Abstract
Background
Therapeutic decisions in atrial fibrillation (AF) are often influenced by assessment of bleeding risk. However, existing bleeding risk scores have limitations.
Objectives
We sought to develop and validate a novel bleeding risk score using routinely available clinical information to predict major bleeding in a large, community-based AF population.
Methods
We analysed data from Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF), a prospective registry that enrolled incident and prevalent AF patients at 176 US sites. Using Cox proportional hazards regression, we identified factors independently associated with major bleeding among patients taking oral anticoagulation (OAC) over a median follow-up of 2 years (interquartile range = 1.6–2.5). We also created a numerical bedside risk score that included the five most predictive risk factors weighted according to their strength of association with major bleeding. The predictive performance of the full model, the simple five-item score, and two existing risk scores (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly, HAS-BLED, and anticoagulation and risk factors in atrial fibrillation, ATRIA) were then assessed in both the ORBIT-AF cohort and a separate clinical trial population, Rivaroxaban Once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET-AF).
Results
Among 7411 ORBIT-AF patients taking OAC, the rate of major bleeding was 4.0/100 person-years. The full continuous model (12 variables) and five-factor ORBIT risk score (older age [75+ years], reduced haemoglobin/haematocrit/history of anaemia, bleeding history, insufficient kidney function, and treatment with antiplatelet) both had good ability to identify those who bled vs. not (C-index 0.69 and 0.67, respectively). These scores both had similar discrimination, but markedly better calibration when compared with the HAS-BLED and ATRIA scores in an external validation population from the ROCKET-AF trial.
Conclusions
The five-element ORBIT bleeding risk score had better ability to predict major bleeding in AF patients when compared with HAS-BLED and ATRIA risk scores. The ORBIT risk score can provide a simple, easily remembered tool to support clinical decision making.
Keywords: Atrial fibrillation, Anticoagulants, Major bleeding, Risk prediction
See page 3265 for the editorial comment on this article (doi:10.1093/eurheartj/ehv415)
Introduction
Anticoagulation therapy can clearly reduce the risk of stroke and systemic emboli when used in atrial fibrillation (AF) patients,1,2 yet clinicians and patients must often consider these benefits vs. the risk of major bleeding.3,4 In clinical practice, simple scores can serve as a useful tool to support providers to estimate the risks of stroke as well as for major bleeding.5–8 However, existing bleeding scores, including hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly (HAS-BLED)9 and anticoagulation and risk factors in atrial fibrillation (ATRIA),10 were based on small numbers of events,11 have shown inconsistent performance in external populations,12,13 and may require data elements that are not accessible for all oral anticoagulation (OAC) users.14–16 Therefore, there remains a need for a simple, accurate risk score that uses readily available clinical information to predict the occurrence of major bleeding in AF patients receiving contemporary anticoagulation.
Using data from the national Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry, we constructed a full continuous predictive model as well as a simple risk score for major bleeding among patients who were taking OAC therapy. We compared the performance of this novel score to that of two other major bleeding models (HAS-BLED and ATRIA) in the ORBIT-AF population as well as in an external validation sample of those enrolled in Rivaroxaban Once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET-AF), a randomized trial of anticoagulation therapy for stroke prevention.
Methods
We used data from ORBIT-AF, a prospective study of 10 132 incident and prevalent AF patients (2010–2012), to construct a risk score for major bleeding.17 Briefly, the ORBIT-AF Registry is a national, outpatient registry of patients with electrocardiographically confirmed AF at 176 sites in the USA. We excluded patients who were not taking OACs at baseline (N = 2419) and patients without follow-up data (N = 302) for a final analytic population of N = 7411. Major bleeding was defined according to International Society on Thrombosis and Haemostasis criteria: (i) fatal bleeding and/or (ii) symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome), and/or (iii) bleeding causing a fall in haemoglobin level of 20 g L−1 (1.24 mmol L−1) or more, or leading to transfusion of two or more units of whole blood or red cells.18 Patient characteristics were described as frequency/percent for categorical variables and medians/interquartile ranges (IQRs) for continuous variables. The characteristics were compared using the chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables.
