Abstract
Reproductive behavior and physiology in adulthood are controlled by hypothalamic sexually dimorphic neuronal networks which are organized under hormonal control during development. These organizing effects may be disturbed by endocrine disrupting chemicals (EDCs). To determine whether developmental exposure to Ethinylestradiol (EE2) may alter reproductive parameters in adult male mice and their progeny, Swiss mice (F1 generation) were exposed from prenatal to peripubertal periods to EE2 (0.1–1 μg/kg/d). Sexual behavior and reproductive physiology were evaluated on F1 males and their F2, F3 and F4 progeny. EE2-exposed F1 males and their F2 to F4 progeny exhibited EE2 dose-dependent increased sexual behavior, with reduced latencies of first mount and intromission, and higher frequencies of intromissions with a receptive female. The EE2 1 μg/kg/d exposed animals and their progeny had more calbindin immunoreactive cells in the medial preoptic area, known to be involved in the control of male sexual behavior in rodents. Despite neuroanatomical modifications in the Gonadotropin-Releasing Hormone neuron population of F1 males exposed to both doses of EE2, no major deleterious effects on reproductive physiology were detected. Therefore EE2 exposure during development may induce a hypermasculinization of the brain, illustrating how widespread exposure of animals and humans to EDCs can impact health and behaviors.
Early life is a period of unique sensitivity during which endogenous hormones exert organizational effects on brain structure and function. During critical developmental periods, embryonic sex hormones promote sexual differentiation and cause permanent changes in the architecture of limbic-hypothalamic circuits by specifying cell number, density of dendritic and axonal connections, and neurotransmitter phenotype1,2. In the male, masculinization and defeminization are the processes whereby the brain is organized to express sex-specific reproductive mating behavior and appropriate hormonal secretion within the hypothalamic-pituitary-gonadal (HPG) axis in adulthood3. These processes result from specific neural networks being induced by androgen production during the perinatal period, notably in rodents4. Testosterone acts mainly indirectly via estrogen receptors (ERs) after its aromatization into estradiol (E2)5. The major brain region mediating masculinization is the preoptic area (POA)6. In the murine POA, a cluster of calbindin-expressing cells containing more cells in males than in females corresponds to the sexually dimorphic nucleus (SDN) in the rat and is involved in the control of male sexual behavior7. The ontogenesis of the hypothalamic sexually dimorphic neuronal networks involved in the control of reproductive behavior and physiology depends on sex hormones, therefore during development the hypothalamus constitutes a key target for endocrine disrupting chemicals (EDCs).
EDCs are natural and synthetic molecules widespread in the environment which interfere with endogenous endocrine processes, and thus alter physiological functions. Pharmaceutical 17α-Ethinylestradiol (EE2) has recently been added to the European Directive 2013/39/EU8 of priority substances found in surface water in the European monitoring list, the so-called “watch list”. EE2 is a potent synthetic estrogen widely used in oral contraceptives, hormone replacement therapy and cancer (breast and prostate) treatments9. Much more resistant to metabolism and inactivation than E2 in humans10, after its excretion EE2 enters the wastewater network and is not completely removed during wastewater treatment processes11. Consequently, EE2 is one of the major pharmaceutical products found as a contaminant in effluent waters. It is much more active than E2 because of the properties of the ethinyl group at the C17α position10, exhibiting a high estrogenic activity and exerting its biological effects via interactions with various ERs through the same mechanisms as E29. Its estrogenic activity is also higher than other estrogenic EDCs such as bisphenol A and phthalates.
Therefore it is reasonable to consider that environmental EE2 contamination could have deleterious effects on animal and human health12, even at concentrations as low as ng.L−1, constituting a potential risk for animal and human populations. To assess the level of this risk, it needs to be evaluated correctly.
There is now compelling evidence that ontogenesis of hypothalamic networks are altered after EDCs exposure during development13,14. We have previously demonstrated that exposure to environmentally relevant doses of EE2 from embryonic day 10 to 14 alters the ontogenesis of the Gonadotropin-Releasing Hormone (GnRH) neurons in the mouse embryo by increasing the number of these neurons15. To assess whether such developmental effects could impact on reproductive function in adulthood, we designed a transversal study in mice to evaluate the consequences of developmental exposure to low doses of EE2 (0.1 and 1 μg/kg (body weight)/day) on reproductive behavior, physiology and neuroanatomy of adult males. The aim was to determine whether exposure to estrogenic EE2 from embryonic development up to the peripubertal period could induce a hypermasculinization of the hypothalamic neuroendocrine networks, leading to alterations in the reproductive function of adult male mice (F1 generation) and their progenies (F2, F3 and F4 generations). Sexual behavior, plasma testosterone concentrations and genital tract anatomy were evaluated. Then the neuroanatomy of GnRH and kisspeptin neurons, key hypothalamic regulators of reproduction, and the sexually dimorphic calbindin cell population were investigated.
Results
Effect of EE2 developmental exposure on sexual behavior
Sexual behavior was evaluated in males of the F1 generation directly exposed to EE2 and in their F2, F3 and F4 progenies. Three discrete components of male sexual behavior enabling sexual motivation and performance to be compared for each F1 to F4 male are presented in Fig. 1: latencies to first mount (A) and to first intromission (B), and frequency of intromissions (C) for each 30-minute test. The mean numbers of ano-genital investigations, attempted mounts and mounts without intromission are presented in Supplemental Material Table S1. As male sexual behavior improves with sexual experience, these parameters were evaluated over three tests. A two-way ANOVA was conducted with treatment and trial number as the two factors.
