Abstract
Ag binding to the BCR is a critical step in B cell development and activation, initiating a cascade of signaling events ultimately leading to proliferation, differentiation, or cell death. A bacterial enzyme, IgG-degrading enzyme of Streptococcus pyogenes (IdeS), was shown to specifically cleave IgG molecules below the hinge region of soluble IgG and when IgG is bound to Ag, resulting in one F(ab′)2 molecule and one homodimeric Fc fragment. Whether IdeS could also cleave the IgG molecule when it is present in the BCR attached to the B cell membrane in a complex with CD79a and CD79b is unknown. In this article, we present human in vitro and ex vivo data showing that IdeS cleaves the IgG present in the BCR complex and very efficiently blocks Ag binding to the BCR. As a consequence of IdeS cleaving the BCR, signaling cascades downstream of the BCR are blocked, and memory B cells are temporarily silenced, preventing them from responding to antigenic stimulation and their transition into Ab-producing cells.
Introduction
The IgG-degrading enzyme of Streptococcus pyogenes (IdeS) is a 35-kDa cysteine protease originally identified in Group A Streptococcus (1). IdeS specifically cleaves IgG molecules at the lower hinge region, generating one F(ab′)2 fragment and one homodimeric Fc fragment. Soluble IgG molecules can be either free proteins or bound to their specific Ags. Furthermore, IgG molecules can be cell-bound, either through their binding to FcγRs or as a transmembrane protein within the BCR complex. Both soluble and Ag-complexed IgG molecules are cleaved with the same efficacy by IdeS, and it was reported that Fc-bound IgG (i.e., attached to S. pyogenes M proteins) can be cleaved, although less efficiently, likely as a result of the competitive interactions between IdeS and the M proteins (2).
The BCR complex contains ligand-binding and signaling elements. The ligand-binding portion consists of an Ab with a transmembrane domain, and the signaling portion consists of a heterodimer called Ig-α/Ig-β (CD79a/CD79b) (3). The CD79 proteins span the plasma membrane and have a cytoplasmic tail bearing ITAM. Upon receptor ligation, ITAM is phosphorylated by the sarcoma family kinase LYN and recruits the spleen tyrosine kinase to the receptor. Activation of spleen tyrosine kinase leads to the formation of a plasma membrane-associated signaling complex, called a signalosome, which assembles signaling molecules, such as phospholipase-Cγ2 (PLC-γ2), PI3K, Bruton’s tyrosine kinase, VAV1, and adaptor molecules (4–7). Two fundamental and intensively studied intermediates in the BCR signaling cascades, PLC-γ2 and PI3K, generate key second messengers, which, in turn, activate IκB kinase and ERK1/2 (aka MAPK3 and MAPK1) (8). B cell fate decisions (i.e., proliferation, survival, differentiation, and cell death) are closely regulated by the balance between these signaling events.
During B cell development, naive mature B cells leave the bone marrow and undergo somatic hypermutation in germinal centers and class switching before becoming high-affinity long-lived plasma cells and memory B cells ready to respond rapidly when activated by antigenic stimulation (9, 10). Knowing that memory B cells respond to Ag through binding to the BCR and that a substantial portion of memory B cells in circulation have an IgG-type of BCR, we set out to address whether IdeS could cleave IgG when it was present in the BCR and whether this had any direct effects on B cell fate.
It was demonstrated recently, as part of a phase I clinical trial, that IdeS efficiently and rapidly cleaves the whole pool of plasma IgG after i.v. administration (0.24 mg/kg body weight [BW]) to healthy human subjects (11). Cleavage of plasma IgG is a multistep process. During administration (14 min from initiation of dosing), plasma IgG was already converted into single-cleaved IgG (scIgG), in which one of the two IgG H chains is cut. Within a few hours, IdeS treatment resulted in complete cleavage of plasma IgG into F(ab′)2 and Fc fragments, with no detectable intact IgG and only low levels of scIgG remaining. No reflux of extravascular IgG was observed after IdeS administration in these healthy volunteers, and newly synthesized IgG could be detected 1 wk after treatment. The level of plasma IgG returned to the normal range between 2 and 8 wk after treatment. The efficient, swift, and temporary removal of IgG opens therapeutic opportunities in several IgG-mediated clinical conditions. For clinical application, it is also important to understand whether IdeS can cleave membrane-bound IgG when it is a component of the BCR, because this might have implications for the efficacy and safety of the therapy.
We present in vitro and ex vivo data showing that the bacterial enzyme IdeS cleaves soluble IgG, as well as releases the F(ab′)2 portion of the BCR complex from surface IgG+ B cells. The truncation of the BCR through IdeS cleavage has marked inhibitory effects on the induction of IgG Ab-secreting cells (ASCs). In contrast, the induction of IgM and IgA ASC is not reduced by treatment with IdeS. These data suggest that treating patients with diseases mediated by pathogenic Abs with IdeS could have therapeutic benefits through the degradation of plasma IgG; additionally, this treatment might delay the replenishing of the plasma cell pool from memory cells. This might be especially attractive in combination with plasma cell toxic treatments, such as proteasome inhibitors. Furthermore, in transplantation, a delay in the production of IgG against newly introduced graft epitopes might help the organ to adapt to its new host.
Materials and Methods
Screening cell lines for surface Ig
Different human B cell lymphoma cell lines (i.e., U-2940 [ACC634], Nu-DUL-1 [ACC579], Raji [CCL-86], and Daudi [CCL-213]) were screened for the presence of membrane-bound IgG or IgM. Briefly, cells were cultured at 37°C in 5% CO2 in RPMI 1640 supplemented with 10% FCS and penicillin/streptomycin (PEST; 50 U/ml, 50 μg/ml). Cells were treated for 30 min at 37°C with PBS or different concentrations of IdeS prior to acid wash (0.1 M glycine [pH 2.7], 0.5 M NaCl) for 5 min on ice. Acid wash removes Abs present in FcγRs or bound to Ag while leaving transmembrane molecules intact (12, 13). Cells were stained with biotinylated Abs specific for the F(ab′)2 portion (Jackson ImmunoResearch, cat. no. 109-066-097; cross-reacts with L chain present in all Ig subclasses) and the Fc portion of IgG (Jackson ImmunoResearch, cat. no. 109-066-098; specific for IgG H chain) or IgM (GM-80A, ICL). Streptavidin-allophycocyanin (Jackson ImmunoResearch, cat. no. 016-130-084) was used to monitor cells in FL4 using an Accuri C6 flow cytometer.
Cell proliferation and viability assays
Proliferation was measured by incorporation of BrdU. Briefly, 5 × 104 cells/well were seeded in 96-well plates and cultured for 24 h after treatment with PBS or different concentrations of IdeS. BrdU was added to each well and incubated for 6 h prior to measuring proliferation, according to the manufacturer’s recommendation (Cell Proliferation ELISA, BrdU colorimetric; Roche, cat. no. 11647229001). Cytochalasin D (C2618; Sigma) and puromycin (Invitrogen) were used at different concentrations as antiproliferative controls.
Cell viability was measured using the sensitive colorimetric assay CCK-8 (Cell Counting Kit-8 [CCK-8]; Dojindo Laboratories). Cells (2 × 104 cells/well) were treated as described for the proliferation assay. CCK-8 was added 24 h after cultivation, and the absorbance at 450 nm was recorded at specified time points. The indicated numbers of Nu-DUL-1 cells were treated with PBS or 30 μg/ml IdeS and seeded into 96-well plates for 24 h prior to the addition of CCK-8. In experiments with enriched B cells, peripheral blood was collected in heparin tubes supplemented with IdeS (30 μg/ml) or PBS and incubated at 37°C, 5% CO2 for 30 min. A total of 250 μl RosetteSep Human B Cell Enrichment mixture (STEMCELL Technologies, cat. no. 07905) was added to 5 ml blood, mixed well, and incubated for 20 min at room temperature. Samples were diluted with an equal volume of PBS supplemented with 2% FCS prior to density gradient separation (Ficoll-Paque PLUS). Harvested B cells were counted and adjusted to 2 × 105 cells/ml in RPMI 1640 supplemented with 10% FCS and PEST. A total of 2 × 104 cells/well was seeded in triplicates in 96-well plates and cultured for 24 h prior to the addition of CCK-8.
