Abstract
Heart failure (HF) and atrial fibrillation (AF) are two conditions that are likely to dominate the next 50 years of cardiovascular (CV) care. Both are increasingly prevalent and associated with high morbidity, mortality, and healthcare cost. They are closely inter-related with similar risk factors and shared pathophysiology. Patients with concomitant HF and AF suffer from even worse symptoms and poorer prognosis, yet evidence-based evaluation and management of this group of patients is lacking. In this review, we evaluate the common mechanisms for the development of AF in HF patients and vice versa, focusing on the evidence for potential treatment strategies. Recent data have suggested that these patients may respond differently than those with HF or AF alone. These results highlight the clear clinical need to identify and treat according to best evidence, in order to prevent adverse outcomes and reduce the huge burden that HF and AF are expected to have on global healthcare systems in the future. We propose an easy-to-use clinical mnemonic to aid the initial management of newly discovered concomitant HF and AF, the CAN-TREAT HFrEF + AF algorithm (Cardioversion if compromised; Anticoagulation unless contraindication; Normalize fluid balance; Target initial heart rate <110 b.p.m.; Renin–angiotensin–aldosterone modification; Early consideration of rhythm control; Advanced HF therapies; Treatment of other CV disease).
Keywords: Heart failure, Atrial fibrillation, Management, Review
Introduction
Heart failure (HF) and atrial fibrillation (AF) were predicted to become epidemics of the 21st century,1 in part due to increased longevity and the success in reducing overall cardiovascular (CV) mortality. Both conditions are increasingly prevalent, with spiralling cost to healthcare services globally.2–4 The incidence of AF is also predicted to double over the next 20 years,5,6 with expectations of 120–215 000 new cases per year by 2030 in Europe alone.7 Rates of HF in a global AF registry were 33% in paroxysmal, 44% in persistent and 56% in permanent AF.8 Thus, the combination of these two conditions will have a dramatic impact on healthcare and require a refocusing of CV care.
The pathophysiology and risk factors for HF and AF are closely aligned, and affected patients are usually elderly with a significant burden of comorbidity.8,9 Atrial fibrillation is both a cause and consequence of HF, with complex interactions leading to impairment of systolic and diastolic function not present in sinus rhythm. Atrial fibrillation is associated with a three-fold increased risk of incident HF.10 Vice versa, the structural and neurohormonal changes in HF make the development and progression of AF much more likely,11 both in heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF). Regardless of which comes first, patients with concomitant HF and AF have significantly worse prognosis.12,13 Given the poor outcomes associated with HF and AF, finding effective therapies for these patients is of paramount importance but also challenging—treatments shown to be effective in one or other of these conditions alone have also been observed to have efficacy or safety concerns in patients with HF and AF combined.14,15 In this review, we summarize the mechanisms and inter-relationship of HF and AF, and provide a state-of-the-art synopsis on optimal management, considering how best to combine therapies and the evidence-base supporting their use.
Mechanisms and pathophysiology of atrial fibrillation in heart failure
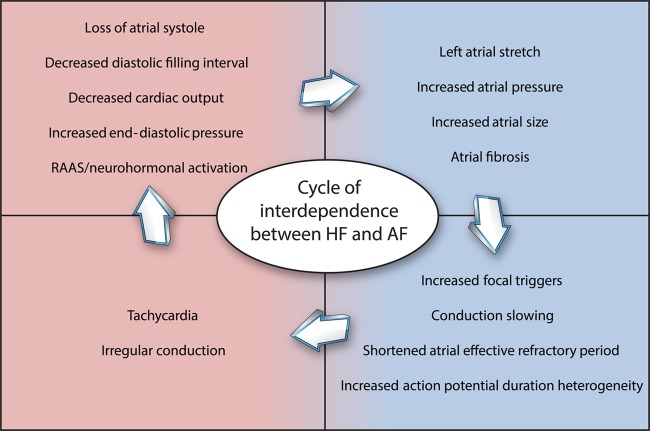
Heart failure and AF share risk factors and common pathophysiologic processes (see Figure 1). Hypertension, smoking, obesity, diabetes, renal impairment, sleep apnoea, and coronary artery disease are all associated with an increased risk of developing both HF and AF.16 In HF, neurohormonal imbalance and activation of the renin–angiotensin–aldosterone system (RAAS) leads to maladaptive physiological changes including increased filling pressures and afterload. These can lead to increased left atrial stretch and fibrosis, contributing to the development of conduction abnormalities and facilitating the initiation and maintenance of AF.17–21 The renin–angiotensin–aldosterone system also directly contributes to proarrhythmic remodelling, with angiotensin II causing atrial fibrosis and anisotropic conduction.22 Patients with HF also demonstrate altered calcium handling and calcium overload, which can lead to after-depolarizations and arrhythmia.23
Figure 1.
Shared and synergistic mechanisms in heart failure and atrial fibrillation. There is a cycle of interdependence between heart failure and atrial fibrillation and each makes the other more likely to occur. RAAS, renin–angiotensin–aldosterone system.
Atrial fibrillation can promote the development of HF by a number of established mechanisms. Loss of atrial systole in AF impairs LV filling and can decrease cardiac output by up to 25%, particularly in patients with diastolic dysfunction.24 Irregular and/or rapid ventricular conduction in AF can lead to LV dysfunction and in some patients, a tachycardia-induced cardiomyopathy.24,25 Restoration of sinus rhythm increases stroke volume and LV emptying even before contractility improves,26 explaining why some patients with HF gain rapid haemodynamic improvement with cardioversion.
Prevention of atrial fibrillation in heart failure (and heart failure in atrial fibrillation)
In the Framingham study, 41% of patients with AF and HF developed HF first, 38% developed AF first, and in the remaining 21% AF and HF occurred at the same time.12 While there are no therapies proven to prevent the risk of incident HF in patients with established AF, the treatment of modifiable CV risk factors (especially hypertension), effective rate control and the diagnosis and treatment of associated comorbidities (e.g. sleep apnoea) would seem to be sensible interventions.