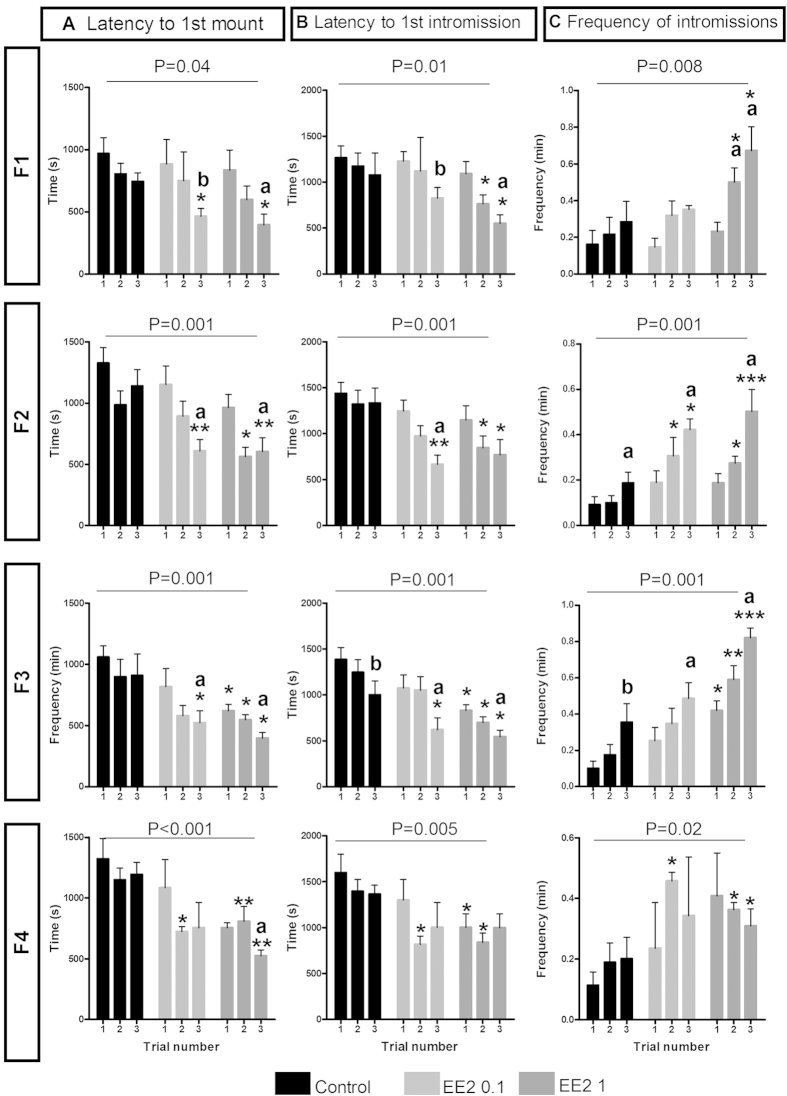
Figure 1. Developmental EE2 exposure affected male sexual behavior of adult F1 mice and their F2 to F4 progenies.
Latencies per second to the first mount (A) and first intromission (B) and frequency of intromissions per minute (C) for each experimental group (Control, EE2 0.1 and EE2 1) (n = 3–5 litters for each group of each generation). Data are expressed as means ± SEM. *p-value < 0.05, **p-value < 0.01 and ***p-value < 0.001; EE2 0.1 or EE2 1 group versus Control group of the same trial test by Bonferroni post-hoc tests after two-way ANOVA. a: p-value < 0.05, aa: p-value < 0.01, aaa: p-value < 0.001, b: p-value = 0.07; significantly different from trial 1 using Friedman tests within each experimental group.
The F1 EE2-exposed males and their F2 to F4 progenies exhibited lower latencies of first mount and intromission, and higher frequencies of intromissions, with the effects being EE2 dose-dependent. The differences increased from the first to the third trial, demonstrating a greater improvement in sexual behavior in the EE2 0.1 and EE2 1 μg/kg/d groups than in the Control animals. These differences between males of the three experimental groups are statistically significant for the second and/or third trials. As an example, for the third trial, F1 male latencies to the first mount and first intromission were 765.3 ± 198.1 and 1078.9 ± 238.9 s for the Control males, and 465.4 ± 118.1 and 827.1 ± 114.0 s, and 395.5 ± 85.0 and 549.4 ± 96.63 s for the EE2 0.1 (p-value < 0.05) and EE2 1 (p-value < 0.05 and p-value < 0.001) groups respectively. In the Control group, the frequency of intromissions was 0.28 ± 0.0 intromission per minute, and 0.35 ± 0.0 and 0.67 ± 0.0 for the EE2 0.1 and EE2 1 groups (p-value < 0.01 and p-value < 0.001) respectively. In some cases, the differences between groups were even significant for the first trial; i.e. in F3 EE2 1 animals, the latency to first mount was 622.1 ± 51.3 s, compared to 1060.2 ± 89.4 s in Control males (p-value < 0.05).
Male fertility
No differences in fertility were detected, since the relative number of litters and litter size did not vary (Supplemental Material Table S2). There was also no difference in sex ratio (number of males/number of females) between the three groups.
EE2 developmental exposure and adult plasma testosterone concentrations
No significant difference was detected in the mean plasma testosterone concentrations between the males of the three experimental groups either for F1, or for F2, F3 or F4 generations (Fig. 2A).
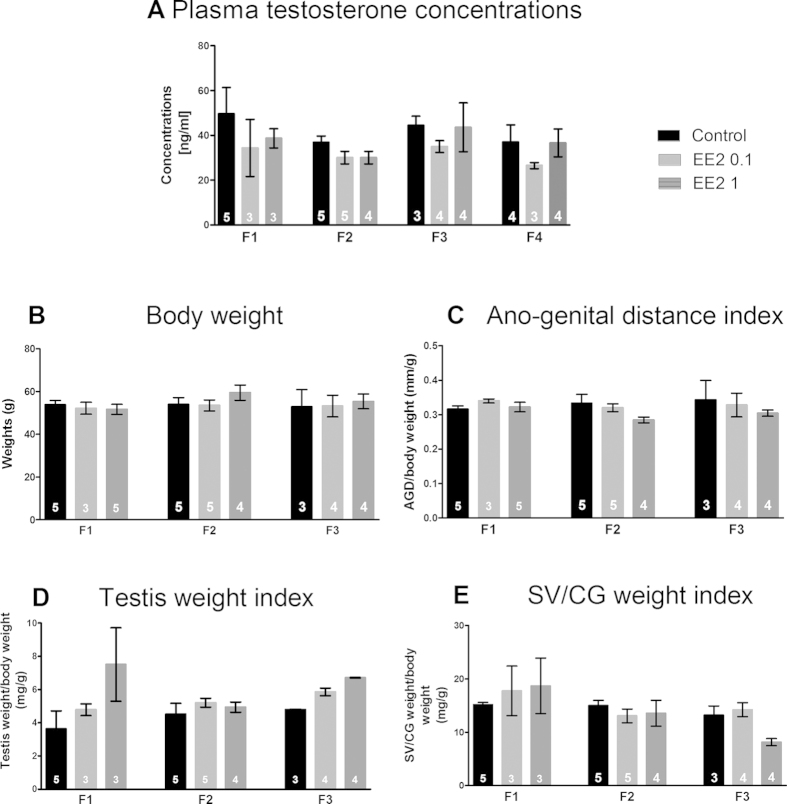
Figure 2. Developmental EE2 exposure did not alter reproductive physiology.
