervention | N/A | N/A | 0.02 | Link et al. JACC 201423 |
| Inverted T waves in 2 of 3 inferior leads | 111 | Composite (6% cardiac death; 8% heart transplantation; 16% VF; 67% sustained VT; 3% arrhythmic syncope) | 2.4 | 1.2–5.2 | 0.02 | Saguner, AJC 201436 | |
| Extent of T-wave inversion | Inverted T waves in ≥3 precordial leads | 215 | Composite arrhythmic (cardiac arrest in 9%, ICD intervention in 22%, sustained VT in 69%) | 4.2 | 1.2–14.5 | 0.03 | Bhonsale et al. Circ AE 201331 |
| QRS fragmentation | Additional deflections/notches at the beginning of the QRS, on top of the R wave, or in the nadir of the S wave in either 1 right precordial lead or in >1 lead including all remaining leads | 111 | Composite (6% cardiac death; 8% heart transplantation; 16% VF; 67% sustained VT; 3% arrhythmic syncope) | 2.7 | 1.1–6.3 | 0.03 | Saguner, AJC 201436 |
| Precordial QRS amplitude ratio | Sum of QRS voltages in V1–V3/sum of QRS voltages in V1–V6 < 0.48 | 111 | Composite (6% cardiac death; 8% heart transplantation; 16% VF; 67% sustained VT; 3% arrhythmic syncope) | 2.9 | 1.4–6.2 | 0.005 | Saguner AJC 201436 |
The list includes predictor variables that have been associated with an increased risk of major arrhythmic events (i.e. SCD, appropriate ICD interventions, or ICD therapy on fast VT/VF) in at least one published multivariable analysis in prospective studies.
FAC, fractional area change; EF, ejection fraction; LV, left ventricle; RV, right ventricle; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
aBorderline statistical significance.
The adverse prognosis of ARVC/D patients has been initially overestimated by reports from tertiary referral centres largely composed of patients referred because of their high-risk status or severe clinical manifestations requiring specialized therapeutic interventions, such as catheter ablation or implantable defibrillator (ICD).21,27,37,38 Studies from community-based patient cohorts and clinical screening of familial ARVC/D reported a much lower overall annual mortality rates (<1%).24,30–32,36,39 These latter data provide a more balanced view of the natural history of ARVC/D, in which the disease may occur with no or relatively mild disability and without the necessity for major therapeutic interventions.10,12–17 The mechanism of SCD in ARVC/D is cardiac arrest due to sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), which may occur as the first manifestation of the disease in young people without previous symptoms.2,7,40
Data from autopsy series and observational clinical studies on ARVC/D have provided a number of clinical predictors of adverse events and death. Table 3 reports the clinical variables identified as independent predictors of poor outcome including malignant arrhythmic events (i.e. SCD, cardiac arrest due to VF, appropriate ICD interventions, or ICD therapy on fast VT/VF), non-SCD, or heart transplantation, which were found in at least one published multivariable analysis. Patients who have experienced sustained VT or VF are at highest risk of experiencing life-threatening arrhythmic events.22–24 Unexplained syncope has been associated with an increased arrhythmic risk in some but not in all studies.22,25,26 Of note, unexplained syncope is defined as a loss of consciousness that: (i) occurs in the absence of documented ventricular arrhythmias and/or circumstances clearly leading to reflex-mediated changes in vascular tone or heart rate such as a micturition, defaecation, cough, or other similar conditions; and (ii) remains unexplained after a detailed clinical evaluation aimed to exclude other cardiac or extracardiac causes.25
Other independent risk factors for adverse events include non-sustained VT on 24-h Holter monitoring’;25,26 dilation/dysfunction of RV, left ventricle (LV), or both;21,22,27–30,39 male gender;31,32 compound and digenic heterozygosity of desmosomal-gene mutations;32 young age at the time of diagnosis;22,23 proband status;31 inducibility at programmed ventricular stimulation;26,28,33 amount of electroanatomic scar34 and electroanatomic scar-related fractionated electrograms;35 extent of T-wave inversion across precordial and inferior leads;23,31,36 low QRS amplitude36 and QRS fragmentation.36
Electrophysiological study
Electrophysiological study (EPS) is a valuable diagnostic test for differential diagnosis between ARVC/D and idiopathic right ventricular outflow tract tachycardia and may provide useful information regarding the VT inducibility for optimization of detection/discrimination algorithms and effective antitachycardia pacing protocols in patients undergoing ICD implantation.11,28 However, conflicting data exist concerning the role of inducibility of sustained VT or VF for prediction of long-term arrhythmic outcome in ARVC/D patients.22,23,25,26,33 Discrepancies between the study results may be explained by differences in arrhythmic endpoints (i.e. life-saving vs. any appropriate ICD discharge).
The largest multicentre studies on ARVC/D patients who received an ICD demonstrated that EPS is of limited value in identifying patients at risk of arrhythmic cardiac arrest because of its low predictive accuracy.22,25 In these studies, the reported incidence of ‘life-saving’ ICD discharges for treatment of fast VT or VF did not differ significantly in patients who were and were not inducible at EPS, regardless of the specific indication for ICD implantation. The study by Corrado et al.25 on the outcome of 106 ARVC/D patients receiving an ICD for primary prevention reported that the positive and negative predictive value of inducibility for VT or VF was 35 and 70%, respectively. In this study, the type of ventricular tachyarrhythmia inducible at the time of EPS (i.e. VT or VF) did not predict a statistically different arrhythmic outcome over the follow-up. The North American Multidisciplinary study on 98 ARVC patients receiving an ICD confirmed that inducible VT or VF at pre-implant EPS did not predict appropriate interventions on fast VT or VF during a mean follow-up of 3.3 years.23 On the contrary, in the cohort of ARVC/D patients reported in the Johns Hopkins studies, inducibility was the most significant independent predictor of appropriate ICD firing. However, in the study by Bhonsale et al.,26 the positive and negative predictive values of inducibility were 65 and 75%, respectively, and a sizeable proportion of patients experienced ICD interventions during follow-up despite a lack of inducibility of VT/VF. Moreover, the predictive value of inducibility for ‘life-saving’ ICD discharges was not demonstrated by either univariate or multivariate analysis. In asymptomatic patients, Bhonsale et al.26 reported that the combination of ≥2 factors such as inducibility at EPS, proband status, non-sustained VT, and PVCs ≥1000/24 h, predicts an incremental risk of appropriate ICD interventions; however, a statistically significant association with life-saving shocks for treatment of rapid VT or VF has not been demonstrated. In the study by Saguner et al.33 inducible VT was an independent predictor of composite endpoint including cardiac death, heart transplantation, unstable VT/VF, and syncope.
