Abstract
AIM: To evaluate the practice of nutritional assessment and management of hospitalised patients with cirrhosis and the impact of malnutrition on their clinical outcome.
METHODS: This was a retrospective cohort study on patients with liver cirrhosis consecutively admitted to the Department of Gastroenterology and Hepatology at the Royal Adelaide Hospital over 24 mo. Details were gathered related to the patients’ demographics, disease severity, nutritional status and assessment, biochemistry and clinical outcomes. Nutritional status was assessed by a dietician and determined by subjective global assessment. Estimated energy and protein requirements were calculated by Simple Ratio Method. Intake was estimated from dietary history and/or food charts, and represented as a percentage of estimated daily requirements. Median duration of follow up was 14.9 (0-41.4) mo.
RESULTS: Of the 231 cirrhotic patients (167 male, age: 56.3 ± 0.9 years, 9% Child-Pugh A, 42% Child-Pugh B and 49% Child-Pugh C), 131 (57%) had formal nutritional assessment during their admission and 74 (56%) were judged to have malnutrition. In-hospital caloric (15.6 ± 1.2 kcal/kg vs 23.7 ± 2.3 kcal/kg, P = 0.0003) and protein intake (0.65 ± 0.06 g/kg vs 1.01 ± 0.07 g/kg, P = 0.0003) was significantly reduced in patients with malnutrition. Of the malnourished cohort, 12 (16%) received enteral nutrition during hospitalisation and only 6 (8%) received ongoing dietetic review and assessment following discharge from hospital. The overall mortality was 51%, and was higher in patients with malnutrition compared to those without (HR = 5.29, 95%CI: 2.31-12.1; P < 0.001).
CONCLUSION: Malnutrition is common in hospitalised patients with cirrhosis and is associated with higher mortality. Formal nutritional assessment, however, is inadequate. This highlights the need for meticulous nutritional evaluation and management in these patients.
Keywords: Liver cirrhosis, Nutrition assessment, Mortality, Malnutrition, Morbidity
Core tip: This is the first study to highlight the lack of nutritional assessment of hospitalised patients with cirrhosis. Despite the well-established prognostic value of nutrition, our study showed that almost half of hospitalized patients with cirrhosis did not have a formal nutritional assessment. The prevalence of malnutrition in this group of patients was high (56%) and in-hospital dietary intake was substantially reduced, even in patients with normal subjective global assessment. We also confirmed that malnutrition was an independent predictor of both short-term and long-term mortality.
INTRODUCTION
Malnutrition is common in chronic liver disease, and occurs in 24%-66% of hospitalised patients with cirrhosis[1-4]. However, in contrast to the common occurrence of malnutrition reported in prospective studies, the largest retrospective population based study of hospitalised patients with cirrhosis reported a substantially lower prevalence of malnutrition at only 6.1%[5]. This suggests that malnutrition is likely under-recognised and inadvertently under-treated in hospitalised patients with liver cirrhosis. Currently, the extent to which nutritional assessment and support is implemented in routine clinical care is unknown.
Malnutrition in patients with liver cirrhosis is associated with increased morbidity including hepatic encephalopathy, variceal bleeding, refractory ascites, spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome (HRS)[6,7]. While a small number of studies have shown that malnutrition is an independent predictor of mortality in patients with liver cirrhosis, the largest multi-centre prospective study demonstrated that reduction in muscle mass was associated with lower cumulative survival only in Childs A and B cirrhosis on univariate but not multivariate analysis[4,8] and survival in Childs C cirrhosis was not associated with nutritional status[8]. Evidence for malnutrition as a prognostic indicator in patients with decompensated cirrhosis has mostly been derived from the transplant population where severe but not mild or moderate malnutrition has been associated with pre-transplant mortality[9]. Pre-transplant reduced nutritional index have also been associated with increased and earlier post-transplant morbidity but not mortality[10,11]. The data relating to the impact of malnutrition on survival in patients with advanced liver disease who are not candidates for liver transplantation however, are limited.
The identification of malnutrition in patients with liver cirrhosis using traditional objective nutritional assessment parameters is often confounded by non-nutritional factors, including liver synthetic function and fluid status. While multi-compartmental body composition analyses have been used to define malnutrition in patients with cirrhosis more accurately, the expense and lack of availability of these techniques limits their use to the research setting. Subjective global assessment (SGA) is often applied to determine nutritional status in routine clinical practice[12]. While SGA may underestimate the frequency and severity of malnutrition[7,13,14], it has been reported to correlate better with disease severity than does body composition analysis[15]. Although nutritional assessment by SGA is a simple bedside tool, it is only one of a large number of undertakings expected of clinicians, and is often forgotten. Furthermore, appropriate nutritional intervention requires the establishment and frequent re-evaluation of nutrient requirements and intake levels which is more efficiently performed by dieticians. Early detection and management of nutritional deficiency by a dietician has been reported to be associated with an improvement in survival, as compared to that by a physician[16]. The aim of this study, therefore, was to evaluate the practice of nutritional assessment and management in hospitalised patients with cirrhosis in a non-liver transplant tertiary hospital, and to assess the impact of malnutrition on their clinical outcomes.