EE2 exposure had no effect on plasma testosterone concentrations of F1 to F4 males (A), or on adult body weight (B), ano-genital distance (AGD) (C), testis weight (D) or seminal vesicles/coagulating glands (SV/CG) weight (E) of F1 to F3 males (n = 3–5 litters for each group of each generation) (Kruskal-wallis’ test). Each parameter measured was reported to the animal’s body weight to be expressed as an index of animal body weight. Since no significant alterations in reproductive tract morphometric parameters were detected, F4 males were not investigated for these analyses.
EE2 developmental exposure and genital tract in adults
There was no significant difference in animal weights between the three experimental groups (Fig. 2B). Figure 2C–E presents the mean indexes for ano-genital distances (AGD) (C), testes weights (D) and seminal vesicles/coagulating glands (SV/CG) weights (E) for F1 to F3 males. No significant difference was detected for any of these parameters. Nevertheless, although there was no significant change in mean VS/CG weights, recurrent abnormalities as right/left asymmetry in VS/CG of EE2 0.1 and EE2 1 males for the F1 and F2 generations were observed (see Supplemental Material Figure S2 for details). Moreover, testes sections counter-stained with eosin-haematoxylin were observed; no gross modification of testes histology was detected, but no quantification was performed (data not shown).
Neuroanatomical studies
Effects of EE2 developmental exposure on hypothalamic GnRH neurons in adults
In F1 adult male mice the hypothalamic GnRH neuron perikarya were assessed, in terms of their numbers and neuroanatomical distribution (Fig. 3A,B) in the main areas where they are scattered (Median Septum (MS), Organum Vasculosum Laminae Terminalis (OVLT) and medial PreOptic Area (mPOA)), and their fiber density was recorded in the Median Eminence (ME), where most of the hypothalamic GnRH neurons project their axons (Fig. 3C).
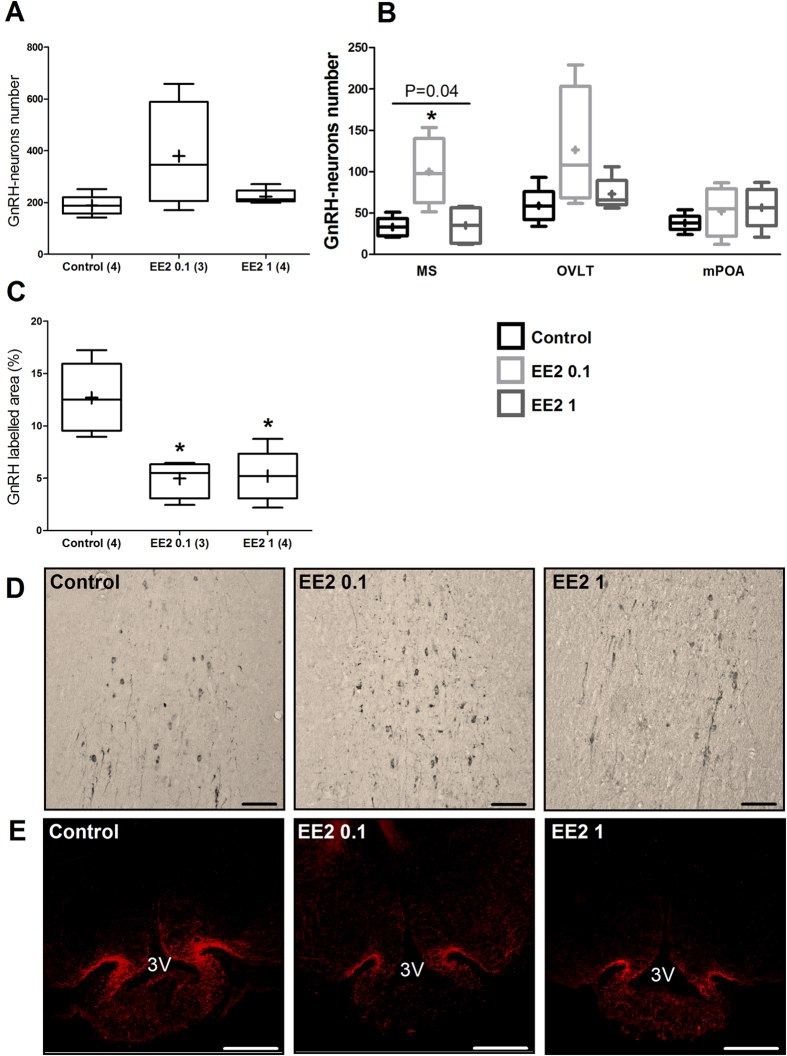
Figure 3. Developmental EE2 exposure disturbed GnRH neuron neuroanatomy in adult F1 male mice.
(A) Tukey’s boxplots of total numbers of GnRH immunoreactive neurons in the hypothalamic POA for each experimental group. (B) Numbers and distribution of GnRH immunoreactive neurons according to the three neuroanatomical areas where cell bodies were counted: Median Septum (MS), Organum Vasculosum Laminae Terminalis (OVLT) and medial PreOptic Area (mPOA). (C) Box-plots of the mean GnRH labeled surface in three areas of the median eminence (ME; anterior, middle and posterior). The line in the middle of the box is plotted at the median and (+) is the mean. Numbers in brackets represent litter numbers. Kruskal-Wallis test with Dunn’s post-test with *p-value < 0.05. (D,E) Representative photographs of immunohistochemical labeling with DAD-Ni staining of GnRH neurons in the MS (D) and immunofluorescent terminal nerves in the ME (E) of Control, EE2 0.1 and EE2 1 males. Scale bars = 100 μm.
