Abstract
The importance of the safety of healthy living liver donors is widely recognized during donor hepatectomy which is associated with blood loss, transfusion, and subsequent post-operative morbidity. Although the low central venous pressure (CVP) technique can still be effective, it may not be advantageous concerning the safety of healthy donors undergoing hepatectomy. Emerging evidence suggests that stroke volume variation (SVV), a simple and useful index for fluid responsiveness and preload status in various clinical situations, can be applied as a guide for fluid management to reduce blood loss during living donor hepatectomy. Synthetic colloid solutions are also associated with serious adverse events such as the use of renal replacement therapy and transfusion in critically ill or septic patients. However, it is uncertain whether the intra-operative use of colloid solution is associated with similarly adverse effects in patients undergoing living donor hepatectomy. In this review article we discuss the recent issues regarding the low CVP technique and the high SVV method, i.e., maintaining 10%-20% of SVV, for fluid management in order to reduce blood loss during living donor hepatectomy. In addition, we briefly discuss the effects of intra-operative colloid or crystalloid administration for surgical rather than septic or critically ill patients.
Keywords: Donor hepatectomy, Central venous pressure, Stroke volume variation, Fluid, Synthetic colloid
Core tip: The low central venous pressure technique can still be effective for reducing blood loss during hepatectomy. However, it may not be advantageous regarding the safety of healthy donors undergoing hepatectomy. Therefore, to reduce blood loss during donor hepatectomy, we propose an alternative fluid management technique using a high stroke volume variation method. For the type of fluid, the use of a non-lactate-containing crystalloid solution is advisable during donor hepatectomy. Colloid administration should be carefully determined depending upon each clinical situation of donor hepatectomy, although future studies will be required to elucidate the effect of colloid solutions on donor outcomes.
INTRODUCTION
Living donor liver transplantation (LDLT) is an established treatment modality for end-stage liver disease and it alleviates the shortage of cadaveric donor organs. This approach to organ procurement greatly benefits the recipient population, although the ethical issues associated with donor hepatectomy and the extreme risks of perioperative morbidity and mortality must always be considered[1-3]. The importance of donor safety during hepatectomy is widely recognized, and potential risks during the perioperative period are a major concern of LDLT. The liver transplantation program at Asan Medical Center began in 1992 with deceased donor liver transplantation and now accomplishes more than 300 LDLTs per year (Figure 1)[4]. At our medical institution, perioperative complications associated with general anesthesia and living donor hepatectomy have been reduced and prevented by careful perioperative management as well as by the clinical experience of the anesthesiologists and the surgical staffs[5].
Figure 1.
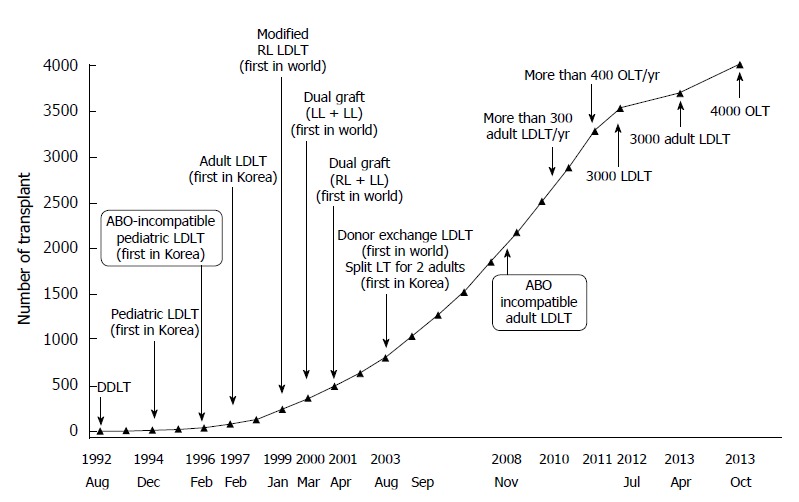
Evolution of liver transplantation at Asan Medical Center, University of Ulsan College of Medicine. From February 1997 to April 2013, 3000 ALDLTs were performed without donor mortality. As of 2010, the annual number of ALDLTs exceeded 300 and with increased performance of ABO-incompatible ALDLT. ALDLT: Adult living donor liver transplantation; DDLT: Deceased donor liver transplantation; LDLT: Living donor liver transplantation; RL: Right-lobe; LL: Left-lobe; LT: Liver transplantation; OLT: Orthotopic liver transplantation. Jan: January; Feb: February; Mar: March; Apr: April; Jul: July; Aug: August; Sep: September; Oct: October; Nov: November; Dec: December. Reprinted with permission[4].
Our article will review the key considerations for the optimal fluid management of living donor hepatectomies for adult-to-adult liver transplantation. These will be based on our clinical experience at Asan Medical Center in Seoul, Korea. We will focus in particular on central venous pressure (CVP) and stroke volume variation (SVV) monitoring and the selection of the appropriate fluid type.
CVP-guided fluid management in living donor hepatectomy
In general, hepatectomy inevitably causes a significant blood loss and transfusion requirement, which are among the major causes of post-operative morbidity and mortality[6-10]. In addition to the many surgical techniques which attempt to reduce blood loss during hepatectomy, fluid management to aid in the reduction of operative bleeding has also been developed. Careful fluid management is believed to be one of the important strategies to minimize blood loss during hepatectomy. Various anesthetic methods to reduce blood loss during living donor hepatectomy include acute normovolemic intraoperative hemodilution with autologous blood donation[11-13], intraoperative cell salvage[13], low CVP technique[14], and the high SVV method[15]. Of these, fluid management, such as lowering CVP and maintaining high SVV, is simpler and easier to perform as it has no requirement for specific devices, equipment or additional personnel.