According to available studies on ARVC/D patients, the protocol of programmed ventricular stimulation should include a minimum of two drive-cycle lengths and three ventricular extrastimuli while pacing from two RV sites (apex and RV outflow tract); inducibility is defined as the induction of either VF or sustained VT, i.e. lasting >30 s or requiring termination because of haemodynamic compromise.21,22,25,41
Recent studies showed that demonstration and quantification of bipolar RV electroanatomic scar area34 as well as identification of scar-related fractionated electrograms and late potentials35 on endocardial voltage mapping during EPS may provide significant added value for arrhythmic risk assessment in ARVC/D. Because endocardial voltage mapping is an invasive, expensive, and highly operator-dependent technique with a significant risk of inaccurate interpretation of low-voltage recordings in areas of normal myocardium due to suboptimal catheter contact, it is not recommended as a routine diagnostic tool.
Recommendations
- EPS should be considered in the diagnosis and/or evaluation of patients with suspected ARVC/D (class IIa).
- Programmed ventricular stimulation may be considered for arrhythmic risk stratification of asymptomatic ARVC/D patients (class IIb).
- Endocardial voltage mapping may be considered in the diagnostic and prognostic evaluation of ARVC/D patients (class IIb).
Follow-up
Patients with ARVC/D should undergo lifelong clinical follow-up to periodically evaluate new onset or worsening of symptoms, progression of morphological and/or functional ventricular abnormalities, and ventricular arrhythmias in order to reassess the risk of SCD and optimize the treatment. Cardiac evaluation of affected patients including resting 12-lead ECG, echocardiography, 24-h Holter monitoring, and exercise testing (for detection of effort-induced ventricular arrhythmias) should be performed on a regular basis (every 1–2 years) depending on the age, symptoms, and disease severity.
Due to the age-related penetrance of ARVC/D, healthy gene carriers and family members should also be offered repeat clinical assessment (every 2–3 years), mostly during adolescence and young adulthood.
Therapy
The most important objectives of clinical management of ARVC/D patients include: (i) reduction of mortality, either by arrhythmic SCD or death from heart failure; (ii) prevention of disease progression leading to RV, LV, or biventricular dysfunction and heart failure; (iii) improvement of symptoms and quality of life by reducing/abolishing palpitations, VT recurrences, or ICD discharges (either appropriate or inappropriate); and (iv) limiting heart failure symptoms and increasing functional capacity. Therapeutic options consist of lifestyle changes, pharmacological treatment, catheter ablation, ICD, and heart transplantation.
Lifestyle changes
A link has been established between SCD and intense exertion in young individuals with ARVC/D. Competitive sports activity has been shown to increase the risk of SCD by five-fold in adolescent and young adults with ARVC/D.42 Early (i.e. pre-symptomatic) identification of affected athletes by pre-participation screening and their disqualification from competitive sports activity may be ‘life-saving’.8,43
In addition, physical exercise has been implicated as a factor promoting development and progression of the ARVC/D phenotype. Kirchhof et al.44 demonstrated that in heterozygous plakoglobin-deficient mice, endurance training accelerated the development of RV dilatation, dysfunction, and ventricular ectopy, suggesting that chronically increased ventricular load might contribute to worsening of the ARVC/D phenotype. It has been postulated that impairment of myocyte cell-to-cell adhesion may lead to tissue and organ vulnerability, which may promote myocyte death especially during mechanical stress, which occurs during competitive sports activity.45,46 Studies in humans confirmed that endurance sports and frequent exercise increase age-related penetrance, risk of VT/VF, and occurrence of heart failure in ARVC/D desmosomal-gene carriers.47,48
Recommendations
- It is recommended that patients with a definite diagnosis of ARVD/C not participate in competitive and/or endurance sports (Class I).
- Patients with a definite diagnosis of ARVD/C should be restricted from participation in athletic activities, with the possible exception of recreational low-intensity sports (Class IIa).
- Restriction from competitive sports activity may be considered in ARVC/D family members with a negative phenotype, either healthy gene carriers (class IIa) or with unknown genotype (class IIb).
Pharmacological therapy
Pharmacological options in ARVC/D treatment consist of antiarrhythmic agents, beta-blockers, and heart failure drug therapy.
Antiarrhythmic drugs
The aim of antiarrhythmic drug (AAD) therapy in patients with ARVC/D is to improve the quality of life by preventing symptomatic ventricular arrhythmias. There are no prospective and randomized trials on AAD therapy in ARVC/D and systematic comparison of treatment strategies.
Moreover, the assessment of efficacy of specific AAD therapy is difficult because ARVC/D patients tend to have multiple arrhythmic events over time and drugs are often changed.41,49 Available data are limited to case–control studies, retrospective analyses, and clinical registries. Hence, indication for AAD therapy and choice of drug are based on an empirical approach resulting from extrapolation from other diseases, personal experience, consensus, and individual decisions.
The available evidence suggests that amiodarone (loading dose of 400–600 mg daily for 3 weeks and then maintenance dose of 200–400 mg daily), alone or in combination with beta-blockers, is the most effective drug for preventing symptomatic ventricular arrhythmias with a relatively low proarrhythmic risk even in patients with ventricular dysfunction, although its ability to prevent SCD is unproved.49 Corrado et al.22 reported that the majority of life-saving ICD interventions in high-risk patients occurred despite concomitant AADs, a finding supporting the concept that AAD therapy may not confer adequate protection against SCD.
Recommendations
- AADs are recommended as an adjunct therapy to ICD in ARVC/D patients with frequent appropriate device discharges (class I).
- The use of AADs should be considered to improve symptoms in patients with frequent premature ventricular beats and/or non-sustained VT (class IIa).
- AADs may be considered as an adjunct therapy to catheter ablation without a back-up ICD in selected ARVC/D patients with recurrent, haemodynamically stable VT (class IIb).
- AAD treatment of asymptomatic ARVC/D patients without documented ventricular arrhythmias and healthy gene carriers is not recommended (class III).
Beta-blockers
Ventricular arrhythmias and cardiac arrest in ARVC/D are frequently triggered by adrenergic stimulation and occur during or immediately after physical exercise.8,40,42,47,48 Autonomic dysfunction with increased sympathetic stimulation of ventricular myocardium and subsequent reduction of beta-adrenoceptor density was demonstrated by Wichter et al.50,51 with the use of radionuclide imaging and quantitative positron emission tomography.
The indication for the use of beta-blocker drugs in ARVC/D relies on their proven efficacy to prevent effort-induced ventricular arrhythmias, their proven efficacy in heart failure management, and their potential but unproven ability to hinder myocardial disease progression by lowering RV wall stress.
Because studies are not available to compare the efficacy of individual beta-blockers and to define the most effective dosage, we recommend using non-vasodilating beta-blockers titrated to maximum tolerated dose for age and weight.
Recommendations
- Beta-blocker therapy is recommended in ARVC/D patients with recurrent VT, appropriate ICD therapies, or inappropriate ICD interventions resulting from sinus tachycardia, supraventricular tachycardia, or atrial fibrillation/flutter with high-ventricular rate (class I).
- Beta-blocker therapy should be considered in all patients with ARVD/C irrespective of arrhythmias (class IIa).
- The prophylactic use of beta-blockers in healthy gene carriers is not recommended (class III).