What about preventing AF in patients with known HF? Meta-analysis of RCTs suggests that angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) reduce the risk of incident AF,27 with RR of 0.79 (95% CI 0.62–1.00) and 0.78 (95% CI 0.66–0.92), respectively.28 Data from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity program (CHARM) show that ARBs can decrease the risk of new-onset AF in patients with HFrEF and HFpEF.29 In the β-blocker vs. placebo trials in HFrEF with baseline sinus rhythm, allocation to β-blockers was associated with a significant reduction in the adjusted odds of incident AF (odds ratio 0.67; 95% CI 0.57–0.79).14 In a small analysis pointing towards the potential benefits of personalized therapy in patients with HF and AF,30 HF patients who were homozygotes for β1 adrenergic receptor 389 Arginine had a 74% reduction in new-onset AF (95% CI 43–88%) when treated with bucindolol vs. placebo.31
Management of concomitant heart failure and reduced ejection fraction and atrial fibrillation
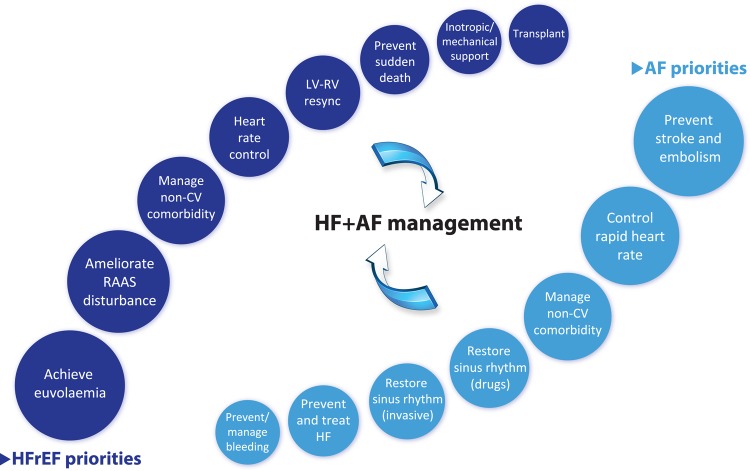
Currently, clinicians often manage patients with combined HFrEF and AF by focusing on particular therapeutic aspects that have an evidence-base in one or other of these conditions (see Figure 2). Researchers have started to investigate if treatment efficacy differs in patients with concomitant disease, but at present these data are limited. In this section, we summarize the evidence-base for common treatment modalities and suggest a simple clinical mnemonic for the initial management of newly diagnosed concomitant HF and AF. The CAN-TREAT HFrEF + AF algorithm (see Figure 3) distinguishes the management of these patients from those with sinus rhythm. The presence of haemodynamic instability should be treated with urgent cardioversion (C). Anticoagulation (A) should be instituted to prevent thromboembolism, and diuretic therapy to normalize (N) fluid balance and reduce symptoms of HF. Subsequent therapy should target (T) an initial heart rate <110 b.p.m. and initiate RAAS antagonism (R), though with limited data on efficacy (see details below). Early (E) rhythm control in patients with symptoms refractory to rate control, and consideration of advanced (A) HF therapies should follow (e.g. cardiac resynchronization therapy), with aggressive treatment (T) of other concomitant CV disease, particularly ischaemia and hypertension.
Figure 2.
Major priorities of management in patients with heart failure and reduced ejection fraction and those with atrial fibrillation. HFrEF, heart failure with reduced ejection fraction; AF, atrial fibrillation; CV, cardiovascular; HF, heart failure; LV, left ventricle; RV, right ventricle.
Figure 3.
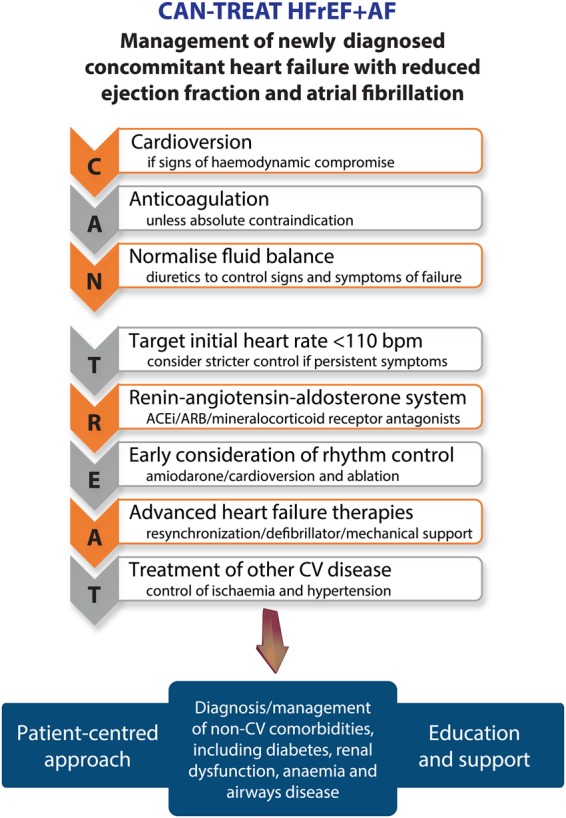
CAN-TREAT initial management algorithm for patients with newly identified heart failure and reduced ejection fraction and atrial fibrillation. ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CV, cardiovascular.
Anticoagulation
Stroke is the most feared complication of AF, most commonly due to embolization of thrombus from the left atrial appendage. Thrombus formation in AF fulfils Virchow's triad of a prothrombotic milieu, stagnant blood flow and endothelial dysfunction. Anticoagulation with vitamin K antagonists (VKA; e.g. warfarin) or non-VKA anticoagulants (NOACs) prevent ∼2/3 of ischaemic strokes in AF patients.32,33 Reduced left ventricular ejection fraction (LVEF) is independently associated with stroke,34 and the combination of HFrEF with AF doubles the risk of stroke compared with AF alone.35 Although no trials have investigated this specific population, indirect sub-group data from the NOAC RCTs suggest the effect of anticoagulation for AF is similar in patients with concomitant HF.36–40 With the combination of higher stroke risk and effective therapy, anticoagulation is essential in all patients with HF and AF that do not have an absolute contraindication, and the NOACs are particularly attractive due to lower rates of intracranial haemorrhage compared to VKA therapy.33
Guideline-recommended heart failure and reduced ejection fraction therapy
Achieving euvolaemia and the resolution of HF symptoms using loop and thiazide diuretics are an important first step in the management of all HF patients, regardless of heart rhythm.
Activation of neurohormonal pathways and RAAS are well described in HF, and the majority of evidence-based therapies target these compensatory mechanisms.41 Angiotensin converting enzyme inhibitors have proven efficacy in HFrEF for significant reduction in mortality, sudden cardiac death, and HF hospitalization,42 but no trials have examined their benefit in concomitant AF. Angiotensin receptor blockers are recommended as alternatives to ACEi in cases of intolerance, and there are numerous RCTs supporting their use in HFrEF.41 In CHARM, randomization to candesartan significantly reduced CV death or HF hospitalization in HFrEF patients with concomitant AF (HR 0.83; 95% CI 0.69–0.99), similar to that observed in patients without AF at baseline (HR 0.84; 95% 0.77–0.92).43 In contrast, irbesartan did not reduce the composite outcome of hospitalization due to HF, stroke, myocardial infarction, or death from vascular causes in AF patients enrolled in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events A or W trials. For those with a history of HF, the HR was 0.90 comparing irbesartan to placebo (95% CI 0.75–1.08).44 It should be pointed out that both of these results were post hoc defined sub-group analyses.
