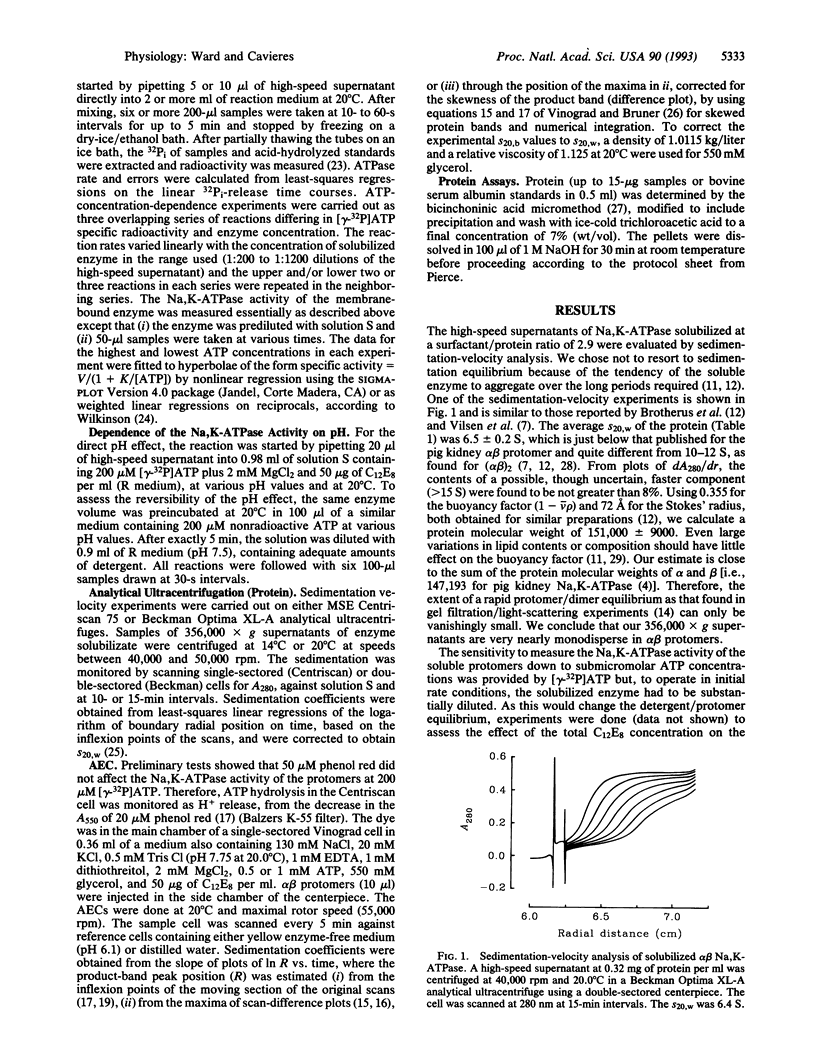
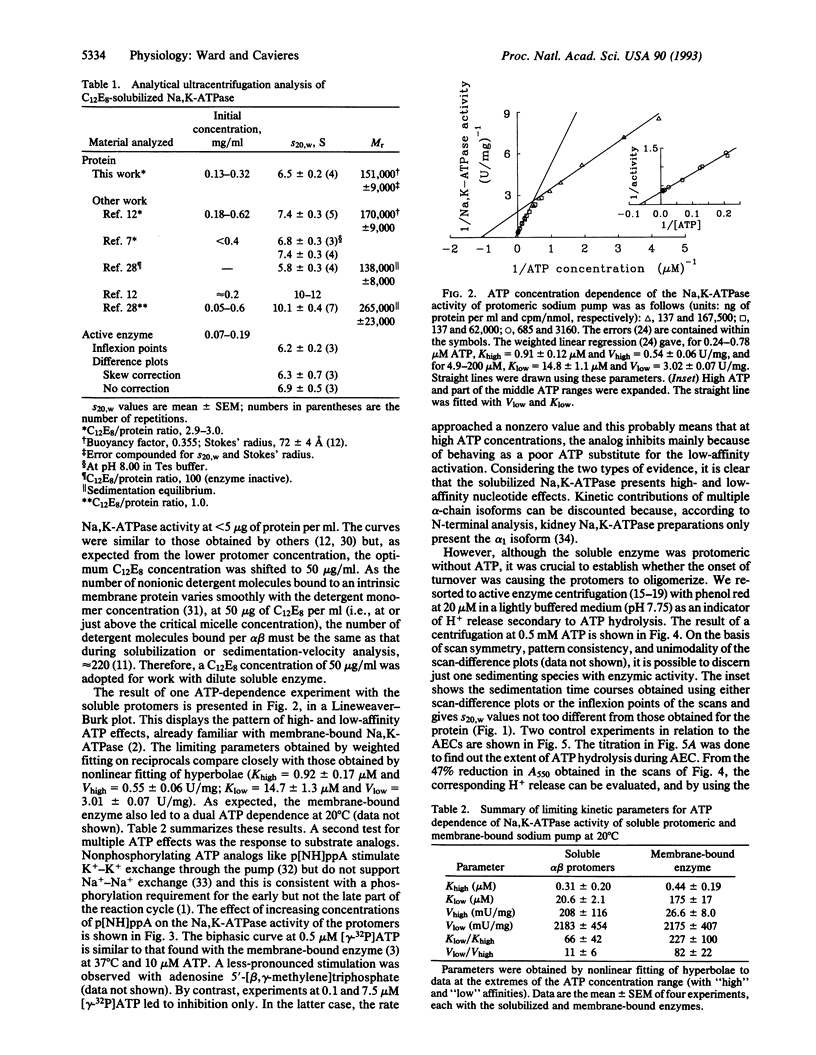
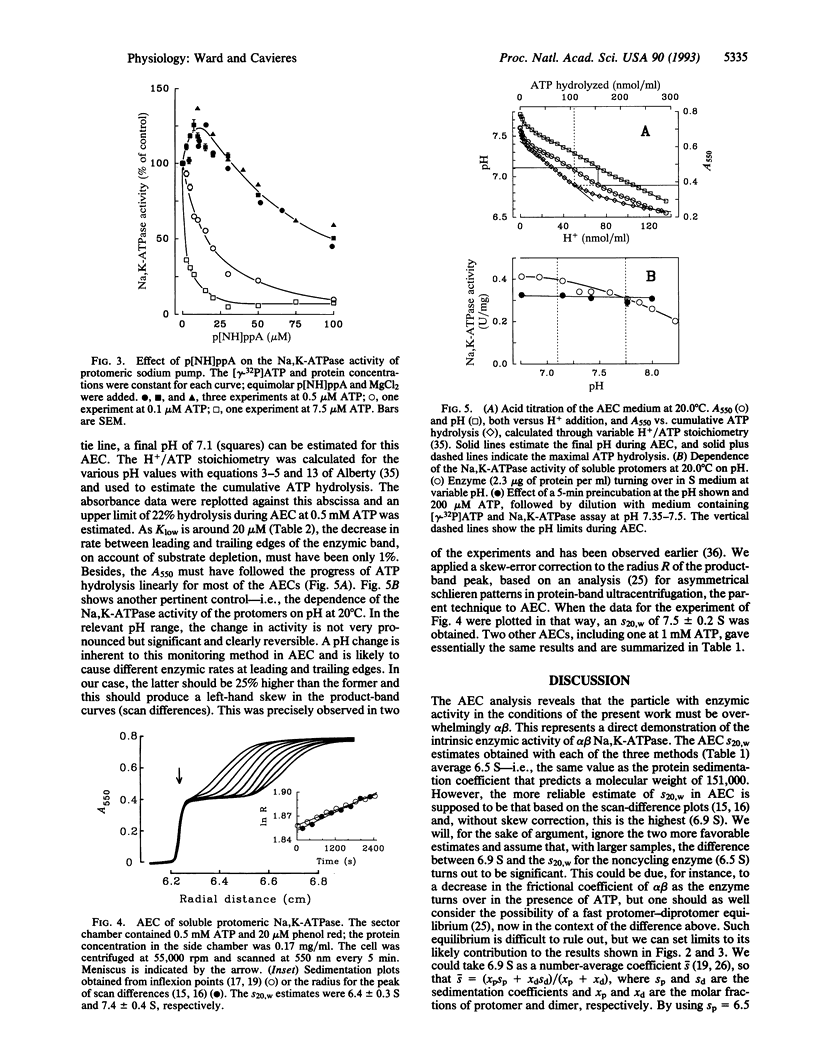
Abstract
A prominent feature of the Na,K-ATPase reaction is an ATP dependence that suggests high- and low-affinity ATP requirements during the enzymic cycle. As only one ATP-binding domain has been identified in the alpha subunit and none has been identified in the beta subunit, it has seemed likely that the apparent negative cooperativity results from subunit interactions in an (alpha beta)2 diprotomer. To test this possibility, we have examined the behavior of solubilized alpha beta protomers of Na,K-ATPase down to 50 nM [gamma-32P]ATP. Active-enzyme analytical ultracentrifugation shows that the protomer is the active species and that no oligomerization occurs during turnover. However, we find that dual ATP effects can be clearly demonstrated and that nonhydrolyzable ATP analogs can stimulate the Na,K-ATPase activity of the soluble protomer. We conclude that the apparent negative cooperativity is inherent to the alpha beta protomer and that this should explain some of the complexities found with membrane-bound Na,K-ATPase and, perhaps, other P-type cation pumps.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Askari A. (Na+ + K+)-ATPase: on the number of the ATP sites of the functional unit. J Bioenerg Biomembr. 1987 Aug;19(4):359–374. doi: 10.1007/BF00768539. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Jacobsen L., Jørgensen P. L. Soluble and enzymatically stable (Na+ + K+)-ATPase from mammalian kidney consisting predominantly of protomer alpha beta-units. Preparation, assay and reconstitution of active Na+, K+ transport. Biochim Biophys Acta. 1983 Jun 10;731(2):290–303. doi: 10.1016/0005-2736(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Møller J. V., Jørgensen P. L. Soluble and active renal Na, K-ATPase with maximum protein molecular mass 170,000 +/- 9,000 daltons; formation of larger units by secondary aggregation. Biochem Biophys Res Commun. 1981 May 15;100(1):146–154. doi: 10.1016/s0006-291x(81)80075-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum E., Schoner W. Phosphate binding and ATP-binding sites coexist in Na+/K(+)-transporting ATPase, as demonstrated by the inactivating MgPO4 complex analogue Co(NH3)4PO4. Eur J Biochem. 1991 Jan 30;195(2):407–419. doi: 10.1111/j.1432-1033.1991.tb15720.x. [DOI] [PubMed] [Google Scholar]