We constructed a multivariable Cox regression model with time to major bleeding over 2 years of follow-up as the outcome. Candidate variables for the model were chosen from the list of variables collected at the baseline study visit based on existing evidence and clinical relevance. The full candidate covariate list is available in Supplementary materials online. Additionally, we accounted for within site clustering of patients using empirical standard errors. All continuous variables were evaluated for non-linearity with the outcome, and those not meeting the linear relationship criterion (P < 0.05) were accounted for using linear splines. Missing data were handled with single imputation. Imputed values were obtained by the Markov chain Monte Carlo method or regression methods.19 We used a backwards selection approach with a stay criterion of P < 0.05 to generate the full continuous predictive model. To create the simple ORBIT score, we retained five predictors from the full model with the highest individual chi-square statistics. Point values were assigned to each predictor according to its strength of association with major bleeding.
We assessed model performance by examining discrimination and calibration at 2 years of follow-up in the ORBIT-AF cohort. Discrimination was evaluated using the C-index,20 which quantifies the ability of the model to correctly distinguish between patients who do and do not experience a major bleeding event. Calibration was evaluated by plotting major bleeding events rates per 100 patient-years and 95% CIs observed in the external validation cohort vs. those previously published from the original derivation cohorts for each discrete score point value. As a sensitivity analysis, we examined discrimination of the ORBIT score for prediction of intracerebral haemorrhage (ICH) using the C-index. Because bleeding rates may be higher among new starts than long-term warfarin users, we conducted a sensitivity analysis assessing ORBIT score performance among patients who were taking warfarin for <6 months.
Comparison with existing scores
We compared the predictive performance of the ORBIT score to that of two existing bleeding scores, HAS-BLED and ATRIA. The HAS-BLED score was derived from 53 major bleeding events occurring in 3978 patients in the EURO Heart Survey on AF.21 The ATRIA score was derived from 461 major bleeds occurring in 9186 adults with AF enrolled in Kaiser Permanente healthcare system in Northern California.10
External validation
We also evaluated the accuracy of the ORBIT, HAS-BLED, and ATRIA scores in an external AF population, ROCKET-AF, an international, randomized, double-blind, event-driven trial of 14 264 patients comparing rivaroxaban (20 mg daily) to dose-adjusted warfarin. Each score was recreated according to definitions given in the original derivation cohorts, using baseline values from the first trial visit, or from the first study visit in ORBIT-AF. Score components not collected in ROCKET-AF or ORBIT-AF were approximated using available data or contributed 0 points to the score if no approximation was available. The full list of definitions used to generate the scores in each dataset is provided in Supplementary materials online.
Statistical analysis was performed using SAS software (version 9.3, Cary, NC). All P-values presented are two sided, and P < 0.05 was considered to be statistically significant for all analyses. All ORBIT-AF study participants provided written informed consent prior to study entry. The ORBIT-AF Registry was approved by the Duke Institutional Review Board (IRB), and participating sites obtained approval from local IRBs as needed prior to entering patient data.
Results
Of 7411 ORBIT-AF patients taking OAC at baseline, the median age was 75 years (IQR 68–82) and 42.4% were female. There were 93.5% treated with warfarin and 6.5% with dabigatran. Over a median of 2 years (IQR 1.6–2.5) of follow-up, 581 (7.8%) major bleeding events occurred. Patients who experienced a major bleeding event during follow-up were on average older, more likely to be white, and more likely to be female than those who did not (Table 1). Patients experiencing a major bleed had a higher comorbidity burden than those who did not, with higher rates of anaemia, chronic obstructive pulmonary disease (COPD), congestive heart failure, hypertension, diabetes, sleep apnoea, and chronic kidney disease. Compared with those without a major bleed, patients with a bleeding event were more likely to be smokers, have a history of frailty, and be living with assistance than those who did not. Estimated CHA2DS2-VASC stroke risk was higher among patients who had a major bleed [median = 5 (IQR 4–6) vs. 4; (IQR = 3–5)]. Use of other antithrombotics was higher among patients who experienced a major bleed than those who did not (49.1 vs. 37.0%), with aspirin representing the majority of other antithrombotic use (34.7% among all patients with no major bleeding and 45.8% among all patients with major bleeding).
