Abstract
AIM: To investigate the effects of biliary tract external drainage (BTED) on intestinal barrier injury in rats with hemorrhagic shock (HS).
METHODS: BTED was performed via cannula insertion into the bile duct of rats. HS was induced by drawing blood from the femoral artery at a rate of 1 mL/min until a mean arterial pressure (MAP) of 40 ± 5 mmHg was achieved. That MAP was maintained for 60 min. A total of 99 Sprague-Dawley rats were randomized into a sham group, an HS group and an HS + BTED group. Nine rats in the sham group were sacrificed 0.5 h after surgery. Nine rats in each of the HS and HS + BTED groups were sacrificed 0.5 h, 1 h, 2 h, 4 h and 6 h after resuscitation. Plasma tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and lipopolysaccharide (LPS) levels were analyzed using enzyme-linked immunosorbent assay. Plasma D-lactate levels were analyzed using colorimetry. The expression levels of occludin and claudin-1 in the ileum were analyzed using Western blot and immunohistochemistry. Histology of the ileum was evaluated by hematoxylin and eosin staining.
RESULTS: Plasma TNF-α levels in the HS + BTED group decreased significantly compared with the HS group at 1 h and 6 h after resuscitation (P < 0.05). Plasma IL-6 levels in the HS + BTED group decreased significantly compared with the HS group at 0.5 h, 1 h and 2 h after resuscitation (P < 0.05). Plasma D-lactate and LPS levels in the HS + BTED group decreased significantly compared with the HS group at 6 h after resuscitation (P < 0.05). The expression levels of occludin in the HS + BTED group increased significantly compared with the HS group at 4 h and 6 h after resuscitation (P < 0.05). The expression levels of claudin-1 in the HS + BTED group increased significantly compared with the HS group at 6 h after resuscitation (P < 0.05). Phenomena of putrescence and desquamation of epithelial cells in the ileal mucosa were attenuated in the HS + BTED group. Ileal histopathologic scores in the HS + BTED group decreased significantly compared with the HS group at 2 h, 4 h and 6 h after resuscitation (P < 0.05).
CONCLUSION: BTED protects against intestinal barrier injury in HS rats.
Keywords: Hemorrhagic shock, Biliary tract external drainage, Occludin, Claudin-1, D-lactate
Core tip: Our previous studies demonstrated that biliary tract external drainage decreased proinflammatory cytokine production and relieved tissue damage in rat models of hemorrhagic shock. In this research, we found that biliary tract external drainage increased the expression levels of occludin and claudin-1 and decreased plasma D-lactate and lipopolysaccharide levels under hemorrhagic shock conditions. These results demonstrate that biliary tract external drainage protects against intestinal barrier injury in hemorrhagic shock rats.
INTRODUCTION
Hemorrhagic shock (HS) induces gut barrier failure, which initiates a systemic inflammatory response[1]. Bile full of proinflammatory mediators enters into the gut following HS, which contributes to tissue injury in the intestine. On one hand, injured gut cells release a large number of inflammatory mediators that cause endothelial dysfunction and activate neutrophils. On the other hand, a large amount of lipopolysaccharide (LPS) is released by gut bacteria through the damaged intestinal barrier into the peripheral blood and may cause distant organ injury[2].
A widespread normal bacterial flora resides in the ileum. D-lactate is the end product of intestinal bacteria. It is neither produced nor metabolized by mammalian cells. During ischemia, as the normal mucosal barrier is damaged and permeability increases, a large amount of D-lactate is released through the damaged intestinal mucosa into the peripheral blood. Thus, D-lactate in peripheral blood can indicate damage situation of the intestinal barrier[3-6]. LPS produced by gut bacteria also enters into the bloodstream and spreads to the entire body under HS conditions. Therefore, the level of LPS in the blood also can reflect the degree of intestinal barrier damage[7-9].
Tight junction (TJ) proteins, including occludin, claudins, and cytoskeleton proteins, play critical roles in the maintenance of the intestinal barrier integrity[10]. Occludin was the first transmembrane TJ protein discovered[11]. Occludin plays a crucial role in the maintenance of epithelial TJs[12-14]. The absence of occludin increases the ion permeability of TJs and causes intestinal barrier dysfunction[15,16]. The claudin family confers barrier functions as constituents of TJ strands, and these proteins directly participate in the transport of materials across epithelia through paracellular pathways by adjusting the tightness and selectivity of TJ strands[17,18]. Claudins determine the paracellular ionic selectivity of the TJ because these proteins have two extracellular loops that display variability in the distribution and number of charged residues[19]. We examined the expression levels of claudin-1 in the ileum for claudin-1 is strongly expressed in rat ileum[20].
Organ function in shock patients with severe acute pancreatitis who accept biliary tract external drainage (BTED) (endoscopic naso-biliary drainage, cholecystostomy or gallbladder percutaneous catheter drainage) rapidly improves in clinical practice. Infection incidence and morbidity of multiple organ dysfunction syndrome (MODS) also significantly decrease. The amelioration of intestinal barrier function may play a vital role in this process. Previous studies also indicated that BTED eased damage of vital organs and improved the survival rate of shock rats[21,22]. However, studies on the relationship between BTED and intestinal barrier function are limited, and most of these studies focused on obstructive jaundice[23-26]. Therefore, we designed this study to observe changes in occludin and claudin-1 in the ileum and D-lactate, LPS, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels in plasma. We explored the effects of BTED on intestinal barrier in HS.
MATERIALS AND METHODS
Ethics statement
This study was carried out in strict accordance with the guidelines for the care and use of laboratory animals established by the Animal Use and Care Committee of the Shanghai Committee on Animal Care. Animal surgical procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Ruijin Hospital, Shanghai Jiao Tong University, Shanghai, China. The animal protocol was designed to minimize pain or discomfort to the animals.