Addressing IdeS efficacy in plasma by SDS-PAGE
Plasma collected during density-gradient separation of heparinized blood treated with PBS or different amounts of IdeS was used to verify the efficacy of IdeS cleavage on soluble IgG. The SDS-PAGE analyses were performed according to the manufacturer’s instructions (Bio-Rad). Briefly, 1 μl plasma was separated on 4–20% Mini-PROTEANTGX precast gels (Bio-Rad) at 200 V for 40 min under nonreduced conditions. The gels were stained with GelCode Blue Stain Reagent (Pierce; Thermo Fisher Scientific), according to the manufacturer’s instructions, and the gels were scanned.
Recovery of cleaved BCR
Nu-DUL-1 cells were treated with PBS or different amounts of IdeS for 1 h at 37°C prior to extensive washing to remove any remaining IdeS. The cells were seeded in 96-well plates in RPMI 1640 supplemented with 10% FCS and PEST. One plate was used immediately for flow cytometry analysis of intact IgG, and the other was cultured (37°C, 5% CO2) for 24 h prior to analysis. Cells were stained with a biotinylated Ab specific for the F(ab′)2 portion (cat. no. 109-066-097), followed by streptavidin-allophycocyanin (cat. no. 016-130-084; both from Jackson ImmunoResearch), and the cells were monitored in FL4 using an Accuri C6 flow cytometer.
Peripheral blood was collected in heparin tubes (BD Vacutainer; cat. no. 367876) from healthy volunteers and treated with either 30 μg/ml IdeS or PBS for 1 h at 37°C prior to isolating PBMCs using density gradient separation (Ficoll-Paque PLUS). The PBMC interface was collected, washed in PBS, and resuspended in culture medium (RPMI 1640 supplemented with 10% FCS and PEST). PBMCs were counted and adjusted to 2 × 106 cells/ml, and a sample was removed, fixed in paraformaldehyde (PFA), washed in PBS supplemented with 0.1% BSA, and stored at 4°C until flow cytometry analysis. The remaining cells were cultured, and samples were removed and fixed in PFA at the indicated time points. Biotinylated anti–CH1-IgG CaptureSelect (BAC, cat. no. 7103202100) was used for detection of the F(ab′)2 portion of IgG, and biotinylated goat anti-human Fc-specific F(ab′)2 fragment (Jackson ImmunoResearch, cat. no. 109-066-098) was used for detection of the Fc portion of IgG. Cells were double stained with PE-conjugated anti-CD19 (Exbio, cat. no. IP-305-T100) and streptavidin-allophycocyanin (Jackson ImmunoResearch, cat. no. 016-130-084). The lymphocyte population was gated using forward-side scatter, and double-positive cells were monitored in FL2 and FL4 using an Accuri C6 flow cytometer.
Intracellular phospho-specific flow cytometry (BCR signaling)
Nu-DUL-1 cells were cultured overnight in serum-free medium to minimize background phosphorylation prior to the start of signaling experiments. The next day, PBS or 30 μg/ml IdeS was added, and the cells were cultured (37°C, 5% CO2) for 30 min. A total of 1 × 106 cells was removed and fixed for 5 min in PFA, followed by a 10-min permeabilization in 70% ethanol on ice. Cells were washed in PBS supplemented with 0.1% BSA and stored at 4°C until analysis (zero sample). After collection of the zero sample, the BCR of the remaining cells was cross-linked by addition of 10 μg/ml goat anti-human F(ab′)2-specific F(ab′)2 (Jackson ImmunoResearch, cat. no. 109-006-097), and cell samples were collected at different time points, fixed, and permeabilized. The fixed cells were stained for flow cytometry analysis using allophycocyanin-conjugated phospho-specific ERK1/2 (eBioscience, cat. no. 17-9109-42) and PE-conjugated phospho-specific PLC-γ2 (BD, cat. no. 558490). Cells were monitored in FL2 and FL4 using an Accuri cytometer C6.
Memory B cell differentiation
Peripheral blood was collected in heparin tubes (BD Vacutainer, cat. no. 367876) from healthy volunteers, and PBMCs were isolated using density gradient separation (Ficoll-Paque PLUS). The PBMC interface was collected, washed in PBS, and resuspended in culture medium (RPMI 1640 supplemented with 10% FCS and PEST). PBMCs were adjusted to 2 × 106 cells/ml and seeded with IdeS (final concentration 0.3, 3, and 30 μg/ml) or PBS. Cells were stimulated with a mixture of rIL-2 and R848, according to the manufacturer’s recommendation (Mabtech), and cultured for 72–96 h. Cells intended for the short-term treatments were left in tubes supplemented with PBS or IdeS and incubated for 1 h at 37°C prior to washing three times with 12 ml PBS and one time with 12 ml in culture medium. These cells were seeded and treated with rIL-2/R848, as above.
ELISPOT filter plates were wetted with 70% ethanol, washed with sterile water, and incubated at 4°C overnight with capture Ab (ELISpot PLUS Mabtech kit, cat. no. 3850-2HW-Plus for monitoring IgG-producing cells; ELISpot PLUS Mabtech kit, cat. no. 3845-2HW-Plus for monitoring IgM-producing cells; and ELISpot BASIC Mabtech kit, cat. no. 3860-2H for monitoring IgA-producing cells). The ELISPOT filter plates were washed and blocked for ≥30 min with culture medium prior to seeding cells.
The cells were transferred to 15-ml tubes, washed extensively, counted, and adjusted to 0.5 × 106 cells/ml. Two-fold dilutions were prepared before the cells were seeded in the prepared ELISPOT filter plates and cultured for 24 h. ELISPOT plates were washed, and biotinylated detection Abs for total IgG, IgM, and IgA analysis (included in the named kits) were added and incubated for 2 h at room temperature. Plates were washed and incubated for 1 h at room temperature with Streptavidin-HRP, and washed and incubated with 3,3′,5,5′-tetramethylbenzidine substrate solution (TMB-D ready-to-use solution; Kem-En-Tec Diagnostics, cat no. 4600H) and developed until distinct spots emerged. The plates were washed in tap water and allowed to dry in the dark, before the filters were documented by photography and the spots were counted manually.
B cell enrichment and flow cytometry
For B cell enrichment, peripheral blood was collected in heparin tubes supplemented with IdeS at 30 μg/ml or PBS and incubated at 37°C, 5% CO2 for 30 min. A total of 250 μl RosetteSep Human B cell Enrichment mixture (STEMCELL Technologies, cat. no. 07905) was added to 5 ml blood, mixed well, and incubated for 20 min at room temperature. Samples were diluted with an equal volume of PBS supplemented with 2% FCS prior to density gradient separation (Ficoll-Paque PLUS). Harvested B cells were counted and adjusted to 1.5 × 105 cells/ml in PBS supplemented 2% FCS prior to seeding in V-shaped 96-well plates for flow cytometry staining. Plates were centrifuged at 1500 rpm for 3 min, and the supernatant was flicked off. A total of 10 μg/ml biotinylated anti–CH1-IgG (BAC, cat. no. 7103202100) was used for detection of the F(ab′)2 portion of IgG, and 0.5 μg/ml biotinylated goat anti-human Fc-specific F(ab′)2 fragment (Jackson ImmunoResearch, cat. no. 109-066-098) was used for detection of the Fc portion of IgG. Cells were double stained with PE-conjugated anti-CD19 (Exbio, cat. no. IP-305-T100) or PE-conjugated anti-CD27 (BD Pharmingen, cat. no. 555441), followed by detection of the biotin-conjugated Abs with streptavidin-allophycocyanin (Jackson ImmunoResearch, cat. no. 016-130-084). Cells were monitored in FL2 and FL4 using an Accuri cytometer C6.