Following the first prospective report of Jones et al[16], maintaining a low CVP is widely used to diminish intraoperative blood loss in some anesthetic protocols of hepatectomy[17-19]. Because CVP is thought to reflect hepatic sinusoid pressure, lowering CVP during liver resection can reduce hepatic parenchymal congestion and subsequent blood loss by helping to control hepatic venous hemorrhage[16,17]. It is known that the target CVP during hepatectomy is 5 mmHg or less, and which has been shown to reduce intraoperative blood loss, the need for blood transfusions, morbidity, and mortality[16-18,20]. A low CVP can be simply produced and maintained by fluid restriction, although there is no study evaluating the lowest safe rate of fluid infusion. For example, Wang et al[20] reported that maintenance of CVP ≤ 4 mmHg by reduction of the fluid infusion rate to near 75 mL/h decreased blood loss during hepatectomy and shortened the length of the patient hospital stay without detrimental effects on hepatic or renal function. A low CVP has also been achieved by elective addition of pharmacologic agents such as nitroglycerin[20-22], morphine[23], milrinone[14], and furosemide[14,20,24]. In addition, the low CVP technique used during the pre-anhepatic phase of liver transplantation surgery reduces intraoperative blood loss as well as protecting the liver function and having no detrimental effects on renal function[25]. On the contrary, a low CVP strategy was associated with higher mortality and morbidity rates among orthotopic liver transplant recipients[23].
However, several lines of evidence have demonstrated that CVP did not correlate with blood loss during hepatectomy of healthy living donors[13,26-28]. For example, CVP during hepatic resection was not associated with intraoperative blood loss in living liver donors[13,27]. In addition, the amount of intraoperative blood loss was not significantly reduced in donors with relatively low CVP during living donor hepatectomy[26,28]. An evaluation of nearly 1000 living liver donors in our medical institution has revealed that factors associated with intraoperative blood loss were patient gender, body weight, and fatty changes in the liver, although not the CVP level during hepatectomy[28]. Furthermore, Niemann et al[27] found that blood loss was similar in donors with and without CVP monitoring, and thus suggesting CVP monitoring may not be necessary in a highly selected patient population, such as living donors[27]. It was also reported that CVP did not correlate with the preload status in normal healthy volunteers[29]. Discrepancies regarding the usefulness of the low CVP technique to reduce blood loss may arise from differences in patient populations. The hemorrhagic tendency of liver tissue in patients with benign or malignant hepatic lesions may differ from that in healthy living liver donors.
In various surgical situations, CVP measurements may be inaccurate, although the correct measurement of CVP is crucial for its proper application[30]. A critical review has assessed the correct placement of pressure transducer for CVP measurement in the physiological context[30]. The authors of this review used a questionnaire among intensivists and anesthesiologists in order to determine the correct alignment of the pressure transducer to the phlebostatic axis (a horizontal plane through the tricuspid valve). Surprisingly, only 3.4% of the respondents identified the correct phlebostatic axis for CVP measurement. Furthermore, 45.6% of the respondents mentioned that CVP had no clear-cut relation to the intravascular volume status or to changes in volume. CVP can be affected by factors, including the patient position, surgical liver manipulation, intrathoracic pressure [including positive end-expiratory pressure (PEEP)], pulmonary disease, tricuspid valve disease, pericardial pressure, and intra-abdominal pressure[30-32]. Indeed, during hepatectomy, liver manipulation, occasional clamping of the inferior vena cava (IVC), hepatic veins or even the portal vein by the surgeon, and relatively frequent patient position changes can cause confusion regarding the correct measurement of the CVP value. It has also been noted that head-down tilting is frequently used during maintenance of low CVP[20,21]. Sand et al[22,33] reported that there was a dissociation between CVP and hepatic venous pressure in a head-down tilt (Trendelenburg’s position) at both 5 cmH2O PEEP and zero end-expiratory pressure. They suggested that CVP cannot be used as a surrogate for both portal and hepatic venous pressures in the head-down position.
More importantly, potential fatal consequences of the low CVP technique during hepatectomy include air embolism and unnecessary hypoperfusion[21,34], although a head-down tilt position is frequently used to prevent possible air embolism and to improve the venous return[21,22]. It can be reasonably expected that a negative CVP can allow large volumes of air through small or unrecognized, opened hepatic veins during hepatic parenchymal resection. In fact, an earlier report by Jones et al[16] also documented that a small air embolism was suspected in two patients (5% of patients maintaining a low CVP). It should be also kept in mind that central venous catheterization is associated with its own morbidity[35,36]. The overall complication rate of central vein catheterization was from 5% to more than 26% in patients who were recommended for treatment or monitoring[36]. Even in living liver donors, central venous catheter-related thrombosis has been observed[37].
Taken together, it is suggested that a low CVP technique used during living donor hepatectomy may not be advantageous regarding the safety of healthy living donors undergoing hepatic resection. Safer, simpler, and more useful fluid management methods are, therefore, required in order to reduce blood loss and subsequent morbidity during living donor hepatectomy.
SVV-guided fluid management during living donor hepatectomy
SVV is one of the dynamic parameters of hemodynamic monitoring used to access the volume status and fluid responsiveness in mechanically ventilated patients. SVV is currently accepted as a simple and sensitive indicator for evaluating fluid responsiveness and the preload status in various clinical settings[38-44]. Our group found that SVV may be an independent hemodynamic predictor of intraoperative blood loss ≥ 700 mL, as well as being a valuable functional preload index during living donor hepatectomy[45]. Dunki-Jacobs et al[46] demonstrated that CVP values significantly correlated to SVV value, thus implying that SVV can be used as an alternative to CVP monitoring during hepatic resection in patients with diseased, but not normal, liver. The usefulness of the SVV value was also reported in patients undergoing laparoscopic liver resection[47]. Kitaguchi et al[47] found that CVP of -1 to 1 mmHg was significantly correlated to an SVV of 18%-21% (R2 = 0.85, P < 0.001), and thus indicating a safe alternative to CVP monitoring during hepatic resection and with equivalent outcomes in blood loss. In addition, when IVC with or without the portal triad was clamped during hepatic resection, the SVV was significantly higher in patients with lower blood loss than in those with higher blood loss[48]. Therefore, SVV may be used as a guide for fluid management in order to reduce blood loss during living donor hepatectomy.