Preload-reducing drug therapy
Fabritz et al.52,53 provided experimental evidence that ventricular preload-reducing therapy prevents or tempers the development of ARVC/D in genetically susceptible murine hearts. Therapy with furosemide and nitrates completely prevented training-induced development of RV enlargement and normalized VT inducibility, thereby rendering treated plakoglobin-deficient mice phenotypically indistinguishable from their trained wild-type littermates.
Preload-reducing drug therapy is not yet part of clinical practice because the results of the animal studies demonstrating its beneficial effects require validation in other ARVC/D models and patients.
Heart failure and antithrombotic drug therapy
The prevalence of RV or biventricular dysfunction leading to progressive heart failure and death in ARVC/D is variable in the published series, mostly depending on the selection criteria of patients, whether referred for arrhythmias or heart failure.20–27,30–33,36,39,44,48,54,55 Left-ventricular involvement was originally considered an end-stage complication of ARVC/D, occurring late during the disease course and leading ultimately to biventricular pump failure.3,4 More recently, genotype–phenotype correlations have shown early and greater LV involvement in genetically predisposed ARVC/D patients.54–58
In ARVC/D, thromboembolic complications may result from intracardiac thrombus formation into ventricular aneurysms, sacculations, or ventricular dilatation due to either global or regional ventricular dysfunction. A retrospective study by Wlodarska et al.59 on 126 ARVC/D patients with severe RV dilatation reported a 0.5% annual incidence rate of thromboembolic complications during a mean follow-up period of 99 ± 64 months.
Recommendations
- For ARVC/D patients who developed right- and/or left-sided heart failure standard pharmacological treatment with angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, and diuretics is recommended (class I).
- Long-term oral anticoagulation is generally indicated for secondary prevention in patients with documented intracavitary thrombosis or venous/systemic thromboembolism (class I).
- For ARVC/D patients with asymptomatic RV and/or LV dysfunction treatment with angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers may be considered (class IIb).
- Prophylactic anticoagulation for primary prevention of thromboembolism on the basis of ventricular dilatation/dysfunction, either global or regional, is not recommended (class III).
Catheter ablation
Catheter ablation is a therapeutic option for ARVC/D patients who have VT. Fibrofatty replacement of RV myocardium creates scar regions that are regarded as arrhythmogenic substrate for VT. Ventricular tachycardia is the result of a scar-related macro-reentry circuit, similar to that observed in the post-myocardial infarction setting, which is suitable for mapping and interruption by catheter ablation. Catheter ablation may be guided by either conventional electrophysiological or substrate-based mapping during sinus rhythm.60–72
Fontaine et al.60,62 first studied the effects of direct current fulguration and demonstrated the feasibility of VT ablation in ARVC/D. Subsequently, several studies have reported on acute and long-term results of endocardial catheter ablation of VT using radiofrequency current.63–73 Overall, acute success was achieved in 60–80% of patients, whereas the recurrence rates during long-term follow-up of 3–5 years were as high as 50–70% (see Supplementary material online, Table S1). The high frequency of VT recurrences and the discrepancy between the successful acute results and the unfavourable long-term outcome have been explained by the progressive nature of the ARVC/D substrate (i.e. fibrofatty scar), which predisposes to the occurrence of multiple reentry circuits and new arrhythmogenic foci over time.10,68 Recently, studies have suggested that epicardial location of some VT reentry circuits, which reflects the propensity of ARVC/D lesion to originate and progress from the epicardium, may partly explain the failure of conventional endocardial mapping/catheter ablation. Garcia et al.70 first reported the feasibility and efficacy of epicardial catheter ablation in ARVC/D patients who underwent an epicardial approach after previously failed endocardial VT mapping/ablation procedures. In these patients, the extent of electroanatomical scar area at voltage mapping was larger on the epicardial side of the RV wall than on the endocardium. Complete success was achieved in 85% of cases (partial success in 92%) and 77% of patients were free of VT during 18 months of follow-up. Phillips et al.72 compared the efficacy of traditional electrophysiological VT mapping/catheter ablation with other strategies including substrate-based and epicardial catheter approaches. The recurrences of VT were significantly reduced irrespective of the mapping/ablation strategy. The cumulative freedom from VT following procedures using 3D-electroanatomical mapping and/or epicardial approach was significantly greater than conventional ablation, although the recurrence rates remain considerable. Freedom from VT after epicardial ablation of 64 and 45% at 1 and 5 years was found, which was significantly improved compared with studies using the endocardial approach. According to Berruezo et al.,71 complete scar de-channelling with elimination of either endo or epicardial scar conducting channels (i.e. intra-scar, inter-scar, or between scar and valvular annuli) in addition to ablation of clinical VT is a promising approach to improve long-term success rate of catheter ablation.
According to available data, catheter ablation of VT in ARVC/D patients should be considered a potentially effective strategy for eliminating frequent VT episodes and ICD shocks rather than a curative therapeutic approach, until long-term efficacy has been consistently documented. Catheter ablation has not been proved to prevent SCD and should not be looked upon as an alternative to ICD therapy in ARVC/D patients with VT, with the exception of selected cases with a drug refractory, haemodynamically stable, single morphology VT.22 Additional AAD therapy and repeated ablation procedures as well as back-up ICD implantation are required to provide clinical control of VT and SCD prevention.
Recommendations
- Catheter ablation of VT is recommended in ARVC/D patients with incessant VT or frequent appropriate ICD interventions on VT despite maximal pharmacological therapy, including amiodarone (class I).
- An epicardial approach to VT ablation is recommended in patients who fail one or more attempts of endocardial VT ablation (class I).
- Catheter ablation of VT should be considered in ARVC/D patients with incessant VT or frequent appropriate ICD interventions on VT who have failed pharmacological therapy other than amiodarone (class IIa).
- A combined endocardial/epicardial VT ablation approach as an initial ablation strategy should be considered, provided that the operator and electrophysiologic laboratory are experienced performing epicardial VT ablation in patients with ARVC/D (class IIa).
- Catheter ablation of VT may be considered in ARVC/D patients with incessant VT or frequent appropriate ICD interventions on VT who have not failed pharmacological therapy and who do not wish to be treated with pharmacological therapy (class IIb).
- Catheter ablation may be indicated as first choice therapy without a back-up ICD for selected patients with drug-refractory, haemodynamically stable, single-morphology VT (class IIb).
- Catheter ablation is not recommended as an alternative to ICD for prevention of SCD in ARVC/D (class III).