Animal model
Ninety-nine adult male Sprague-Dawley rats (250-300 g) were purchased from the Experimental Animal Center of Ruijin Hospital. The animals were acclimatized to laboratory conditions (25 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for one week prior to experimentation. Rats were randomly divided into 3 groups after adaptation: sham group, HS group, and HS + BTED group. Rats were fasted overnight before the experiment, but rats were allowed to drink water. Rats in the HS + BTED group were intraperitoneally anesthetized with 3% sodium pentobarbital (0.2 mL/100 g), and laparotomies were performed after shaving and sterilization. Catheters were placed in both femoral arteries for blood pressure measurement and blood withdrawal. The bile duct was exposed long enough for BTED. Rats were subjected to HS by slowly withdrawing blood at a rate of 1 mL/min until a mean arterial pressure (MAP) of 40 ± 5 mmHg was achieved. A catheter (inner diameter, 0.4 mm; outer diameter, 0.8 mm; length, 20 cm) was inserted into the bile duct. The distal end of the bile duct was ligated, and the catheter was passed through the rat flank to avoid bile passage into the gut and allow the external collection of bile. The abdomen was closed subsequently. An MAP of 40 ± 5 mmHg was maintained for 1 h. Rats were resuscitated using their shed blood and an equal volume of normal saline at the end of shock period. HS rats underwent pentobarbital anesthesia, laparotomy, vascular cannulation, blood withdrawal and suturing but no BTED. Sham rats underwent pentobarbital anesthesia, laparotomy, vascular cannulation and suturing, but no blood withdrawal or BTED. Nine rats in the sham group were sacrificed 0.5 h after surgery. Nine rats in the HS group and the HS + BTED group were sacrificed 0.5 h, 1 h, 2 h, 4 h and 6 h after resuscitation.
Enzyme-linked immunosorbent assay
Plasma TNF-α, IL-6, and LPS levels were quantified using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. The absorbance from each sample was normalized for the actual concentration.
D-lactate colorimetric assay
Plasma D-lactate levels were quantified using a D-lactate colorimetric assay kit according to the manufacturer’s instructions. The absorbance from each sample was normalized for the D-lactate concentration.
Western blot analysis
Intestinal mucosal scrapings from all animals were stored at -80 °C for Western blot analysis. RIPA lysis buffer and 5 × loading buffer were prepared. Briefly, samples were homogenized in RIPA lysis buffer. Tissues were frozen immediately in liquid nitrogen and placed in a mortar for pulverization. Total protein was extracted using tissue total protein lysis buffer, and protein concentration was measured using a BCA Protein Assay Kit.
Proteins were separated using SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidenedifluofide (PVDF) membranes. The blot was immune probed using primary antibody overnight at 4 °C. Primary antibodies for Western blotting were a rabbit polyclonal antibody to occludin (1:250), a rabbit polyclonal antibody to claudin-1 (1:500) and a mouse monoclonal antibody to GAPDH (1:2000). The blots were incubated with an HRP-conjugated secondary antibody for 1 h at room temperature and reacted with an enhanced chemiluminescence substrate. The resulting chemiluminescence was recorded using an imaging system (Imagequant LAS 400, GE, United States). The enhanced chemiluminescence signals were digitized using Photoshop CS6 software (Adobe, United States) to quantify the expression levels of occludin and claudin-1. Relative occludin and claudin-1 protein expression was normalized to respective values for GAPDH, and the results are described as fold increases relative to baseline levels in negative control.
Immunohistochemistry
The samples were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at 4 μm. Sections were mounted onto APES-coated slides, deparaffinized, rehydrated, incubated in 3% hydrogen peroxide to quench any endogenous peroxidase activity, and washed with distilled water and PBS for 5 min. Sections were placed in 3% citrate buffer to repair antigens. The buffer was heated to a temperature of 92 °C-98 °C using microwave, and the temperature was maintained for 10 min. Sections were cooled to room temperature. A 10% nonimmune goat serum was applied to eliminate nonspecific staining. Sections were incubated overnight at 4 °C with an optimally diluted rabbit polyclonal anti-rat occludin antibody (1:100) or rabbit polyclonal anti-rat claudin-1 antibody (1:100). The sections were washed with PBS and incubated with a broad-spectrum secondary antibody for 30 min, rewashed, and incubated with peroxidase-conjugated streptavidin for 15 min. Peroxidation activity was visualized by incubation with DAB solution. The sections were counterstained with hematoxylin.
Hematoxylin and eosin staining
The samples were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at 4 μm. Sections were mounted onto APES-coated slides. After deparaffinization and dehydration, the sections were stained with hematoxylin and eosin for microscopic examination. The severity of ileal injury was scored from 0 to 3 as follows: 0, normal (no damage); 1, mild (focal epithelial edema and necrosis); 2, moderate (diffuse swelling or necrosis of the villi); 3, severe (diffuse necrosis of the villi with evidence of neutrophil infiltration in the submucosa and/or hemorrhage). All evaluations were made on six fields per section and six sections under 100 × magnification[27,28].
Reagents
The rat TNF-α ELISA kit was purchased from the MaibioCompany (MHK0008, Shanghai, China). The rat IL-6 ELISA kit was purchased from the MaibioCompany (MRK0004, Shanghai, China). The rat LPS ELISA kit was purchased from the CusabioCompany (CSB-E14247r, Wuhan, China). The D-lactate Colorimetric Assay Kit was purchased from the BioVision Company (K667-100, Milpitas, United States). RIPA lysis buffer, BCA Protein Assay Kit and 5 × loading buffer were purchased from the Beyotime Institute of Biotechnology (Jiangsu, China). The rabbit polyclonal antibody to occludin was purchased from the Abcam Company (ab31721, Cambridge, MA, United States). The rabbit polyclonal antibody to claudin-1 was purchased from the Biorbyt Company (Ab-210, Cambridge, Cambridgeshire, United Kingdom). The mouse monoclonal antibody to GAPDH was purchased from the Abcam Company (ab8245, Cambridge, MA, United States). The enhanced chemiluminescence substrate was purchased from the ComWin Biotechnology Company (Beijing, China). The immunohistochemistry kit was purchased from the InvitrogenCompany (Frederick, United States).