Table 1.
Baseline characteristics by major bleeding during follow-upa
| Variable | No major bleed (N = 6830; 92.2%) | Major bleed (N = 581; 7.8%) | P-value** |
|---|---|---|---|
| Demographics | |||
| Age (years) (median) IQR | 75 (67–81) | 78 (71–83) | <0.0001 |
| Male gender | 57.9 | 53.9 | 0.06 |
| White race | 89.4 | 91.6 | 0.06 |
| Comorbidities | |||
| Anaemia/abnormal Hgb/Hct | 34.8 | 57.5 | <0.0001 |
| Hypertension | 84.5 | 89.3 | 0.0019 |
| Diabetes | 30.3 | 33.7 | 0.08 |
| Current smoker | 5.3 | 6.5 | <0.0001 |
| GI bleed | 7.4 | 15.5 | <0.0001 |
| Prior stroke | 9.2 | 13.1 | 0.002 |
| CHF | 33.8 | 44.9 | <0.0001 |
| MI | 15.4 | 20.5 | 0.001 |
| Osteoporosis/hip fracture | 14.4 | 20.3 | 0.0001 |
| COPD | 15.7 | 24.4 | <0.0001 |
| History of cancer | 23.3 | 30.8 | <0.0001 |
| Antithrombotic therapy | |||
| Antiplatelets | 37.0 | 49.1 | <0.0001 |
| Warfarin | 93.4 | 95.0 | 0.14 |
| Dabigatran | 6.6 | 5.2 | 0.18 |
| eGFR<60 mg/dL/1.73 m2 | 34.0 | 48.4 | <0.0001 |
| CHA2DS2-VASC, median (IQR) | 4.0 (3.0–5.0) | 5.0 (4.0–6.0) | <0.0001 |
| HAS-BLED, median (IQR) | 2.0 (1.0–2.0) | 2.0 (2.0–3.0) | <0.0001 |
| ATRIA bleeding score, median (IQR) | 3.0 (1.0–4.0) | 4.0 (3.0–6.0) | <0.0001 |
IQR, interquartile range; hgb, haemoglobin; hct, haematocrit; GI, gastrointestinal; CHF, congestive heart failure; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
aPer cent or value.
**P-values from chi-squared tests for categorical variables and Kruskal–Wallis tests for continuous variables.
The full continuous ORBIT bleeding model included the following independent predictors of major bleeding: antiplatelet therapy (aspirin, ticagrelor, prasugrel, clopidogrel, fixed dose combination aspirin-dipyridamole), prior bleeding (any history of gastrointestinal, intracranial or haemorrhagic stroke documented at the baseline study visit), age, estimated glomerular filtration rate (eGFR), haematocrit, history of CHF, history of cancer, COPD, history of anaemia/abnormal haemoglobin (<13 mg/dL for males and <12 mg/dL for females) or haematocrit (<40% for males and <36% for females), history of hip fracture or osteoporosis, and smoking status (recent/former or current). Following construction of the full model, we created a simple, five-factor numerical bleeding risk score from the five strongest predictors termed ORBIT (older (75 years or older); reduced haemoglobin (<13 mg/dL in men and <12 mg/dL in women), haematocrit (<40% in men and <36% in women) or history of anaemia; bleeding history; insufficient kidney function (eGFR < 60 mg/dL/1.73 m2); and treatment with an antiplatelet agent. We assigned point values based on the log scale according to the magnitude of modelling coefficient representing each variable's association with major bleeding.22 Reduced haemoglobin/haematocrit/history of anaemia and bleeding history received two points, and insufficient kidney function, treatment with antiplatelets, and older age received one point. Table 2 shows the associations between each score component and major bleeding risk in the multivariable model. Reduced haemoglobin/anaemia was most strongly associated with major bleeding, followed by bleeding history, treatment with antiplatelets, insufficient kidney function, and older age. As shown in Table 3, there was a broad distribution of ORBIT major bleeding risk scores among patients in the study population, with a bleeding risk score of 1 (22.9%) being the most common. Observed major bleeding rates increased with increasing risk score.