Implantable defibrillator therapy
Implantable defibrillator therapy is the most logical therapeutic strategy for patients with ARVC/D, because the natural history is primarily characterized by the risk of SCD and, only secondarily, by contractile dysfunction leading to progressive heart failure. Prospective randomized trials are currently not available for ethical reasons and because of practical limitations predominantly linked to relatively low disease prevalence and low event rate. The available data, coming from observational studies/registries of large populations of ARVC/D patients, have established efficacy and safety of ICD therapy.22,25,26,28,74–81 The main results of available studies on ICD therapy in ARVC/D are summarized in Supplementary material online, Table S2. These studies consistently document that ICD successfully interrupts lethal ventricular tachyarrhythmias and improves long-term outcome of selected high-risk ARVC/D patients. Overall, between 48 and 78% of patients received appropriate ICD interventions during a mean follow-up period of 2–7 years after implantation. Many of these patients experienced multiple ICD discharges during this period and VT storm was not infrequently reported. In most studies, the survival benefit of the ICD was evaluated by comparing the actual patient survival rate with the projected freedom of ICD interventions for fast VT (>240 b.p.m.) or VF (i.e. ‘life- saving’ ICD interventions), which were used as a surrogate for aborted SCD, based on the assumption that these tachyarrhythmias would have been fatal without termination by the device.22,25,28,77 The endpoint was reached by device interrogation and review of stored electrocardiograms regarding ICD interventions in response to fast VT/VF during follow-up. In the largest multicentre study, the fast VT/VF-free survival rate was 72% at 36 months compared with the actual patient survival of 98%, with an estimated survival benefit of 26%.22 The largest single-centre experience found an estimated improvement of overall survival of 23, 32 and 35% after 1, 3 and 7 years of follow-up, respectively.28 These results were confirmed by other series reporting rates of life-saving ICD interventions in 30–50% of patients during follow-up. Despite the relatively short follow-up of the available studies, the time between implantation and the first appropriate discharge was ≥1 year in a large proportion of patients with a maximal interval of 5.5 years.25,26,74–81 This finding suggests that ICD implantation is a lifelong preventive measure with life-saving interventions occurring even after particularly long phases of dormant ventricular electrical instability.
It is important to recognize that survival benefit of ICD treatment is obtained at the expense of significant complications during follow-up, with estimated rates of lead/device related complications and inappropriate ICD therapies of 3.7%/year and 4.4%/year, respectively (see Supplementary material online, Table S2). Detailed information on ICD-related complications in the published ICD studies is provided by the recent meta-analysis by Schinkel.82 In the long-term study (80 ± 43 months) by Wichter et al.,28 37 of 60 (62%) ARVC/D patients had a total of 53 serious adverse events (31 lead-related), 10 occurring during the perioperative phase and 43 during follow-up. This high rate of lead-related adverse events may be explained by the peculiar ARVC/D pathobiology which leads to progressive loss of myocardium with fibrofatty replacement, also affecting the site of RV lead implantation. In this regard, Corrado et al. reported that ∼4% of ARVC/D patients required an additional septal lead owing to loss of ventricular sensing/pacing functions at the apical RV free wall during a follow-up of 3.3 years.22 Therefore, particular attention should be paid to progressive loss of R-wave sensing amplitude during follow-up, which may compromise adequate device function and may indicate disease progression.
Inappropriate ICD interventions occur in 10–25% of patients with ARVC/D, mostly at young age,77 and are usually caused by sinus tachycardia or atrial tachyarrhythmia (see Supplementary material online, Table S2). Inappropriate interventions are painful and may have a profound clinical and psychological impact on patients.83 The incidence of inappropriate ICD discharges can be lowered by appropriate ICD programming84 and administration of beta-blockers or sotalol. Although the use of dual-chamber detection algorithms offers the potential to reduce the number of inappropriate interventions by improving discrimination of ventricular from supraventricular arrhythmias, an additional lead in atrium predisposes to a higher incidence of early and late post-operative complications.
Indications for ICD implantation
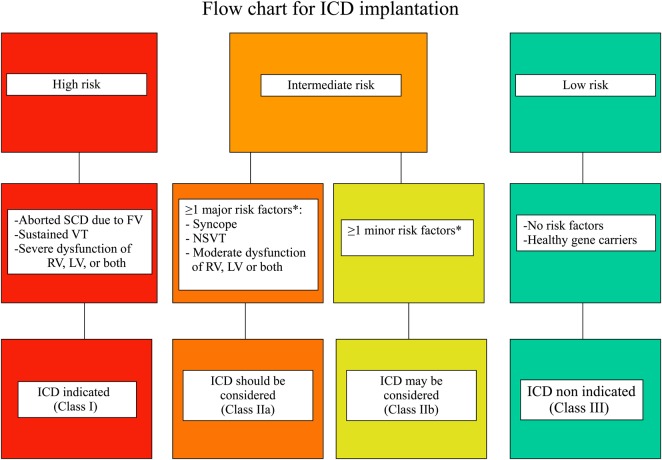
The published studies on ARVC/D patients that provided information about the independent predictors for major arrhythmic events (i.e. SCD, cardiac arrest due to VF, sustained VT, and appropriate ICD interventions) during follow-up (Table 3), have been used to construct three categories of risk for SCD (high, intermediate, and low) that were determined by consensus (Figure 1). The recommendations for ICD implantation for each risk category were based not only on the statistical risk, but also on the general health, socioeconomic factors, the psychological impact and the adverse effects of the device.
Figure 1.
Flow chart of risk stratification and indications to ICD implantation in ARVC/D. Based on the available data on annual mortality rates associated to specific risk factors, the estimated risk of major arrhythmic events in the high-risk category is >10%/year, in the intermediate ranges from 1 to 10%/year, and in the low-risk category is <1%/year. Indications to ICD implantation were determined by consensus taking into account not only the statistical risk, but also the general health, socioeconomic factors, the psychological impact and the adverse effects of the device. SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia; RV, right ventricle; LV, left ventricle. *See the text for distinction between major and minor risk factors.
The high-risk category includes patients who experienced cardiac arrest due to VF or sustained VT. This group of patients has an estimated rate of life-threatening arrhythmic events >10%/year and most benefits for ICD therapy.22–24 A prophylactic ICD implantation is also recommended in patients with severe RV dysfunction (RV fractional area change ≤17% or RV EF ≤35%) or LV dysfunction (LV EF ≤35%) who are considered at high risk by consensus, even in the absence of life-threatening ventricular arrhythmias.21,22,27,28,30,39 Because the specific arrhythmic risk of ventricular dysfunction is still undermined for patients with ARVC/D, the inclusion of this clinical variable into the high-risk category was based on extrapolation from other cardiomyopathies and personal experience.
The low-risk category comprises probands and relatives without risk factors as well as healthy gene carriers who show a low rate of malignant arrhythmic events (estimated annual event rate <1%/year25,31) over a long-term follow-up and do not require any treatment, including ICD therapy.