Statistical analysis
Data were analyzed using SPSS 16.0 software. All data are expressed as mean ± SE and compared using the unpaired Student’s t-test. P < 0.05 was considered to be statistically significant.
RESULTS
Effect of BTED on plasma TNF-α levels
Plasma TNF-α levels in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h and 2 h after resuscitation. Plasma TNF-α levels in the HS + BTED group decreased significantly compared with the HS group at 1 h and 6 h after resuscitation (P < 0.05). Plasma TNF-α levels in the HS + BTED group increased significantly compared with the HS group at 4 h after resuscitation (P < 0.05) (Figure 1A).
Figure 1.
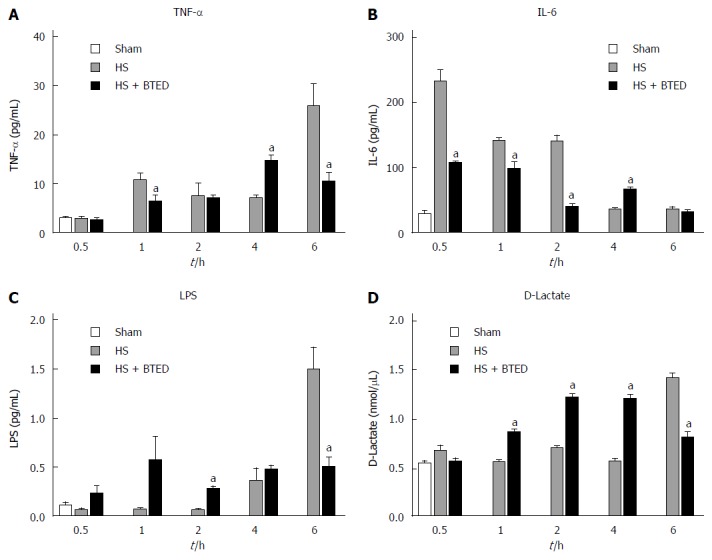
Plasma tumor necrosis factor-α (A), interleukin-6 (B), lipopolysaccharide (C), and D-lactate levels (D). Results are presented as mean ± SE (n = 6). aP < 0.05, vs the HS group at the same time point. HS: Hemorrhagic shock; BTED: Biliary tract external drainage; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; LPS: Lipopolysaccharide.
Effect of BTED on plasma IL-6 levels
Plasma IL-6 levels in the HS + BTED group showed no significant differences compared with the HS group at 6 h after resuscitation. Plasma IL-6 levels in the HS + BTED group decreased significantly compared with the HS group at 0.5 h, 1 h and 2 h after resuscitation (P < 0.05). Plasma IL-6 levels in the HS + BTED group increased significantly compared with the HS group at 4 h after resuscitation (P < 0.05) (Figure 1B).
Effect of BTED on plasma LPS levels
Plasma LPS levels in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h, 1 h and 4 h after resuscitation. Plasma LPS levels in the HS + BTED group increased significantly compared with the HS group at 2 h after resuscitation (P < 0.05). Plasma LPS levels in the HS + BTED group decreased significantly compared with the HS group at 6 h after resuscitation (P < 0.05) (Figure 1C).
Effect of BTED on plasma D-lactate levels
Plasma D-lactate levels in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h after resuscitation. Plasma D-lactate levels in the HS + BTED group increased significantly compared with the HS group at 1 h, 2 h and 4 h after resuscitation (P < 0.05). Plasma D-lactate levels in the HS + BTED group decreased significantly compared with the HS group at 6 h after resuscitation (P < 0.05) (Figure 1D).
Western blot analysis of expression of occludin and claudin-1 in the ileum
The expression levels of occludin in the ileum of the HS + BTED group did not show significant differences compared with the HS group at 0.5 h, 1 h and 2 h after resuscitation. The expression levels of occludin in the ileum in the HS + BTED group increased significantly compared with the HS group at 4 h and 6 h after resuscitation (P < 0.05) (Figure 2A and B). The expression levels of claudin-1 in the ileum in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h, 1 h, 2 h and 4 h after resuscitation. The expression levels of claudin-1 in the ileum in the HS + BTED group increased significantly compared with the HS group at 6 h after resuscitation (P < 0.05) (Figure 2A and C).
Figure 2.
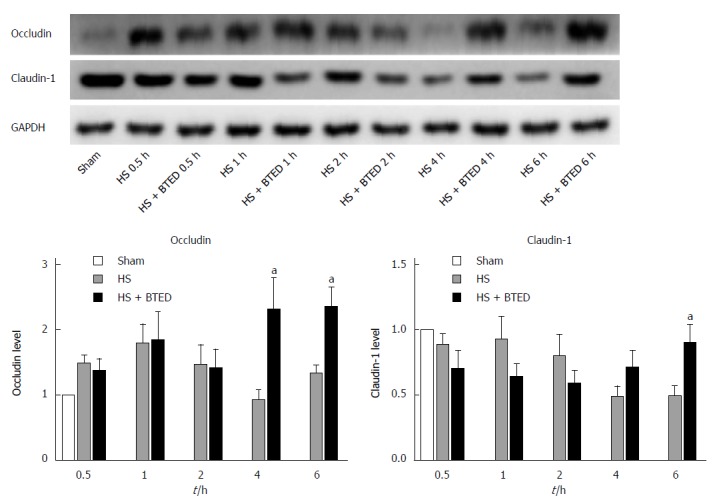
Western blot analysis of expression of occludin (A and B) and claudin-1 (A and C) in the ileum. Results are presented as mean ± SE (n = 9). aP < 0.05 vs the HS group at the same time point. HS: Hemorrhagic shock; BTED: Biliary tract external drainage.