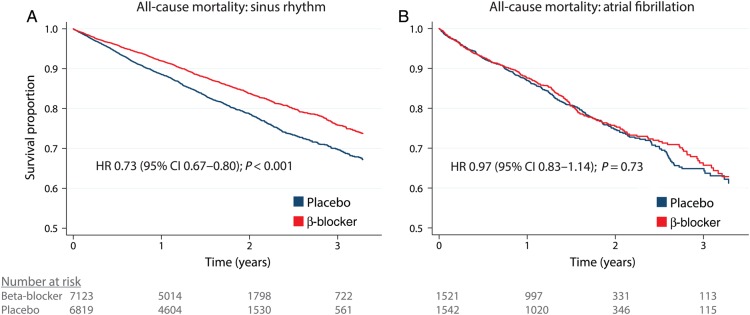
β-Blockers are now a standardized part of treatment in HFrEF following numerous RCTs describing a substantial reduction in all-cause mortality, CV death and hospitalization compared with placebo. In these trials, between 8 and 23% of enrolled participants were in AF at baseline.14 Pooling individual patient data from 11 RCTs (with 96% of recruited participants ever enrolled in such trials), the adjusted HR for all-cause mortality for β-blockers vs. placebo was 0.73 (95% CI 0.67–0.80) in sinus rhythm. In patients with AF the HR was 0.97 (95% CI 0.83–1.14), with the interaction P-value for baseline rhythm highly significant at 0.002 with no heterogeneity (see Figure 4).14 The lack of benefit in AF was consistent across all sub-groups, including age categories, gender, NYHA class, and baseline heart rate. There were also no significant reductions in a range of secondary outcomes despite a sample size of over 3000 patients, including CV hospitalization and composite clinical outcomes.14 Again, these data are based on sub-group analysis and the patients with AF differed to those in sinus rhythm. However, pooling individual patient data allows more robust handling and statistical analysis, as well as sufficient power.45 Hence clinicians should not expect a prognostic benefit from β-blockers in HFrEF patients with concomitant AF; however, there was no apparent harm and patients may have other indications, such as symptom or heart rate control. We are currently exploring whether this variance in efficacy is due to heart rate, LVEF, or other fundamental differences in how AF patients respond to β-blockers.46
Figure 4.
β-Blockers in heart failure and reduced ejection fraction with sinus rhythm and atrial fibrillation. Kaplan–Meier survival curves for β-blocker vs. placebo in heart failure patients with (A) sinus rhythm and (B) atrial fibrillation. Data are unadjusted survival curves for all reported deaths. Hazard ratios are derived from an adjusted one-stage Cox regression model, stratified by study and censored at 1200 days (3.3 years). Reproduced from Kotecha et al.14
Mineralocorticoid receptor antagonists (MRAs), such as spironolactone and eplerenone, are recommended in all HFrEF patients with persisting symptoms (NYHA classes II–IV) after treatment with ACEi and β-blockers.41 Although the majority of data on MRA are positive, in a post hoc analysis of the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial evaluating rate and rhythm-control strategies, spironolactone was associated with increased mortality (HR 1.4, 95% CI 1.1–1.8).47 Despite a propensity-matched statistical model, it is not possible to exclude residual confounding as an explanation for this unexpected finding (i.e. sicker patients receiving MRA). Baseline AF was not reported in the Randomized Aldactone Evaluation Study of spironolactone vs. placebo.48 In the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure trial, the reduction in CV death or HF hospitalization was similar for HFrEF patients with or without a history of AF (P for interaction 0.59).49
To summarize, there are scarce data on the efficacy of ACEi, ARBs, or MRA in HFrEF with concomitant AF to decrease morbidity or mortality; however, their use is still recommended to reduce adverse remodelling in HF. The totality of RCT data on β-blockers in HFrEF patients with AF have now been analysed, and suggest that β-blockers have a neutral effect on death and hospitalization in these patients.
Rate vs. rhythm control of atrial fibrillation
Although sub-group data suggest that sinus rhythm is associated with improved outcomes in patients with AF (including all-cause survival),50 clinical trials have failed to demonstrate superiority of either a rate or rhythm-control strategy. For example in the AF-CHF trial, there was no difference in CV death when comparing a rate vs. rhythm-control strategy in patients with HFrEF and NYHA classes II–IV (HR 1.06, 95% CI 0.86–1.30, P = 0.59), with similar findings for all-cause mortality and worsening HF.51 There are several reasons that rhythm control has failed to improve survival in clinical trials, including limited efficacy and adverse effects of available treatments, or delayed intervention such that the cumulative effects of AF are already unable to be reversed. Sinus rhythm can be difficult to achieve and maintain, particularly in patients with HF. In the rhythm control arm of AF-CHF, 21% crossed over to rate control, 82% were taking amiodarone, 27% were in AF at 4-year follow-up, and 58% had at least one episode of AF during the trial.51 On the other hand, in studies of catheter ablation of AF, restoration of sinus rhythm is associated with significant improvement in left ventricular function (11% increase in LVEF on average).52 While there are no clear differences in CV outcomes, patients with AF and HF who spend a higher proportion of time in sinus rhythm suffer less severe functional impairment (NYHA class III symptoms in 27 vs. 35%, P < 0.0001).53 Based on these and other data, current guidelines reserve rhythm-control therapy for those patients who experience AF-related symptoms or worsening HF despite adequate rate control.54
Specific rate-control therapies
The three available therapies for rate control of AF in the context of HFrEF are discussed below and summarized in Table 1.
Table 1.