MATERIALS AND METHODS
Study population
A retrospective analysis was performed of all patients with chronic liver disease and cirrhosis, who had been admitted to the Department of Gastroenterology and Hepatology in the Royal Adelaide Hospital, the largest tertiary referral hospital in South Australia, between January 2010 and December 2011. The patients were identified from a prospectively collected hospital database of all patients admitted with chronic liver disease. Cirrhosis was proven histologically or defined by evidence of chronic liver disease with the presence of portal hypertension or complications of hepatic decompensation on the basis of clinical, biochemical and radiological findings. The only exclusion criterion was elective admission for only day procedures (paracentesis or endoscopy). The project was approved by the Royal Adelaide Hospital Research Ethics Committee, and all patient data were de-identified to maintain confidentiality.
Data collection
Detailed demographic and disease-specific data relating to the patients with liver cirrhosis were collected from careful review of case notes, electronic medical records, and prospectively compiled Gastroenterological departmental databases. The disease-specific variables recorded were: aetiology of liver disease, severity of liver disease as graded by Child-Pugh classification[17] and Model for End-Stage Liver Disease (MELD) score[18] and laboratory data including bilirubin, albumin, serum creatinine and international normalised ratio.
In addition to the hospital patient records, an independent record of nutritional assessment was prospectively maintained by the Dietetics Department. The evaluation of nutritional status was performed by a dedicated dietician specialized in gastrointestinal and liver disease, using the Subjective Global Assessment (SGA). The SGA entailed a medical history including weight change, dietary change, gastrointestinal symptoms, and functional impairment, and physical examination for subcutaneous fat store, muscle wasting, peripheral oedema and ascites and body mass index (BMI). BMI was calculated from dry weight following paracentesis or estimated by adjusting for ascites and peripheral oedema according to Mendenhall, 1992[19]. The recommended caloric and protein requirements were calculated using the simple ratio method. In accordance with the European Society of Clinical Nutrition and Metabolism guidelines[20], energy requirement was calculated as 35-40 kcal/kg per day and protein requirement as 1.2-1.5 g/kg/d[20]. In-hospital dietary intake was calculated from detailed 3 d dietary recall by the patient and daily food charts. Nutritional intervention and the route of nutritional supplementation (enteral, parenteral or oral) were recorded.
Clinical details relating to the hospital admission, patient progress and outcomes from the hospitalization, associated morbidities, and mortality were obtained from extensive review and cross check of hospital medical record, outpatient records, and electronic patient database (covering all major metropolitan hospitals and the 8 largest rural centres). If there was uncertainty, the patients as well as their primary care physician were contacted via telephone. Follow-up was from the time of the index admission until time of death, or censored at the date of last clinical encounter alive.
The primary outcomes of this study were: (1) the proportion of patients who had dietetic assessment and interventions; and (2) the impact of nutrition status on mortality. Secondary outcomes were the impact of nutritional status on hospital length of stay, infectious complications, liver-related morbidity and hospital readmission.
Statistical analysis
Statistical analysis was carried out using SPSS version 22.0.0 for Windows. Parametric data are presented as mean ± SEM and non-parametric data as median (range). Categorical variables were compared using Chi-square and Fisher’s exact test where appropriate. Quantitative variables were tested by t-test and, when the assumption for normality was not met, the Mann Whitney U test. Univariate analysis for survival was performed using Kaplan Meier method and differences in Kaplan Meier curves were tested for statistical significance using the log rank test. A multivariate Cox Proportional Hazards regression analysis of nutritional status on survival time was performed, controlling for age, disease severity (MELD score), the presence of hepatocellular carcinoma (HCC) and creatinine. As the proportional effect of malnutrition on the risk of death was not constant over time, the proportional hazards assumption of the standard Cox regression was not met for the malnutrition variable. Instead, the Cox regression model that allowed for a time-dependent effect of malnutrition on survival was utilized. P < 0.05 was considered statistically significant.