Analysis of IgG-type of BCR in a first-in-man clinical study
A phase I, double-blind, randomized study with single ascending doses of IdeS was conducted at the Phase I Unit, Lund, after approval from Swedish regulatory and ethical authorities (ClinicalTrials.gov Identifier: NCT01802697). All subjects signed written informed consent before any study-related procedures were initiated. As an exploratory part of the study, the integrity of the IgG-type of BCR on CD19+ cells was monitored at different time points after i.v. treatment with 0.12 or 0.24 mg/kg BW IdeS. Peripheral blood was collected in heparin tubes, and PBMCs were isolated within 2 h of collection using density gradient separation (Ficoll-Paque PLUS). The PBMC interface was collected, washed in PBS, and fixed in PFA for 30 min on ice. Cells were washed and stored in PBS supplemented with 0.5% BSA until all time points were collected. Cells were stained with 10 μg/ml biotinylated anti–CH1-IgG (BAC, cat. no. 7103202100) for detection of the F(ab′)2 portion of IgGl; 0.5 μg/ml biotinylated goat anti-human Fc-specific F(ab′)2 fragment (cat. no. 109-066-098, Jackson ImmunoResearch) was used for detection of the Fc portion of IgG. Cells were double stained with PE-conjugated anti-CD19 (Immunotools, cat. no. 21270194) and streptavidin-allophycocyanin (Jackson ImmunoResearch, cat. no. 016-130-084). The lymphocyte population was gated in the predose sample for each individual, and this gate was used for all time points for a subject. CD19+ cells were monitored in FL2, and the F(ab′)2/Fc signal was monitored in FL4. CD19+ cells were monitored in M1 (FL2), and these cells were further monitored for presence of a signal upon anti-Fc and anti-Fab staining (FL4). In each sample the cell counts in R1 (double-positive cells), as well as mean fluorescent intensity (MFI), were determined.
Furthermore, the frequency of double-positive cells was calculated using the following equation:
This formula was used to be able to appreciate the difference in MFI when only low cell counts were present in the R1.
Results
IdeS cleaves human IgG-type of BCR with similar efficacy as soluble IgG
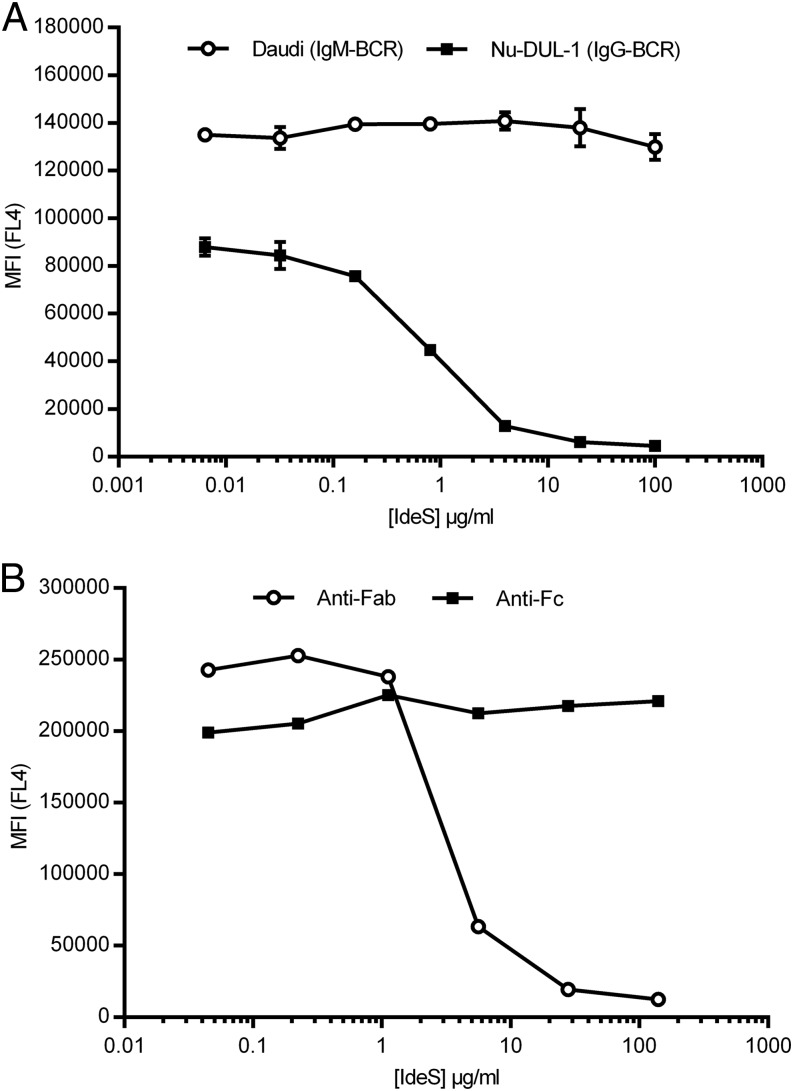
Four human B-lymphoma cell lines were screened for the presence of transmembrane IgG or IgM using a flow cytometry approach, including an acid wash step to remove IgG or IgM not attached to the membrane via a transmembrane domain (12, 13). After verifying the presence of the IgG- or IgM-type of BCR on the cell lines, Nu-DUL-1 (IgG-type of BCR) and Daudi (IgM-type of BCR) were selected as models for further analysis. A Fab-specific F(ab′)2 Ab was used to detect the presence of the Fab portion of the BCR because the Ab cross-reacts with the L chain present in both IgG and IgM. Membrane-bound IgG could no longer be detected on the cell surface of Nu-DUL-1 cells following treatment with IdeS concentrations > 4 μg/ml. In contrast, Daudi cells having an IgM-type of BCR were not affected, even at high concentrations of IdeS (>100 μg/ml) (Fig. 1A). Nu-DUL-1 cells were probed with an anti-IgG Fc-specific Ab to determine whether the Fc portion of the BCR remained attached to the cell surface after IdeS treatment. Although IdeS efficiently removes the F(ab′)2 portion of the BCR, the Fc portion is not affected and remains attached to the membrane (Fig. 1B).
FIGURE 1.
IdeS cleaves IgG-type, but not IgM-type, of BCR on B cells. (A) Flow cytometry analysis of cells stained with biotinylated anti-Fab Ab, followed by streptavidin-allophycocyanin, after treatment of Nu-DUL-1 cells (IgG-type) and Daudi cells (IgM-type) with the indicated concentrations of IdeS. The y-axis shows MFI of duplicate samples in FL4, and vertical lines represent SD. The data are representative of two independent experiments. (B) Flow cytometry analysis of Nu-DUL-1 cells (IgG-type) stained with either biotin-conjugated anti-Fab or anti-Fc, followed by streptavidin-allophycocyanin, after treatment with the indicated concentrations of IdeS. The y-axis shows MFI in FL4. The data are representative of two independent experiments.