Rate control of atrial fibrillation in heart failure with reduced ejection fraction
| Guidelines | Agent | Safety | Efficacy |
|---|---|---|---|
| Recommended | β-Blockers | Individual patient data sub-group meta-analysis of RCTs suggests no safety concerns.14 | Individual patient data sub-group meta-analysis of RCTs shows no impact on mortality or hospitalization in concomitant HFrEF and AF.14 |
| Recommended as second line | Digoxin | Systematic review suggests no increase in mortality in concomitant HF and AF; higher mortality in AF patients in observational studies is likely due to residual confounding.55 | No RCTs vs. placebo in AF patients;55 combined therapy with β-blockers improves LVEF.56 |
| Avoid/use caution | Non-dihydropyridine calcium channel blockers | Limited sub-group data in post-MI patients only; suggestive of increased death, re-infarction, and HF.57 | None demonstrated.58 |
β-Blockers
As previously discussed, β-blockers in HFrEF patients with AF do not appear to improve mortality or reduce hospital admissions.14 However, their use is widespread for control of heart rate in AF, both acutely and for long-term management. In the acute setting of HF with rapid AF, β-blockers are useful for rate-reduction and preferred to digoxin due to their effectiveness at high sympathetic tone.59 As β-blockers can initially be negatively inotropic, initiation of β-blockers requires a measured approach using incremental dosage to achieve a heart rate that balances the need for rate control with other haemodynamic parameters. For long-term control of heart rate, β-blockers are traditionally the first-line choice for clinicians.60
Digoxin
Cardiac glycosides, such as digoxin and digitoxin, have seen recent declines in use after the publication of the DIG trial which showed no mortality benefit from digoxin in HF patients with sinus rhythm.61,62 Importantly, patients randomized to digoxin suffered less hospitalizations. In observational studies and post hoc analysis of RCTs, there have been concerns about increased mortality with digoxin,63 but equally a number of studies have found no association.64–67 As clearly demonstrated in a systematic review of all digoxin vs. control studies, the main problem with non-randomized assessment is that clinicians are more likely to prescribe digoxin to the sickest patients with HF and/or AF, which results in bias that cannot be adjusted for, even with complex statistical modelling.55 Unfortunately, there are currently no direct RCT comparisons of digoxin use in patients with AF. Until further evidence becomes available, digoxin should be used cautiously in appropriate patients, with no expectation of any effect on mortality.55 In a crossover mechanistic RCT of 47 patients with HFrEF and AF, there were no differences in heart rate, blood pressure, walk distance, or LVEF comparing carvedilol and digoxin, although β-blockers did result in higher BNP levels (183 pg/mL with carvedilol vs. 79.5 with digoxin; P = 0.03). There was a small and marginally significant improvement in LVEF with combination β-blocker/digoxin compared with placebo/digoxin after 4 months of treatment (30.6 ± 9.6% vs. 26.0 ± 12.4%; P = 0.048).56
Calcium channel blockers
Non-dihydropyridine calcium channel blockers (verapamil or diltiazem) are not recommended in patients with significantly impaired left ventricular function due to their negative inotropic effects, although specific data are limited.41,68 In the Multicenter Diltiazem Postinfarction Trial, patients were randomized to diltiazem or placebo 3–15 days after the onset of myocardial infarction.69 HF patients, including 490 with evidence of pulmonary congestion, had an increase in the composite of cardiac death or non-fatal re-infarction (HR 1.41, 95% CI 1.01–1.96). In subsequent analysis, diltiazem was found to increase late-onset HF in those with LVEF <40%.57 Verapamil did not improve outcomes after myocardial infarction in patients who developed HF in the Danish Verapamil Infarction Trials.58
Heart rate targets for atrial fibrillation in the context of heart failure and reduced ejection fraction
Following the publication of the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) trial, patients with AF can initially be treated to a more lenient heart rate regime (<110 beats/min resting heart rate). To summarize, 614 patients with permanent AF were randomized to a heart rate <80 b.p.m. at rest and <110 b.p.m. during moderate exercise or lenient control, with results showing a similar rate of composite clinical events in each arm.70 There were also no differences in functional outcomes, hospital admissions, or symptoms.71 Fifteen percent of the population had LVEF <40% but it remains unclear if these targets apply to AF patients with HFrEF. However, these findings are consistent with other results14,72,73 and would suggest that heart rate in AF is more a marker of disease than a therapeutic target and that a lenient heart rate may be acceptable if symptoms are controlled and tachycardia is avoided. It is worth noting that other guideline recommendations continue to advocate a resting heart rate <80 b.p.m. in patients with symptomatic AF and left ventricular dysfunction.74
Specific rhythm-control strategies
Cardioversion
The first step in rhythm control is the restoration of sinus rhythm, which often requires cardioversion. Urgent cardioversion is recommended in any patient with significant haemodynamic impairment secondary to AF. Elective cardioversion is indicated in individuals with symptomatic persistent AF. Unfortunately, recurrence of AF after successful cardioversion is a frequent problem (∼50% at 6 months), particularly in patients with HF.75,76 Over half of inpatients undergoing cardioversion for atrial arrhythmias have HF.77
Antiarrhythmic drugs
Antiarrhythmic drugs (AAD) for the maintenance of sinus rhythm in patients with AF and HFrEF are limited to dofetilide or amiodarone; however, dofetilide is not approved in Europe. Both drugs have associated safety concerns (see Table 2). Other AAD are not recommended for general use in HFrEF. When antiarrhythmic medications are used to treat AF in patients with HF, every effort should be made to avoid toxicity.
Table 2.
Antiarrhythmic drug therapy for atrial fibrillation in heart failure
| Guidelines | Agent | Class | Safety | Efficacy |
|---|---|---|---|---|
| Recommended | Amiodarone | Mixed channel blockade | Risks of toxicity, including thyroid, hepatic, pulmonary, and neurological.78 | Superior efficacy for maintenance of sinus rhythm vs. placebo: odds ratio 0.15 (95% CI 0.10–0.22).79 |
| Dofetilide | III | Requires inpatient stay for loading. Risk of torsades 0.8–3.3%. Not approved in EU. | Lower risk of all-cause rehospitalization in patients with AF at baseline vs. placebo: relative risk 0.70 (95% CI 0.56–0.89).80 | |
| Caution required | Dronedarone | Mixed channel blockade | Increased mortality in patients with HF and permanent AF.15,81 | Decreased risk of CV hospitalization or death in patients with AF and no recent HF decompensation vs. placebo: 0.76 (95% CI 0.69–0.84).82 |
| Sotalol | III | Concern for excess proarrhythmia in patients with acute myocardial infarction or LVEF ≤40%: relative risk 1.65 (95% CI 1.15–2.36) for all-cause mortality.83a | Sotalol was inferior to amiodarone in patients with AF (28% had NYHA class I/II HF).84 | |
| Contraindicated | Flecainide and Propafenone | I | Flecainide, encainide and moracizine increased mortality in patients with myocardial infarction.85 Propafenone can precipitate decompensated HF, particularly in CYP 2D6 slow-metabolizers. |
aSWORD evaluated d-sotalol rather than d,l-sotalol.
Catheter ablation
Catheter ablation has been shown to significantly improve freedom from AF in patients who have failed AAD,86 and avoids their toxicity. Accordingly, the use of catheter ablation has increased in clinical practice.87 Observational data suggest that in patients with AF and HF, LVEF improves by 11.1% after ablation (95% CI 7.1–15.2).52 Higher recurrence rates of AF are seen after ablation in patients with HF,88 leading to a need for additional ablation procedures.89,90 The PABA-CHF pilot study compared a rate-control approach with atrioventricular (AV) node ablation and cardiac resynchronization therapy (CRT), vs. catheter ablation in 81 patients with drug-refractory AF and mild-to-moderate HF.91 In the ablation group, 71% maintained sinus rhythm without AAD. The patients randomized to ablation had better HF-related quality of life, better 6-min walk times, and greater improvement in LVEF. Similar findings were reported in the ARC-HF trial of 52 HFrEF patients randomized to ablation or rate control with β-blockers/digoxin.92 More recently, pre-publication results from the Ablation vs. Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD trial suggest promising results for catheter ablation in this patient group, including greater maintenance of sinus rhythm.93 While there is debate as to which ablative approach is most effective for restoring sinus rhythm in patients with persistent forms of AF, the Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II demonstrated that neither additional linear ablation nor ablation of complex fractionated electrograms improves efficacy.94 Larger, more definitive trials are underway to help clarify whether ablation leads to improved CV outcomes in patients with AF and HF.