To demonstrate this concept, we recently performed a randomized controlled study and compared the effects of SVV 10%-20% (high SVV) and < 10% SVV (control) on blood loss during living donor right hepatectomy[15]. The mean blood loss during living donor right hepatectomy was remarkably lower in the high SVV group than in the control group (475.5 ± 131.2 mL vs 835.5 ± 341.1 mL, P < 0.001; Figure 2). There was also no significant between-group difference observed in the perioperative laboratory values such as hemoglobin, the lactate and creatinine levels, liver function test, and coagulation profiles, the incidence of intraoperative hypotensive episode and mean arterial blood pressure during hepatectomy. Neither group of donors experienced renal failure or death. All donors were discharged from the hospital without any major complications.
Figure 2.
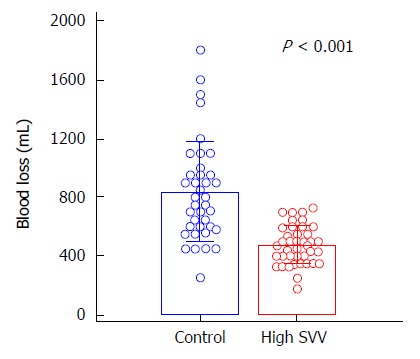
Effect of stroke volume variation-directed fluid management on blood loss during living donor right hepatectomy. The control group (< 10% SVV) received usual fluid management (6-10 mL/kg per hour during surgery), whereas the high SVV group underwent fluid restriction (2-4 mL/kg per hour until completion of hepatectomy) with SVV ranging from 10% to 20%. Blood loss was significantly lower in the high SVV than in the control group. SVV: Stroke volume variation. Reproduced data with permission[15].
We determined high SVV as 10%-20% based on previous studies[41,44] and our numerous experiences with living donor hepatectomy over 20 years. In our previous study, the median SVV of the high SVV group was 11.7% (11.0%-13.0%) [vs 5.3% (4.7%-6.3%) in the control group] and was safely maintained[15]. Therefore, in order to reduce blood loss during living donor hepatectomy, we propose a fluid management technique using a high SVV method.
Proposed algorithm of SVV-guided fluid management
SVV can be safely maintained at 10%-20% with simple fluid restriction and optional administration of a small dose of diuretics during living donor hepatectomy, in accordance with our previous study results[15] and clinical experience. A proposed fluid management algorithm during living donor hepatectomy is shown in Figure 3.
Figure 3.
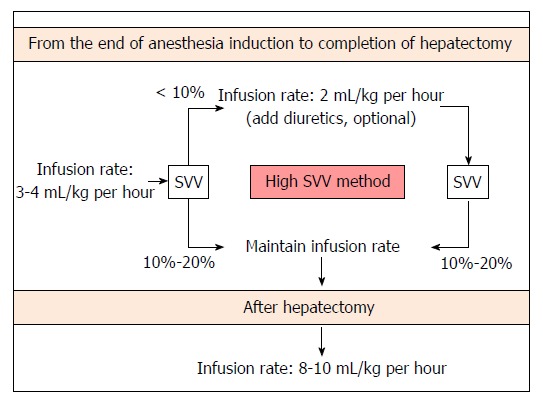
Proposed algorithm of stroke volume variation-guided fluid management during living donor hepatectomy. SVV: Stroke volume variation. Modified with permission[15].
Neither replacement of a volume deficit from fasting nor compensatory intravascular volume expansion is conducted prior to induction of anesthesia when using our protocol. Donors’ lungs are mechanically ventilated in order to maintain a constant tidal volume of 8-10 mL/kg. We do not apply PEEP during mechanical ventilation because SVV is obviously influenced by PEEP[49,50]. After anesthesia induction, fluid restriction is begun and is continued until the completion of hepatectomy. The fluid is administered intraoperatively using crystalloid (plasmalyte) and colloid solutions (albumin). Colloid solution is administered only after hepatectomy for volume replacement. At first, fluid administration is restricted with the infusion rate of 3-4 mL/kg per hour until completion of hepatectomy in order to maintain an SVV of 10%-20%. If the donor’s SVV was < 10% during this period, the infusion rate was decreased to 2 mL/kg per hour and furosemide (max. 40 mg) was optionally administered according to the clinicians’ decision. After completion of hepatectomy, fluid replacement is maintained with the infusion of a colloid or crystalloid solution at a rate of 8-10 mL/kg per hour. Systolic arterial blood pressure is maintained at ≥ 90 mmHg. If systolic arterial blood pressure decreased further < 80 mmHg during donor hepatectomy, ephedrine and additional fluid are administered. Urine output is maintained at ≥ 0.5 mL/kg per hour. Hemoglobin concentration is maintained at ≥ 7 g/dL; if the hemoglobin concentration is < 7 g/dL, transfusion of packed red blood cells is planned.
Furosemide may be optionally administered in order to achieve high SVV according to our protocol. However, furosemide may induce acute kidney injury. Several reviews in diseased subjects have found that rapid or early excessive fluid removal using diuretics, and which leads to hypovolemia, may be associated with an increased risk for acute kidney injury[51,52]. In addition, furosemide has not shown significant clinical benefits in the prevention or treatment of acute kidney injury[51,52]. In our recent clinical trial, healthy living liver donors showed no significant changes in perioperative serum creatinine levels regardless of furosemide administration[15]. This discrepancy may be related, at least in part, to the investigated population. Although a relatively small dose of adjuvant furosemide may be used with care in order to maintain high SVV in healthy living donors, further studies concerning effective and safe alternative drugs for maintaining high SVV of 10%-20% will be required.