Between the two categories there are ARVC/D patients with ≥1 risk factors who are deemed to have an intermediate risk (estimated annual event rate between 1 and 10%25,26). Among the consensus experts there was general agreement that syncope, non sustained ventricular tachycardia (NSVT), or moderate ventricular dysfunction, either RV (RV fractional area change between 24 and 17% or RV EF between 40 and 36%), or left-ventricular (LV EF between 45 and 36%), are ‘major’ risk factor that justify (weight of opinion in favour of ICD) a prophylactic ICD. On the other hand, there was general consensus that the other factors reported in Table 3 (‘minor’ risk factors) are associated with a risk of major arrhythmic events not sufficiently high (or controversial) to warrant systematic ICD implantation for primary prevention (weight of opinion against ICD). The decision to implant an ICD in patients of this category should be made on individual basis, by assessing the overall clinical profile, the age, the strength of the risk factor identified, the level of SCD risk that is acceptable to the patient, and the potential risk of inappropriate interventions and complications.
It is noteworthy that indications for ICD implantation may vary in different countries as a consequence of several non-clinical factors such as cultural background, socio-economic conditions, health system, availability of advanced technology, cost–benefit considerations, and liability. Compared with the conservative approach of many European countries, the current threshold for decision to implant an ICD in the USA is lower.13 It is particularly important to outline the potential risk of inappropriate ICD implants due to a false diagnosis of ARVC/D based on misinterpretation of imaging studies including cardiac magnetic resonance.85
Recommendations
- Implantation of an ICD is recommended in ARVC/D patients who have experienced ≥1 episodes of haemodynamically unstable, sustained VT or VF (class I).
- Implantation of an ICD is recommended in ARVC/D patients with severe systolic dysfunction of the RV, LV, or both, irrespective of arrhythmias (class I).
- Implantation of an ICD should be considered in ARVC/D patients who have experienced ≥1 episodes of haemodynamically stable, sustained VT (class IIa).
- Implantation of an ICD should be considered in patients who have ‘major’ risk factors such as unexplained syncope, moderate ventricular dysfunction, or NSVT (class IIa).
- Implantation of an ICD may be considered in patients with ‘minor’ risk factors after a careful discussion of the long-term risks and benefits of ICD implantation (class IIb).
- Prophylactic ICD implantation is not recommended in asymptomatic ARVC/D patients with no risk factors or healthy gene carriers (class III).
Device selection
A single-chamber ICD system is recommended in order to minimize the incidence of long-term lead-related complications, mostly in young patients.
Experience with ICD therapy consistently highlights the beneficial effect of antitachycardia pacing which is highly effective in terminating VT episodes in ARVC/D patients. The precise clinical role of leadless subcutaneous ICD in patients with ARVC/D remains to be defined. A decision whether to implant a leadless device needs to be patient specific, balancing lead-related complications with the likelihood of recurrent VT that may be effectively pace-terminated.
Additional cardiac resynchronization therapy appears reasonable for those ARVC/D patients with a LV EF ≤35% and a wide QRS with a left bundle branch block pattern, even though clinical benefit is extrapolated from resynchronization therapy in other disease states.86 Right-ventricular resynchronization therapy has been proposed as a therapy for patients with congenital heart disease and chronic RV heart failure;87 however, no data are available concerning the clinical and haemodynamic effects of RV pacing in ARVC/D patients with RV dysfunction and a wide QRS with right bundle branch block pattern.
Heart transplantation
Arrhythmogenic right-ventricular cardiomyopathy/dysplasia patients with untreatable heart failure or uncontrollable ventricular tachyarrhythmias may require heart transplantation. Tedford et al.88 reported the Johns Hopkins Registry experience with 18 ARVC/D patients (61% males; mean age 40 ± 14 year) undergoing heart transplantation. The most common indication for cardiac transplantation was heart failure, with less than one-third of patients receiving transplants for intractable ventricular arrhythmias. Patients who received heart transplants were significantly younger (mean age at the time of first symptoms 24 ± 13 years) and had a more prolonged clinical course (time from first symptoms to transplant ∼15 years) compared with other ARVC/D registry participants. One-year after transplant, the survival was 94 and 88% of patients were alive at an average post-transplant follow-up of 6.2 ± 4.8 years.
Heart transplantation is recommended as a final therapeutic option in ARVC/D patients with either severe, unresponsive congestive heart failure or recurrent episodes of VT/VF which are refractory to catheter (and surgical) ablation in experienced centres and/or ICD therapy.
Other surgical therapies
There is currently no clinical role of surgical therapies such as RV cardiomyoplasty,89 RV disarticulation,90 beating heart cryoablation,91 and left cardiac sympathetic denervation92 in the treatment of patients with ARVC/D.
Conclusions
The therapeutic management of patients with ARVC/D has evolved over the years and continues to be an important challenge. To further improve risk stratification and treatment of patients, more information is needed on the natural history, long-term prognosis, and risk assessment. Special attention should be focused on the identification of patients who would benefit from ICD implantation in comparison to pharmacological and other non-pharmacological approaches. Data from prospective studies/registries with larger number of patients and longer follow-up as well as data obtained from multicentre randomized controlled trials are required to provide evidence-based recommendations for the best care of ARVC/D patients.
Current therapeutic and preventive measures are palliative, not curative. The definitive cure of ARVC/D will be based on the discovery of the molecular mechanisms that are involved in the aetiology and pathogenesis of the disease.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported in part by the TRANSAC Research Grant of the University of Padua, Italy.
Conflict of interest: H.C. reports grants from Medtronic and St. Jude Medical, during the conduct of the study; F.D. and C.B. report grants from the Georg and Bertha Schwyzer-Winker Foundation; M.N.A.M.E. reports personal fees from Boston Scientific Corporation, St. Jude Medical, and from Medtronic. The other authors report no conflicts of interest.