Recognizing the limitations of percutaneous ablation in patients with more advanced forms of AF, there are emerging alternative approaches to catheter ablation, including both surgical ablation and hybrid ablation. In the ‘convergent’ procedure, a surgical transdiaphragmatic endoscopic approach is used to make non-contiguous epicardial ablation lesions. These lesions are later completed with catheter ablation to produce complete pulmonary vein isolation. In one study of 101 patients, in which 30% had symptomatic HF, freedom from AF was 66% at 1 year with a major complication rate of 6%.95 More traditional surgical ablation, like the Cox-Maze procedure, also has a role, particularly when HF patients with symptomatic AF are undergoing surgery for valvular disease or revascularization.96 Preliminary data in patients with concomitant HF suggests that Cox-Maze procedures may be effective and safe in those with LVEF <40% and symptomatic HF, with sinus rhythm maintained in >80% at 6-month follow-up.97
Device therapy and management of advanced heart failure
Cardiac resynchronization therapy decreases mortality and prevents hospitalizations in patients with symptomatic HF, LVEF ≤35%, and QRS duration ≥120 ms.98 Between 25 and 50% of patients eligible for CRT have AF, although patients with AF are not well represented in randomized trials of CRT.99 At present, CRT is a class IIa recommended therapy in patients with HF and concomitant AF.100,101 Loss of AV synchrony and rapid ventricular rates in AF may impair benefit from CRT and small studies have suggested that the beneficial effect is rather due to AV node ablation.102,103 A meta-analysis of 23 observational studies including 7495 recipients suggests that patients with AF have a higher rate of non-response to CRT (35 vs. 28%, P = 0.001).102 Recent data from 8951 patients with AF and HF in the US NCDR registry demonstrated that CRT-D therapy was associated with lower mortality, all-cause, and HF readmissions compared with ICD therapy alone.104 Thus while response rates are lower in patients with AF, CRT should still be pursued in appropriate patients and every attempt should be made to ensure aggressive rate control. This is important to ensure biventricular pacing is as close to 100% as possible105,106 and avoid inappropriate shocks. AV node ablation should be considered in cases of tachycardia refractory to medical therapy.
Patients with HFrEF and AF often present unique challenges when implanted pump-support is required. Increased hospitalization and mortality have been observed in patients with the HeartMate II left ventricular assist device (adjusted HR for persistent AF 3.54; 95% CI 1.52–8.25; P < 0.01), with more frequent thromboembolic events in AF despite higher INR.107
Tachycardiomyopathy
Tachycardia-induced cardiomyopathy is a long-recognized complication of AF, affecting as few as 3% and as many as 25% of patients with atrial tachyarrhythmias.25,108 Several mechanisms have been proposed to contribute to tachycardia-induced cardiomyopathy, including decreased density of l-type calcium channels and β-adrenergic receptors, increased intracellular calcium and diastolic contracture, impaired myocardial blood flow due to raised left ventricular diastolic pressure, oxidative stress, and even deleterious polymorphisms in angiotensin converting enzyme.109
The diagnosis should be considered in a patient with no prior CV history who presents with new-onset HF in the setting of AF with rapid ventricular conduction. When evaluating patients with AF and left ventricular dysfunction, it is paramount to exclude other underlying causes of ventricular dysfunction, including ischaemia. Once ischaemia has been ruled out, other indicators of non-ischaemic cardiomyopathy should also assessed (e.g. left ventricular hypertrophy, alcohol/drug use, infiltrative disorders, etc.). It is important to emphasize that there are no established diagnostic criteria for tachycardia-induced cardiomyopathy and the diagnosis can be elusive in the majority of patients with established CV disease or when the initial presentation is missed.109
Once anticoagulation has been initiated and the risk of thrombus has been addressed, sinus rhythm should be restored with cardioversion. Several methods can be used to maintain sinus rhythm; short-term amiodarone (3 months) is often helpful and allows for recovery before deploying a more durable treatment modality such as catheter ablation. Recovery of ventricular function confirms the diagnosis and may take up to 6 weeks.110 Patients with tachycardia-induced cardiomyopathy have similar outcomes following catheter ablation compared with patients without structural heart disease.111
Heart failure with preserved ejection fraction and atrial fibrillation
Heart failure with preserved ejection fraction is common, responsible for over half of prevalent HF, yet no therapies have yet been shown to reduce mortality or morbidity.41 Atrial fibrillation and HFpEF are closely linked, with similar risks and mechanisms as discussed above.112 Current management is no different to that in sinus rhythm; diuretics to reduce signs and symptoms of fluid overload, and optimisation of hypertension and other comorbidities. Whether MRA have a specific role in improving exercise capacity and diastolic function by reducing fibrosis is currently under investigation.113 The risk of stroke in AF with HFpEF is similar to HFrEF, and therefore all suitable patients require anticoagulation.114
Future directions
Given the limited treatment options for patients with HF and AF, there is a clear unmet need in this important patient population. Future investigation is particularly required for rate control, optimal methods of rhythm control, and prevention. There is also a need to stratify the use of various treatments to improve efficacy and safety, and a number of studies are addressing whether genetic profiling can help to personalize our therapeutic approach.
Despite advances in many areas of AF, it seems that the evidence to guide best practices for rate control is more uncertain than ever. Adequately powered prospective clinical trials are needed to clarify optimal rate-control targets and the best pharmacologic treatments to achieve rate control in patients with HF and AF. While ablation represents a promising alternative to AAD, clinicians require more information on the balance between effectiveness, complications, and potential prognostic benefits. Finally, given the poor outcomes observed in patients with HF and AF, perhaps the best treatment strategy is to prevent AF from occurring in the first place. Clinical trials of interventions targeted at left atrial substrate and preventing disease progression may have an important role in this regard. Table 3 presents some of the main studies currently recruiting that will assess patients with HF and AF. It is clear that the combination of these two common CV conditions will continue to challenge physicians both in CV and general medicine for many years to come.
Table 3.
Upcoming trials relating to heart failure and atrial fibrillation
| Trial | Objective | Status | Further information |
|---|---|---|---|
| CASTLE-AF | Catheter ablation for AF and HFrEF | Funded, recruiting | https://clinicaltrials.gov/ct2/show/NCT00643188 |
| RAFT AF | Rate vs. rhythm control for AF and HFrEF | Funded, recruiting | https://clinicaltrials.gov/ct2/show/NCT01420393 |
| GENETIC AF | Genetically targeted AF therapy in HF | Funded, recruiting | https://clinicaltrials.gov/ct2/show/NCT01970501 |
| IMPRESS-AF | Spironolactone in AF with HFpEF | Funded, recruiting | http://www.isrctn.com/ISRCTN10259346 |
| EAST | Early rhythm control for AF | Funded, recruiting | https://clinicaltrials.gov/ct2/show/NCT01288352 |
| CABANA | Early rhythm control for AF | Funded, recruiting | https://clinicaltrials.gov/ct2/show/NCT00911508 |
| CATCH ME | AF genetics and tissue profiling | Funded, recruiting | http://www.catch-me.info |
| DIGIT-HF | Digitoxin vs. placebo in HFrEF | Funded, recruiting | https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-005326-38/DE |
| RATE-AF | Digoxin vs. β-blockers in AF | Funded, not started | https://clinicaltrials.gov/ct2/show/NCT02391337 |
Funding
Funding to pay the Open Access publication charges for this article was provided by the University of Birmingham.