The statistical methods of this study was reviewed by Kylie Lange, Biostatistician, Centre of Research Excellence (CRE) in Translating Nutritional Science to Good Health, Discipline of Medicine, The University of Adelaide
RESULTS
A total of 231 patients [167 male (72%); 56.3 ± 0.9 years] with cirrhosis were admitted over the 24 mo. The most common aetiologies of cirrhosis were alcoholic liver disease (56%), a combination of alcoholic liver disease and hepatitis C (12%) and hepatitis C (10%).The mean MELD score was 17.0 ± 0.5, with the majority of patients having Child-Pugh B (42%) and C (49%) disease. Only 9% (n = 21) patients had Child-Pugh A cirrhosis. The median length of hospital stay was 7 d (range, 1-116 d). Other characteristics and details relating to the hospitalization are summarized in Table 1.
Table 1.
Baseline characteristics of hospitalised patients with liver cirrhosis n (%)
| Variable | n = 231 |
| Gender | 167 M; 64 F |
| Age (yr) | 56.3 ± 0.9 |
| Aetiology | |
| Alcohol | 130 (56) |
| Alcohol and HCV | 28 (12) |
| HCV | 23 (10) |
| NAFLD | 19 (8) |
| Others | 31 (14) |
| Child-Pugh classification | |
| Childs A | 21 (9) |
| Childs B | 96 (42) |
| Childs C | 114 (49) |
| MELD Score | 17.0 ± 0.5 |
| Ascites | 135 (71) |
| Hepatic encephalopathy | 88 (38) |
| Albumin | 24.9 ± 0.3 |
| Bilirubin | 80.3 ± 7.1 |
| INR | 1.6 ± 0.04 |
| Median LOS (d) | 7 (1-116) |
| Admission with variceal bleeding | 30 (13) |
| 28-d mortality | 31 (13) |
| In-hospital mortality | 33 (14) |
HCV: Hepatitis C virus; NAFLD: Non-alcoholic liver disease; MELD: Model for End-Stage Liver Disease; INR: International normalised ratio; LOS: Length of stay.
After a median follow-up period of 14.9 mo (range, 0.03-40.8 mo), 117 (51%) patients died, with a 1-year survival rate of 61% and a 3-year survival of 42%. The in-hospital mortality rate was 14% and the 28 d mortality rate was 13%. On multivariate Cox regression analysis, reduced survival was related to older age (P < 0.001), presence of HCC (P < 0.001) and greater disease severity as determined by MELD score (P = 0.01).
Prevalence of nutritional assessment, malnutrition and interventions
Of the 231 hospitalised patients with cirrhosis, only 131 (57%) had dietetic assessment during their admission. On univariate analysis, Child-Pugh score (9.1 ± 0.9 vs 9.9 ± 0.2, P = 0.01), serum albumin (26.3 ± 0.7 vs 23.1 ± 0.4, P = 0.004) and the presence of ascites [OR 2.0 (95%CI: 1.1-3.5), P = 0.02] were factors that associated with referral for a formal dietetic evaluation. There were no differences in the length of hospital stay, in-hospital mortality and 28-d mortality between the patients assessed by dieticians and those who were not (Table 2). Of the 131 patients who had an initial nutritional assessment, 71 (54%) were subsequently reviewed by a dietician during their inpatient encounter. More importantly, only 6 (8%) of the malnourished patients attended outpatient dietetic clinic after discharge from hospital.
Table 2.
Characteristics of hospitalised patients assessed by dietician n (%)
| Variable | No nutritional assessment (n = 100) | Nutritional assessment (n = 131) | P value |
| Gender | 75M; 25F | 92M; 39F | 0.46 |
| Age | 57.6 ± 1.3 | 55.3 ± 1.2 | 0.23 |
| Aetiology-alcohol | 66 (66) | 99 (76) | 0.14 |
| Child-Pugh score | 9.1 ± 0.9 | 9.9 ± 0.2 | 0.01 |
| MELD score | 16.6 ± 0.8 | 17.4 ± 0.6 | 0.20 |
| Ascites | 63 (63) | 102 (78) | 0.02 |
| Hepatic encephalopathy | 35 (35) | 53 (40) | 0.40 |
| Albumin | 26.3 ± 0.7 | 23.1 ± 0.4 | 0.004 |
| Bilirubin | 71.4 ± 10.6 | 87.1 ± 9.6 | 0.15 |
| INR | 1.6 ± 0.05 | 1.7 ± 0.05 | 0.43 |
| Median LOS | 5 (1-116) | 9 (1-100) | 0.09 |
| Admission with variceal bleeding | 15 (15) | 15 (11) | 0.44 |
| 28-d mortality | 12 (12) | 19 (15) | 0.55 |
| In-hospital mortality | 12 (12) | 21 (16) | 0.45 |
MELD: Model for End-Stage Liver Disease; INR: International normalised ratio; LOS: Length of stay.