No significant effect of EE2 treatment on the number of perikarya neurons was detected (Fig. 3A; Kruskal-Wallis test; p-value > 0.05). The neuroanatomical distribution of the GnRH neuron’ cell bodies through the MS, OVLT and mPOA revealed that in the Control group the majority of neurons were located in the OVLT (Fig. 3B). This distribution was not altered in the EE2 1 group but was significantly different in the MS of the EE2 0.1 group compared to the Control group (Kruskal-Wallis test with Dunn’s multiple comparison; p-value < 0.05) (Fig. 3B illustrated in Fig. 3D).
In males developmentally exposed to EE2, the average percentage of the GnRH labeled area in the ME was significantly lower in the EE2 0.1 (Kruskal-Wallis test; p-value = 0.02) and EE2 1 (Dunn’s multiple comparisons; p-values < 0.05) groups than in the Control group (Fig. 3C,E).
As F1 males exhibited no defect in fertility, the GnRH neuron network was not studied in their progeny.
EE2 developmental exposure and hypothalamic kisspeptin neurons in adults
The hypothalamic kisspeptin neurons are key regulators of GnRH neuron activity. In males, during development, these neurons are organized in the Periventricular preoptic (PVpo) nucleus by gonadal steroids16 rendering them potentially sensitive to xenoestrogens such as EE2. We therefore compared the numbers of immunoreactive kisspeptin neurons in the PVpo nuclei of Control and EE2-exposed males and no significant differences were observed for F1 generation animals or their F3 and F4 progeny (Fig. 4A).
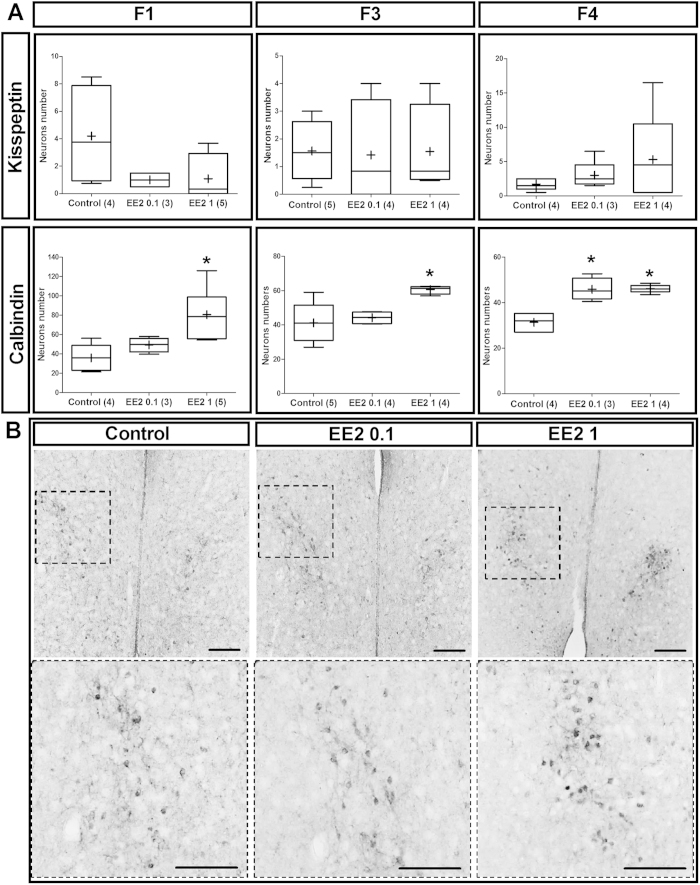
Figure 4. Unlike kisspeptin neurons, developmental EE2 exposure disturbed sexually dimorphic calbindin immunoreactive neurons in the medial PreOptic Area (mPOA).
(A) Tukey’s boxplots of the number of kisspeptin and calbindin-immunoreactive neurons per brain section in mPOA of F1, F3 and F4 males. The line in the middle of the box is plotted at the median and (+) is the mean. Numbers in brackets represent litter numbers. Kruskal-Wallis test with Dunn’s post-test with *p-value < 0.05. (B) Representative anti-calbindin immunostaining in the mPOA showing the location of the sexually dimorphic calbindin population (NDS-POA). Images of the first panel present a low magnification giving an overview of the location of NDS-POA broadly delimited by the dotted squares magnified in the panel below. Scale bars = 100 μm.
Effects of EE2 developmental exposure on hypothalamic calbindin cells
Developmental exposure to EE2 significantly increased the number of calbindin immunoreactive (calb-ir) cells in the mPOA of F1 males of the EE2 1 group (80.5 ± 13.1) compared to the Control group (35.8 ± 8.0) (Kruskal-Wallis test; p-value = 0.04) (Fig. 4A), as illustrated in Fig. 4B. Similarly, in the mPOA of F3 males, EE2 increased the number of calb-ir neurons in the EE2 1 group (60.5 ± 1.2) compared to the Control group (41.2 ± 5.3) (Kruskal-Wallis test; p-value = 0.02). In F4 males, there were more calb-ir cells in both the EE2 0.1 (45.8 ± 2.5) and EE2 1 (46.1 ± 0.7) groups than in the Control group (31.3 ± 1.8) (Kruskal-Wallis test; p-value < 0.0001).
Discussion
We demonstrated in the mouse a crucial impact of developmental exposure to both doses of EE2 (0.1 and 1 μg/kg/day) on male sexual behavior and calbindin neuronal population in the hypothalamic mPOA. EE2-exposed males expressed increased sexual behavior and had more calbindin cells in the mPOA. These effects were EE2 dose-dependent and were transmitted up to the F4 generation. Despite neuroanatomical modifications observed in the GnRH neuron population of F1 males under both doses of EE2 exposure, no major deleterious effects on reproductive physiology was detected in F1 adult males or their progenies.