- Cavieres J. D. Calmodulin and the target size of the (Ca2+ + Mg2+)-ATPase of human red-cell ghosts. Biochim Biophys Acta. 1984 Apr 11;771(2):241–244. doi: 10.1016/0005-2736(84)90539-x. [DOI] [PubMed] [Google Scholar]
- Cavieres J. D., Glynn I. M. Sodium-sodium exchange through the sodium pump: the roles of ATP and ADP. J Physiol. 1979 Dec;297(0):637–645. doi: 10.1113/jphysiol.1979.sp013061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavieres J. D. The molecular size required varies according to the reaction step round the sodium pump cycle. FEBS Lett. 1987 Dec 10;225(1-2):145–150. doi: 10.1016/0014-5793(87)81147-x. [DOI] [PubMed] [Google Scholar]
- Cohen R., Giraud B., Messiah A. Theory and practice of the analytical centrifugation of an active substrate-enzyme complex. Biopolymers. 1967 Feb;5(2):203–225. doi: 10.1002/bip.1967.360050208. [DOI] [PubMed] [Google Scholar]
- Cohen R., Mire M. Analytical-band centrifugation of an active enzyme-substrate complex. 1. Principle and practice of the centrifugation. Eur J Biochem. 1971 Nov 11;23(2):267–275. doi: 10.1111/j.1432-1033.1971.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Cortas N., Elstein D., Markowitz D., Edelman I. S. Anomalous mobilities of Na,K-ATPase alpha subunit isoforms in SDS-PAGE: identification by N-terminal sequencing. Biochim Biophys Acta. 1991 Nov 18;1070(1):223–228. doi: 10.1016/0005-2736(91)90168-8. [DOI] [PubMed] [Google Scholar]
- Craig W. S. Monomer of sodium and potassium ion activated adenosinetriphosphatase displays complete enzymatic function. Biochemistry. 1982 Oct 26;21(22):5707–5717. doi: 10.1021/bi00265a049. [DOI] [PubMed] [Google Scholar]
- Esmann M., Christiansen C., Karlsson K. A., Hansson G. C., Skou J. C. Hydrodynamic properties of solubilized (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. Biochim Biophys Acta. 1980 Dec 2;603(1):1–12. doi: 10.1016/0005-2736(80)90386-7. [DOI] [PubMed] [Google Scholar]