According to the SGA, 74/131 (56%) of the assessed patients were identified as “malnourished”. Malnutrition was significantly associated with lower BMI (23.2 ± 0.8 kg/m2 vs 26.3 ± 0.7 kg/m2, P = 0.005) and poorer in-hospital oral caloric intake (1080 ± 91 kcal per day vs 1674 ± 114 kcal per day, P = 0.0003), and protein intake (99 ± 7 g vs 45 ± 4 g, P < 0.0001). When, dietary intake was measured as a percentage of daily requirements calculated from body weight, malnourished patients only met 45% (15.6 ± 1.2 kcal/kg) of their total caloric requirements compared to 68% (23.7 ± 2.3 kcal/kg) those that were not malnourished (P = 0.0003), and 54% (0.65 ± 0.06 g/kg) vs 84% (1.01 ± 0.07 g/kg) of their protein requirements in the malnourish and not malnourished cohort respectively (P = 0.0003). There was no association between malnutrition and age, gender, aetiology of liver disease, disease severity, the presence of ascites, and the presence of hepatic encephalopathy (Table 3).
Table 3.
Characteristics of malnourished vs well-nourished cirrhotic patients
| Normal nutritional status (n = 57) | Malnutrition (n = 74) | P value | |
| Gender | 39M:18F | 53M:21F | 0.89 |
| Age | 54.9 ± 1.9 | 55.6 ± 1.6 | 0.89 |
| Aetiology-alcohol | 41 | 58 | 0.41 |
| Child-Pugh Classification | |||
| Childs A | 3 | 4 | 1.00 |
| Childs B | 23 | 28 | 0.86 |
| Childs C | 31 | 42 | 0.70 |
| Child Pugh score | 9.72 ± 0.3 | 9.99 ± 0.3 | 0.48 |
| MELD score | 16.9 ± 0.8 | 17.8 ± 0.9 | 0.87 |
| Ascites | 44 | 58 | 1.00 |
| Hepatic encephalopathy | 23 | 30 | 1.00 |
| Albumin | 24.5 ± 0.8 | 23.3 ± 0.6 | 0.25 |
| Bilirubin | 85.3 ± 12.9 | 88.5 ± 13.9 | 0.90 |
| INR | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.38 |
| Creatinine | 84.9 ± 8.6 | 91.6 ± 8.6 | 0.70 |
| BMI | 26.3 ± 0.7 | 23.2 ± 0.8 | 0.005 |
| 28-d mortality | 3 | 16 | 0.011 |
| In-hospital mortality | 3 | 18 | 0.0035 |
MELD: Model for End-Stage Liver Disease; INR: International normalised ratio; LOS: Length of stay; BMI: Body mass index.
Of the patients assessed by a dietician and determined to be malnourished, only 28 (38%) patients received supplementation with oral nutritional supplements (n = 14), enteral nutrition (n = 12), parenteral nutrition (n = 1) and combined enteral-parenteral nutrition (n = 1). The remaining 46 (62%) patients received dietary advice alone. A low sodium diet was prescribed for 23 (31%) patients. Of the 12 patients who were enterally fed, four had a percutaneous endoscopic gastrostomy (PEG) inserted for long-term feeding. All four patients did not have ascites at the time of PEG insertion. For the remaining patients, the median duration of enteral feeding was 5 d (1-50 d). There were no complications relating to PEG or enteral feeding.
Impact of malnutrition on clinical outcomes
In-hospital mortality was significantly higher in cirrhotic patients who were malnourished compared to those who were not [24.3% vs 5.3%, P = 0.0035; HR = 5.79 (95%CI: 1.61-20.77)]. On univariate analysis, malnutrition was associated with shortened survival (P = 0.001; Figure 1). On multivariate Cox regression analysis, adjusting for age, presence of HCC, serum bilirubin, serum creatinine and disease severity, malnutrition remained an independent risk factor for reduced survival (HR = 5.29, 95%CI: 2.31-12.1; P < 0.001). In-hospital caloric and protein intake of less than 25% of the calculated daily requirements was associated with shortened survival on univariate analysis (Figure 2), but was not an independent prognostic factor on multivariate analysis.
Figure 1.
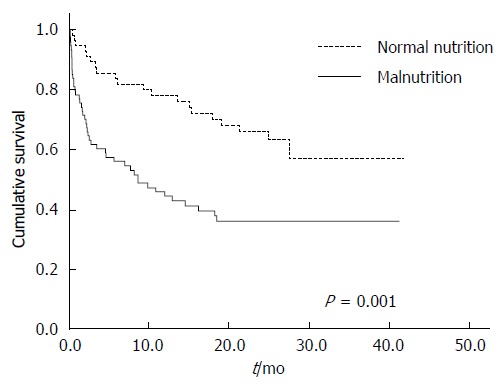
Relationship between overall survival and the presence of malnutrition in patients with liver cirrhosis.