Immunohistochemical analysis of expression of occludin in the ileum
Occludin in the sham group was expressed as cytoplasmic granules located mostly at the apical part of epithelial cells. The total epithelial cells lining the villi exhibited positive immunostaining for occludin in the sham group (Figure 3A). There was loss of occludin expression by most epithelial cells lining the villi in the HS group (Figure 3B-F). Occludin expression in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h, 1 h and 2 h after resuscitation (Figure 3B-D and G-I). Occludin expression in the epithelial cells lining the villi was enhanced significantly in the HS + BTED group compared with the HS group at 4 h and 6 h after resuscitation (Figure 3 E, F, J and K).
Figure 3.
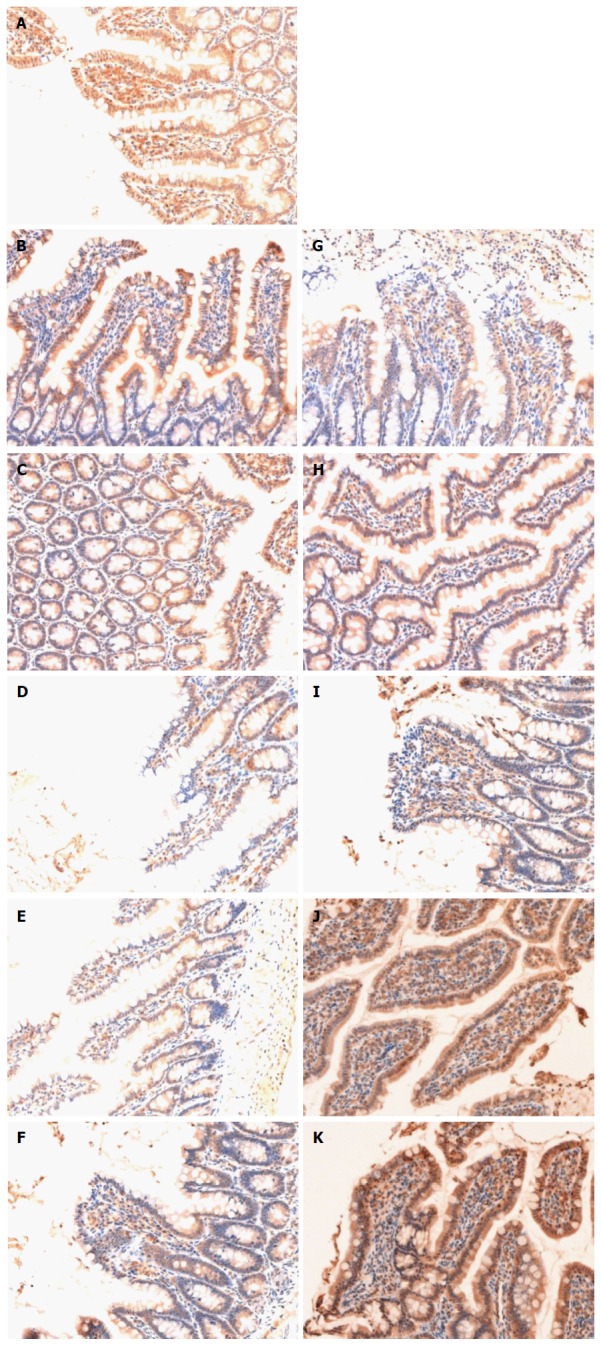
Immunohistochemical analysis of expression of occludin in the ileum. Sham group (A), HS group (B: 0.5 h after resuscitation; C: 1 h after resuscitation; D: 2 h after resuscitation; E: 4 h after resuscitation; F: 6 h after resuscitation) and HS + BTED group (G: 0.5 h after resuscitation; H: 1 h after resuscitation; I: 2 h after resuscitation; J: 4 h after resuscitation; K: 6 h after resuscitation). The expression levels of occludin in the ileum in the HS + BTED group were enhanced significantly compared with the HS group at 4 h and 6 h after resuscitation. HS: Hemorrhagic shock; BTED: Biliary tract external drainage.
Immunohistochemical analysis of expression of claudin-1 in the ileum
The epithelial cells lining the villi exhibited positive immunostaining for claudin-1 in the sham group (Figure 4A). There was loss of claudin-1 expression by most epithelial cells lining the villi in the HS group (Figure 4B-F). Claudin-1 expression in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h, 1 h, 2 h and 4 h after resuscitation (Figure 4B-E and G-J). Claudin-1 expression was enhanced significantly in the HS + BTED group compared with the HS group at 6 h after resuscitation (Figure 4F and K).
Figure 4.