We reported in a large cohort study that the preoperative hemoglobin level and graft-to-donor weight ratio were useful independent predictors of blood transfusion in living donor hepatectomy[53]. Preoperatively moderate anemia (hemoglobin < 11 g/dL, but ≥ 8 g/dL) was observed in 1.4% of living liver donors[53]. Thus, the high SVV method can be recommended for living liver donors with risk factors such as anemia in order to reduce blood loss during donor hepatectomy as well as subsequent blood transfusion requirements.
However, this parameter has several possible limitations because SVV utilizes cardiorespiratory interaction over a mechanically ventilated respiratory cycle[43,54-56]. First, the patient must be mechanically ventilated without any spontaneous breathing. Second, the tidal volume should be ≥ 8 mL/kg of the ideal body weight. Third, several clinical conditions, such as cardiac arrhythmia, preclude the use of this parameter.
Types of fluids in living donor hepatectomy
Numerous recent reviews and meta-analyses have reported that synthetic colloids than crystalloids or albumin seemed to increase the need for renal replacement therapy and blood transfusion and resulted in more serious adverse events while providing no overall clinical benefits for critically ill or septic patients[57-63]. However, it is uncertain whether the intraoperative use of colloid solution is associated with similarly adverse effects in patients undergoing surgery, as shown Table 1. A meta-analysis of 17 randomized studies evaluating the renal safety of hydroxyethyl starches (HES) 130/0.40 in surgical patients observed no evidence of renal dysfunction[64]. Another meta-analysis from Gillies et al[65] found no difference in the incidence of death or AKI in surgical patients receiving 6% HES. However, they did not recommend the use of 6% HES solution in surgical patients because of the absence of demonstrable benefits[65]. Cytokines and other inflammatory mediators produced in a septic condition can induce gaps between endothelial cells, thus resulting in microvascular leak and tissue edema[66]. Because the endothelial permeability of septic patients differs from that in the healthy population, harmful or no beneficial results from these critically ill patients cannot be extended in living liver donors or elective surgical patients. In trauma patients having similar pathophysiology as that of surgical patients, it has been reported that fluid resuscitation with colloid solution decreased both the morbidity and mortality[67,68]. Currently, there is few report evaluating the outcomes of the intraoperative use of colloid solutions in living donors undergoing hepatectomy. Further studies will be required in order to demonstrate either the significant benefits or harms associated with the administration of synthetic colloid solutions in living liver donors. Therefore, administration of colloid solution should be carefully determined depending upon each clinical situation of living donor hepatectomy.
Table 1.
Controversial effects of intraoperative colloid administration on the outcomes of surgical patients
| Ref. | Study design | Types of surgery (n) | Types of colloid | Clinical outcomes of colloid |
| Mukhtar et al[77] | RCT | Living donor liver transplantation (40) | 6% HES 130/0.4 vs Alb | No difference in renal outcome |
| Rioux et al[78] | Retrospective, propensity score matching | Cardiac surgery (254) | 10% pentastarch 250/0.45 (< 14 mL/kg vs ≥ 14 mL/kg) | Dose-dependent renal toxicity |
| Hokema et al[79] | Retrospective | Renal transplantation (113) | 6% HES 130/0.4 vs NS or RA | No difference in delayed renal graft function |
| Martin et al[64] | Meta-analysis | Various surgical procedures (1230) | 6% HES 130/0.4 vs comparators1 | No differences in renal outcome and length of hospital stay |
| Canet et al[80] | Prospective observational, propensity score matching, multicenter | General surgery (2462) | Colloids2 vs crystalloids | Increased pulmonary or cardiovascular complications and length of hospital stay |
| No difference in mortality | ||||
| Van der Linden et al[81] | RCT, double-blind, two-center | Pediatric cardiac surgery (61) | 6% HES 130/0.4 vs Alb | No differences in complications |
| Skhirtladze et al[82] | RCT, double-blind | Cardiac surgery (236) | 6% HES 130/0.4 vs Alb vs RL | Both colloids increased blood transfusion, decreased coagulation and increased the creatinine level |
| Rasmussen et al[83] | RCT | Cystectomy (33) | 6% HES 130/0.4 vs RL | Increased blood loss and decreased coagulation |
| No differences in re-operation and hospital stay | ||||
| Kancir et al[84] | RCT | Hip arthroplasty (38) | 6% HES 130/0.4 vs NS | No harmful effect on renal function |
| Lindroos et al[85] | RCT | Neurosurgery (30) | 6% HES 130/0.4 vs RA | Decreased coagulation |
| No differences in blood loss | ||||
| Yates et al[86] | RCT, double-blind | Colorectal surgery (202) | 6% HES 130/0.4 vs RL | No differences in complications |
| Gillies et al[65] | Meta-analysis | Non-cardiac surgery (1567) | 6% HES vs comparators1 | No differences in mortality or AKI |
| Kashy et al[87] | Retrospective, propensity score matching | Non-cardiac and non-urological surgery (29360) | Colloids3 vs crystalloids | Dose-dependent renal toxicity |
| Mercier et al[88] | RCT, double-blind, multicenter | Caesarean section (167) | 6% HES 130/0.4 vs RL | Same effects in both mother and neonate |
| Hand et al[89] | Retrospective, propensity score matching | Deceased donor liver transplantation (174) | 6% HES 130/0.4 vs Alb | 6% HES increase risk of AKI |
| Kancir et al[90] | RCT, double-blind | Radical prostatectomy (36) | 6% HES 130/0.4 vs NS | No difference in renal outcome |
| Choi et al[91] | Retrospective | Living donor hepatectomy (1969 ) | 6% HES 130/0.4 vs crystalloids | Postoperative delayed recovery of hepatic function |
Comparators included crystalloid solutions, gelatin solutions, and albumin;
Mainly 6% HES 130/0.4;
Mainly 6% hexastarch 670/0.75. AKI: Acute kidney injury; Alb: 5% human albumin; HES: Hydroxyethyl starch; NS: Normal saline; RA: Ringer’s acetate solution; RCT: Randomized controlled trial; RL: Ringer’s lactate solution.