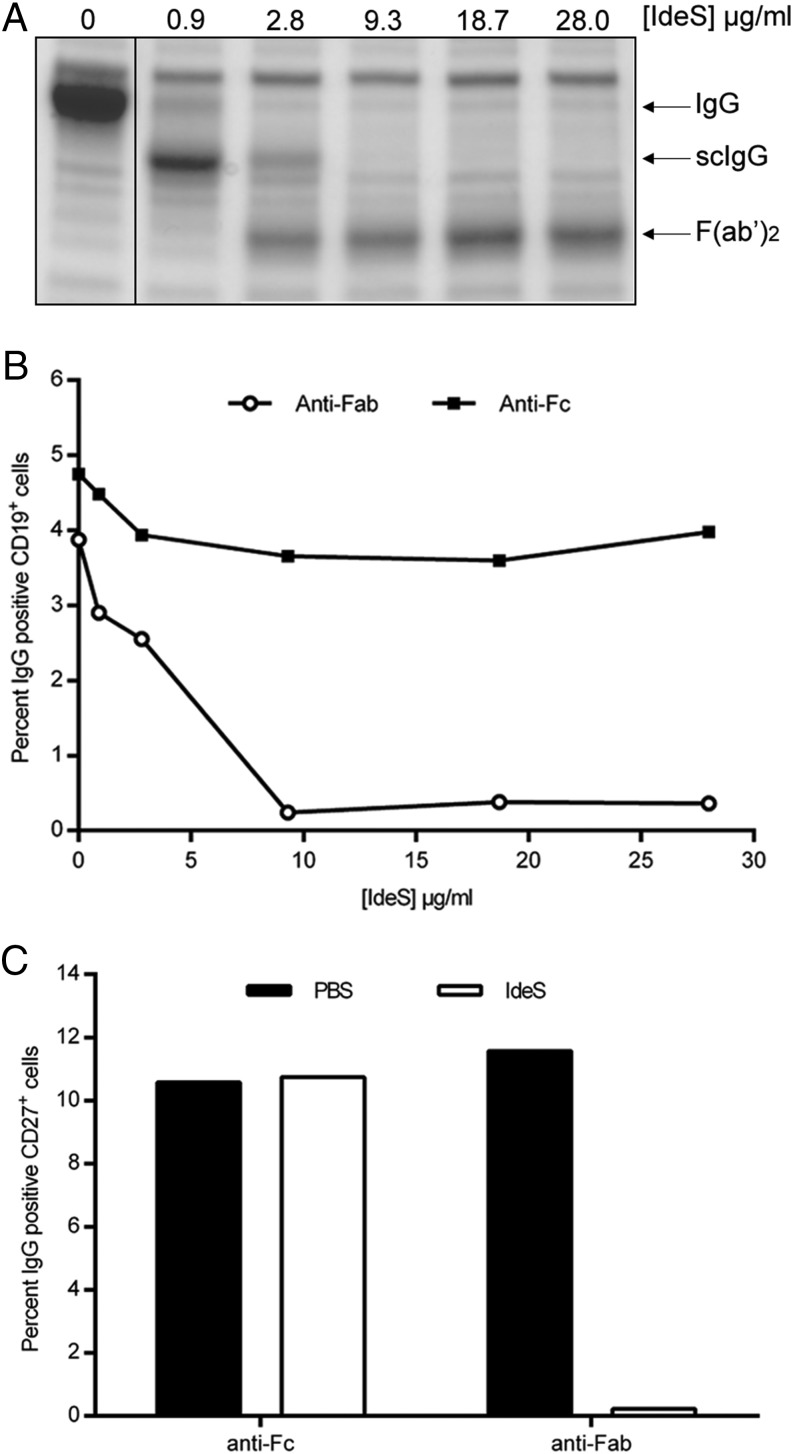
Next, we addressed whether the same pattern of BCR cleavage by IdeS was observed in fresh PBMCs purified from healthy volunteers. Due to the cross-reactivity of the Fab-specific Ab with L chains on IgM, the anti–IgG-CH1 domain–specific CaptureSelect fragment was used for staining the heterogenic PBMC population. Blood collected in heparin tubes was treated with increasing concentrations of IdeS for 30 min (37°C) before separation by density gradient centrifugation; both plasma and PBMCs were collected. Analysis of the plasma by SDS-PAGE confirmed the efficacy of IdeS to cleave soluble IgG (Fig. 2A). scIgG was detectable following IdeS treatment at a concentration of 0.9 μg/ml, whereas complete cleavage was achieved at 9 μg/ml.
FIGURE 2.
IdeS cleaves the IgG-type of BCR with similar efficacy as soluble IgG. (A) Heparinized peripheral blood was treated with PBS or different concentrations of IdeS. After an incubation period, plasma was isolated and separated by SDS-PAGE. Intact IgG, scIgG, and F(ab′)2 fragments are indicated to the right. (B) PBMCs purified from the same PBS- or IdeS-treated blood were double stained for CD19+ (PE-coupled Ab) and anti-Fc or anti-Fab (biotin-conjugated Abs, followed by streptavidin-allophycocyanin) and analyzed by flow cytometry. Lymphocytes were gated using forward scatter/side scatter, and CD19+ cells within the lymphocyte gate were monitored for anti-Fab and anti-Fc signal. The data are representative of two independent experiments. (C) B cells were negatively selected (RosetteSep) after PBS or IdeS treatment (30 μg/ml). This CD19+-enriched population was double stained for CD27+ (PE-conjugated Ab) and anti-Fab or anti-Fc (biotin-conjugated Abs, followed by streptavidin-allophycocyanin) and analyzed by flow cytometry. The y-axis shows the percentage of CD27+ and IgG+ double-positive cells. The data are representative of two independent experiments.
FACS analysis of CD19+ PBMCs showed that IdeS could remove the F(ab′)2 portion of IgG from the BCR while leaving the Fc portion in the B cell membrane (Fig. 2B). The effect of IdeS treatment is not fully visible by flow cytometry as long as the scIgG product is present and attached to the membrane through its second intact H chain. Full cleavage of membrane-bound IgG was achieved at 9 μg/ml IdeS. To our knowledge, these results show for the first time IdeS efficacy on membrane-bound IgG present in the BCR complex.
To further define the effect of IdeS on the memory subset of B cells, the effect on CD19+/CD27+ memory B cells was investigated. CD19+ B cells constitute only a small percentage of the total PBMC population. Hence, CD19+ B cells were enriched to >90% purity using negative selection (RosetteSep) (data not shown). Approximately 10% of this population stained double positive for surface IgG and CD27 prior to IdeS treatment (Fig. 2C). After IdeS treatment, <1% of the CD19+/CD27+ cells stained positive for cell surface IgG (Fig. 2C). Thus, these data demonstrate that the BCR on class-switched memory B cells (i.e., CD19+/CD27+/surface IgG+ cells) is efficiently cleaved by IdeS.
Cleaved BCR is rapidly regenerated on both cell lines and PBMCs and has no effect on cell viability
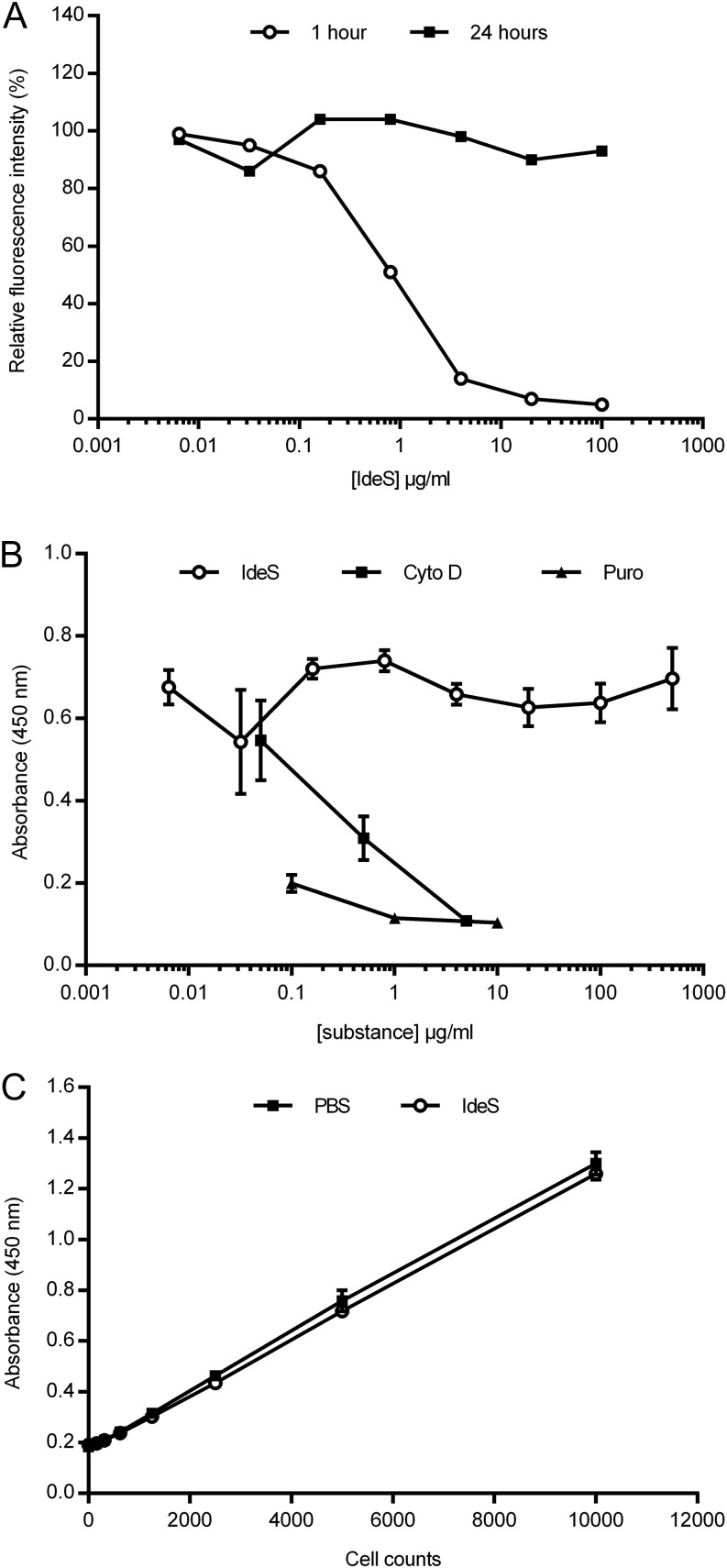
To investigate membrane turnover of the IgG-type of BCR after IdeS treatment, Nu-DUL-1 cells were treated with different concentrations of IdeS, washed to remove IdeS, and cultured. One hour after treatment with IdeS > 4 μg/ml, membrane IgG was undetectable; however, by 24 h after treatment, the Fab-specific signal had returned to the original levels, demonstrating that the membrane-bound IgG had recovered (Fig. 3A). Nu-DUL-1 cells were also analyzed for proliferative capacity using BrdU incorporation; there was no difference in proliferation after cleaving the IgG-type of BCR, even when IdeS treatment was continued over 24 h (Fig. 3B). Substances with known antiproliferative capacity (puromycin and cytochalasin D) had a strong antiproliferative effect. Furthermore, there was no effect on cell viability after IdeS treatment (Fig. 3C).