Figure 2.
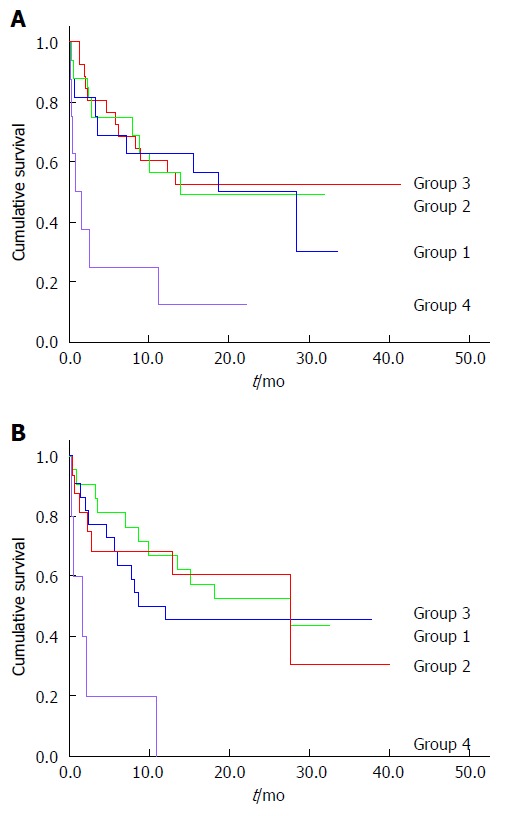
Caloric and protein intake and survival. A: Caloric intake and survival. Group 1: Caloric intake > 75% of recommended; Group 2: Caloric intake 50%-75% of recommended; Group 3: Caloric intake 25%-50% of recommended; Group 4: Caloric intake < 25% of recommended. Lower survival rates in group 4 vs 1 (P = 0.014), group 4 vs 2 (P = 0.017) and group 4 vs 3 (P = 0.002). Pairwise comparisons between groups 1, 2 and 3 (not significant); B: Protein intake and survival. Group 1: Protein intake > 75% of recommended; Group 2: Protein intake 50%-75% of recommended; Group 3: Protein intake 25%-50% of recommended; Group 4: Protein intake < 25% of recommended. Lower survival rates in group 4 vs 1 (P < 0.001), group 4 vs 2 (P = 0.003) and group 4 vs 3 (P = 0.005). Pairwise comparisons between groups 1, 2 and 3 (not significant).
There was no relationship between malnutrition and the length of hospital stay (malnourished vs normal: 9 (1-100) d vs 9 (1-66) d, P = 0.6), development of ascites (3.5% vs 2.7%, P = 1.0), or encephalopathy (14% vs 12%, P = 0.8). After discharge, there was a trend for more malnourished patients to be re-admitted for major liver related complications, such as spontaneous bacterial peritonitis, hepatorenal syndrome, and/or variceal bleeding than those without malnutrition (10.8% vs 5.6%, P = 0.13).
DISCUSSION
In addition to reinforcing the notion that malnutrition is common in cirrhotic patients, the current study also highlights the following important issues: (1) formal dietetic assessment and intervention are not routine for hospitalized patients with liver cirrhosis and up to 43% of patients were not assessed; (2) daily caloric and protein intake in these patients was sub-optimal, even in patients who had normal SGA; (3) only one third of patients who were identified to have malnutrition and poor caloric intake were actively given nutritional supplement; (4) less than 10% of the identified malnourished patients had outpatient follow-up and, most importantly; and (5) the presence of “malnutrition” was associated with higher mortality. Together, our findings strongly support for a need of early dietetic evaluation in all hospitalized patients with chronic liver disease in order to identify subjects at-risk for “malnutrition”, so that early nutritional intervention can be instituted during hospital admission and also after discharge.
To our knowledge, this is the first study to highlight the lack of dietetic assessment in hospitalised patients with liver cirrhosis. Despite the well-established prognostic value of nutrition, our study showed that almost half of hospitalized patients with cirrhosis did not have a formal nutritional assessment. While patients with hypoalbuminaemia and ascites were more likely to receive formal dietetic input, neither of these factors was predictive of nutritional status. More importantly, the prevalence of malnutrition in this group of patients is high (56%) and is in keeping with previously reports[3,4], which would support the notion that malnutrition is under-diagnosed in these patients in routine clinical care.