Table 2.
Association between outcomes registry for better informed treatment risk score components and major bleeding
| Variable | Hazard ratioa | 95% CI HR | Chi-square-value | Points |
|---|---|---|---|---|
| Older age | 1.38 | 1.17–1.61 | 15 | 1 |
| Reduced haemoglobin/Hct/anaemia | 2.07 | 1.74–2.47 | 66 | 2 |
| Bleeding history | 1.73 | 1.34–2.23 | 18 | 2 |
| Insufficient kidney function | 1.44 | 1.21–1.72 | 17 | 1 |
| Treatment with antiplatelets | 1.51 | 1.30–1.75 | 30 | 1 |
aOutcomes registry for better informed treatment bleeding risk score components (point value) = older than 74 (1), reduced haemoglobin/anaemia (2), bleeding history (2), insufficient kidney function (<60 mL/min/1.73 m2) (1), treatment with antiplatelet (1). Abnormal haemoglobin (<13 mg/dL for males and <12 mg/dL for females) or haematocrit (<40% for males and <36% for females).
Table 3.
Outcomes registry for better informed treatment bleeding risk score and observed major bleeding rates
| ORBIT bleeding score | Total number | Number of major bleeds | Patient-years | Bleeds per 100 patient-years (95% CI) | ORBIT bleeding score* category | Total number | Number of major bleeds | Patient-years | Bleeds per 100 patient-years |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1064 | 37 | 2154 | 1.7 (1.2–2.4) | Low (0–2) | 4341 | 206 | 8711 | 2.4 |
| 1 | 1701 | 79 | 3426 | 2.3 (1.9–2.9) | |||||
| 2 | 1576 | 90 | 3131 | 2.9 (2.3–3.5) | |||||
| 3 | 1351 | 123 | 2593 | 4.7 (4.0–5.6) | Medium (3) | 1351 | 123 | 2593 | 4.7 |
| 4 | 1038 | 130 | 1901 | 6.8 (5.8–8.1) | High (≥4) | 1719 | 252 | 3096 | 8.1 |
| 5 | 458 | 74 | 822 | 9.0 (7.2–11.2) | |||||
| 6 | 173 | 36 | 293 | 12.3 (9.0–16.7) | |||||
| 7 | 50 | 12 | 80 | 14.9 (8.9–25.3) | |||||
| Overall | 7411 | 581 | 14 399 | 4.0 |
*ORBIT bleeding risk score components (point value) = Older than 74 (1), Reduced hemoglobin/Anemia (2), Bleeding history (2), Insufficient kidney function [<60 ml/min/1.73 meters2] (1), Treatment with Antiplatelet (1). Abnormal hemoglobin (<13 mg/dL for males and <12 mg/dL for females) or hematocrit (<40% for males and <36% for females).
After classifying patients into low (ORBIT scores 0–2), medium (ORBIT score 3), and high (ORBIT score 4 or greater) categories, we compared bleeding rates within estimated risk groups. The largest proportion of patients were classified as low bleeding risk (58.6%), followed by high risk (23.2%), and medium risk (18.2%). Observed bleeding rates per 100 patient-years increased with increasing risk group, from 2.4 in the low-risk group, to 4.7 in the medium risk group, and 8.1 in the high-risk group (Table 3).