Conflict of interest: D.K. has received research grants from Menarini, professional development support from Daiitchi Sankyo and is the lead for the Beta-blockers in Heart Failure Collaborative Group (BB-meta-HF) and the RAte-control Therapy Evaluation in Atrial Fibrillation trial group (RATE-AF). J.P.P. receives funding for clinical research from ARCA Biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, ResMed, and St Jude Medical and serves as a consultant to Johnson & Johnson, Laguna Pharmaceuticals, Medtronic, and Spectranetics.
Acknowledgements
With thanks to Gregory YH Lip (Centre for Cardiovascular Sciences, University of Birmingham, UK), Paulus Kirchhof (Centre for Cardiovascular Sciences, University of Birmingham, UK), and Frank Ruschitzka (Cardiovascular Division, Heart Failure/Heart Transplant Unit, University Hospital Zurich, Switzerland).
References
- 1.Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997;337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 2.Wodchis WP, Bhatia RS, Leblanc K, Meshkat N, Morra D. A review of the cost of atrial fibrillation. Value Health 2012;15:240–248. [DOI] [PubMed] [Google Scholar]
- 3.Guha K, McDonagh T. Heart failure epidemiology: European perspective. Curr Cardiol Rev 2013;9:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace 2011;13(Suppl. 2):ii13–ii17. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 7.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O'Neill J, Silva-Cardoso J, Zharinov O, Gamra H, Alam S, Ponikowski P, Lewalter T, Rosenqvist M, Steg PG. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632–639. [DOI] [PubMed] [Google Scholar]
- 9.van Deursen VM, Urso R, Laroche C, Damman K, Dahlstrom U, Tavazzi L, Maggioni AP, Voors AA. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014;16:103–111. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Hart CL, Hole DJ, McMurray JJV. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 11.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D–8D. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 13.Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail 2009;11:676–683. [DOI] [PubMed] [Google Scholar]
- 14.Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD, Beta-Blockers in Heart Failure Collaborative Group. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 2014;384:2235–2243. [DOI] [PubMed] [Google Scholar]
- 15.Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 16.Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014;64:710–721. [DOI] [PubMed] [Google Scholar]
- 17.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation 2003;108:668–671. [DOI] [PubMed] [Google Scholar]
- 18.Lalani GG, Schricker A, Gibson M, Rostamian A, Krummen DE, Narayan SM. Atrial conduction slows immediately before the onset of human atrial fibrillation: a bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol 2012;59:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills RW, Narayan SM, McCulloch AD. Mechanisms of conduction slowing during myocardial stretch by ventricular volume loading in the rabbit. Am J Physiol Heart Circ Physiol 2008;295:H1270–H1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts-Thomson KC, Wilson L, De Sciscio P, Young GD, Sanders P. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the ‘second factor’. J Am Coll Cardiol 2009;53:1182–1191. [DOI] [PubMed] [Google Scholar]
- 21.Healey JS, Israel CW, Connolly SJ, Hohnloser SH, Nair GM, Divakaramenon S, Capucci A, Van Gelder IC, Lau CP, Gold MR, Carlson M, Themeles E, Morillo CA. Relevance of electrical remodeling in human atrial fibrillation: results of the asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and the atrial fibrillation reduction atrial pacing trial mechanisms of atrial fibrillation study. Circ Arrhythm Electrophysiol 2012;5:626–631. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 2001;104:2608–2614. [DOI] [PubMed] [Google Scholar]
- 23.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation 2011;123:2922–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deedwania PC, Lardizabal JA. Atrial fibrillation in heart failure: a comprehensive review. Am J Med 2010;123:198–204. [DOI] [PubMed] [Google Scholar]
- 25.Nerheim P, Birger-Botkin S, Piracha L, Olshansky B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation 2004;110:247–252. [DOI] [PubMed] [Google Scholar]
- 26.Raymond RJ, Lee AJ, Messineo FC, Manning WJ, Silverman DI. Cardiac performance early after cardioversion from atrial fibrillation. Am Heart J 1998;136:435–442. [DOI] [PubMed] [Google Scholar]
- 27.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 28.Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 29.Ducharme A, Swedberg K, Pfeffer MA, Cohen-Solal A, Granger CB, Maggioni AP, Michelson EL, McMurray JJ, Olsson L, Rouleau JL, Young JB, Olofsson B, Puu M, Yusuf S, Investigators C. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J 2006;152:86–92. [PubMed] [Google Scholar]
- 30.Kirchhof P, Sipido KR, Cowie MR, Eschenhagen T, Fox KA, Katus H, Schroeder S, Schunkert H, Priori S. The continuum of personalized cardiovascular medicine: a position paper of the European Society of Cardiology. Eur Heart J 2014;35:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aleong RG, Sauer WH, Davis G, Murphy GA, Port JD, Anand IS, Fiuzat M, O'Connor CM, Abraham WT, Liggett SB, Bristow MR. Prevention of atrial fibrillation by bucindolol is dependent on the Beta389 Arg/Gly adrenergic receptor polymorphism. JACC Heart Fail 2013;1:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 33.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 34.Hays AG, Sacco RL, Rundek T, Sciacca RR, Jin Z, Liu R, Homma S, Di Tullio MR. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal M, Apostolakis S, Lane DA, Lip GY. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin Ther 2014;36:1135–1144. [DOI] [PubMed] [Google Scholar]
- 36.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S, AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 37.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 38.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 39.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 40.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 41.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira RKF, Ferreira EVM, Ramos RP, Messina CMS, Kapins CEB, Silva CMC, Ota-Arakaki JS. Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J Heart Lung Transplant 2014;33:157–162. [DOI] [PubMed] [Google Scholar]
- 43.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and Morbidity (CHARM) program. J Am Coll Cardiol 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 44.Active I Investigators Yusuf S, Healey JS, Pogue J, Chrolavicius S, Flather M, Hart RG, Hohnloser SH, Joyner CD, Pfeffer MA, Connolly SJ. Irbesartan in patients with atrial fibrillation. N Engl J Med 2011;364:928–938. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Ioannidis JA, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405–411. [DOI] [PubMed] [Google Scholar]
- 46.Kotecha D, Manzano L, Altman DG, Krum H, Erdem G, Williams N, Flather MD, Beta-Blockers in Heart Failure Collaborative Group. Individual patient data meta-analysis of beta-blockers in heart failure: rationale and design. Syst Rev 2013;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Meara E, Khairy P, Blanchet MC, de Denus S, Pedersen OD, Levesque S, Talajic M, Ducharme A, White M, Racine N, Rouleau JL, Tardif JC, Roy D. Mineralocorticoid receptor antagonists and cardiovascular mortality in patients with atrial fibrillation and left ventricular dysfunction: insights from the Atrial Fibrillation and Congestive Heart Failure Trial. Circ Heart Fail 2012;5:586–593. [DOI] [PubMed] [Google Scholar]
- 48.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 49.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 50.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG, AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 51.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL, Atrial F, Congestive Heart Failure I. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 52.Dagres N, Varounis C, Gaspar T, Piorkowski C, Eitel C, Iliodromitis EK, Lekakis JP, Flevari P, Simeonidou E, Rallidis LS, Tsougos E, Hindricks G, Sommer P, Anastasiou-Nana M. Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta-analysis. J Card Fail 2011;17:964–970. [DOI] [PubMed] [Google Scholar]
- 53.Suman-Horduna I, Roy D, Frasure-Smith N, Talajic M, Lesperance F, Blondeau L, Dorian P, Khairy P, Investigators A-CT. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol 2013;61:455–460. [DOI] [PubMed] [Google Scholar]