Poor nutrient intake is likely to be a major factor responsible for the development of malnutrition in these patients. Consistent with finding of a previous study[21], dietary intake was substantially reduced in our patients with liver cirrhosis, even in patients who had normal SGA (only 68% daily carbohydrate and 85% protein requirements), resulting in a negative energy balance, weight loss and the development of malnutrition. This reduction in nutrient intake in these patients is likely to be multi-factorial. Patients with cirrhosis frequently experience gastrointestinal symptoms[22,23] which may contribute to reduced nutrient intake[24]. Delayed gastric emptying has been reported in patients with cirrhosis and has been associated with post-prandial fullness and bloating[25]. Alteration in taste acuity has been associated with deficiencies in trace elements including zinc, magnesium and vitamin A[26,27], which are common in patients with cirrhosis. Appetite is also reduced owing to increased inflammatory cytokines and alterations in appetite regulating hormones in cirrhotic patient including leptin, ghrelin, PYY and CCK[25,28-32].
Paradoxically, the lack of nutrient intake also influences the function of the gastrointestinal tract. In healthy and critically ill subjects, enteral nutrient deprivation is associated with mucosal atrophy, increased intestinal permeability and infections[33,34]. The use of enteral nutrition in critically ill and surgical patients has been shown to prevent the development of these adverse changes to the gastrointestinal tract[34,35], and has been associated with reduced morbidity, particularly septic complications[36,37]. Given such poor oral intake in cirrhotic patients, it is very likely that similar gastrointestinal changes of nutritional deprivation will be observed. This may explain the increase in intestinal permeability seen in cirrhotic patients with malnutrition[38], which subjects these patients to a higher risk of infectious complications and hepatic encephalopathy[39]. Given our finding of increased mortality in malnourished patients and in patients with poor in-hospital caloric and protein intake, the role of routine oral nutritional supplement or even aggressive enteral nutrition on gut functions and defence needs further evaluation.
Another key finding in our study was that nutritional status as determined by subjective global assessment had a major impact on short-term and long-term mortality in hospitalised patients with liver cirrhosis. Studies examining the association between nutrition and survival have yielded different results. Compared to other studies, our patient population had more advanced liver disease with over ninety percent having decompensated cirrhosis, and a high mortality rate during hospital stay and the follow-up period.
Our findings are consistent with other studies that involved hospitalized patients with mostly decompensated cirrhosis[4,5,9,40], with similar hazard ratios of 2-5.3 for mortality[9,40]. Conversely, studies enrolling stable, and mostly compensated cirrhotic patients in the outpatient setting have failed to demonstrate an association between malnutrition and survival[7,8,21]. A potential explanation for the difference in the impact of nutritional status on outcomes between inpatients and outpatients is the lack of room for compensation in the already decompensated inpatients.
Despite the higher mortality in cirrhotic patients with malnutrition, we found no significant difference in liver related morbidity. This is contrary to previous studies which have found increased variceal bleeding, refractory ascites, spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome (HRS) in malnourished patients[6,7]. The failure to find any difference in liver related morbidity in our study may be due to the patient population with advanced, decompensated cirrhosis, high prevalence of pre-existing morbidity, and the small number of subsequent new liver related complications.
We acknowledge that this is a retrospective review with the potential limitations associated with the study design. Nevertheless, the data were thoroughly collected and cross-checked from a number of prospective databases. Furthermore, all patients with liver cirrhosis who were admitted to the Department of Gastroenterology and Hepatology in the Royal Adelaide Hospital were included in the study and the sample size was sufficient to demonstrate differences in the clinical outcomes. The strength of this study is that it provides information on nutritional care and the impact of malnutrition on clinical outcomes in patients with cirrhosis in the real-world setting. Although SGA may have a lower sensitivity for the detection of malnutrition compared to hand grip strength[7,41] and multi-compartmental body composition analysis[13], SGA is a simple, inexpensive and reliable bed-side tool to assess for malnutrition with an 80% inter-observer reproducibility rate[42].
In conclusion, although malnutrition is common in hospitalised patients with liver cirrhosis and is associated with higher short- and long-term mortality, nearly half of these patients have no dietetic assessment and intervention. These findings suggest that routine dietetic assessment should be performed on all patients who are admitted with chronic liver disease and related complication, so that at-risk subjects for “malnutrition” can be identified and early nutritional intervention can be instituted during hospitalisation but also after discharge.
ACKNOWLEDGMENTS
The authors thank Dr Rahul Solanki for his help with the collection of data, and Kylie Lange for her expertise and assistance in performing the statistical analysis.
COMMENTS
Background
Malnutrition is common in patients with liver cirrhosis and is associated with increased morbidity including hepatic encephalopathy, variceal bleeding, refractory ascites, spontaneous bacterial peritonitis and hepatorenal syndrome. Malnutrition is also associated with increased mortality. Early detection and nutritional intervention by a dietician can improve survival.