Table 4 displays the discrimination of each of the four models (full continuous ORBIT model, the five-item ORBIT score, HAS-BLED, and ATRIA) in the ORBIT-AF cohort and the ROCKET-AF trial population. In the ORBIT-AF cohort, the full continuous ORBIT model showed the best discrimination, followed by the simple ORBIT score, the ATRIA score, and the HAS-BLED score. In a sensitivity analysis, the five-item ORBIT score showed good performance for prediction of ICH (C-index = 0.69; 95% CI = 0.63, 0.74). In a second sensitivity analysis restricting the population to patients taking warfarin for <6 months, ORBIT score performance was similar (0.65; 0.57, 0.74) to that observed in the overall population.
Table 4.
C-statistics* (95% confidence intervals) for score discrimination by study cohort
| Score | ORBIT-AF cohort | ROCKET-AF cohort | Risk categories |
|---|---|---|---|
| Full continuous model | 0.69 (0.67, 0.72) | 0.63 (0.61, 0.65) | – |
| ORBIT score | 0.67 (0.64, 0.69) | 0.62 (0.60, 0.64) | 0–7 |
| HAS-BLED score | 0.64 (0.62, 0.67) | 0.59 (0.57, 0.61) | 0–9 |
| ATRIA score | 0.66 (0.63, 0.68) | 0.60 (0.58, 0.62) | 0–10 |
*C-index is calculated at 2 years.
We used the ROCKET-AF study as a validation sample. Over 21 769 person-years of follow-up (median = 1.9 years), 772 major bleeds occurred in ROCKET-AF (3.5/100 person-years). Discrimination was slightly reduced for all scores when assessed in the ROCKET-AF trial population than seen in the ORBIT-AF community-based cohort. However, predictive accuracy patterns for the various scores were similar in ROCKET-AF population as seen in the ORBIT-AF population. The highest discrimination was seen with the full continuous ORBIT model, followed by the simple ORBIT score, ATRIA and HAS-BLED.
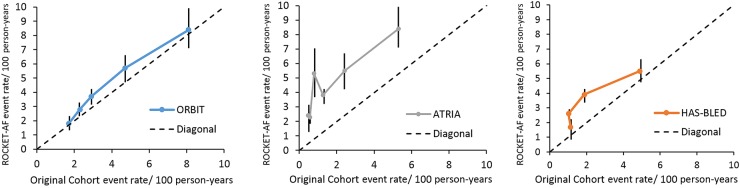
Results from the model calibration analysis comparing observed bleeding rates in ROCKET-AF with reported bleeding rates in the original derivation populations for ORBIT, HAS-BLED, and ATRIA scores are displayed in Figure 1. The ORBIT score displayed superior calibration compared with the remaining two scores, followed by HAS-BLED and ATRIA. The HAS-BLED score showed relatively poor calibration for low-risk score strata. The ATRIA score showed poor calibration for most risk groups.
Figure 1.
Calibration plot of outcomes registry for better informed treatment, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly, and anticoagulation and risk factors in atrial fibrillation in the rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation external validation cohort. This figure displays the major bleeding events rates per 100 patient-years and 95% confidence intervals observed in the external validation rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation cohort vs. those previously published from the original derivation cohorts for each discrete score point value. The highest risk categories for each score were combined to promote stable estimates as follows: outcomes registry for better informed treatment (0, 1, 2, 3, ≥4), anticoagulation and risk factors in atrial fibrillation (0, 1, 2, 3, ≥4), and hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly (0, 1, 2, ≥3). ORBIT-AF; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; ROCKET-AF, Rivaroxaban Once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation; ATRIA, anticoagulation and risk factors in atrial fibrillation.