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation 1982;65:384–398. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–133. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 1996;94:983–991. [DOI] [PubMed] [Google Scholar]
- 4.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol 1997;30:1512–1520. [DOI] [PubMed] [Google Scholar]
- 5.Corrado D, Basso C, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis, and treatment. Heart 2000;83:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen-Chowdhry S, Lowe MD, Sporton SC, McKenna WJ. Arrhythmogenic right ventricular cardiomyopathy: clinical presentation, diagnosis, and management. Am J Med 2004;117:685–695. [DOI] [PubMed] [Google Scholar]
- 7.Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation 2005;112:3823–3832. [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 2006;296:1593–1601. [DOI] [PubMed] [Google Scholar]
- 9.Sen-Chowdhry S, Prasad SK, Syrris P, Wage R, Ward D, Merrifield R, Smith GC, Firmin DN, Pennell DJ, McKenna WJ. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol 2006;48:2132–2140. [DOI] [PubMed] [Google Scholar]
- 10.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009;373:1289–1300. [DOI] [PubMed] [Google Scholar]
- 11.Corrado D, Basso C, Leoni L, Tokajuk B, Turrini P, Bauce B, Migliore F, Pavei A, Tarantini G, Napodano M, Ramondo A, Buja G, Iliceto S, Thiene G. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol 2008;51:731–739. [DOI] [PubMed] [Google Scholar]
- 12.Herren T, Gerber PA, Duru F. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: a not so rare ‘disease of the desmosome’ with multiple clinical presentations. Clin Res Cardiol 2009;98:141–158. [DOI] [PubMed] [Google Scholar]
- 13.Patel HC, Calkins H. Arrhythmogenic right ventricular dysplasia. Curr Treat Options Cardiovasc Med 2010;12:598–613. [DOI] [PubMed] [Google Scholar]
- 14.Sen-Chowdhry S, Morgan RD, Chambers JC, McKenna WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med 2010;61:233–253. [DOI] [PubMed] [Google Scholar]
- 15.Basso C, Corrado D, Bauce B, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2012;5:1233–1246. [DOI] [PubMed] [Google Scholar]
- 16.Paul M, Wichter T, Fabritz L, Waltenberger J, Schulze-Bahr E, Kirchhof P. Arrhythmogenic right ventricular cardiomyopathy: an update on pathophysiology, genetics, diagnosis, and risk stratification. Herzschrittmacherther Elektrophysiol 2012;23:186–195. [DOI] [PubMed] [Google Scholar]
- 17.Calkins H. Arrhythmogenic right ventricular dysplasia. Curr Probl Cardiol 2013;38:103–123. [DOI] [PubMed] [Google Scholar]
- 18.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nava A, Bauce B, Basso C, Muriago M, Rampazzo A, Villanova C, Daliento L, Buja G, Corrado D, Danieli GA, Thiene G. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2000;36:2226–2233. [DOI] [PubMed] [Google Scholar]
- 21.Lemola K, Brunckhorst C, Helfenstein U, Oechslin E, Jenni R, Duru F. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: long term experience of a tertiary care centre. Heart 2005;91:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, Zanotto G, Verlato R, Vergara G, Delise P, Turrini P, Basso C, Naccarella F, Maddalena F, Estes NA, III, Buja G, Thiene G. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003;108:3084–3091. [DOI] [PubMed] [Google Scholar]
- 23.Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NA., III Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins DA, Hendricks N, Shaboodien G, Mbele M, Parker M, Vezi BZ, Latib A, Chin A, Little F, Badri M, Moolman-Smook JC, Okreglicki A, Mayosi BM. Clinical features, survival experience, and profile of plakophylin-2 gene mutations in participants of the arrhythmogenic right ventricular cardiomyopathy registry of South Africa. Heart Rhythm 2009;6(suppl):S10–S17. [DOI] [PubMed] [Google Scholar]
- 25.Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes NA, III, Wichter T, McKenna WJ, Thiene G, Marcus FI. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation 2010;122:1144–1152. [DOI] [PubMed] [Google Scholar]
- 26.Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, Dalal D, Tedford R, Russell SD, Abraham T, Tandri H, Judge DP, Calkins H. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol 2011;58:1485–1496. [DOI] [PubMed] [Google Scholar]
- 27.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2004;110:1879–1884. [DOI] [PubMed] [Google Scholar]
- 28.Wichter T, Paul M, Wollmann C, Acil T, Gerdes P, Ashraf O, Tjan TD, Soeparwata R, Block M, Borggrefe M, Scheld HH, Breithardt G, Bocker D. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy: single-center experience of long-term follow-up and complications in 60 patients. Circulation 2004;109:1503–1508. [DOI] [PubMed] [Google Scholar]
- 29.Saguner AM, Vecchiati A, Baldinger SH, Rueger S, Medeiros-Domingo A, Mueller-Burri AS, Haegeli LM, Biaggi P, Manka R, Luscher TF, Fontaine G, Delacretaz E, Jenni R, Held L, Brunckhorst C, Duru F, Tanner FC. Different prognostic value of functional right ventricular parameters in Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Cardiovasc Imaging 2014;7:230–239. [DOI] [PubMed] [Google Scholar]
- 30.Pinamonti B, Dragos AM, Pyxaras SA, Merlo M, Pivetta A, Barbati G, Di Lenarda A, Morgera T, Mestroni L, Sinagra G. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J 2011;32:1105–1113. [DOI] [PubMed] [Google Scholar]
- 31.Bhonsale A, James CA, Tichnell C, Murray B, Madhavan S, Philips B, Russell SD, Abraham T, Tandri H, Judge DP, Calkins H. Risk stratification in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. Circ Arrhythm Electrophysiol 2013;6:569–578. [DOI] [PubMed] [Google Scholar]
- 32.Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, Migliore F, Marra MP, Lorenzon A, De Bortoli M, Calore M, Nava A, Daliento L, Gregori D, Iliceto S, Thiene G, Basso C, Corrado D. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2013;6:533–542. [DOI] [PubMed] [Google Scholar]
- 33.Saguner AM, Medeiros-Domingo A, Schwyzer MA, On CJ, Haegeli LM, Wolber T, Hurlimann D, Steffel J, Krasniqi N, Rueger S, Held L, Luscher TF, Brunckhorst C, Duru F. Usefulness of inducible ventricular tachycardia to predict long-term adverse outcomes in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2013;111:250–257. [DOI] [PubMed] [Google Scholar]
- 34.Migliore F, Zorzi A, Silvano M, Bevilacqua M, Leoni L, Marra MP, Elmaghawry M, Brugnaro L, Dal Lin C, Bauce B, Rigato I, Tarantini G, Basso C, Buja G, Thiene G, Iliceto S, Corrado D. Prognostic value of endocardial voltage mapping in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Arrhythm Electrophysiol 2013;6:167–176. [DOI] [PubMed] [Google Scholar]
- 35.Santangeli P, Dello Russo A, Pieroni M, Casella M, Di Biase L, Burkhardt JD, Sanchez J, Lakkireddy D, Carbucicchio C, Zucchetti M, Pelargonio G, Themistoclakis S, Camporeale A, Rossillo A, Beheiry S, Hongo R, Bellocci F, Tondo C, Natale A. Fragmented and delayed electrograms within fibrofatty scar predict arrhythmic events in arrhythmogenic right ventricular cardiomyopathy: results from a prospective risk stratification study. Heart Rhythm 2012;9:1200–1206. [DOI] [PubMed] [Google Scholar]