- Esmann M., Skou J. C., Christiansen C. Solubilization and molecular weight determination of the (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. Biochim Biophys Acta. 1979 Apr 12;567(2):410–420. doi: 10.1016/0005-2744(79)90127-x. [DOI] [PubMed] [Google Scholar]
- Esmann M., Skou J. C. Kinetic properties of C12E8-solubilized (Na+ + K+)-ATPase. Biochim Biophys Acta. 1984 May 31;787(1):71–80. doi: 10.1016/0167-4838(84)90109-2. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Mimura K., Matsui H., Takagi T. Minimum enzyme unit for Na+/K+-ATPase is the alpha beta-protomer. Determination by low-angle laser light scattering photometry coupled with high-performance gel chromatography for substantially simultaneous measurement of ATPase activity and molecular weight. Biochim Biophys Acta. 1989 Aug 7;983(2):217–229. doi: 10.1016/0005-2736(89)90237-x. [DOI] [PubMed] [Google Scholar]
- Huang W. H., Kakar S. S., Askari A. Mechanisms of detergent effects on membrane-bound (Na+ + K+)-ATPase. J Biol Chem. 1985 Jun 25;260(12):7356–7361. [PubMed] [Google Scholar]
- Jensen J., Ottolenghi P. ATP binding to solubilized (Na+ + K+)-ATPase. The abolition of subunit-subunit interaction and the maximum weight of the nucleotide-binding unit. Biochim Biophys Acta. 1983 Jun 10;731(2):282–289. doi: 10.1016/0005-2736(83)90020-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Kemper D. L., Everse J. Active enzyme centrifugation. Methods Enzymol. 1973;27:67–82. doi: 10.1016/s0076-6879(73)27005-2. [DOI] [PubMed] [Google Scholar]
- Llewellyn D. J., Smith G. D. An evaluation of active enzyme centrifugation as a zonal and boundary technique by the analysis of simulated data. Arch Biochem Biophys. 1978 Oct;190(2):483–494. doi: 10.1016/0003-9861(78)90302-8. [DOI] [PubMed] [Google Scholar]
- Martin D. W. Active unit of solubilized sarcoplasmic reticulum calcium adenosinetriphosphatase: an active enzyme centrifugation analysis. Biochemistry. 1983 Apr 26;22(9):2276–2282. doi: 10.1021/bi00278a034. [DOI] [PubMed] [Google Scholar]