The ability of an individual to recognize and adequately interact with a breeding partner constitutes a key step in reproductive success and species survival. The competence to respond to hormonal and social stimuli of breeding partners through appropriate behavior strongly depends on key developmental events during which neural circuits controlling these behaviors are correctly implemented17. We demonstrated that EE2-exposed animals exhibited a significant EE2 dose-dependent decrease in the latency to initiate sexual behavior and in the frequency of intromissions. This phenotype reflects an increase in the sexual behavior of EE2-exposed males. Differences in testosterone production of males from the three experimental groups could explain such increased male sexual behavior, but the plasma testosterone concentrations after hCG stimulation in EE2-exposed and Control males were unchanged. However, we did not evaluate whether the circulating levels of testosterone or the concentration of biologically active hormone in the bloodstream changed.
Beyond its major role in regulating adult male reproduction, testosterone plays a crucial part during development, both in the differentiation of the genital tract and of the brain17,18. The developmental sexual differentiation of specific brain areas such as the mPOA, involving local aromatization of testosterone into E2, is essential for the masculinization of neural networks by E2, producing permanent behavioral changes in adulthood. EE2 has a strong estrogenic effect and this could have induced a hypermasculinization of the brain leading to increased sexual behavior in EE2-exposed mice. To test this hypothesis of an effect of EE2 on the organization of brain neuroanatomy, independently of variations in testosterone levels, the sexual behavior of adult castrated males exposed to EE2 during development and treated with a same dose of exogenous testosterone should be investigated. This could illustrate an organizational effect of EE2 rather than a physiological influence due to the circulating level of testosterone.
The mPOA constitutes the primary neuroendocrine structure regulating male sexual behavior in different species7,18. Within the mPOA, the sexually dimorphic nucleus (SDN-POA) has been largely studied in the rat19 and other rodents20,21,22,23 and has been shown to be highly sensitive to aromatized androgens during the perinatal period in the male24,25,26. This structure is characterized by a cluster of calbindin cells whose number is greater in males than in females. It has recently been demonstrated that the mPOA might be a target for EDCs27. Our study found more calbindin immunoreactive cells in the mPOA of the F1 generation of EE2-exposed males and of their F3 and F4 offspring than in Control males, mainly in the EE2 1 group. This increase supports the hypothesis of a hypermasculinization effect of EE2 on brain structures involved in the control of male sexual behavior.
The modifications in male sexual behavior and calbindin cell numbers in the mPOA were observed in adulthood in F1 males exposed to EE2 during development. These changes, established early in life during the perinatal period, endure until adulthood. Moreover, these modifications were also observed in their progeny up to the F4 generation. Epigenetic processes constitute a mechanism by which endogenous and exogenous cues can control gene expression in the long-term. Notably, DNA methylation is associated with long-term DNA transcriptional repression. In the mouse, Nugent et al.28 demonstrated that masculinization of the POA and male sexual behavior lead to E2-mediated decreases in DNA methyltransferase enzymes. Moreover, in the rat, Anway et al.29 and Skinner & Guerrero-Bosagna30 showed that developmental exposure to the fungicide vinclozolin was able to alter mate preference and sperm epigenome, associated with epigenetic transgenerational inheritance for three generations. We can thus hypothesize that the increased male sexual behavior and the modifications in calbindin cells in the mPOA observed in our study over four generations may result from epigenetic processes, involving alterations in the sperm epigenome, which could be responsible for the transgenerational inheritance of the phenotypes. However these mechanisms remain to be characterized.
Hypothalamic GnRH neurons constitute the major center for controlling the HPG axis and thus the reproductive function31. In the current study, developmental exposure to EE2 did not modify the overall number of GnRH cell bodies in the POA, but induced a differential increase in immunoreactivity of GnRH neurons in the rostral region with more perikarya in the MS of the adult EE2 0.1 group males. This enhanced immunoreactivity may be due to a greater GnRH peptide content in perikarya leading to more neurons being detected by immunohistochemistry. However, we cannot exclude defects in the neuronal migration occurring between embryonic day 11 and 16 leading to misallocated neurons32,15. Furthermore, the dramatic decrease in the immunoreactivity of the GnRH terminal nerves in the ME in the EE2 0.1 and EE2 1groups could be explained either by a modification in GnRH peptide storage or transport from perikarya to terminal nerves, or by increased exocytotic release of peptide detected as neuropeptide depletion within the neurons. Clements et al.33 demonstrated in the rat that developmental exposure to dioxin induced changes in GnRH secretion in the ME linked to GnRH retention in perikarya. The major estrogen sensitive input onto GnRH neurons is the estrogen-sensitive kisspeptin neuronal network, but no effect on the dimorphic kisspeptin neuron population in the PVpo was observed in the present study. Altogether, despite the changes within the GnRH neuronal network, there was no major impact on reproductive physiology, fertility and genital tract anatomy.
One objective of this study was to determine whether oral exposure to low doses of EE2, below those tested in classical toxicology assays34 would disrupt neuroendocrine and behavioral outputs of male reproductive function. Two dose levels were tested; the lowest (0.1 μg/kg of body weight per day) corresponded to an environmental pollution occasionally found in aquatic environments35, and the highest (1 μk/kg of body weight per day) was within the range of a pharmacological dose9. An intake of EE2 at both 0.1 and 1 μkg/kg/day produced a dose-dependent effect on sexual behavior, calbindin cell number in the POA and GnRH fiber density in the ME. However, an effect on the number of GnRH soma was only observed at the lowest dose. This suggests that EE2 acts via different mechanisms depending on the target and the exposure time. It has been shown that EE2 has greater affinity to estrogen receptor ERα than ERβ36, but we know that hormone concentration or binding affinity does not necessarily predict the severity of the in vivo effect37,38,39. The POA highly expresses ERα which is strongly implicated in the sexual differentiation of neuroendocrine networks40. Moreover, the fact that ERβ-KO males show normal, strong sexual behavior41 implies that only ERα is important for sexual behavior. Together these data strongly suggest that the effects of EE2 on POA differentiation and sexual behavior are mediated by ERα rather than ERβ. However, during development, GnRH neurons express ERβ42, which has not been confirmed for ERα43, supporting the idea of a possible direct effect of EE2 on these neurons via ERβ. The absence of an effect at the highest EE2 dose could be related to differences in signaling pathways activated by different estrogen receptors at different EE2 concentrations. This hypothesis is in line with the well-established “low dose hypothesis” described in many EDCs studies and currently largely documented44. Several studies have reported that the effect of a low dose may be more potent than that of a higher dose for a specific parameter monitored towards a given endocrine active compound, particular regarding the risk assessment of reproductive parameters45. The nonlinear dose-response effect of endocrine-active molecules thus appears as the most significant and extensively peer-reviewed issue within the field of EDCs and it remains poorly understood (for review see refs 45,46).