Discussion
Physician concerns about major bleeding represent a key barrier to optimal anticoagulation use in AF.23 However, prior studies have clearly demonstrated that physician estimates of bleeding risk tend to be inaccurate and lower than existing scores.24 Using the ORBIT-AF registry community-based population, we identified factors associated with major bleeding on OAC and created a simple five-factor risk score with the acronym ORBIT. The predictive accuracy of the novel risk score performed well relative to two other major bleeding models (HAS-BLED and ATRIA) in an external clinical trial population, ROCKET-AF. Combined, we believe the ORBIT bleeding risk score could have application as a simple aid to clinical decision making in routine practice.
The recent introduction of simple tools for estimation of bleeding risk in AF has resulted in heightened interest in their comparative statistical performance and clinical utility. Two prior studies examined the prognostic utility of the two commonly used AF bleeding risk scores, ATRIA and HAS-BLED, in the warfarin14 and idraparinux arms25 of the Evaluating the Use of SR34006 Compared with Warfarin or Acenocoumarol in Patients With Atrial Fibrillation trial. In both studies, each score showed only modest discrimination (c-indices ∼0.6) for clinically relevant bleeding, major bleeding, or death. While study authors concluded that HAS-BLED showed superior performance for clinically relevant bleeding, predictive performance was similar across scores for the major bleeding endpoint. As has been noted previously,15 risk of clinically relevant bleeding has may have less impact on anticoagulation decisions than risk of major bleeding and may represent a less specific endpoint for evaluation of score performance than major bleeding events. In addition, AF clinical trial populations are highly selected, and may exclude patients at the upper end of the bleeding risk spectrum. In our analysis, all three risk scores showed better discriminative performance in ORBIT-AF than in ROCKET-AF, likely due to more stringent trial inclusion criteria that results in a more homogenous, less representative patient population. The ATRIA and HAS-BLED scores also showed poor calibration in ROCKET-AF, indicating these scores may have less predictive accuracy in more narrow patient populations.
Our results are similar to those from prior studies showing similar, but modest performance of bleeding risk scores in observational cohorts. In an analysis of 7156 patients diagnosed with non-valvular AF in a four-hospital institution, Lip and colleagues reported that discrimination was modest and similar across six bleeding risk scores, including ATRIA and HAS-BLED (C-index ≈0.6).26 The ATRIA and HAS-BLED scores also performed similarly in a small study of 937 patients in an outpatient anticoagulation clinic11 and in a recent meta-analysis, which reported low sensitivity for major bleeding events in high-risk categories for all three scores.12 The suboptimal performance observed when applying existing scores to external observational populations may in part stem from methodological limitations of the original derivation studies. The HAS-BLED score, for example, was derived based on only 53 major bleeding events, and a sizable proportion (25%) of information was missing on major bleeds during follow-up.9 Anticoagulation and risk factors in atrial fibrillation study authors noted that their score was based on limited covariates from a computerized database that lacked information on potentially important risk factors such as blood pressure and antiplatelet use.10 Additionally, the claims data used in the ATRIA study may be less sensitive for identifying major bleeds, which may in part explain the substantially higher event rates observed in ROCKET at every level of the ATRIA score. Existing scores are further limited by elements that may not be readily accessible by the practitioner. Complete calculation of HAS-BLED, for example, requires information on ‘labile INR’, which is difficult to measure and not relevant to patients taking novel oral anticoagulants or to those who have not had prior anticoagulation. The ORBIT score addresses the limitations of existing scores through inclusion of known risk factors for severe bleeding, elements that are relevant to all patients taking OAC, and derivation in a large, national, contemporary population of AF with a large number of major bleeding events.4,23,24
While bleeding risk estimation can be helpful in identifying high-risk AF patients for closer monitoring, it is important to note that prior work has demonstrated a net clinical benefit of OAC even in patients with high estimated bleeding risk.27,28 Further, while risk scores provide important information to the clinician for estimating risk of adverse events, they represent only one consideration relevant to therapeutic decision making. While several variables identified as risk factors for major bleeding in prior studies were either unavailable (labile INR) or not associated with major bleeding in this cohort (alcohol abuse), these may be important for the provider to consider on the individual patient level. Optimal treatment strategies incorporate patient preferences and values, which may differ from that of the physician. Results from a recent survey suggest that patients are prepared to sustain four major bleeds to avoid a single stroke.29 However, as highlighted in the European Society of Cardiology Consensus document on bleeding risk assessment,5 much of the existing evidence on patient preferences comes from small studies with heterogeneous methods. Further work is needed to describe patient preferences, perception of risk, and how the format of information provided influences these.