FIGURE 3.
Recovery of surface BCR expression on B cells after IdeS treatment. (A) Flow cytometry analysis of the presence of F(ab′)2 fragments on the surface of Nu-DUL-1 cells after treatment with different amounts of IdeS. IdeS was removed, and cells were cultured and analyzed after 1 and 24 h. Cells were stained with biotin-conjugated anti-Fab Ab, followed by streptavidin-allophycocyanin. The y-axis shows relative fluorescent intensity for which non-IdeS–treated signals were assigned 100%. The data are representative of two independent experiments. (B) Nu-DUL-1 cells were treated with different amounts of IdeS or antiproliferative control substances (cytochalasin D and puromycin) and cultured for 24 h prior to a 6-h BrdU incorporation. Data are mean ± SD of triplicate samples. The data are representative of two independent experiments. (C) Nu-DUL-1 cells were treated with PBS or IdeS (30 μg/ml) for 24 h before an intracellular hydrogenase activity–based viability assay (CCK-8) was used as read-out. The data are representative of two independent experiments.
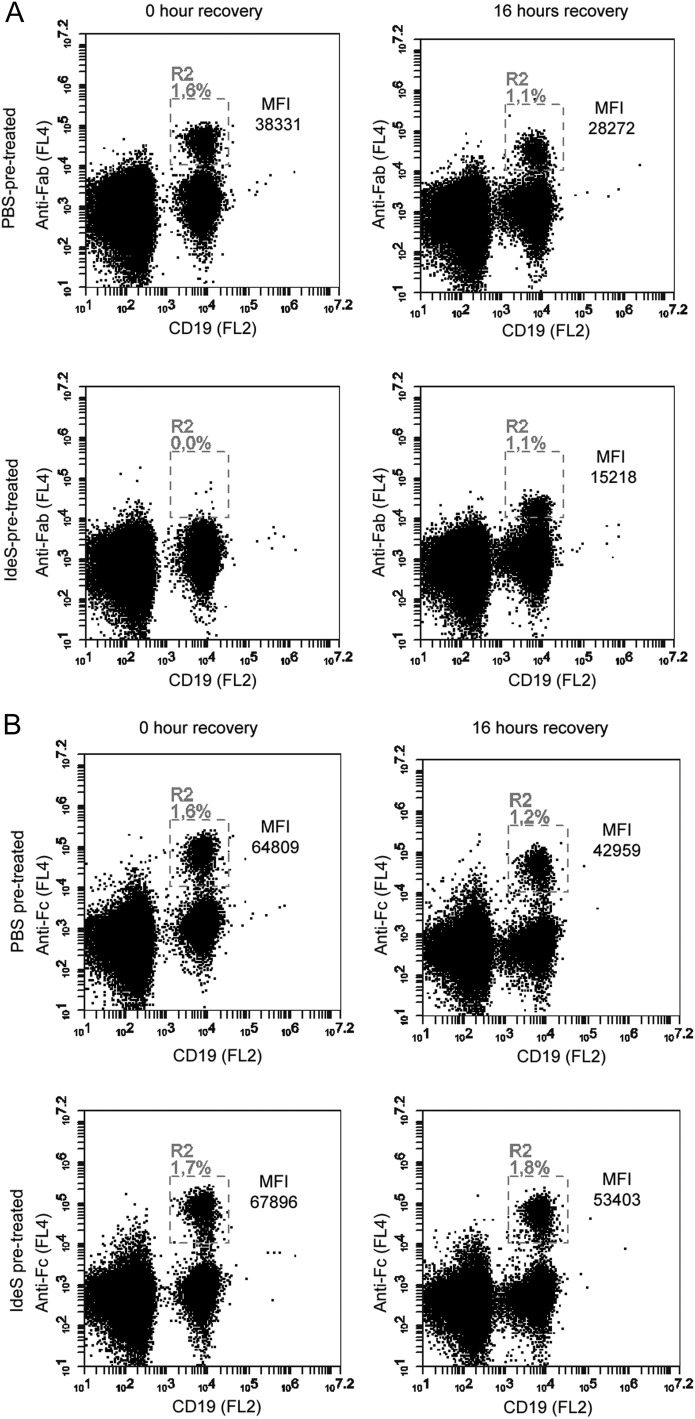
The regeneration of the IgG-type of BCR within 24 h on a highly proliferating lymphoma cell line was not unexpected because membrane turnover on such proliferating cells is usually very high. To investigate whether the turnover of the IgG-type of BCR was similar on primary B cells, blood from healthy volunteers was collected in heparin tubes and treated with a high dose (30 μg/ml) of IdeS. A sample of cells was removed from the cultures at different time points, fixed, stained with anti-CD19 for B cell lineage, and further stained with anti-Fab or anti-Fc to monitor IgG-BCR. The IgG-type of BCR was found to be rapidly regenerated on normal human CD19+ cells; within 16 h after cleavage by IdeS, the number of Fab+ cells had returned to pretreatment levels, although the level of expression/cell did not reach the full MFI as observed before IdeS treatment. This indicates that, 16 h post-IdeS treatment, intact IgG-BCR is detectable on the surface of PBMCs, even though all IgG-BCRs have not yet been replaced (Fig. 4A). Fc portions of membrane-anchored IgG-BCR remained detectable throughout the time course, demonstrating that IdeS treatment results only in loss of the F(ab′)2 (Fig. 4B). Because B cells only account for ∼10% of the total PBMC population, we also investigated the ability of IdeS to cleave IgG-BCR on an enriched population of B cells (>90% CD19+ cells). Approximately 20% of the CD19+ cells were also shown to be positive for surface IgG using either F(ab′)2- or Fc-specific reagents (data not shown). IdeS treatment efficiently removed the F(ab′)2 portion of membrane-bound IgG, leaving behind the intact Fc. Again, the turnover was rapid: cell surface IgG recovered within 16 h after the B cells were treated with IdeS. Once more, cleavage of IgG-BCR had no significant effect on cell viability during the monitored period, as measured by CCK-8 (data not shown).