- 36.Saguner AM, Ganahl S, Baldinger SH, Kraus A, Medeiros-Domingo A, Nordbeck S, Saguner AR, Mueller-Burri AS, Haegeli LM, Wolber T, Steffel J, Krasniqi N, Delacretaz E, Luscher TF, Held L, Brunckhorst CB, Duru F. Usefulness of electrocardiographic parameters for risk prediction in arrhythmogenic right ventricular dysplasia. Am J Cardiol 2014;113:1728–1734. [DOI] [PubMed] [Google Scholar]
- 37.Blomstrom-Lundqvist C, Sabel KG, Olsson SB. A long term follow up of 15 patients with arrhythmogenic right ventricular dysplasia. Br Heart J 1987;58:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus FI, Fontaine GH, Frank R, Gallagher JJ, Reiter MJ. Long-term follow-up in patients with arrhythmogenic right ventricular disease. Eur Heart J 1989;10(Suppl. D):68–73. [DOI] [PubMed] [Google Scholar]
- 39.Peters S. Long-term follow-up and risk assessment of arrhythmogenic right ventricular dysplasia/cardiomyopathy: personal experience from different primary and tertiary centres. J Cardiovasc Med (Hagerstown) 2007;8:521–526. [DOI] [PubMed] [Google Scholar]
- 40.Corrado D, Thiene G, Nava A, Rossi L, Pennelli N. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am J Med 1990;89:588–596. [DOI] [PubMed] [Google Scholar]
- 41.Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation 1992;86:29–37. [DOI] [PubMed] [Google Scholar]
- 42.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol 2003;42:1959–1963. [DOI] [PubMed] [Google Scholar]
- 43.Corrado D, Schmied C, Basso C, Borjesson M, Schiavon M, Pelliccia A, Vanhees L, Thiene G. Risk of sports: do we need a pre-participation screening for competitive and leisure athletes? Eur Heart J 2011;32:934–944. [DOI] [PubMed] [Google Scholar]
- 44.Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, Breithardt G, Ruiz P, Wichter T, Levkau B. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 2006;114:1799–1806. [DOI] [PubMed] [Google Scholar]
- 45.Corrado D, Thiene G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: clinical impact of molecular genetic studies. Circulation 2006;113:1634–1637. [DOI] [PubMed] [Google Scholar]
- 46.Sen-Chowdhry S, Syrris P, McKenna WJ. Role of genetic analysis in the management of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2007;50:1813–1821. [DOI] [PubMed] [Google Scholar]
- 47.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, Ribe M, Holst AG, Edvardsen T, Haugaa KH. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, Estes NA, III, Marcus F, Scheinman MM. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol 2009;54:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wichter T, Hindricks G, Lerch H, Bartenstein P, Borggrefe M, Schober O, Breithardt G. Regional myocardial sympathetic dysinnervation in arrhythmogenic right ventricular cardiomyopathy. An analysis using 123I-meta-iodobenzylguanidine scintigraphy. Circulation 1994;89:667–683. [DOI] [PubMed] [Google Scholar]
- 51.Wichter T, Schafers M, Rhodes CG, Borggrefe M, Lerch H, Lammertsma AA, Hermansen F, Schober O, Breithardt G, Camici PG. Abnormalities of cardiac sympathetic innervation in arrhythmogenic right ventricular cardiomyopathy: quantitative assessment of presynaptic norepinephrine reuptake and postsynaptic beta-adrenergic receptor density with positron emission tomography. Circulation 2000;101:1552–1558. [DOI] [PubMed] [Google Scholar]
- 52.Fabritz L, Fortmuller L, Yu TY, Paul M, Kirchhof P. Can preload-reducing therapy prevent disease progression in arrhythmogenic right ventricular cardiomyopathy? Experimental evidence and concept for a clinical trial. Prog Biophys Mol Biol 2012;110:340–346. [DOI] [PubMed] [Google Scholar]
- 53.Fabritz L, Hoogendijk MG, Scicluna BP, van Amersfoorth SC, Fortmueller L, Wolf S, Laakmann S, Kreienkamp N, Piccini I, Breithardt G, Noppinger PR, Witt H, Ebnet K, Wichter T, Levkau B, Franke WW, Pieperhoff S, de Bakker JM, Coronel R, Kirchhof P. Load-reducing therapy prevents development of arrhythmogenic right ventricular cardiomyopathy in plakoglobin-deficient mice. J Am Coll Cardiol 2011;57:740–750. [DOI] [PubMed] [Google Scholar]
- 54.Bauce B, Basso C, Rampazzo A, Beffagna G, Daliento L, Frigo G, Malacrida S, Settimo L, Danieli G, Thiene G, Nava A. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J 2005;26:1666–1675. [DOI] [PubMed] [Google Scholar]
- 55.Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation 2007;115:1710–1720. [DOI] [PubMed] [Google Scholar]
- 56.Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–2187. [DOI] [PubMed] [Google Scholar]
- 57.Marra MP, Leoni L, Bauce B, Corbetti F, Zorzi A, Migliore F, Silvano M, Rigato I, Tona F, Tarantini G, Cacciavillani L, Basso C, Buja G, Thiene G, Iliceto S, Corrado D. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol 2012;5:91–100. [DOI] [PubMed] [Google Scholar]
- 58.te Riele AS, Bhonsale A, James CA, Rastegar N, Murray B, Burt JR, Tichnell C, Madhavan S, Judge DP, Bluemke DA, Zimmerman SL, Kamel IR, Calkins H, Tandri H. Incremental value of cardiac magnetic resonance imaging in arrhythmic risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wlodarska EK, Wozniak O, Konka M, Rydlewska-Sadowska W, Biederman A, Hoffman P. Thromboembolic complications in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Europace 2006;8:596–600. [DOI] [PubMed] [Google Scholar]
- 60.Fontaine G, Frank R, Rougier I, Tonet JL, Gallais Y, Farenq G, Lascault G, Lilamand M, Fontaliran F, Chomette G, Grosgogeat Y. Electrode catheter ablation of resistant ventricular tachycardia in arrhythmogenic right ventricular dysplasia: experience of 13 patients with a mean follow-up of 45 months. Eur Heart J 1989;10(Suppl. D):74–81. [DOI] [PubMed] [Google Scholar]
- 61.Ellison KE, Friedman PL, Ganz LI, Stevenson WG. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. J Am Coll Cardiol 1998;32:724–728. [DOI] [PubMed] [Google Scholar]
- 62.Fontaine G, Tonet J, Gallais Y, Lascault G, Hidden-Lucet F, Aouate P, Halimi F, Poulain F, Johnson N, Charfeddine H, Frank R. Ventricular tachycardia catheter ablation in arrhythmogenic right ventricular dysplasia: a 16-year experience. Curr Cardiol Rep 2000;2:498–506. [DOI] [PubMed] [Google Scholar]
- 63.Reithmann C, Hahnefeld A, Remp T, Dorwarth U, Dugas M, Steinbeck G, Hoffmann E. Electroanatomic mapping of endocardial right ventricular activation as a guide for catheter ablation in patients with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol 2003;26:1308–1316. [DOI] [PubMed] [Google Scholar]
- 64.Marchlinski FE, Zado E, Dixit S, Gerstenfeld E, Callans DJ, Hsia H, Lin D, Nayak H, Russo A, Pulliam W. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation 2004;110:2293–2298. [DOI] [PubMed] [Google Scholar]