- 54.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al-Attar N, Alfieri O, Angelini A, Blomstrom-Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbuchel H, Heldal M, Kristensen SD, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FW. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 55.Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GYH, Steeds RP, Townend JN, Kotecha D. Safety and efficacy of digoxin therapy: a systematic review and meta-analysis of observational and controlled trial data. BMJ 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khand AU, Rankin AC, Martin W, Taylor J, Gemmell I, Cleland JG. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol 2003;42:1944–1951. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein RE, Boccuzzi SJ, Cruess D, Nattel S. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation 1991;83:52–60. [DOI] [PubMed] [Google Scholar]
- 58.The Danish Study Group on Verapamil in Myocardial Infarction. Secondary prevention with verapamil after myocardial infarction. Am J Cardiol 1990;66:33–40. [DOI] [PubMed] [Google Scholar]
- 59.Segal JB, McNamara RL, Miller MR, Kim N, Goodman SN, Powe NR, Robinson K, Yu D, Bass EB. The evidence regarding the drugs used for ventricular rate control. J Fam Practice 2000;49:47–59. [PubMed] [Google Scholar]
- 60.National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation. NICE Clinical Guideline 180; 2014. http://www.nice.org.uk/guidance/cg180/ (15 September 2014). [PubMed] [Google Scholar]
- 61.Goldberger ZD, Alexander GC. Digitalis use in contemporary clinical practice: refitting the foxglove. JAMA Intern Med 2014;174:151–154. [DOI] [PubMed] [Google Scholar]
- 62.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 63.Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 2015. doi:10.1093/eurheartj/ehv143. [DOI] [PubMed] [Google Scholar]
- 64.Flory JH, Ky B, Haynes K, Brunelli SM, Munson J, Rowan C, Strom BL, Hennessy S. Observational cohort study of the safety of digoxin use in women with heart failure. BMJ Open 2012;2:e000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gheorghiade M, Fonarow GC, van Veldhuisen DJ, Cleland JG, Butler J, Epstein AE, Patel K, Aban IB, Aronow WS, Anker SD, Ahmed A. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur Heart J 2013;34:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrey JL, Romero S, Garcia-Egido A, Escobar MA, Corzo R, Garcia-Dominguez G, Lechuga V, Gomez F. Mortality and morbidity of heart failure treated with digoxin. A propensity-matched study. Int J Clin Pract 2011;65:1250–1258. [DOI] [PubMed] [Google Scholar]
- 67.Allen LA, Fonarow GC, Simon DN, Thomas LE, Marzec LN, Pokorney SD, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Chang P, Peterson ED, Piccini JP, ORBIT-AF Investigators. Digoxin use and subsequent outcomes among patients in a contemporary atrial fibrillation cohort. J Am Coll Cardiol 2015;65:2691–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elkayam U. Calcium channel blockers in heart failure. Cardiology 1998;89(Suppl. 1):38–46. [DOI] [PubMed] [Google Scholar]
- 69.The effect of diltiazem on mortality and reinfarction after myocardial infarction. The Multicenter Diltiazem Postinfarction Trial Research Group. N Engl J Med 1988;319:385–392. [DOI] [PubMed] [Google Scholar]
- 70.Van Gelder IC, Groenveld HF, Crijns HJGM, Tuininga YS, Tijssen JGP, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 71.Groenveld HF, Crijns HJGM, Van den Berg MP, Van Sonderen E, Alings AM, Tijssen JGP, Hillege HL, Tuininga YS, Van Veldhuisen DJ, Ranchor AV, Van Gelder IC. The effect of rate control on quality of life in patients with permanent atrial fibrillation: data from the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) Study. J Am Coll Cardiol 2011;58:1795–1803. [DOI] [PubMed] [Google Scholar]
- 72.Silvet H, Hawkins LA, Jacobson AK. Heart rate control in patients with chronic atrial fibrillation and heart failure. Congest Heart Fail 2013;19:25–28. [DOI] [PubMed] [Google Scholar]
- 73.Cullington D, Goode KM, Zhang J, Cleland JG, Clark AL. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail 2014;2:213–220. [DOI] [PubMed] [Google Scholar]
- 74.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 75.Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, Darabantiu D, Crijns HJ, Kirchhof P, Vardas P, Tavazzi L, Maggioni AP, Boriani G. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J 2014;35:3365–3376. [DOI] [PubMed] [Google Scholar]
- 76.Nergardh AK, Rosenqvist M, Nordlander R, Frick M. Maintenance of sinus rhythm with metoprolol CR initiated before cardioversion and repeated cardioversion of atrial fibrillation: a randomized double-blind placebo-controlled study. Eur Heart J 2007;28:1351–1357. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg BA, Schulte PJ, Hofmann P, Ersboll M, Alexander JH, Broderick-Forsgren K, Anstrom KJ, Granger CB, Piccini JP, Velazquez EJ, Shah BR. Outcomes after nonemergent electrical cardioversion for atrial arrhythmias. Am J Cardiol 2015; doi:10.1016/j.amjcard.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piccini JP, Berger JS, O'Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J 2009;30:1245–1253. [DOI] [PubMed] [Google Scholar]
- 79.Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011;13:329–345. [DOI] [PubMed] [Google Scholar]
- 80.Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001;104:292–296. [DOI] [PubMed] [Google Scholar]
- 81.Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, Atar D, Avezum A, Blomstrom P, Borggrefe M, Budaj A, Chen SA, Ching CK, Commerford P, Dans A, Davy JM, Delacretaz E, Di Pasquale G, Diaz R, Dorian P, Flaker G, Golitsyn S, Gonzalez-Hermosillo A, Granger CB, Heidbuchel H, Kautzner J, Kim JS, Lanas F, Lewis BS, Merino JL, Morillo C, Murin J, Narasimhan C, Paolasso E, Parkhomenko A, Peters NS, Sim KH, Stiles MK, Tanomsup S, Toivonen L, Tomcsanyi J, Torp-Pedersen C, Tse HF, Vardas P, Vinereanu D, Xavier D, Zhu J, Zhu JR, Baret-Cormel L, Weinling E, Staiger C, Yusuf S, Chrolavicius S, Afzal R, Hohnloser SH, PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 82.Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–678. [DOI] [PubMed] [Google Scholar]
- 83.Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The Lancet 1996;348:7–12. [DOI] [PubMed] [Google Scholar]
- 84.Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD, Jr, Raisch DW, Ezekowitz MD, The Sotalol Amiodarone Atrial Fibrillation Efficacy Trial I. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005;352:1861–1872. [DOI] [PubMed] [Google Scholar]
- 85.Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The cardiac arrhythmia suppression trial (CAST) investigators. N Engl J Med 1989;321:406–412. [DOI] [PubMed] [Google Scholar]
- 86.Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349–361. [DOI] [PubMed] [Google Scholar]
- 87.Piccini JP, Sinner MF, Greiner MA, Hammill BG, Fontes JD, Daubert JP, Ellinor PT, Hernandez AF, Walkey AJ, Heckbert SR, Benjamin EJ, Curtis LH. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation 2012;126:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen MS, Marrouche NF, Khaykin Y, Gillinov AM, Wazni O, Martin DO, Rossillo A, Verma A, Cummings J, Erciyes D, Saad E, Bhargava M, Bash D, Schweikert R, Burkhardt D, Williams-Andrews M, Perez-Lugones A, Abdul-Karim A, Saliba W, Natale A. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol 2004;43:1004–1009. [DOI] [PubMed] [Google Scholar]
- 89.Gentlesk PJ, Sauer WH, Gerstenfeld EP, Lin D, Dixit S, Zado E, Callans D, Marchlinski FE. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:9–14. [DOI] [PubMed] [Google Scholar]
- 90.Wilton SB, Fundytus A, Ghali WA, Veenhuyzen GD, Quinn FR, Mitchell LB, Hill MD, Faris P, Exner DV. Meta-analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol 2010;106:1284–1291. [DOI] [PubMed] [Google Scholar]
- 91.Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al-Ahmad A, Beheiry S, Santarelli P, Starling RC, Dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haissaguerre M, Natale A, PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008;359:1778–1785. [DOI] [PubMed] [Google Scholar]
- 92.Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, McDonagh TA, Underwood SR, Markides V, Wong T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 93.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci M, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A. Ablation vs. amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: Results from the AATAC multicenter randomized trial. Conference proceedings from the American College of Cardiology Scientific Session March 16 2015, San Diego, 2015. http://www.abstractsonline.com/pp8/#!/3658/presentation/37598. (17 September 2015).