FIGURE 4.
Recovery of IgG-type BCR expression on ex vivo IdeS-treated PBMCs. PBMCs were treated with PBS or IdeS (30 μg/ml) prior to the start of staining. Lymphocytes were gated using forward scatter/side scatter (data not shown), and CD19+ cells (PE-conjugated Ab) within the lymphocyte gate were monitored for anti-Fab or anti-Fc signal using biotin-conjugated fragment-specific Abs, followed by streptavidin-allophycocyanin. (A) Flow cytometry analysis of anti-Fab signal on CD19+ cells immediately after PBS or IdeS treatment and after 16 h of IdeS-free culturing. Double-positive cells are found in R2 and expressed as the percentage of cells in the lymphocyte gate. MFI (anti-Fab) in R2 is shown. (B) Flow cytometry analysis of anti-Fc signal on CD19+ cells immediately after PBS or IdeS treatment and after 16 h of IdeS-free culturing. Double-positive cells are found in R2 and expressed as the percentage of cells in the lymphocyte gate. MFI (anti-Fc) in R2 is shown. The data are representative of two independent experiments.
IdeS treatment inhibits BCR signaling
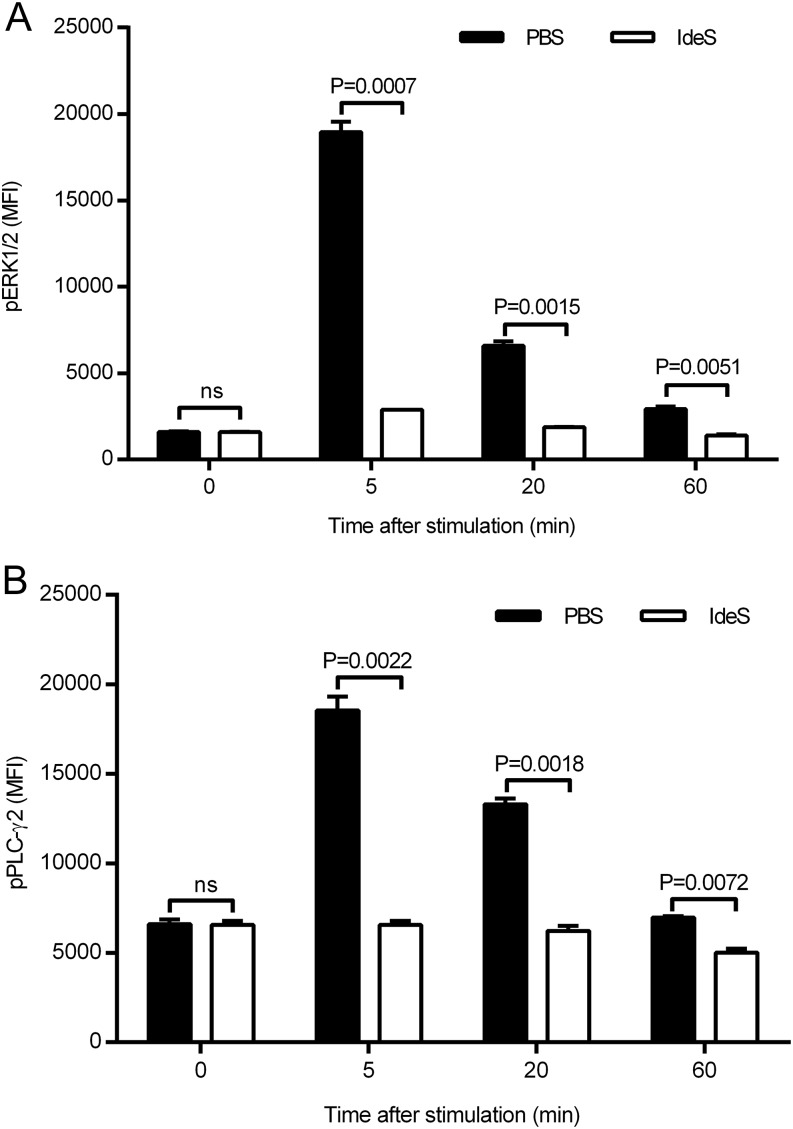
BCR signaling is important in the activation, survival, and differentiation of B lymphocytes. The initial event after BCR engagement is the activation of Lyn and Syk, which results in additional signal propagated via PLC-γ2 and ERK1/2 activation. PLC-γ2 and ERK1/2 phosphorylation was monitored as downstream indicators for the BCR signaling cascade to investigate the effect of IdeS cleavage of the F(ab′)2 portion of the BCR. Cross-linking of the BCR on Nu-DUL-1 cells was achieved using a F(ab′)2-specific Ab as surrogate Ag. The cells were unable to signal through their BCR after IdeS treatment (Fig. 5), as demonstrated by the absence of PLC-γ2 and ERK1/2 phosphorylation. The mock-treated cells responded normally. These data demonstrate that IdeS-treated cells with a cleaved IgG-type of BCR cannot respond to antigenic stimulation.
FIGURE 5.
IdeS treatment inhibits BCR signaling. Nu-DUL-1 cells were treated with PBS or IdeS (30 μg/ml) prior to cross-linking using a F(ab′)2-specific Ab. (A) ERK1/2 phosphorylation was followed at different time points after stimulation using a phospho-specific Ab in flow cytometry. MFI of duplicate samples (mean ± SD) is shown for each time point. (B) PLC-γ2 phosphorylation was followed at different time points after stimulation using a phospho-specific Ab in flow cytometry. MFI of duplicate samples (mean ± SD) is shown for each time point. The p values were calculated using GraphPad Prism and an unpaired, two-tailed t test. All data are representative of three independent experiments. ns, not significant.
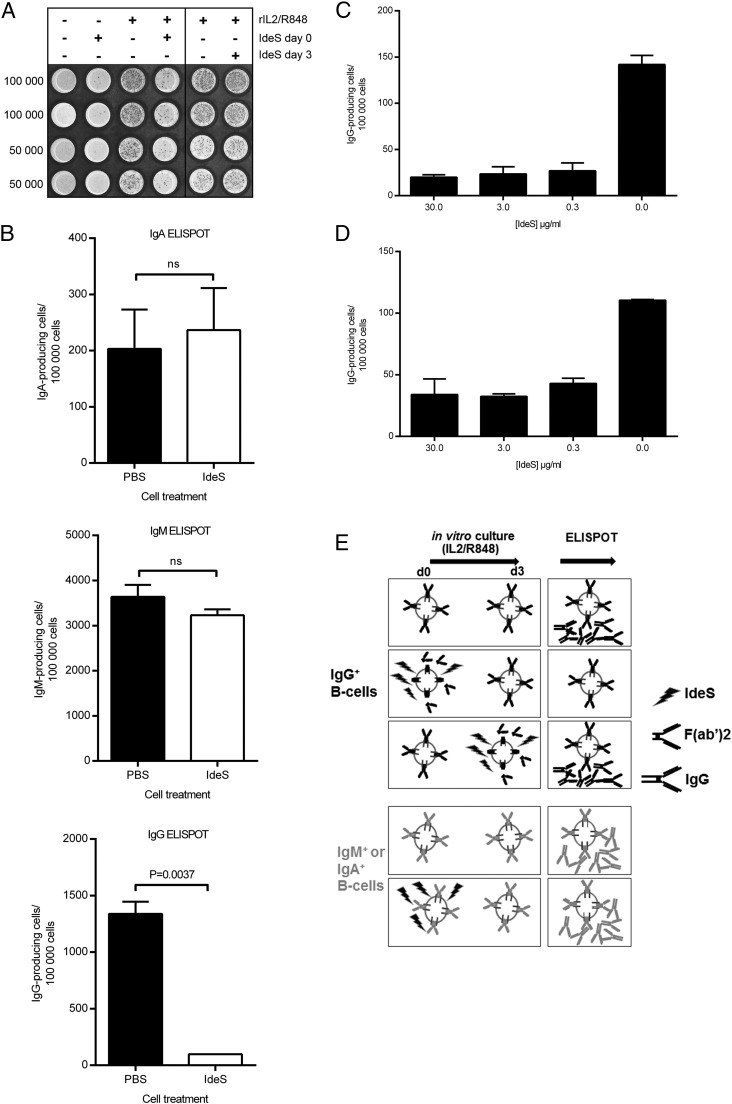
IdeS blocks B cell differentiation
As a result of the finding that IdeS does not affect cell viability but does render them unable to respond to Ag, we next explored the functionality of primary memory B cells. Thus, PBMCs were collected, treated with IdeS, and stimulated with rIL-2 and R848 to activate memory B cells and differentiate them into Ig-producing cells (14). After 72–96 h, the cells were washed extensively to remove IdeS and then analyzed for the frequency of Ig-producing cells. In some experiments, IdeS was not added until day 3 of IL-2/R848 culture; we found that at this time point it was not possible to stop the secretion of IgG from IL-2/R848–differentiated B cells. This also shows that the loss of signal in the ELISPOT assay, when IdeS was added from the start, is not due to a carryover effect of IdeS interfering with the Abs of the ELISPOT assay (Fig. 6A). In most experiments, IdeS was present throughout the stimulation period (96 h), and the results showed that IdeS treatment inhibited memory B cell differentiation, reducing the number of IgG-producing cells, whereas it had no effect on the differentiation of IgM- or IgA-producing cells (Fig. 6B). There was a significant effect on B cell differentiation at all tested IdeS concentrations from 0.3 to 30 μg/ml (Fig. 6C). A second set of experiments in which IdeS was washed away prior to stimulation with IL-2 and R848 also resulted in a significant reduction in the number of IgG-producing cells (Fig. 6D), showing that the initial removal of the Ag-binding portion of IgG-BCR is an important step in inhibiting memory B cell activation into IgG-producing cells. Fig. 6E illustrates that removal of the IgG-type of BCR through IdeS treatment prior to polyclonal in vitro stimulation prevents B cells from differentiating into IgG-secreting cells, even when using the strong activator combination rIL-2/R848. IdeS treatment does not prevent IgG secretion when given at the end of the stimulation period. Furthermore, the presence of IdeS does not prevent differentiation of B cells carrying an IdeS-insensitive BCR (IgM or IgA). This clearly indicates that an intact IgG-type of BCR is necessary during the early events of rIL-2/R848 stimulation.