Crystalloid solutions are extensively used to maintain and replace the intravascular volume in a variety of surgical procedures performed under general anesthesia. When a large amount of normal saline containing no buffer or other electrolytes is administered during surgery, a hyperchloremic metabolic acidosis can result, thus causing increased morbidity and a longer hospital stay following major surgery[69-71]. In living liver donors, normal saline administration during hepatectomy may be detrimental due to its association with hyperchloremic metabolic acidosis[26,72]. Ringer’s lactate (RL) solution which contains 28 mmol/L of lactate and has a pH of 6.5, is one of the most preferred crystalloids due to its similar electrolyte composition to extracellular fluid as well as its buffering effect[72]. However, it has been shown that the infusion of a large volume of RL solution during major spine surgery caused postoperative mild hyponatremia and respiratory acidosis, although it did not induce hyperchloremic metabolic acidosis as does normal saline[73]. A recent report conducted following major abdominal surgery found that total infused saline and the lactate level were independent factors related to metabolic acidosis[71]. Due to the increased concentration of lactate after living donor hepatectomy[74], the RL solution should be carefully infused in living donors undergoing extensive hepatic dissection. Because hepatic blood flow and the capacity of the liver to metabolize lactate may change during and after hepatectomy[75], additional administration of exogenous lactate may increase the lactate concentration[5]. Shin et al[76] reported a prospective study comparing the effects of two, crystalloid solutions with and without lactate on the liver function test data and the serum lactate level in living donors undergoing right hepatectomy. In that study, median lactate concentrations 1 h after hepatectomy were significantly higher in donors with RL solution than with the plasmalyte, a balanced solution of pH 7.4 and containing acetate and gluconate (rather than lactate) as bicarbonate precursors [4.2 (3.2-5.7) mmol/L vs 3.3 (2.6-4.6) mmol/L, respectively][76]. Therefore, the use of non-lactate-containing crystalloid solution, such as plasmalyte, is advisable during living donor hepatectomy in order to avoid induction of hyperlactatemia and hyperchloremia[5,76].
CONCLUSION
The importance of precise fluid management in order to reduce blood loss during hepatectomy in healthy living liver donors cannot be overemphasized. Although a low CVP technique may be still effective, we propose the high SVV method, i.e., maintaining 10%-20% of SVV for fluid management in order to reduce blood loss during living donor hepatectomy. The non-lactate-containing crystalloid solution is also advisable for use during living donor hepatectomy. However, intraoperative administration of synthetic colloid solution should be carefully determined depending on each clinical situation of living donor hepatectomy. Future studies will be required in order to elucidate the effect of colloid solutions on the outcomes of living liver donors undergoing hepatectomy.
ACKNOWLEDGMENTS
The authors thank Professors, Gyu-Sam Hwang (Department of Anesthesiology and Pain Medicine), Sung-Gyu Lee, Shin Hwang, and Clinical Associate Professor, Tae-Yong Ha (Division of Liver Transplantation and Hepatobiliary Surgery, Department of Surgery, Asan Medical Center, Seoul, South Korea) for preparing this review article.
Footnotes
Conflict-of-interest statement: The authors report no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 7, 2015
First decision: June 2, 2015
Article in press: October 20, 2015
P- Reviewer: De Raffele E S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Abecassis MM, Fisher RA, Olthoff KM, Freise CE, Rodrigo DR, Samstein B, Kam I, Merion RM; A2ALL Study Group. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant. 2012;12:1208–1217. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coelho JC, de Freitas AC, Matias JE, de Godoy JL, Zeni Neto C, Parolin MB, Okawa L. Donor complications including the report of one death in right-lobe living-donor liver transplantation. Dig Surg. 2007;24:191–196. doi: 10.1159/000102898. [DOI] [PubMed] [Google Scholar]
- 3.Iida T, Ogura Y, Oike F, Hatano E, Kaido T, Egawa H, Takada Y, Uemoto S. Surgery-related morbidity in living donors for liver transplantation. Transplantation. 2010;89:1276–1282. doi: 10.1097/TP.0b013e3181d66c55. [DOI] [PubMed] [Google Scholar]
- 4.Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17–38. doi: 10.1111/ajt.12907. [DOI] [PubMed] [Google Scholar]
- 5.Hwang GS, McCluskey SA. Anesthesia and outcome after partial hepatectomy for adult-to-adult donor transplantation. Curr Opin Organ Transplant. 2010;15:377–382. doi: 10.1097/MOT.0b013e3283387f75. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 8.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, Liu YW, Yong CC, Concejero A, Jawan B, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transpl. 2006;12:950–957. doi: 10.1002/lt.20746. [DOI] [PubMed] [Google Scholar]