- Ottolenghi P., Jensen J. The K+-induced apparent heterogeneity of high-affinity nucleotide-binding sites in (Na+ + K+)-ATPase can only be due to the oligomeric structure of the enzyme. Biochim Biophys Acta. 1983 Jan 5;727(1):89–100. doi: 10.1016/0005-2736(83)90372-3. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Modyanov N. N., Broude N. E., Petrukhin K. E., Grishin A. V., Arzamazova N. M., Aldanova N. A., Monastyrskaya G. S., Sverdlov E. D. Pig kidney Na+,K+-ATPase. Primary structure and spatial organization. FEBS Lett. 1986 Jun 9;201(2):237–245. doi: 10.1016/0014-5793(86)80616-0. [DOI] [PubMed] [Google Scholar]
- Plesner I. W., Plesner L., Nørby J. G., Klodos I. The steady-state kinetic mechanism of ATP hydrolysis catalyzed by membrane-bound (Na+ + K+)-ATPase from ox brain. III. A minimal model. Biochim Biophys Acta. 1981 May 6;643(2):483–494. doi: 10.1016/0005-2736(81)90090-0. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Substrate sites for the (Na+ + K+)-dependent ATPase. Biochim Biophys Acta. 1976 May 13;429(3):1006–1019. doi: 10.1016/0005-2744(76)90345-4. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. The calcium pump of the surface membrane and of the sarcoplasmic reticulum. Annu Rev Physiol. 1989;51:473–485. doi: 10.1146/annurev.ph.51.030189.002353. [DOI] [PubMed] [Google Scholar]
- Simons T. J. The interaction of ATP-analogues possessing a blocked gamma-phosphate group with the sodium pump in human red cells. J Physiol. 1975 Jan;244(3):731–739. doi: 10.1113/jphysiol.1975.sp010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Barden R. E., Utter M. F. Identification of the reacting form of pyruvate carboxylase. J Biol Chem. 1972 Nov 25;247(22):7383–7390. [PubMed] [Google Scholar]
- Vilsen B., Andersen J. P., Petersen J., Jørgensen P. L. Occlusion of 22Na+ and 86Rb+ in membrane-bound and soluble protomeric alpha beta-units of Na,K-ATPase. J Biol Chem. 1987 Aug 5;262(22):10511–10517. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Tonomura Y. Binding of monovalent cations to Na+,K+-dependent ATPase purified from porcine kidney. II. Acceleration of transition from a K+-bound form to a Na+-bound form by binding of ATP to a regulatory site of the enzyme. J Biochem. 1980 Nov;88(5):1377–1385. doi: 10.1093/oxfordjournals.jbchem.a133106. [DOI] [PubMed] [Google Scholar]