Conclusions
This study demonstrated that although developmental exposure to low doses of EE2 resulted in no overall deleterious effect on reproduction in adult male mice, sexual behavior and neuronal networks were impacted. These effects were transmitted to the progeny up to the F4 animals. Here we were able to highlight some effects which would not have been detected with toxicological regulatory studies34, since sexual behavior is not a parameter monitored per se. Such deep and transgenerational modifications of sexual behavior necessarily impact on individual and species fitness. Although care must obviously be taken when extrapolating from animal studies, it is possible to imagine the effect of such alterations induced by EE2 or other estrogenic EDCs on human health and the implied societal consequences. The relevance for effective health and safety of such new non-adverse, but biologically significant changes has to be seriously considered to understand the threats and to prevent risks to human health. Moreover, such long-term alterations in neuronal networks induced by developmental steroid perturbations may affect different social behaviors or even be involved in neurodegenerative diseases47. Demonstrating a significant elevation in progesterone and testosterone concentrations in amniotic fluids in the males with autism compared with controls, Baron-Cohen et al.48 suggest links between the prenatal hormone environment, sexually dimorphic behaviors and autism. This concern therefore needs to be considered by stakeholders and politicians to prevent long-term deleterious consequences.
Methods
Animals
Animals were obtained from fifteen pregnant Swiss mice (5 dams per experimental group; F0 generation) purchased from a commercial breeder (Charles Rivers, France). Mice were housed in individual standard cages during gestation and with their pups for lactation, and each male was housed with three brothers from weaning up to the beginning of the sexual behavior tests. Animals were given a free access to food and water under controlled temperature (22 °C) and photoperiod cycle (12 h light/12 h dark). All experiments were performed according to the European directive 2010/63/EU on the protection of animals used for scientific purposes and approved by an ethical committee for animal experimentation (CEEA Val-de-Loire, Tours, France, n°00302-01).
Ethinylestradiol exposure
A stock solution of EE2 (Sigma Aldrich) was prepared in absolute ethanol (1 μg/mL) and dilutions to final doses of exposure were performed directly in drinking water. The daily dose was adjusted according to animals’ weights and water consumption. To estimate EE2 exposure, we previously evaluated the volume of drinking water ingested by a Swiss mouse per day (around 5 mL for an adult mouse). Animals were exposed to EE2 from embryonic day 10 until postnatal day (PND) 40 (F1 generation). This exposure period covered the whole developmental period, including in utero life from mid-gestation and the perinatal period until juvenile life, corresponding to the organizational window of neuroendocrine networks, and extended up to puberty, during which these neuroendocrine networks are activated2. The animals were divided into three groups. The first (EE2 0.1 group) was exposed to 0.1 μg of EE2 per kg body weight per day, a dose compatible with an exposure range found in highly polluted environments35. The second (EE2 1 group) was exposed to 1 μg of EE2 per kg body weight per day, corresponding to pharmacological exposure with a contraceptive pill or hormone replacement therapy9, and defined as the lowest observed adverse effect level (LOAEL) in humans34,49. The third (Control group) received vehicle. In order to avoid bias on animal growth due to litter size, each litter was reduced to 4 males and 4 females at PND4. Analyses were carried out on adult animals (from 8 weeks of age). The males tested for sexual behavior were different from the males included in the neuroanatomical studies.
Animals of the F2, F3 and F4 generations were obtained by crossing females and males within each experimental group. Thus F2, F3 and F4 males of the EE2 0.1 and EE2 1 groups originated from EE2-exposed parents, grand- and great-grand-parents respectively.
Each experimental group of each F1 to F4 generation included 12-20 males originating from 3 to 12 litters.
Sexual behavior
Tests were performed in a dark room under red light. Naive males were individually housed for one week before the first test in a room devoid of the presence of any females. Each male of the F1, F2, F3 and F4 generations (n = 12–14 for each group of each generation obtained from 3 to 5 different litters per group) was tested three times, corresponding to the three trials, with a time interval of two weeks between trials. Males were tested in a cage containing fresh litter. After 10 min habituation an estrous female was introduced and the test lasted for 30 minutes. A mount involved a male placing its two anterior paws on either sides of the flank of the receptive female. Intromission was determined when the male carried out back and forth thrusting movements of the pelvic region. The numbers of ano-genital investigations and mount attempts, the latencies and the frequencies of mounts and intromissions were scored41.
Estrous females used as stimuli were ovariectomized under general anesthesia (xylazine/ketamine) and implanted with Silastic implants (Dow Corning) containing 50 μg of E2-benzoate (Sigma-Aldrich). Four hours before the tests, females were given a subcutaneous injection of 1 mg of progesterone (Sigma-Aldrich) diluted in 100 μL of oil to induce receptivity.
Fertility study
Male fertility was assessed at the end of the behavioral analyses. A male mouse was housed with a female for 1 to 14 days until a vaginal plug was observed. The number of litters and pups per litter were recorded for each experimental group of males.
Plasma testosterone concentrations
Two hours before being euthanized, males of the F1 to F4 generations were injected intraperitoneally with 15 UI of human chorionic gonadotropin (hCG) (Intervet, France) diluted in physiological serum50. Blood was collected at death, plasma separated and stored at −20 °C until testosterone assays. Plasma testosterone concentrations were evaluated through a RIA using 3H testosterone as previously described51. The sensitivity of the assay was 0.115 ng/mL and the intra-assay coefficient of variation was 7%.
Genital tract analysis
When F1, F2 and F3 animals were euthanized, each male was weighted, the ano-genital distance (AGD) was measured, and testes, seminal vesicles and coagulating glands (SV/CG) were weighted.