- 10.Kusano T, Sasaki A, Kai S, Endo Y, Iwaki K, Shibata K, Ohta M, Kitano S. Predictors and prognostic significance of operative complications in patients with hepatocellular carcinoma who underwent hepatic resection. Eur J Surg Oncol. 2009;35:1179–1185. doi: 10.1016/j.ejso.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Balci ST, Pirat A, Torgay A, Cinar O, Sevmis S, Arslan G. Effect of restrictive fluid management and acute normovolemic intraoperative hemodilution on transfusion requirements during living donor hepatectomy. Transplant Proc. 2008;40:224–227. doi: 10.1016/j.transproceed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Jawan B, Cheng YF, Tseng CC, Chen YS, Wang CC, Huang TL, Eng HL, Liu PP, Chiu KW, Wang SH, et al. Effect of autologous blood donation on the central venous pressure, blood loss and blood transfusion during living donor left hepatectomy. World J Gastroenterol. 2005;11:4233–4236. doi: 10.3748/wjg.v11.i27.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz JT, Valentín-Gamazo C, Görlinger K, Malagó M, Peters J. Blood-transfusion requirements and blood salvage in donors undergoing right hepatectomy for living related liver transplantation. Anesth Analg. 2003;96:351–355. doi: 10.1097/00000539-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Ryu HG, Nahm FS, Sohn HM, Jeong EJ, Jung CW. Low central venous pressure with milrinone during living donor hepatectomy. Am J Transplant. 2010;10:877–882. doi: 10.1111/j.1600-6143.2010.03051.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi SS, Jun IG, Cho SS, Kim SK, Hwang GS, Kim YK. Effect of stroke volume variation-directed fluid management on blood loss during living-donor right hepatectomy: a randomised controlled study. Anaesthesia. 2015;70:1250–1258. doi: 10.1111/anae.13155. [DOI] [PubMed] [Google Scholar]
- 16.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–1060. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Sun YM, Wu FX, Yang LQ, Lu ZJ, Yu WF. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol. 2014;20:303–309. doi: 10.3748/wjg.v20.i1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81–88. doi: 10.1002/jso.23455. [DOI] [PubMed] [Google Scholar]
- 19.Mise Y, Sakamoto Y, Ishizawa T, Kaneko J, Aoki T, Hasegawa K, Sugawara Y, Kokudo N. A worldwide survey of the current daily practice in liver surgery. Liver Cancer. 2013;2:55–66. doi: 10.1159/000346225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–939. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 22.Sand L, Lundin S, Rizell M, Wiklund J, Stenqvist O, Houltz E. Nitroglycerine and patient position effect on central, hepatic and portal venous pressures during liver surgery. Acta Anaesthesiol Scand. 2014;58:961–967. doi: 10.1111/aas.12349. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder RA, Collins BH, Tuttle-Newhall E, Robertson K, Plotkin J, Johnson LB, Kuo PC. Intraoperative fluid management during orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2004;18:438–441. doi: 10.1053/j.jvca.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Kim YK, Shin WJ, Song JG, Jun IG, Kim HY, Seong SH, Sang BH, Hwang GS. Factors associated with changes in coagulation profiles after living donor hepatectomy. Transplant Proc. 2010;42:2430–2435. doi: 10.1016/j.transproceed.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 25.Feng ZY, Xu X, Zhu SM, Bein B, Zheng SS. Effects of low central venous pressure during preanhepatic phase on blood loss and liver and renal function in liver transplantation. World J Surg. 2010;34:1864–1873. doi: 10.1007/s00268-010-0544-y. [DOI] [PubMed] [Google Scholar]
- 26.Chhibber A, Dziak J, Kolano J, Norton JR, Lustik S. Anesthesia care for adult live donor hepatectomy: our experiences with 100 cases. Liver Transpl. 2007;13:537–542. doi: 10.1002/lt.21074. [DOI] [PubMed] [Google Scholar]
- 27.Niemann CU, Feiner J, Behrends M, Eilers H, Ascher NL, Roberts JP. Central venous pressure monitoring during living right donor hepatectomy. Liver Transpl. 2007;13:266–271. doi: 10.1002/lt.21051. [DOI] [PubMed] [Google Scholar]
- 28.Kim YK, Chin JH, Kang SJ, Jun IG, Song JG, Jeong SM, Park JY, Hwang GS. Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol Scand. 2009;53:601–606. doi: 10.1111/j.1399-6576.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–699. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 30.Sondergaard S, Parkin G, Aneman A. Central venous pressure: we need to bring clinical use into physiological context. Acta Anaesthesiol Scand. 2015;59:552–560. doi: 10.1111/aas.12490. [DOI] [PubMed] [Google Scholar]
- 31.Giordano C, Deitte LA, Gravenstein N, Rice MJ. What is the preferred central venous pressure zero reference for hepatic resection? Anesth Analg. 2010;111:660–664. doi: 10.1213/ANE.0b013e3181c76d3e. [DOI] [PubMed] [Google Scholar]
- 32.Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–748. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- 33.Sand L, Rizell M, Houltz E, Karlsen K, Wiklund J, Odenstedt Hergès H, Stenqvist O, Lundin S. Effect of patient position and PEEP on hepatic, portal and central venous pressures during liver resection. Acta Anaesthesiol Scand. 2011;55:1106–1112. doi: 10.1111/j.1399-6576.2011.02502.x. [DOI] [PubMed] [Google Scholar]
- 34.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Kusminsky RE. Complications of central venous catheterization. J Am Coll Surg. 2007;204:681–696. doi: 10.1016/j.jamcollsurg.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 36.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 37.Hata T, Fujimoto Y, Suzuki K, Kim B, Ishigami M, Ogawa H, Arikawa T, Nagai S, Kamei H, Nakamura T, et al. Two cases of central venous catheter-related thrombosis in living liver donors: how can the risk be minimized? Clin Transplant. 2009;23:289–293. doi: 10.1111/j.1399-0012.2008.00939.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25:904–916. doi: 10.1007/s00540-011-1217-1. [DOI] [PubMed] [Google Scholar]
- 39.Kim YK, Shin WJ, Song JG, Jun IG, Kim HY, Seong SH, Hwang GS. Comparison of stroke volume variations derived from radial and femoral arterial pressure waveforms during liver transplantation. Transplant Proc. 2009;41:4220–4228. doi: 10.1016/j.transproceed.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 40.Xia J, He Z, Cao X, Che X, Chen L, Zhang J, Liang W. The brain relaxation and cerebral metabolism in stroke volume variation-directed fluid therapy during supratentorial tumors resection: crystalloid solution versus colloid solution. J Neurosurg Anesthesiol. 2014;26:320–327. doi: 10.1097/ANA.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 41.Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F. Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 2008;101:761–768. doi: 10.1093/bja/aen277. [DOI] [PubMed] [Google Scholar]