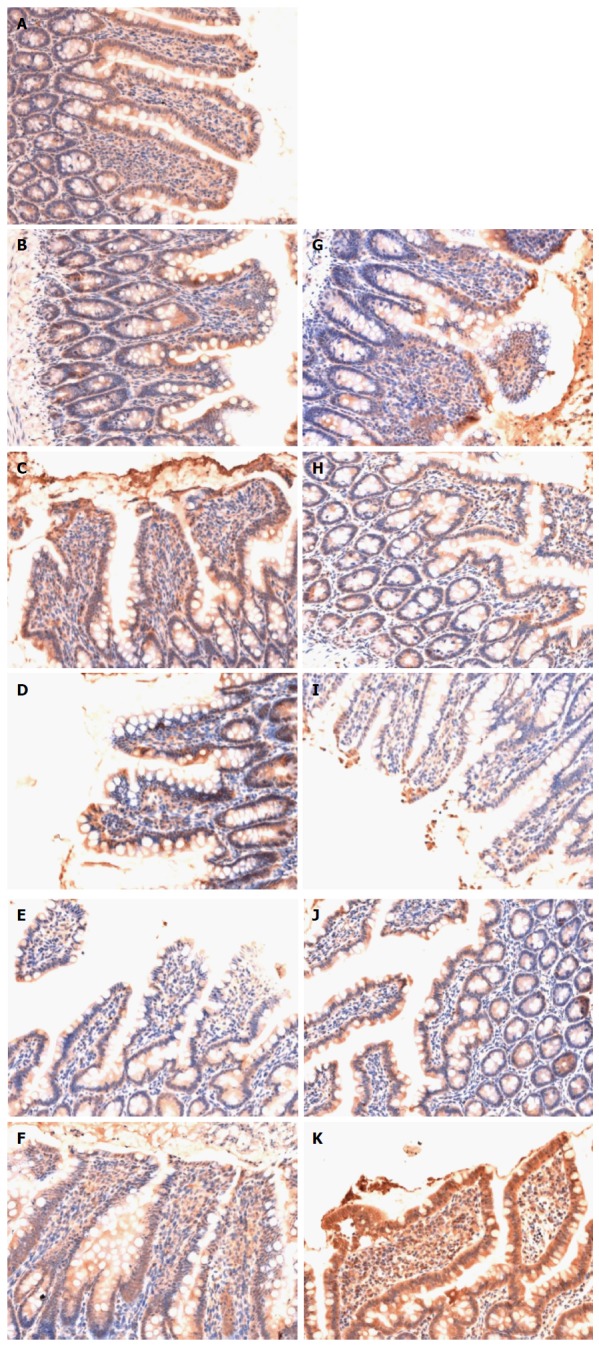
Immunohistochemical analysis of expression of claudin-1 in the ileum. Sham group (A), HS group (B: 0.5 h after resuscitation; C: 1 h after resuscitation; D: 2 h after resuscitation; E: 4 h after resuscitation; F: 6 h after resuscitation) and HS + BTED group (G: 0.5 h after resuscitation; H: 1 h after resuscitation; I: 2 h after resuscitation; J: 4 h after resuscitation; K: 6 h after resuscitation). The expression levels of claudin-1 in the ileum in the HS + BTED group were enhanced significantly compared with the HS group at 6 h after resuscitation. HS: Hemorrhagic shock; BTED: Biliary tract external drainage.
Histomorphology of the ileum
No obvious tissue damage of the ileum was shown in the sham group (Figure 5Aa). Epithelial cells of small intestinal villi of rats in the HS group showed necrosis and exfoliation. Inflammatory cell infiltration was observed (Figure 5Ab-f). The tissue damage in the ileum of the HS + BTED group was significantly alleviated compared with the HS group. Phenomena of putrescence and desquamation of epithelial cells in the intestinal mucosa were attenuated (Figure 5Ag-k). Histopathologic scores in the HS + BTED group showed no significant differences compared with the HS group at 0.5 h and 1 h after resuscitation. Histopathologic scores in the HS + BTED group decreased significantly compared with the HS group at 2 h, 4 h and 6 h after resuscitation (P < 0.05) (Figure 5B).
Figure 5.
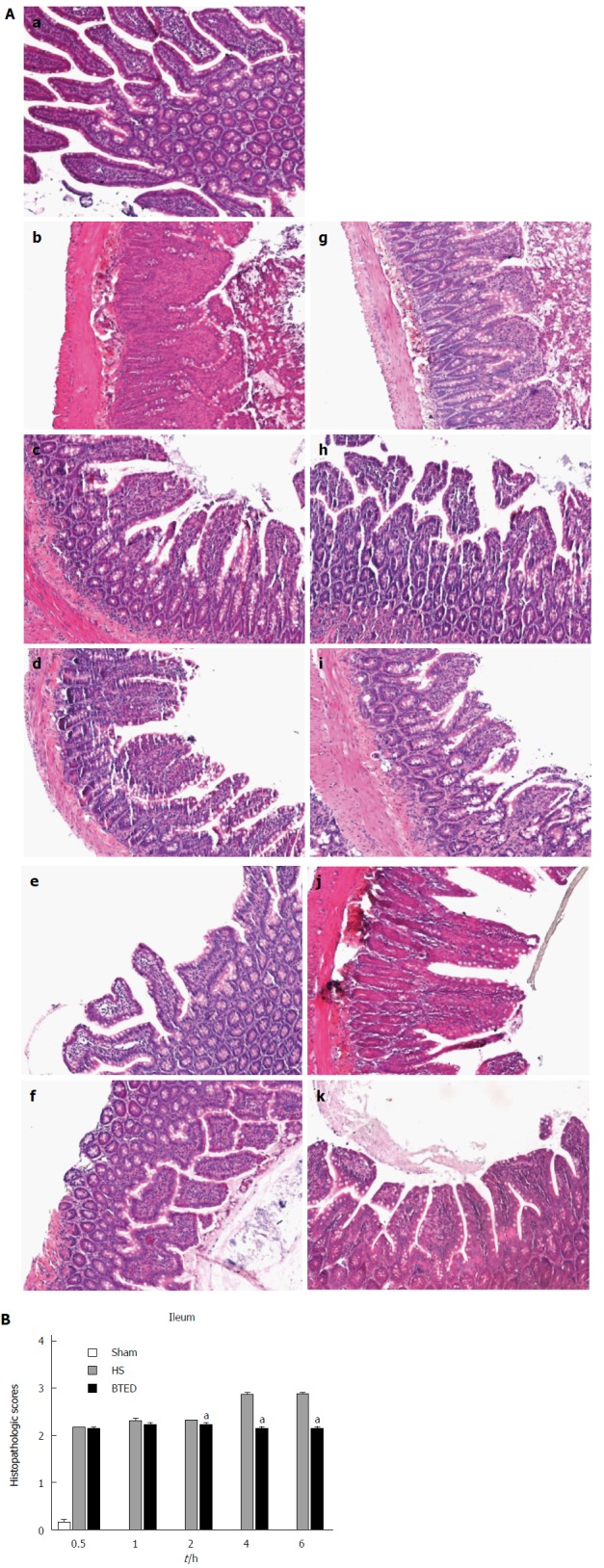
Hematoxylin and eosin staining (A) and histopathologic scores (B) of the ileum. Sham group (a), HS group (b: 0.5 h after resuscitation; c: 1 h after resuscitation; d: 2 h after resuscitation; e: 4 h after resuscitation; f: 6 h after resuscitation) and HS + BTED group (g: 0.5 h after resuscitation; h: 1 h after resuscitation; i: 2 h after resuscitation; j: 4 h after resuscitation; k: 6 h after resuscitation). Histopathologic scores are presented as mean ± SE (n = 6). aP < 0.05 vs the HS group at the same time point. HS: Hemorrhagic shock; BTED: Biliary tract external drainage.