Immunohistochemistry
Brains were collected according to the protocol described in Geller et al.52. After embedding in TissuTek®, brains were cut with a cryostat (Leica) into 20 μm coronal slices, stored at -20 °C until immunohistochemistry. Slices were processed for immunostaining with rabbit polyclonal anti-GnRH antibody (1:3000; ref. 52), sheep anti-kisspeptin antibody (1:2000; ref. 53) or mouse monoclonal anti-calbindin antibody (1:5000; C8948; clone CB-955; Sigma-Aldrich). GnRH immunostaining was performed in the POA and ME of F1 animals, and kisspeptin and calbindin immunostainings were performed respectively in the PVpo nucleus and mPOA of F1, F3 and F4 animals. Details of all the procedures are provided in Supplemental Material Part1. No immunohistochemical analysis could be performed on F2 animals because of technical troubles.
Analysis and quantification of immunohistochemical studies
GnRH cell numbers were analyzed under an optical microscope at 20X magnification in three neuroanatomical regions: Median Septum (MS), Organum Vasculosum Laminae Terminalis (OVLT) and medial PreOptic Area (mPOA). In each region, GnRH immunoreactive cell bodies were counted on 10 consecutive coronal sections. The mean numbers of GnRH immunoreactive neurons were compared between the experimental groups both overall by summing the numbers determined in the three regions and within each neuroanatomical region.
Analysis of GnRH terminal nerves in the ME was performed using epifluorescence microscopic images computerized with Mercator Software (Explora Nova, La Rochelle, France). Three coronal slices corresponding to the anterior, middle and posterior parts of the ME were selected according to Paxinos & Franklin’s brain atlas54 (Supplemental Material Figure S1). The proportion of the area of GnRH fibers which were immunoreactive was quantified in 10.000 μm2 taken from and centered on the ME (20X magnification). Results are presented as the distribution of mean labeled surface area per litter (1–2 animals per litter) for each group.
Kisspeptin neurons were counted on confocal images (LSM 700, 20X magnification) with Zen® software. For each animal, two brain coronal slices corresponding to the PVpo were analyzed. All the labeled neurons distributed around the third ventricle were counted and a mean number of neurons per section and per animal was obtained. Counting of calbindin positive cells in the mPOA was carried out on images taken at 20X magnification according to a method adapted from ref. 26. Details are described in Supplemental Material Part2. The values represent the mean numbers of cells counted on one side of the third ventricle.
Statistical analyses
Results are presented as average values per litter for each parameter monitored and obtained from values of animals from the same litter. For each litter one to two animals were used for neuroanatomical studies and two to four animals were analyzed for behavior. Statistical analyses were performed with GraphPad Prism5® software (GraphPad Software, San Diego, CA). Data were compared using a non-parametric Kruskal Wallis test with a Dunn’s post-test. Behavioral parameters were analyzed using a two-way ANOVA test with a Bonferroni post-test. A Friedman test was used for intragroup comparisons (trial number effect). A Chi-square test was used to compare frequencies. Differences were considered significant for p-value < 0.05. Data presented in the histograms are means ± Standard Error of the Mean (SEM).
Additional Information
How to cite this article: Derouiche, L. et al. Developmental exposure to Ethinylestradiol affects transgenerationally sexual behavior and neuroendocrine networks in male mice. Sci. Rep. 5, 17457; doi: 10.1038/srep17457 (2015).
Supplementary Material
Acknowledgments
This work has been supported by the French National Research Agency (ANR) grant NEED (#AF08CES 011). Mr Derouiche is a Ph.D. student supported by the French MESR. We thank Mr. Cahier and Ms. Crespin and Ms. Cirot for the care of the animals at the Experimental unit PAO n°1297 (EU0028), INRA Centre Val-de-Loire, and the INRA PRC hormonal assay platform for testosterone assays. We are grateful to Dr Fouchécourt for the discussion on the hCG injection protocol, Dr Martini for her participation in testing mouse behavior and Ms. Torchy for the kisspeptin study.
Footnotes
Author Contributions D.P. designed the study. L.D. and D.P. performed the experiments, analyzed the data and drafted the manuscript. M.K. and A.H.D. supervised the design of the study and revised the manuscript. All authors critically reviewed the manuscript for intellectual content and gave final approval for the version to be submitted for publication.
References
- Bakker J. et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci. 9, 220–226 (2006). [DOI] [PubMed] [Google Scholar]
- McCarthy M. M. Estradiol and the developing brain. Physiol. Rev. 88, 91–124 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. M. How it’s made: organisational effects of hormones on the developing brain. J. Neuroendocrinol. 22, 736–742 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. M. & McCarthy M. M. Steroid-induced sexual differentiation of the developing brain: multiple pathways, one goal. J. Neurochem. 105, 1561–1572 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J. Steroid control and sexual differentiation of brain aromatase. J. Steroid Biochem. Mol. Biol. 61, 323–339 (1997). [PubMed] [Google Scholar]
- Simerly R. B. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536 (2002). [DOI] [PubMed] [Google Scholar]
- Coolen L. M., Peters H. J. & Veening J. G. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J. Comp. Neurol. 397, 421–435 (1998). [DOI] [PubMed] [Google Scholar]
- European Union. [Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy] Off. J. Eur. Union L 226 56 [E.P.a.C.o.t.E. Union (ed.)] [1–17] (2013).