Research frontiers
Despite the prognostic importance of malnutrition in liver cirrhosis, little is known about the current practice of nutritional management in hospitalized patients with liver cirrhosis.
Innovations and breakthroughs
This study shows that malnutrition is common in hospitalized patients with cirrhosis and is associated with increased mortality. However, this is the first study to highlight the lack of formal dietetic assessment and nutritional intervention in these patients.
Applications
The study results suggest the need for more conscientious nutritional assessment and management in the routine clinical care of hospitalised patients with liver cirrhosis.
Terminology
SGA is a simple bedside tool for nutritional assessment which entails a medical history including weight change, dietary change, gastrointestinal symptoms, and functional impairment, and physical examination for subcutaneous fat store, muscle wasting, peripheral oedema and ascites and body mass index.
Peer-review
This study evaluated the practice of nutritional assessment and management of hospitalised patients with cirrhosis and the impact of malnutrition on their clinical outcome. The author found that malnutrition was common in hospitalised patients with cirrhosis and is associated with higher mortality. However, the formal nutritional assessment was inadequate currently. This highlights the need for meticulous nutritional evaluation and management in these patients. This is a well conducted and well written study. The experiments are described in detail, the results are shown nicely and the figures are impressive.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Royal Adelaide Hospital Research Ethics Committee.
Informed consent statement: The need to obtain informed consent from subjects was waived by the Royal Adelaide Hospital Research Ethics Committee.
Conflict-of-interest statement: The authors do not have any conflict of interest to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 18, 2015
First decision: May 18, 2015
Article in press: September 2, 2015
P- Reviewer: Gong ZG, Liang XS S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Teiusanu A, Andrei M, Arbanas T, Nicolaie T, Diculescu M. Nutritional status in cirrhotic patients. Maedica (Buchar) 2012;7:284–289. [PMC free article] [PubMed] [Google Scholar]
- 2.Guglielmi FW, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, Conte D, Federico A, Gasbarrini G, Guglielmi A, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE) Dig Liver Dis. 2005;37:681–688. doi: 10.1016/j.dld.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19:515–521. doi: 10.1016/s0899-9007(02)01071-7. [DOI] [PubMed] [Google Scholar]
- 4.Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–450. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 5.Sam J, Nguyen GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29:1396–1402. doi: 10.1111/j.1478-3231.2009.02077.x. [DOI] [PubMed] [Google Scholar]
- 6.Møller S, Bendtsen F, Christensen E, Henriksen JH. Prognostic variables in patients with cirrhosis and oesophageal varices without prior bleeding. J Hepatol. 1994;21:940–946. doi: 10.1016/s0168-8278(05)80599-9. [DOI] [PubMed] [Google Scholar]
- 7.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi) Hepatology. 1996;23:1041–1046. doi: 10.1002/hep.510230516. [DOI] [PubMed] [Google Scholar]
- 9.Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, Sabin C, Burroughs AK. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563–572. doi: 10.1111/j.1365-2036.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, DiCecco S, Francisco-Ziller N, Charlton M. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000;70:1347–1352. doi: 10.1097/00007890-200011150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo FA, Perez RM, Freitas MM, Kondo M. Comparison of three methods of nutritional assessment in liver cirrhosis: subjective global assessment, traditional nutritional parameters, and body composition analysis. J Gastroenterol. 2006;41:476–482. doi: 10.1007/s00535-006-1794-1. [DOI] [PubMed] [Google Scholar]
- 14.Naveau S, Belda E, Borotto E, Genuist F, Chaput JC. Comparison of clinical judgment and anthropometric parameters for evaluating nutritional status in patients with alcoholic liver disease. J Hepatol. 1995;23:234–235. doi: 10.1016/0168-8278(95)80344-0. [DOI] [PubMed] [Google Scholar]
- 15.Vulcano DS, Carvalhaes MA, Bakonyi Neto A. Evaluation of nutritional indicators and body composition in patients with advanced liver disease enrolled for liver transplantation. Acta Cir Bras. 2013;28:733–739. doi: 10.1590/s0102-86502013001000008. [DOI] [PubMed] [Google Scholar]
- 16.Iwasa M, Iwata K, Hara N, Hattori A, Ishidome M, Sekoguchi-Fujikawa N, Mifuji-Moroka R, Sugimoto R, Fujita N, Kobayashi Y, et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition. 2006;29:1418–1421. doi: 10.1016/j.nut.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 18.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 19.Mendenhall CL. Protein-calorie malnutrition in alcholic liver disease. In: Wartson RR, Watz IB, editors. Nutrition and Alcohol. Boca Raton: CRC Press; 1992. pp. 363–384. [Google Scholar]