Limitations
Our study has several limitations. First, not all components of each score we evaluated were available in the ORBIT-AF or ROCKET-AF databases. However, lack of data availability also limits application of risk scores in clinical practice. Furthermore, HAS-BLED score authors have promoted the score's utility in non-VKA anticoagulated patients and others for whom INR data are not available.30 Second, while the ORBIT-AF study collects detailed clinical data on the majority of known risk factors for major bleeding, it is possible that other important risk factors exist and were not captured in our dataset. Third, while the ORBIT score includes the five risk factors that were most strongly associated with major bleeding in our study, additional factors may also be important in estimating patient-specific risk. Additionally, we validated the ORBIT score in a clinical trial population, which represents a selected patient population; validation in broader international populations to assess generalizability is warranted. Finally, ORBIT-AF participating sites were selected to be representative of the US national AF population; however, the cohort may not be fully representative of all community practice. As such, further external validation to assure the generalizability of the ORBIT risk score would be informative.
Conclusions
The ORBIT bleeding score is a novel, user-friendly score to estimate major bleeding risk among patients with AF and exhibits similar performance compared with a full predictive model. While ORBIT, HAS-BLED, and ATRIA scores showed similar discrimination in an external validation population, the ORBIT score showed superior calibration and is more widely applicable than existing bleeding scores.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
D.S., L.T.: performed statistical analysis. J.P., E.P.: handled funding and supervision. J.P., E.P., E.H., J.A., B.G., P.K., G.F., K.M., P.C.: acquired the data. E.O., D.S., L.T., E.H., J.A., B.G., P.K., G.F., K.M., P.C., M.P., E.P., J.P.: conceived and designed the research. E.O., D.S., L.T., J.P., E.P.: drafted the manuscript. E.O., D.S., L.T., E.H., J.A., B.G., P.K., G.F., K.M., M.P., E.P., J.P.: made critical revision of the manuscript for key intellectual content.
Funding
The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. This project was supported (in part) by funding from the Agency of Healthcare Research and Quality through cooperative agreement number 1U19 HS021092. E.C.O.: received research funding from Merck and served as a Consultant/Advisory Board member for Portola (modest). J.E.A.: received Consultant/Advisory Board fees from Bristol-Myers-Squibb, Pfizer, Janssen, Daiichi, Boehringer-Ingelheim, Alere (all modest). E.M.H.: a member of the Consultant/Advisory Boards (modest) for Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, Daiichi Sankyo, Janssen, Medtronic, Pfizer. P.R.K.: served as a Consultant/Advisory Board for Boehringer-Ingelheim, Bristol-Myers-Squibb, Johnson & Johnson, Portola, Merck, Sanofi, Daiichi Sankyo (all modest). B.G.: a member of the advisory board for Boston Scientific, Medtronic, Johnson and Johnson and St Jude Medical (both modest) and has served as an advisory board member for Ortho McNeil. G.C.F.: served as a Consultant/Advisory Board member for Ortho McNeil. K.W.M.: received research support from AstraZeneca, Amgen, Bayer, Boehringer-Ingleheim, Bristol-Myers-Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Portola, POZEN Pharmaceutical, Schering-Plough, and The Medicines Company, and has consulting agreements with Amgen, AstraZeneca, Glaxo SmithKline, Johnson & Johnson, and Merck. P.C.: an employee of Janssen. E.D.P.: received research support from Eli Lilly & Company and Janssen. J.P.P.: received research support from Boston Scientific Corporation and Janssen and consultancies to Forest Laboratories, Janssen, and Medtronic. Funding to pay the Open Access publication charges for this article was provided by Janssen Scientific Affairs, LLC.