DISCUSSION
This study demonstrated that plasma TNF-α, IL-6, LPS, and D-lactate levels decreased significantly after BTED under HS conditions. The expression levels of occludin and claudin-1 in the ileum increased significantly after BTED under HS conditions. Phenomena of putrescence and desquamation of epithelial cells in the intestinal mucosa were attenuated after BTED. Ileal histopathologic scores decreased significantly after BTED under HS conditions.
Activated Kupffer cells produce proinflammatory TNF-α and IL-6 during the pathogenesis of HS, which induces the release of additional proinflammatory mediators from hepatocytes[29,30]. Bile full of proinflammatory mediators enters into the gut, which aggravates tissue injury in the ileum and induces the release of additional proinflammatory mediators from intestinal cells. Gut-derived cytokines, such as TNF-α and IL-6, enter into the liver via the portal vein, which aggravates liver injury and induces the release of more proinflammatory mediators from Kupffer cells to complete the inflammatory loop of the gut-liver axis[31-34]. This vicious cycle eventually leads to MODS.
The initial application of biliary drainage was to temporarily relief patient’s biliary obstruction[35,36]. This technique is widely used clinically because it is less invasive and exhibits fewer complications. Many methods are used, such as nasal biliary drainage, percutaneous transhepaticcholangial drainage, gallbladder fistula and so on, with the development of this technology[37,38]. Previous studies demonstrated that the inflammatory cytokine TNF-α in bile increased significantly in HS rats[22]. BTED blocks the entry of inflammatory cytokines into the ileum with bile, which reduces inflammatory cytokines that enter into the blood through the gut and avoids intestinal cell release of more inflammatory cytokines after stimulation by inflammatory cytokines. BTED blockade of the vicious cycle of the gut-liver axis may play an important role in the prevention and treatment of MODS. TNF-α and IL-6 are key mediators involved in many physiologic processes including immunity, inflammation, and metabolism. Plasma TNF-α levels rose within 10 min after hemorrhage, and peaked at 30 min after hemorrhage during HS. Higher concentrations of TNF-α and IL-6 were associated, not only with an increased mortality rate, but also with an increased risk for subsequent adult respiratory distress syndrome and multiple organ failure in HS patients. In our study, plasma TNF-α levels in the HS + BTED group decreased significantly compared with the HS group at 1 h and 6 h after resuscitation. Plasma IL-6 levels in the HS + BTED group decreased significantly compared with the HS group at 0.5 h, 1 h and 2 h after resuscitation. These results showed that BTED reduces the body’s inflammatory reaction in HS. However, plasma TNF-α and IL-6 levels in the HS + BTED group increased significantly compared with the HS group at 4 h after resuscitation. The increases of plasma TNF-α and IL-6 levels may be because BTED reduced inflammation of intestinal villi and retained more capillaries. Blood supply in the HS + BTED group was better after resuscitation. TNF-α and IL-6 accumulated in the ileum and were released into the bloodstream faster.
In our earlier study, necrosis and exfoliation of epithelial cells of small intestinal villi of rats with severe acute pancreatitis were attenuated after BTED, which suggests that BTED plays a protective role on the ileum of rats with severe acute pancreatitis. BTED attenuated the phenomena of putrescence and desquamation of epithelial cells in the intestinal mucosa of HS rats[22]. BTED reduced neutrophil infiltration, superficial necrosis and sloughing of epithelium of intestinal villus and improved survival rates of the LPS treated rats[21]. BTED improved intestinal barrier function in obstructive jaundice models[24]. The tissue damage to the ileum in the HS + BTED group was significantly relieved compared with the HS group in this study. Phenomena of putrescence and desquamation of epithelial cells in the intestinal mucosa were attenuated. Ileal histopathologic scores decreased significantly after BTED under HS conditions in this study. These results showed that BTED protects against intestinal injury in HS.
The severity of hemorrhagic/traumatic shock affected plasma D-lactate concentrations in rats[16]. Increased plasma D-lactate levels predict an increased risk of mortality after hemorrhage and trauma[39]. A rapid decrease in plasma D-lactate could indicate reduced 28-d mortality in critically ill septic shock patients[40]. Ethyl pyruvate can lessen intestinal permeability and protect intestinal barrier function in dogs with septic shock via decreasing the levels of plasma D-lactate and reducing inflammation of the small intestinal mucosa[41]. Plasma D-lactate levels in the HS + BTED group decreased significantly compared with the HS group at 6 h after resuscitation in this study. Plasma LPS levels showed the same variation trend. These results showed that BTED protects against intestinal barrier injury in HS. However, plasma D-lactate levels in the HS + BTED group increased significantly compared with the HS group at 1 h, 2 h and 4 h after resuscitation. Plasma LPS levels in the HS + BTED group increased significantly compared with the HS group at 2 h after resuscitation. These results may be caused by the following reasons. On one hand, BTED reduced inflammation of intestinal villi and retained more capillaries. Intestinal villus blood supply was better in the HS + BTED group. Therefore, more D-lactate and LPS were absorbed through the damaged intestinal mucosa into the peripheral blood. On the other hand, BTED may aggravate intestinal barrier damage as an invasive operation in early stages.
Increasing occludin content in the small intestine enhances the intestinal barrier[42-44]. In our study, the expression levels of occludin in the ileum of the HS + BTED group increased significantly compared with the HS group at 4 h after resuscitation. Fish oil enhanced intestinal integrity by increasing protein expression of the intestinal TJ protein claudin-1 in weaned pigs after LPS challenge[45]. In our study, the expression levels of claudin-1 in the ileum of the HS + BTED group increased significantly compared with the HS group at 6 h after resuscitation. These results showed that BTED increases the expression of occludin and claudin-1 under HS conditions.