FIGURE 6.
IdeS specifically blocks B cell maturation of IgG-producing cells. PBMCs were treated with PBS or IdeS and stimulated with rIL-2 and R848 to activate memory B cells and differentiate them into Ig-producing cells. ELISPOT filter plates were evaluated for the number of IgG-producing cells. (A) The filter plate was seeded with 50,000 or 100,000 cells that were treated or not with rIL-2/R848 on day 0 and with/without IdeS (30 μg/ml) on day 0 or 3. (B) Number of IgA-, IgM-, and IgG-producing cells after stimulation with rIL-2/R848 in the presence or absence of 30 μg/ml IdeS for 96 h. Data are mean (± SD) of four to six wells. The p value was calculated using GraphPad Prism and an unpaired, two-tailed t test. (C) Number of IgG-producing cells after stimulation with rIL-2/R848 in the presence or absence of 0.3–30 μg/ml IdeS for 72 h. Data are mean (± SD) of two to four wells. (D) Number of IgG-producing cells after pretreating cells for 1 h with 0.3–30 μg/ml IdeS prior to removing IdeS and subjecting cells to 72 h of stimulation with rIL-2/R848. Data are mean (± SD) of two to four wells. (E) Graphical illustration of the ELISPOT experiments in which IdeS was added from the start or at the end of the culturing period. Data in (B)–(D) are representative of two independent experiments. ns, not significant.
IdeS cleaves the IgG-type of BCR in vivo in humans
IdeS was recently tested in a first-in-man study in which healthy human subjects were given single ascending i.v. doses (ClinicalTrials.gov Identifier: NCT01802697) (11). The highest tested dose given to four subjects was 0.24 mg/kg BW. An exploratory part of the trial was designed to analyze the integrity of the IgG-BCR on circulating CD19+ lymphocytes at different time points after IdeS administration. Peripheral blood was collected, and PBMCs were purified at predose and at 2, 24, 48, and 96 h postadministration. Cells were fixed immediately to prevent further cell metabolism and stored until all time points from each subject could be analyzed. The PBMCs were double stained for CD19 and either F(ab′)2-specific or Fc-specific Abs and analyzed using flow cytometry to determine both the frequency and MFI of cells expressing F(ab′)2 (i.e., intact IgG-type of BCR) and Fc on their cell surface. It is important to note that this method cannot discriminate between intact and scIgG-BCR (i.e., scIgG will still stain positive for the Fab portion of the IgG-BCR).
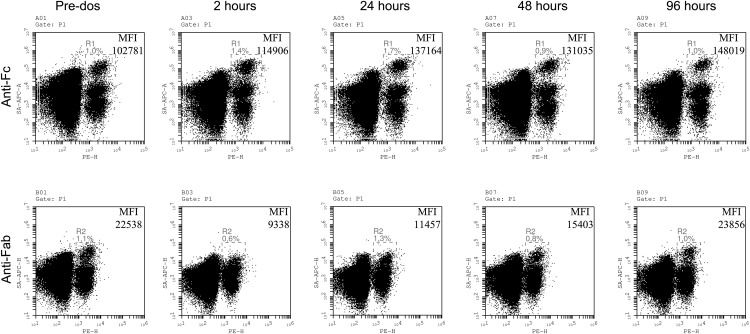
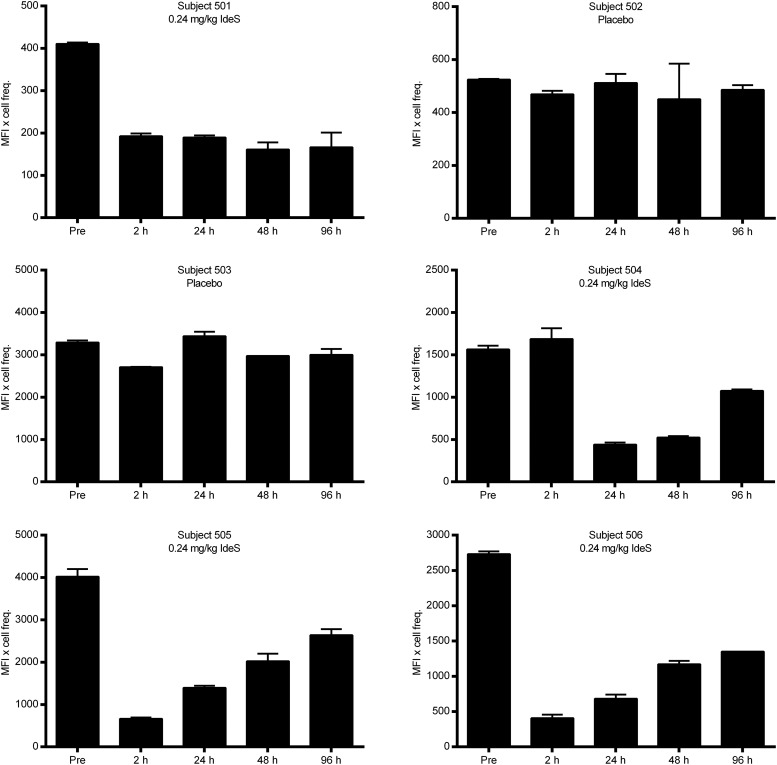
The results demonstrated that the number of CD19+ cells that stained positive for F(ab′)2 was reduced as early as 2 h after treatment with IdeS (0.24 mg/kg BW), whereas the number of cells that stained positive for Fc was unchanged (Fig. 7). This clearly proves that IdeS in vivo cleaved the surface IgG on CD19+ cells, resulting in the loss of the F(ab′)2 fragment. Data from the subjects enrolled in the study, four of whom were treated with IdeS and two of whom received placebo, demonstrate that IdeS efficiently cleaved the IgG-BCR in humans and that the frequency of CD19+ cells that were positive for surface IgG gradually recovered over the days following treatment (Fig. 8).