Acknowledgements
The authors thank Daniel E. Singer, MD, for his valuable insight into the study design and manuscript revisions, and the ORBIT-AF Registry staff and participants for their important contributions to this work.
References
- 1.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009;151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Lane DA, Torp-Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost 2012;107:584–589. [DOI] [PubMed] [Google Scholar]
- 3.Man-Son-Hing M, Laupacis A, O'Connor AM, Biggs J, Drake E, Yetisir E, Hart RG. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA 1999;282:737–743. [DOI] [PubMed] [Google Scholar]
- 4.Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, Nagpal S, Cox JL. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lip GY, Andreotti F, Fauchier L, Huber K, Hylek E, Knight E, Lane D, Levi M, Marin F, Palareti G, Kirchhof P. Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] Working Group on Thrombosis. Thromb Haemost 2011;106:997–1011. [DOI] [PubMed] [Google Scholar]
- 6.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 7.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 8.Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology 2007;69:546–554. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 10.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roldan V, Marin F, Fernandez H, Manzano-Fernandez S, Gallego P, Valdes M, Vicente V, Lip GY. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a ‘real-world’ population with atrial fibrillation receiving anticoagulant therapy. Chest 2013;143:179–184. [DOI] [PubMed] [Google Scholar]
- 12.Caldeira D, Costa J, Fernandes RM, Pinto FJ, Ferreira JJ. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–284. [DOI] [PubMed] [Google Scholar]
- 13.Donze J, Rodondi N, Waeber G, Monney P, Cornuz J, Aujesky D. Scores to predict major bleeding risk during oral anticoagulation therapy: a prospective validation study. Am J Med 2012;125:1095–1102. [DOI] [PubMed] [Google Scholar]
- 14.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol 2012;60:861–867. [DOI] [PubMed] [Google Scholar]
- 15.Singer DE. Methodologic problems in the assessment of bleed scores. J Am Coll Cardiol 2013;61:481. [DOI] [PubMed] [Google Scholar]
- 16.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 17.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J 2011;162:606–612 e601. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 19.Enders CK. Applied Missing Data Analysis. New York: Guilford Press; 2010. [Google Scholar]
- 20.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 21.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 22.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 23.Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011;40:675–683. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg BA, Kim S, Thomas L, Fonarow GC, Hylek E, Ansell J, Go AS, Chang P, Kowey P, Gersh BJ, Mahaffey KW, Singer DE, Piccini JP, Peterson ED. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation 2014;129:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR 2 HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J Am Coll Cardiol 2013;61:386–387. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Banerjee A, Lagrenade I, Lane DA, Taillandier S, Fauchier L. Assessing the risk of bleeding in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation project. Circ Arrhythm Electrophysiol 2012;5:941–948. [DOI] [PubMed] [Google Scholar]
- 27.Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, Raunso J, Tolstrup JS, Hansen PR, Gislason GH, Torp-Pedersen C. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost 2011;106:739–749. [DOI] [PubMed] [Google Scholar]
- 28.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 29.Lahaye S, Regpala S, Lacombe S, Sharma M, Gibbens S, Ball D, Francis K. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost 2014;111:465–473. [DOI] [PubMed] [Google Scholar]
- 30.Lip GY, Larsen TB, Skjoth F, Rasmussen LH. Reply: to PMID 22575324. J Am Coll Cardiol 2013;61:596. [DOI] [PubMed] [Google Scholar]