There are some limitations in our study. First, the observation time was relatively short so that we are not sure how long the effect of BTED lasts. Second, there are many kinds of TJ proteins expressed in the ileal epithelium. Changes of occludin and claudin-1 may not reflect the real changes of all TJ proteins.
In summary, BTED decreases plasma TNF-α and IL-6 levels and ileal histopathologic scores under HS conditions. BTED increases the expression levels of occludin and claudin-1 and decreases plasma D-lactate and LPS levels under HS conditions. These results show that BTED protects against intestinal barrier injury in HS. Specific mechanisms require further research.
ACKNOWLEDGMENTS
We thank the staff of Shanghai Institute of Traumatology and Orthopaedics for their technical support. We also thank Lin Zhang from Department of Epidemiology and Biostatistics, Shanghai Jiaotong University School of Public Health, for her statistical support.
COMMENTS
Background
Hemorrhagic shock (HS) induces gut barrier failure, which initiates a systemic inflammatory response. Bile full of proinflammatory mediators enters into the gut following HS, which contributes to tissue injury in the intestine. A large amount of lipopolysaccharide (LPS) is released by gut bacteria through the damaged intestinal barrier into the peripheral blood and may cause distant organ injury. Studies on the relationship between biliary tract external drainage (BTED) and intestinal barrier function are limited, and most of these studies focused on obstructive jaundice.
Research frontiers
D-lactate is the end product of intestinal bacteria. It is neither produced nor metabolized by mammalian cells. During ischemia, as the normal mucosal barrier is damaged and permeability increases, a large amount of D-lactate is released through the damaged intestinal mucosa into the peripheral blood. Thus, D-lactate in peripheral blood can indicate damage situation of intestinal barrier. Tight junction (TJ) proteins, including occludin, claudins, and cytoskeleton proteins, play critical roles in the maintenance of the intestinal barrier integrity.
Innovations and breakthroughs
BTED significantly decreased plasma TNF-α, IL-6, LPS, and D-lactate levels and increased the expression levels of occludin and claudin-1 in the ileum under HS conditions. Phenomena of putrescence and desquamation of epithelial cells in the intestinal mucosa were attenuated after BTED. Ileum histopathologic scores decreased significantly after BTED under HS conditions.
Applications
These results show that BTED protects against intestinal barrier injury in HS and provide a new choice for the treatment of HS.
Terminology
The initial application of BTED was to temporarily relief patient’s biliary obstruction. This technique is widely used clinically because it is less invasive and exhibits fewer complications. Many methods are used, such as nasal biliary drainage, percutaneous transhepaticcholangial drainage, gallbladder fistula and so on, with the development of this technology. BTED blocks the entry of inflammatory cytokines into the ileum with bile, which reduces inflammatory cytokines that enter into the blood through the gut and avoids intestinal cell release of more inflammatory cytokines after stimulation by inflammatory cytokines.
Peer-review
This manuscript is quite well written.
Footnotes
Supported by National Natural Science Foundation of China, No. 81171789.
Institutional review board statement: This study has been reviewed and approved by Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China [IACUC protocol number: SYXK (Shanghai) 2011-0113].
Conflict-of-interest statement: We declare no conflicts of interest regarding this study.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 6, 2015
First decision: July 10, 2015
Article in press: September 15, 2015
P- Reviewer: Cerwenka HR S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Reys LG, Ortiz-Pomales YT, Lopez N, Cheadle G, de Oliveira PG, Eliceiri B, Bansal V, Costantini TW, Coimbra R. Uncovering the neuroenteric-pulmonary axis: vagal nerve stimulation prevents acute lung injury following hemorrhagic shock. Life Sci. 2013;92:783–792. doi: 10.1016/j.lfs.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Welch M. D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg. 1999;86:712. doi: 10.1046/j.1365-2168.1999.1104a.x. [DOI] [PubMed] [Google Scholar]
- 4.Ruh J, Vogel F, Schmidt E, Werner M, Klar E, Secchi A, Gebhard MM, Glaser F, Herfarth C. Effects of hydrogen peroxide scavenger Catalase on villous microcirculation in the rat small intestine in a model of inflammatory bowel disease. Microvasc Res. 2000;59:329–337. doi: 10.1006/mvre.1999.2201. [DOI] [PubMed] [Google Scholar]
- 5.Szalay L, Umar F, Khadem A, Jafarmadar M, Fürst W, Ohlinger W, Redl H, Bahrami S. Increased plasma D-lactate is associated with the severity of hemorrhagic/traumatic shock in rats. Shock. 2003;20:245–250. doi: 10.1097/00024382-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Murray MJ, Barbose JJ, Cobb CF. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J Surg Res. 1993;54:507–509. doi: 10.1006/jsre.1993.1078. [DOI] [PubMed] [Google Scholar]