- 65.Miljoen H, State S, de Chillou C, Magnin-Poull I, Dotto P, Andronache M, Abdelaal A, Aliot E. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 2005;7:516–524. [DOI] [PubMed] [Google Scholar]
- 66.Verma A, Kilicaslan F, Schweikert RA, Tomassoni G, Rossillo A, Marrouche NF, Ozduran V, Wazni OM, Elayi SC, Saenz LC, Minor S, Cummings JE, Burkhardt JD, Hao S, Beheiry S, Tchou PJ, Natale A. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation 2005;111:3209–3216. [DOI] [PubMed] [Google Scholar]
- 67.Satomi K, Kurita T, Suyama K, Noda T, Okamura H, Otomo K, Shimizu W, Aihara N, Kamakura S. Catheter ablation of stable and unstable ventricular tachycardias in patients with arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol 2006;17:469–476. [DOI] [PubMed] [Google Scholar]
- 68.Dalal D, Jain R, Tandri H, Dong J, Eid SM, Prakasa K, Tichnell C, James C, Abraham T, Russell SD, Sinha S, Judge DP, Bluemke DA, Marine JE, Calkins H. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2007;50:432–440. [DOI] [PubMed] [Google Scholar]
- 69.Nogami A, Sugiyasu A, Tada H, Kurosaki K, Sakamaki M, Kowase S, Oginosawa Y, Kubota S, Usui T, Naito S. Changes in the isolated delayed component as an endpoint of catheter ablation in arrhythmogenic right ventricular cardiomyopathy: predictor for long-term success. J Cardiovasc Electrophysiol 2008;19:681–688. [DOI] [PubMed] [Google Scholar]
- 70.Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366–375. [DOI] [PubMed] [Google Scholar]
- 71.Berruezo A, Fernandez-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, Boussy T, Tolosana JM, Arbelo E, Brugada J. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol 2012;5:111–121. [DOI] [PubMed] [Google Scholar]
- 72.Philips B, Madhavan S, James C, Tichnell C, Murray B, Dalal D, Bhonsale A, Nazarian S, Judge DP, Russell SD, Abraham T, Calkins H, Tandri H. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol 2012;5:499–505. [DOI] [PubMed] [Google Scholar]
- 73.Tschabrunn CM, Marchlinski FE. Ventricular tachycardia mapping and ablation in arrhythmogenic right ventricular cardiomyopathy/dysplasia: Lessons Learned. World J Cardiol 2014;6:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boriani G, Artale P, Biffi M, Martignani C, Frabetti L, Valzania C, Diemberger I, Ziacchi M, Bertini M, Rapezzi C, Parlapiano M, Branzi A. Outcome of cardioverter-defibrillator implant in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Vessels 2007;22:184–192. [DOI] [PubMed] [Google Scholar]
- 75.Breithardt G, Wichter T, Haverkamp W, Borggrefe M, Block M, Hammel D, Scheld HH. Implantable cardioverter defibrillator therapy in patients with arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, or no structural heart disease. Am Heart J 1994;127(Pt 2):1151–1158. [DOI] [PubMed] [Google Scholar]
- 76.Hodgkinson KA, Parfrey PS, Bassett AS, Kupprion C, Drenckhahn J, Norman MW, Thierfelder L, Stuckless SN, Dicks EL, McKenna WJ, Connors SP. The impact of implantable cardioverter-defibrillator therapy on survival in autosomal-dominant arrhythmogenic right ventricular cardiomyopathy (ARVD5). J Am Coll Cardiol 2005;45:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Link MS, Wang PJ, Haugh CJ, Homoud MK, Foote CB, Costeas XB, Estes NA., III Arrhythmogenic right ventricular dysplasia: clinical results with implantable cardioverter defibrillators. J Interv Card Electrophysiol 1997;1:41–48. [DOI] [PubMed] [Google Scholar]
- 78.Piccini JP, Dalal D, Roguin A, Bomma C, Cheng A, Prakasa K, Dong J, Tichnell C, James C, Russell S, Crosson J, Berger RD, Marine JE, Tomaselli G, Calkins H. Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia. Heart Rhythm 2005;2:1188–1194. [DOI] [PubMed] [Google Scholar]
- 79.Roguin A, Bomma CS, Nasir K, Tandri H, Tichnell C, James C, Rutberg J, Crosson J, Spevak PJ, Berger RD, Halperin HR, Calkins H. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2004;43:1843–1852. [DOI] [PubMed] [Google Scholar]
- 80.Schuler PK, Haegeli LM, Saguner AM, Wolber T, Tanner FC, Jenni R, Corti N, Luscher TF, Brunckhorst C, Duru F. Predictors of appropriate ICD therapy in patients with arrhythmogenic right ventricular cardiomyopathy: long term experience of a tertiary care center. PLoS One 2012;7:e39584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tavernier R, Gevaert S, De Sutter J, De Clercq A, Rottiers H, Jordaens L, Fonteyne W. Long term results of cardioverter-defibrillator implantation in patients with right ventricular dysplasia and malignant ventricular tachyarrhythmias. Heart 2001;85:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schinkel AF. Implantable cardioverter defibrillators in arrhythmogenic right ventricular dysplasia/cardiomyopathy: patient outcomes, incidence of appropriate and inappropriate interventions, and complications. Circ Arrhythm Electrophysiol 2013;6:562–568. [DOI] [PubMed] [Google Scholar]
- 83.Ingles J, Sarina T, Kasparian N, Semsarian C. Psychological wellbeing and posttraumatic stress associated with implantable cardioverter defibrillator therapy in young adults with genetic heart disease. Int J Cardiol 2013;168:3779–3784. [DOI] [PubMed] [Google Scholar]
- 84.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, III, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 85.Marcus F, Basso C, Gear K, Sorrell VL. Pitfalls in the diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Am J Cardiol 2010;105:1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 87.Dubin AM, Feinstein JA, Reddy VM, Hanley FL, Van Hare GF, Rosenthal DN. Electrical resynchronization: a novel therapy for the failing right ventricle. Circulation 2003;107:2287–2289. [DOI] [PubMed] [Google Scholar]
- 88.Tedford RJ, James C, Judge DP, Tichnell C, Murray B, Bhonsale A, Philips B, Abraham T, Dalal D, Halushka MK, Tandri H, Calkins H, Russell SD. Cardiac transplantation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2012;59:289–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chachques JC, Argyriadis PG, Fontaine G, Hebert JL, Frank RA, D'Attellis N, Fabiani JN, Carpentier AF. Right ventricular cardiomyoplasty: 10-year follow-up. Ann Thorac Surg 2003;75:1464–1468. [DOI] [PubMed] [Google Scholar]
- 90.Zacharias J, Forty J, Doig JC, Bourke JP, Hilton CJ. Right ventricular disarticulation. An 18-year single centre experience . Eur J Cardiothorac Surg 2005;27:1000–1004. [DOI] [PubMed] [Google Scholar]
- 91.Bakir I, Brugada P, Sarkozy A, Vandepitte C, Wellens F. A novel treatment strategy for therapy refractory ventricular arrhythmias in the setting of arrhythmogenic right ventricular dysplasia. Europace 2007;9:267–269. [DOI] [PubMed] [Google Scholar]
- 92.Coleman MA, Bos JM, Johnson JN, Owen HJ, Deschamps C, Moir C, Ackerman MJ. Videoscopic left cardiac sympathetic denervation for patients with recurrent ventricular fibrillation/malignant ventricular arrhythmia syndromes besides congenital long-QT syndrome. Circ Arrhythm Electrophysiol 2012;5:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]