- 42.Biais M, Nouette-Gaulain K, Roullet S, Quinart A, Revel P, Sztark F. A comparison of stroke volume variation measured by Vigileo/FloTrac system and aortic Doppler echocardiography. Anesth Analg. 2009;109:466–469. doi: 10.1213/ane.0b013e3181ac6dac. [DOI] [PubMed] [Google Scholar]
- 43.Slagt C, Malagon I, Groeneveld AB. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth. 2014;112:626–637. doi: 10.1093/bja/aet429. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Hwang GS, Kim SO, Kim YK. Is stroke volume variation a useful preload index in liver transplant recipients? A retrospective analysis. Int J Med Sci. 2013;10:751–757. doi: 10.7150/ijms.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YK, Shin WJ, Song JG, Jun IG, Hwang GS. Does stroke volume variation predict intraoperative blood loss in living right donor hepatectomy? Transplant Proc. 2011;43:1407–1411. doi: 10.1016/j.transproceed.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 46.Dunki-Jacobs EM, Philips P, Scoggins CR, McMasters KM, Martin RC. Stroke volume variation in hepatic resection: a replacement for standard central venous pressure monitoring. Ann Surg Oncol. 2014;21:473–478. doi: 10.1245/s10434-013-3323-9. [DOI] [PubMed] [Google Scholar]
- 47.Kitaguchi K, Gotohda N, Yamamoto H, Kato Y, Takahashi S, Konishi M, Hayashi R. Intraoperative circulatory management using the FloTrac™ system in laparoscopic liver resection. Asian J Endosc Surg. 2015;8:164–170. doi: 10.1111/ases.12158. [DOI] [PubMed] [Google Scholar]
- 48.Harimoto N, Matsuyama H, Kajiyama K, Nagaie T, Ikegami T, Yoshizumi T, Soejima Y, Shirabe K, Ikeda T, Kawanaka H, et al. Significance of stroke volume variation during hepatic resection under infrahepatic inferior vena cava and portal triad clamping. Fukuoka Igaku Zasshi. 2013;104:362–369. [PubMed] [Google Scholar]
- 49.Kubitz JC, Annecke T, Kemming GI, Forkl S, Kronas N, Goetz AE, Reuter DA. The influence of positive end-expiratory pressure on stroke volume variation and central blood volume during open and closed chest conditions. Eur J Cardiothorac Surg. 2006;30:90–95. doi: 10.1016/j.ejcts.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Michard F, Chemla D, Richard C, Wysocki M, Pinsky MR, Lecarpentier Y, Teboul JL. Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med. 1999;159:935–939. doi: 10.1164/ajrccm.159.3.9805077. [DOI] [PubMed] [Google Scholar]
- 51.Ejaz AA, Mohandas R. Are diuretics harmful in the management of acute kidney injury? Curr Opin Nephrol Hypertens. 2014;23:155–160. doi: 10.1097/01.mnh.0000441150.17202.be. [DOI] [PubMed] [Google Scholar]
- 52.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 53.Choi SS, Cho SS, Kim SH, Jun IG, Hwang GS, Kim YK. Factors associated with blood transfusion in donor hepatectomy: results from 2344 donors at a large single center. Transplantation. 2013;96:1000–1007. doi: 10.1097/TP.0b013e3182a41937. [DOI] [PubMed] [Google Scholar]
- 54.Michard F, Biais M. Rational fluid management: dissecting facts from fiction. Br J Anaesth. 2012;108:369–371. doi: 10.1093/bja/aer511. [DOI] [PubMed] [Google Scholar]
- 55.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 56.McGee WT, Raghunathan K. Physiologic goal-directed therapy in the perioperative period: the volume prescription for high-risk patients. J Cardiothorac Vasc Anesth. 2013;27:1079–1086. doi: 10.1053/j.jvca.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 57.Patel A, Laffan MA, Waheed U, Brett SJ. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014;349:g4561. doi: 10.1136/bmj.g4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel A, Waheed U, Brett SJ. Randomised trials of 6% tetrastarch (hydroxyethyl starch 130/0.4 or 0.42) for severe sepsis reporting mortality: systematic review and meta-analysis. Intensive Care Med. 2013;39:811–822. doi: 10.1007/s00134-013-2863-6. [DOI] [PubMed] [Google Scholar]
- 59.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;2:CD000567. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 60.Serpa Neto A, Veelo DP, Peireira VG, de Assunção MS, Manetta JA, Espósito DC, Schultz MJ. Fluid resuscitation with hydroxyethyl starches in patients with sepsis is associated with an increased incidence of acute kidney injury and use of renal replacement therapy: a systematic review and meta-analysis of the literature. J Crit Care. 2014;29:185.e1–185.e7. doi: 10.1016/j.jcrc.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 61.Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, Fergusson DA. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 62.Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, Wetterslev J. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346:f839. doi: 10.1136/bmj.f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2012;144:223–230. doi: 10.1016/j.jtcvs.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Martin C, Jacob M, Vicaut E, Guidet B, Van Aken H, Kurz A. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology. 2013;118:387–394. doi: 10.1097/ALN.0b013e31827e5569. [DOI] [PubMed] [Google Scholar]
- 65.Gillies MA, Habicher M, Jhanji S, Sander M, Mythen M, Hamilton M, Pearse RM. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis. Br J Anaesth. 2014;112:25–34. doi: 10.1093/bja/aet303. [DOI] [PubMed] [Google Scholar]
- 66.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 67.James MF, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma) Br J Anaesth. 2011;107:693–702. doi: 10.1093/bja/aer229. [DOI] [PubMed] [Google Scholar]