- 94.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 95.Gehi AK, Mounsey JP, Pursell I, Landers M, Boyce K, Chung EH, Schwartz J, Walker TJ, Guise K, Kiser AC. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm 2013;10:22–28. [DOI] [PubMed] [Google Scholar]
- 96.Kong MH, Lopes RD, Piccini JP, Hasselblad V, Bahnson TD, Al-Khatib SM. Surgical Maze procedure as a treatment for atrial fibrillation: a meta-analysis of randomized controlled trials. Cardiovasc Ther 2010;28:311–326. [DOI] [PubMed] [Google Scholar]
- 97.Ad N, Henry L, Hunt S. The impact of surgical ablation in patients with low ejection fraction, heart failure, and atrial fibrillation. Eur J Cardiothorac Surg 2011;40:70–76. [DOI] [PubMed] [Google Scholar]
- 98.McAlister FA, Ezekowitz JA, Wiebe N, Rowe B, Spooner C, Crumley E, Hartling L, Klassen T, Abraham W. Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med 2004;141:381–390. [DOI] [PubMed] [Google Scholar]
- 99.Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S, Peterson J, Yetisir E, Theoret-Patrick P, Luce M, Tang AS. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ 2011;183:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–e319. [DOI] [PubMed] [Google Scholar]
- 101.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, III, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Creager MA, Demets D, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013;127:e283–e352. [DOI] [PubMed] [Google Scholar]
- 102.Wilton SB, Leung AA, Ghali WA, Faris P, Exner DV. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm 2011;8:1088–1094. [DOI] [PubMed] [Google Scholar]
- 103.Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WH. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol 2009;53:765–773. [DOI] [PubMed] [Google Scholar]
- 104.Khazanie P, Greiner MA, Al-Khatib SM, Piccini JP, Turakhia MP, Varosy PD, Masoudi FA, Curtis L, Hernandez A. Use and comparative effectiveness of cardiac resynchronization therapy among patients with heart failure and atrial fibrillation: data from the NCDR registry. J Am Coll Cardiol 2014;63:A315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hayes DL, Boehmer JP, Day JD, Gilliam FR, III, Heidenreich PA, Seth M, Jones PW, Saxon LA. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm 2011;8:1469–1475. [DOI] [PubMed] [Google Scholar]
- 106.Boriani G, Gasparini M, Landolina M, Lunati M, Proclemer A, Lonardi G, Iacopino S, Rahue W, Biffi M, DiStefano P, Grammatico A, Santini M, ClinicalService cardiac c. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail 2011;13:868–876. [DOI] [PubMed] [Google Scholar]
- 107.Enriquez AD, Calenda B, Gandhi PU, Nair AP, Anyanwu AC, Pinney SP. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol 2014;64:1883–1890. [DOI] [PubMed] [Google Scholar]
- 108.Donghua Z, Jian P, Zhongbo X, Feifei Z, Xinhui P, Hao Y, Fuqiang L, Yan L, Yong X, Xinfu H, Surong M, Muli W, Dingli X. Reversal of cardiomyopathy in patients with congestive heart failure secondary to tachycardia. J Interv Card Electrophysiol 2013;36:27–32. [DOI] [PubMed] [Google Scholar]
- 109.Gupta S, Figueredo VM. Tachycardia mediated cardiomyopathy: pathophysiology, mechanisms, clinical features and management. Int J Cardiol 2014;172:40–46. [DOI] [PubMed] [Google Scholar]
- 110.Zimmermann AJ, Bossard M, Aeschbacher S, Schoen T, Voellmin G, Suter Y, Lehmann A, Hochgruber T, Pumpol K, Sticherling C, Kuhne M, Conen D, Kaufmann BA. Effects of sinus rhythm maintenance on left heart function after electrical cardioversion of atrial fibrillation: implications for tachycardia-induced cardiomyopathy. Can J Cardiol 2015;31:36–43. [DOI] [PubMed] [Google Scholar]
- 111.Calvo N, Bisbal F, Guiu E, Ramos P, Nadal M, Tolosana JM, Arbelo E, Berruezo A, Sitges M, Brugada J, Mont L. Impact of atrial fibrillation-induced tachycardiomyopathy in patients undergoing pulmonary vein isolation. Int J Cardiol 2013;168:4093–4097. [DOI] [PubMed] [Google Scholar]
- 112.Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation 2012;126:2353–2362. [DOI] [PubMed] [Google Scholar]
- 113.Lip GY, Gill P, Kirchhof P, Shantsila E, Holder R, Calvert M, Fisher J, Mostafa A. IMPRESS-AF: improved exercise tolerance in heart failure with preserved ejection fraction by spironolactone on myocardial fibrosis in atrial fibrillation. National Institute for Health Research Project Portfolio 2014. http://www.nets.nihr.ac.uk/projects/eme/121019. (20 October 14). [DOI] [PMC free article] [PubMed]
- 114.Kotecha D, Banerjee A, Lip GY. Increased stroke risk in atrial fibrillation patients with heart failure: does ejection fraction matter? Stroke 2015;46:608–609. [DOI] [PubMed] [Google Scholar]