- 7.Ding LA, Li JS, Li YS, Zhu NT, Liu FN, Tan L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J Gastroenterol. 2004;10:2373–2378. doi: 10.3748/wjg.v10.i16.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–453. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 9.Velloso LA, Folli F, Saad MJ. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr Rev. 2015;36:245–271. doi: 10.1210/er.2014-1100. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94:386–393. doi: 10.1111/j.1651-2227.2005.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 11.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 13.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109(Pt 9):2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 15.Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, Utusnomiya T, Shimada M. Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res. 2012;173:341–347. doi: 10.1016/j.jss.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–F876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion. Int J Pharm. 2012;426:82–89. doi: 10.1016/j.ijpharm.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Jackson GD, Dai Y, Sewell WA. Bile mediates intestinal pathology in endotoxemia in rats. Infect Immun. 2000;68:4714–4719. doi: 10.1128/iai.68.8.4714-4719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YJ, Mao EQ, Ouyang B, Chen J, Tang YQ, Huang SW, Guan XD. Effect of biliary tract external drainage on cytokine expression and histomorphology of intestine, liver, and lung in rats with hemorrhagic shock. Crit Care Med. 2009;37:2800–2806. doi: 10.1097/CCM.0b013e3181a59469. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida Y, Ajiki T, Ueno K, Shinozaki K, Murakami S, Okazaki T, Matsumoto T, Matsumoto I, Fukumoto T, Usami M, et al. Preoperative bile replacement improves immune function for jaundiced patients treated with external biliary drainage. J Gastrointest Surg. 2014;18:2095–2104. doi: 10.1007/s11605-014-2674-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang SZ, Wang XB. Effects of biliary drainage on the intestinal barrier function in obstructive jaundice. Hepatogastroenterology. 2013;60:1284–1288. doi: 10.5754/hge13236. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya S, Nagino M, Kanazawa H, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg. 2004;239:510–517. doi: 10.1097/01.sla.0000118594.23874.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–1349. doi: 10.1002/bjs.1800831007. [DOI] [PubMed] [Google Scholar]
- 27.Yang FL, Subeq YM, Lee CJ, Lee RP, Peng TC, Hsu BG. Melatonin ameliorates hemorrhagic shock-induced organ damage in rats. J Surg Res. 2011;167:e315–e321. doi: 10.1016/j.jss.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Lee CC, Lee RP, Subeq YM, Lee CJ, Chen TM, Hsu BG. Fluvastatin attenuates severe hemorrhagic shock-induced organ damage in rats. Resuscitation. 2009;80:372–378. doi: 10.1016/j.resuscitation.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Hsu JT, Chen TH, Chiang KC, Kuo CJ, Lin CJ, Yeh TS. Role of p38 MAPK pathway in 17β-estradiol-mediated attenuation of hemorrhagic shock-induced hepatic injury. J Appl Physiol (1985) 2015;118:187–192. doi: 10.1152/japplphysiol.00464.2014. [DOI] [PubMed] [Google Scholar]
- 30.Jiang JX, Diao YF, Tian KL, Chen HS, Zhu PF, Wang ZG. Effect of hemorrhagic shock on endotoxin-inducing TNF production and intra-tissue lipopolysaccharide-binding protein mRNA expression and their relationship. Shock. 1997;7:206–210. doi: 10.1097/00024382-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navaneethan U. Hepatobiliary manifestations of ulcerative colitis: an example of gut-liver crosstalk. Gastroenterol Rep (Oxf) 2014;2:193–200. doi: 10.1093/gastro/gou036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381–7391. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visschers RG, Luyer MD, Schaap FG, Olde Damink SW, Soeters PB. The gut-liver axis. Curr Opin Clin Nutr Metab Care. 2013;16:576–581. doi: 10.1097/MCO.0b013e32836410a4. [DOI] [PubMed] [Google Scholar]
- 35.Wurbs D, Phillip J, Classen M. Experiences with the long standing nasobiliary tube in biliary diseases. Endoscopy. 1980;12:219–223. doi: 10.1055/s-2007-1021747. [DOI] [PubMed] [Google Scholar]
- 36.Nagai N, Toli F, Oi I, Suzuki H, Kozu T. Continuous endoscopic pancreatocholedochal catheterization. Gastrointest Endosc. 1976;23:78–81. doi: 10.1016/s0016-5107(76)73596-x. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami H, Kuwatani M, Abe Y, Kubota Y, Kawakubo K, Kubo K, Kawahata S, Sakamoto N. Direct peroral ultraslim endoscopy-guided biliary drainage in a patient with cystic duct carcinoma and an occluded self-expandable metallic stent. Endoscopy. 2015;47 Suppl 1 UCTN:E43–E44. doi: 10.1055/s-0034-1391258. [DOI] [PubMed] [Google Scholar]
- 38.Prachayakul V, Aswakul P. Endoscopic ultrasound-guided biliary drainage as an alternative to percutaneous drainage and surgical bypass. World J Gastrointest Endosc. 2015;7:37–44. doi: 10.4253/wjge.v7.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu CL, Sun R, Qiao XJ, Xu CC, Shang XY, Niu WN. Protective effect of glutamine on intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J Gastroenterol. 2014;20:4662–4674. doi: 10.3748/wjg.v20.i16.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huygelen V, De Vos M, Willemen S, Tambuyzer B, Casteleyn C, Knapen D, Van Cruchten S, Van Ginneken C. Increased intestinal barrier function in the small intestine of formula-fed neonatal piglets. J Anim Sci. 2012;90 Suppl 4:315–317. doi: 10.2527/jas.53731. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Chen F, Odle J, Lin X, Jacobi SK, Zhu H, Wu Z, Hou Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- 42.Sobhian B, Kröpfl A, Hölzenbein T, Khadem A, Redl H, Bahrami S. Increased circulating D-lactate levels predict risk of mortality after hemorrhage and surgical trauma in baboons. Shock. 2012;37:473–477. doi: 10.1097/SHK.0b013e318249cb96. [DOI] [PubMed] [Google Scholar]
- 43.Sapin V, Nicolet L, Aublet-Cuvelier B, Sangline F, Roszyk L, Dastugue B, Gazuy N, Deteix P, Souweine B. Rapid decrease in plasma D-lactate as an early potential predictor of diminished 28-day mortality in critically ill septic shock patients. Clin Chem Lab Med. 2006;44:492–496. doi: 10.1515/CCLM.2006.086. [DOI] [PubMed] [Google Scholar]
- 44.Kou QY, Guan XD. [Protective effect of ethyl pyruvate on barrier function of intestinal mucosa in dogs with septic shock] Zhonghua Weichang Waike Zazhi. 2008;11:177–180. [PubMed] [Google Scholar]
- 45.De Vos M, Huygelen V, Van Raemdonck G, Willemen S, Fransen E, Van Ostade X, Casteleyn C, Van Cruchten S, Van Ginneken C. Supplementing formula-fed piglets with a low molecular weight fraction of bovine colostrum whey results in an improved intestinal barrier. J Anim Sci. 2014;92:3491–3501. doi: 10.2527/jas.2013-6437. [DOI] [PubMed] [Google Scholar]