- 68.Ogilvie MP, Pereira BM, McKenney MG, McMahon PJ, Manning RJ, Namias N, Livingstone AS, Schulman CI, Proctor KG. First report on safety and efficacy of hetastarch solution for initial fluid resuscitation at a level 1 trauma center. J Am Coll Surg. 2010;210:870–880. doi: 10.1016/j.jamcollsurg.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 69.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 70.Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Park CM, Chun HK, Jeon K, Suh GY, Choi DW, Kim S. Factors related to post-operative metabolic acidosis following major abdominal surgery. ANZ J Surg. 2014;84:574–580. doi: 10.1111/j.1445-2197.2012.06235.x. [DOI] [PubMed] [Google Scholar]
- 72.Stephens R, Mythen M. Optimizing intraoperative fluid therapy. Curr Opin Anaesthesiol. 2003;16:385–392. doi: 10.1097/01.aco.0000084478.59960.76. [DOI] [PubMed] [Google Scholar]
- 73.Takil A, Eti Z, Irmak P, Yilmaz Göğüş F. Early postoperative respiratory acidosis after large intravascular volume infusion of lactated ringer’s solution during major spine surgery. Anesth Analg. 2002;95:294–298. doi: 10.1097/00000539-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Khalaf H, Al-Sofayan M, El-Sheikh Y, Al-Bahili H, Al-Sagheir M, Al-Sebayel M. Donor outcome after living liver donation: a single-center experience. Transplant Proc. 2007;39:829–834. doi: 10.1016/j.transproceed.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Chioléro R, Tappy L, Gillet M, Revelly JP, Roth H, Cayeux C, Schneiter P, Leverve X. Effect of major hepatectomy on glucose and lactate metabolism. Ann Surg. 1999;229:505–513. doi: 10.1097/00000658-199904000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests after living donor right hepatectomy: a comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011;55:558–564. doi: 10.1111/j.1399-6576.2011.02398.x. [DOI] [PubMed] [Google Scholar]
- 77.Mukhtar A, Aboulfetouh F, Obayah G, Salah M, Emam M, Khater Y, Akram R, Hoballah A, Bahaa M, Elmeteini M, et al. The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg. 2009;109:924–930. doi: 10.1213/ane.0b013e3181aed54f. [DOI] [PubMed] [Google Scholar]
- 78.Rioux JP, Lessard M, De Bortoli B, Roy P, Albert M, Verdant C, Madore F, Troyanov S. Pentastarch 10% (250 kDa/0.45) is an independent risk factor of acute kidney injury following cardiac surgery. Crit Care Med. 2009;37:1293–1298. doi: 10.1097/CCM.0b013e31819cc1a0. [DOI] [PubMed] [Google Scholar]
- 79.Hokema F, Ziganshyna S, Bartels M, Pietsch UC, Busch T, Jonas S, Kaisers U. Is perioperative low molecular weight hydroxyethyl starch infusion a risk factor for delayed graft function in renal transplant recipients? Nephrol Dial Transplant. 2011;26:3373–3378. doi: 10.1093/ndt/gfr017. [DOI] [PubMed] [Google Scholar]
- 80.Canet J, Sabaté S, Mazo V. Effects of intraoperative colloid administration on outcome in a population-based general surgical cohort: a propensity score analysis. Minerva Anestesiol. 2013;79:891–905. [PubMed] [Google Scholar]
- 81.Van der Linden P, De Villé A, Hofer A, Heschl M, Gombotz H. Six percent hydroxyethyl starch 130/0.4 (Voluven®) versus 5% human serum albumin for volume replacement therapy during elective open-heart surgery in pediatric patients. Anesthesiology. 2013;119:1296–1309. doi: 10.1097/ALN.0b013e3182a6b387. [DOI] [PubMed] [Google Scholar]
- 82.Skhirtladze K, Base EM, Lassnigg A, Kaider A, Linke S, Dworschak M, Hiesmayr MJ. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer’s lactate on blood loss and coagulation after cardiac surgery. Br J Anaesth. 2014;112:255–264. doi: 10.1093/bja/aet348. [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen KC, Johansson PI, Højskov M, Kridina I, Kistorp T, Thind P, Nielsen HB, Ruhnau B, Pedersen T, Secher NH. Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg. 2014;259:249–254. doi: 10.1097/SLA.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 84.Kancir AS, Pleckaitiene L, Hansen TB, Ekeløf NP, Pedersen EB. Lack of nephrotoxicity by 6% hydroxyethyl starch 130/0.4 during hip arthroplasty: a randomized controlled trial. Anesthesiology. 2014;121:948–958. doi: 10.1097/ALN.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 85.Lindroos AC, Niiya T, Randell T, Niemi TT. Stroke volume-directed administration of hydroxyethyl starch (HES 130/0.4) and Ringer’s acetate in prone position during neurosurgery: a randomized controlled trial. J Anesth. 2014;28:189–197. doi: 10.1007/s00540-013-1711-8. [DOI] [PubMed] [Google Scholar]
- 86.Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–289. doi: 10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 87.Kashy BK, Podolyak A, Makarova N, Dalton JE, Sessler DI, Kurz A. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology. 2014;121:730–739. doi: 10.1097/ALN.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mercier FJ, Diemunsch P, Ducloy-Bouthors AS, Mignon A, Fischler M, Malinovsky JM, Bolandard F, Aya AG, Raucoules-Aimé M, Chassard D, et al. 6% Hydroxyethyl starch (130/0.4) vs Ringer’s lactate preloading before spinal anaesthesia for Caesarean delivery: the randomized, double-blind, multicentre CAESAR trial. Br J Anaesth. 2014;113:459–467. doi: 10.1093/bja/aeu103. [DOI] [PubMed] [Google Scholar]
- 89.Hand WR, Whiteley JR, Epperson TI, Tam L, Crego H, Wolf B, Chavin KD, Taber DJ. Hydroxyethyl starch and acute kidney injury in orthotopic liver transplantation: a single-center retrospective review. Anesth Analg. 2015;120:619–626. doi: 10.1213/ANE.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kancir AS, Johansen JK, Ekeloef NP, Pedersen EB. The effect of 6% hydroxyethyl starch 130/0.4 on renal function, arterial blood pressure, and vasoactive hormones during radical prostatectomy: a randomized controlled trial. Anesth Analg. 2015;120:608–618. doi: 10.1213/ANE.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 91.Choi SS, Cho SS, Ha TY, Hwang S, Lee SG, Kim YK. Intraoperative factors associated with delayed recovery of liver function after hepatectomy: analysis of 1969 living donors. Acta Anaesthesiol Scand (Epub) 2015 doi: 10.1111/aas.12630. [DOI] [PubMed] [Google Scholar]