- Stanczyk F. Z., Archer D. F. & Bhavnani B. R. Ethinylestradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception. 87, 706–727 (2013). [DOI] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 8 (Suppl 1), 3–63 (2005). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci. Total Environ. 511C, 54–62 (2014). [DOI] [PubMed] [Google Scholar]
- Jobling S. & Owen R. [Ethinyl estradiol: Bitter Pill for the Pre-cautionary Principle] Late Lessons from Early Warnings: Science, Precaution, Innovation [Gee D. (ed.)]. [331–339] (European Environment Agency, Copenhagen, 2013). [Google Scholar]
- Gore A. C. & Patisaul H. B. Neuroendocrine disruption: historical roots, current progress, questions for the future. Front. Neuroendocrinol. 31, 395–399 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Olea M. et al. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 203, 158–173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon D., Cadiou V., Angulo L. & Duittoz A. H. Maternal exposure to 17-alpha-ethinylestradiol alters embryonic development of GnRH-1 neurons in mouse. Brain Res. 1433, 29–37 (2012). [DOI] [PubMed] [Google Scholar]
- Semaan S. J. Tolson K. P. & Kauffman A. S. The development of kisspeptin circuits in the Mammalian brain. Adv Exp Med Biol 784, 221–252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K. M., Nugent B. M. & McCarthy M. M. Sexual differentiation of the rodent brain: dogma and beyond. Front. Neurosci. 6, 26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J., Absil P., Gérard M., Appeltants D. & Ball G. F. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J. Neurosci. 18, 6512–6527 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. & Field P. M. Sexual dimorphism in the preoptic area of the rat. Science 173, 731–733 (1971). [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Carter C. S., Steerman C. & DeVoogd T. J. Sex differences in dentritic patterns in hamster preoptic area. Brain Res. 126, 63–72 (1977). [DOI] [PubMed] [Google Scholar]
- Tobet S. A., Zahniser D. J. & Baum M. J. Differentiation in male ferrets of a sexually dimorphic nucleus of the preoptic/anterior hypothalamic area requires prenatal estrogen. Neuroendocrinology 44, 299–308 (1986). [DOI] [PubMed] [Google Scholar]
- Bodo C. & Rissman E. F. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology 149, 4142–4150 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C. & Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J. Comp. Neurol. 518, 3618–3629 (2010). [DOI] [PubMed] [Google Scholar]
- Gorski R. A. Sexual differentiation of the brain. Hosp. Pract. 13, 55–62 (1978). [DOI] [PubMed] [Google Scholar]
- Arendash G. W. & Gorski R. A. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res. Bull. 10, 147–154 (1983). [DOI] [PubMed] [Google Scholar]
- Gilmore R. F., Varnum M. M. & Forger N. G. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol. Sex. Differ. 12, 3–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M. et al. Vulnerability of the neural circuitry underlying sexual behavior to chronic adult exposure to oral bisphenol A in male mice. Endocrinology 155, 502–512 (2014). [DOI] [PubMed] [Google Scholar]
- Nugent B. M. et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 18, 690–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway M. D., Cupp A. S., Uzumcu M. & Skinner M. K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K. & Guerrero-Bosagna C. Role of CpG deserts in the epigenetic transgenerational inheritance of differential DNA methylation regions. BMC Genomics. 15, 692 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison A. E. [Physiology of the gonadotropin-releasing hormone neuronal network] Knobil and Neill’s Physiology of Reproduction [Neill J. D. (ed.)] [1415–1482] (Academic Press, San Diego, 2006). [Google Scholar]
- Schwanzel-Fukuda M. & Pfaff D. W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 338, 161–164 (1989). [DOI] [PubMed] [Google Scholar]
- Clements R. J., Lawrence R. C. & Blank J. L. Effects of intrauterine 2,3,7,8-tetrachlorodibenzo-p-dioxin on the development and function of the gonadotrophin releasing hormone neuronal system in the male rat. Reprod. Toxicol. 28, 38–45 (2009). [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Multigenerational reproductive toxicology study of ethinyl estradiol (CAS No. 57-63-6) in Sprague-Dawley rats [Natl Toxicol Program Tech Rep Ser] [547:1-312] (2010). [PubMed]
- Zhou Y., Zha J., Xu Y., Lei B. & Wang Z. Occurrences of six steroid estrogens from different effluents in Beijing, China. Environ Monit Assess 184, 1719–1729 (2012). [DOI] [PubMed] [Google Scholar]
- Kunz P. Y., Kienle C., Carere M., Homazava N. & Kase R. In vitro bioassays to screen for endocrine active pharmaceuticals in surface and waste waters. J. Pharm. Biomed. Anal. pii : S0731-7085(14) 00545–7 (2014). [DOI] [PubMed] [Google Scholar]
- Simon J. et al. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology 62, 265–278 (2015). [DOI] [PubMed] [Google Scholar]
- Melnick R. et al. Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review. Environ Health Perspect 110, 427–431 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N. Low-dose effects of hormones and endocrine disruptors. Vitam Horm 94, 129–165 (2014). [DOI] [PubMed] [Google Scholar]
- Sato T. et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA 101, 1673–1678 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. et al. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc. Natl. Acad. Sci. USA 26, 12887–12892 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N., Reuss A. E. & Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology 143, 2503–2507 (2002). [DOI] [PubMed] [Google Scholar]
- Herbison A. E. & Pape J. R. New Evidence for Estrogen Receptors in Gonadotropin-Releasing Hormone Neurons. Frontiers in Neuroendocrinology 22, 292–308 (2001). [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N. et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 3, 378–455 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil C. et al. Low dose effects and non-monotonic dose responses for endocrine active chemicals: Science to practice workshop: Workshop summary. Chemosphere 93, 847–856 (2013). [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N. et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol 8, 1–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A. C., Martien K. M., Gagnidze K. & Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr. Rev. 35, 961–991 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. et al. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry. 20, 369–376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashchak C. A. et al. Comparison of pharmacodynamic properties of various estrogen formulations. Am. J. Obstet. Gynecol. 144, 511–518 (1982). [DOI] [PubMed] [Google Scholar]
- Lécureuil C. et al. Transferrin overexpression alters testicular function in aged mice. Mol. Reprod. Dev. 74, 197–206 (2007). [DOI] [PubMed] [Google Scholar]
- Hochereau-de Reviers M. T., Perreau C., Pisselet C., Fontaine I. & Monet-Kuntz C. Comparisons of endocrinological and testis parameters in 18-month-old Ile de France and Romanov rams. Domest. Anim. Endocrinol. 7, 63–73 (1990). [DOI] [PubMed] [Google Scholar]
- Geller S., Kolasa E., Tillet Y., Duittoz A. & Vaudin P. Olfactory ensheathing cells form the microenvironment of migrating GnRH-1 neurons during mouse development. Glia. 61, 550–566 (2013). [DOI] [PubMed] [Google Scholar]
- Franceschini I. et al. Immunohistochemical evidence for the presence of various kisspeptin isoforms in the mammalian brain. J Neuroendocrino. 25, 839–851 (2013). [DOI] [PubMed] [Google Scholar]
- Paxinos G. & Franklin K. B. J. The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, 1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.