- 20.Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, Ferenci P, Holm E, Vom Dahl S, Müller MJ, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285–294. doi: 10.1016/j.clnu.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 22.Galati JS, Monsour HP, Dyer CH, Seagren S, Quigley EMM. A survey or the frequency of gastrointestinal complaints in patients with chronic liver disease. Gastroenterology. 1995;108:A1068. [Google Scholar]
- 23.Kalaitzakis E, Simrén M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Björnsson E. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41:1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 24.Kalaitzakis E, Josefsson A, Castedal M, Henfridsson P, Bengtsson M, Andersson B, Björnsson E. Gastrointestinal symptoms in patients with cirrhosis: a longitudinal study before and after liver transplantation. Scand J Gastroenterol. 2013;48:1308–1316. doi: 10.3109/00365521.2013.836755. [DOI] [PubMed] [Google Scholar]
- 25.Kalaitzakis E, Sadik R, Holst JJ, Ohman L, Björnsson E. Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:346–352. doi: 10.1016/j.cgh.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Madden AM, Bradbury W, Morgan MY. Taste perception in cirrhosis: its relationship to circulating micronutrients and food preferences. Hepatology. 1997;26:40–48. doi: 10.1002/hep.510260106. [DOI] [PubMed] [Google Scholar]
- 27.Russell RM, Cox ME, Solomons N. Zinc and the special senses. Ann Intern Med. 1983;99:227–239. doi: 10.7326/0003-4819-99-2-227. [DOI] [PubMed] [Google Scholar]
- 28.Kalaitzakis E, Bosaeus I, Ohman L, Björnsson E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: correlations with energy intake and resting energy expenditure. Am J Clin Nutr. 2007;85:808–815. doi: 10.1093/ajcn/85.3.808. [DOI] [PubMed] [Google Scholar]
- 29.Campillo B, Sherman E, Richardet JP, Bories PN. Serum leptin levels in alcoholic liver cirrhosis: relationship with gender, nutritional status, liver function and energy metabolism. Eur J Clin Nutr. 2001;55:980–988. doi: 10.1038/sj.ejcn.1601255. [DOI] [PubMed] [Google Scholar]
- 30.Valentini L, Schuetz T, Omar A, Gläser S, Kasim E, Nowotny P, Kroencke T, Ockenga J. Abnormal plasma peptide YY(3-36) levels in patients with liver cirrhosis. Nutrition. 2011;27:880–884. doi: 10.1016/j.nut.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Paloheimo LI, Clemmesen O, Dalhoff K, Rehfeld JF. Plasma cholecystokinin and its precursors in hepatic cirrhosis. J Hepatol. 1997;27:299–305. doi: 10.1016/s0168-8278(97)80175-4. [DOI] [PubMed] [Google Scholar]
- 32.Kanayama S, Himeno S, Higashimoto Y, Yamasaki Y, Kitani T, Tarui S. Plasma cholecystokinin-octapeptide like immunoreactivity in patients with hepatic cirrhosis. Life Sci. 1987;41:1915–1920. doi: 10.1016/0024-3205(87)90743-0. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, Maiz A, Lopez F, Guzman S, Vargas C. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care. 1999;14:73–77. doi: 10.1016/s0883-9441(99)90017-5. [DOI] [PubMed] [Google Scholar]
- 34.Kudsk KA. Effect of route and type of nutrition on intestine-derived inflammatory responses. Am J Surg. 2003;185:16–21. doi: 10.1016/s0002-9610(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 35.McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305–315. doi: 10.1177/0884533609335176. [DOI] [PubMed] [Google Scholar]
- 36.Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K, Oreskovich MR, Simonowitz D, Johansen K. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma. 1986;26:882–891. doi: 10.1097/00005373-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29:916–922; discussion 922-923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Norman K, Pirlich M, Schulzke JD, Smoliner C, Lochs H, Valentini L, Bühner S. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66:1116–1119. doi: 10.1038/ejcn.2012.104. [DOI] [PubMed] [Google Scholar]
- 39.Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Björnsson E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194–1201. doi: 10.1111/j.1478-3231.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 40.Abad-Lacruz A, Cabré E, González-Huix F, Fernández-Bañares F, Esteve M, Planas R, Llovet JM, Quer JC, Gassull MA. Routine tests of renal function, alcoholism, and nutrition improve the prognostic accuracy of Child-Pugh score in nonbleeding advanced cirrhotics. Am J Gastroenterol. 1993;88:382–387. [PubMed] [Google Scholar]
- 41.Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant. 2011;25:248–254. doi: 10.1111/j.1399-0012.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- 42.Hasse J, Strong S, Gorman MA, Liepa G. Subjective global assessment: alternative nutrition-assessment technique for liver-transplant candidates. Nutrition. 1993;9:339–343. [PubMed] [Google Scholar]