FIGURE 7.
Recovery of IgG-type BCR expression on PBMCs collected after a single i.v. dose of 0.24 mg/kg BW IdeS in a human healthy subject. Flow cytometry analysis of CD19+/IgG+ cells at different time points after a single dose of IdeS. PBMCs were purified, and lymphocytes gated using forward scatter/side scatter (P1). B cells (CD19+) were monitored in FL2 (PE), and IgG was monitored in FL4 (allophycocyanin). Double-positive cells for CD19 (PE) and the Fc portion of IgG (FL4) predose and up to 96 h postdosing (upper panels). Double-positive cells for CD19 (FL2) and the Fab portion of IgG (FL4) predose and up to 96 h postdosing (lower panels). MFI for double-positive cells are given for R1 (CD19/anti-Fc) and R2 (CD19/anti-Fab). The data are representative of two staining experiments.
FIGURE 8.
IdeS cleaves the IgG-type of BCR in vivo in humans. Healthy human subjects were dosed with 0.24 mg/kg BW IdeS, and PBMCs were collected at different time points after dosing. The percentage of cells double positive for CD19 (PE) and F(ab′)2 (allophycocyanin) was analyzed using flow cytometry. Hours postdosing are shown on the x-axes and MFI × cell frequency is shown on the y-axes. Data are the mean (± SD) of two independent flow cytometry experiments for each patient.
Discussion
It is well known that IdeS cleaves soluble IgG and even Ag-bound IgG in vitro (1, 15, 16) and in vivo (17–20); however, whether IdeS can also cleave transmembrane IgG present in the BCR complex has never been addressed. Although the Fc and Fab of the BCR are identical to their soluble IgG counterparts, it was unknown whether the BCR was protected from IdeS cleavage (e.g., as a result of steric hindrance by other molecules in the BCR complex). In a series of experiments, we were able to show that the IgG-BCR is a sensitive substrate for IdeS and that the efficacy of IdeS on cell surface IgG and soluble IgG is similar (Fig. 2A, 2B). The methods used for addressing IdeS activity on the IgG-type of BCR are based on the fact that the IgG molecule is anchored to the cell membrane via a transmembrane domain and that IdeS cuts the F(ab′)2 portion of the Ab. Because the H chains are covalently linked, this method cannot discriminate between intact IgG and the intermediate scIgG generated upon IdeS cleaving the first H chain because the scIgG remains attached to the membrane through its intact second H chain. However, it is reasonable to anticipate that scIgG is generated from membrane-bound IgG at low concentrations of IdeS, as seen with soluble IgG as a substrate. The data clearly show that within 4 h IdeS cleaves the IgG-BCR and completely prevents BCR signaling in response to receptor ligation. Thus, B cells with IdeS-cleaved IgG-BCR are rendered incapable of Ag binding, resulting in loss of the major cellular events downstream of receptor ligation (i.e., internalization, processing, and presentation on MHC class II molecules). B cells are very potent APCs (21, 22) after specific BCR-mediated endocytosis and can present an Ag in association with HLA after it has been processed into peptides. Therefore, the loss of the Fab portion of the BCR upon IdeS cleavage is likely to have an impact on Ag presentation to CD4+ T cells. We were unable to determine whether the signaling capacity of scIgG-BCR, generated early in the time course after IdeS treatment, is impaired as a consequence of conformational changes induced in the molecule following cleavage of a single H chain or whether it remains fully functional until both H chains have been cleaved.
B cell presentation of processed Ag to T cells can induce B cell proliferation and differentiation (23). IdeS cleavage of IgG-BCR on memory B cells blocks the differentiation into IgG-secreting cells. Nevertheless, IgA or IgM ASCs are unaffected when treated with IdeS prior to activation with polyclonal stimulators (IL-2 and R848). However, IdeS has no blocking effect in IgG-BCR–type cells if it is used after initiation of activation.
Cleaving the IgG-type of BCR has no impact on cell viability in this context (Fig. 3). However, cells may recover their IgG-type BCR more slowly after IdeS treatment in vivo (Fig. 7). The fate of B cell subpopulations could not be analyzed in this exploratory part of the clinical study because of the limited numbers of cells available for analysis. We speculate that the IgG-type of BCR is recovered on the cleaved cells by means of membrane turnover in a similar manner to that observed in vitro; however, it cannot be ruled out that the slight delay in recovery seen in vivo is due to maturation of new B cells rather than mere membrane turnover.
Additionally, IdeS effect on memory B-cells points toward an important role for the IgG–BCR complex in responding to stimulation, even in the absence of Ag (i.e., in maintaining tonic signals). A single amino acid mutation in the extracellular region of CD79b of the BCR results in agammaglobulinemia (24). This finding indicates that the interaction between the proteins in the extracellular portion of the BCR complex is required for cell activation, and we speculate that the interaction between CD79a/b and IgG is important for the generation of a proper signalosome in response to external stimuli. Further support for this hypothesis comes from studies targeting the CD79b extracellular domain using a nonlytic Ab, which resulted in B cell anergy and the loss of IgG production (25). We propose that disrupting the BCR by cleaving off the F(ab′)2 portion results in unresponsiveness due to improper assembly of intracellular proteins and, therefore, loss of the formation of a functional signalosome.
IdeS is being developed for removal of donor HLA-specific Abs in highly sensitized patients on the waiting list for kidney transplantation. These patients have developed Abs against most organ donors; without elimination of these Abs or desensitization before transplantation, the kidney allograft will be rejected. By removing donor-specific Abs (DSAs) using IdeS prior to transplantation, we believe that patients can be made eligible for transplantation, despite a positive cross-match test before treatment. An additional effect of IdeS treatment is the instant generation of free F(ab′)2 fragments from DSAs with retained binding capacity. These F(ab′)2 fragments may bind and block epitopes in the graft; because the F(ab′)2 fragments have lost their Fc-mediated functions, such as complement-dependent cytotoxicity, Ab-dependent cellular cytotoxicity, and Ab-dependent cellular phagocytosis, they may have the capacity to block out IgM and newly formed DSAs and, thereby, provide an additional protection to the graft. The results presented in this article show that IdeS cleaves the IgG-type of BCR on CD27+ memory B cells and renders them incapable of answering to antigenic stimulation. Thus, we speculate that preformed DSAs are removed by IdeS and, furthermore, that the DSA-specific memory B cells are initially incapable of responding to donor Ags, giving the graft more time to adapt to the host environment. This may have positive long-term effects on the outcome of graft survival, because the initial activation of memory B cells and generation of plasma cells are likely to be delayed by IdeS treatment. The demonstration that a single IdeS treatment creates a window during which a patient is deficient in functional IgG and memory B cells are unable to respond to their Ag is intriguing. The concept indicates therapeutic possibilities for desensitization in transplantation, as well as in other situations in which a recall response must be prevented or delayed.
Acknowledgments
We thank Kathryn Wood (Professor of Immunology, University of Oxford) for her expert support and guidance in reviewing the manuscript.
Footnotes
- ASC
- Ab-secreting cell
- BW
- body weight
- DSA
- donor-specific Ab
- IdeS
- IgG-degrading enzyme of Streptococcus pyogenes
- MFI
- mean fluorescent intensity
- PEST
- penicillin/streptomycin
- PFA
- paraformaldehyde
- PLC-γ2
- phospholipase-Cγ2
- scIgG
- single-cleaved IgG.
Disclosures
S.J., L.W., and C.K. are inventors on a pending patent application (PCT/EP2015/065895).
References
- 1.von Pawel-Rammingen U., Johansson B. P., Björck L. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21: 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su Y. F., Chuang W. J., Wang S. M., Chen W. Y., Chiang-Ni C., Lin Y. S., Wu J. J., Liu C. C. 2011. The deficient cleavage of M protein-bound IgG by IdeS: insight into the escape of Streptococcus pyogenes from antibody-mediated immunity. Mol. Immunol. 49: 134–142. [DOI] [PubMed] [Google Scholar]
- 3.Reth M. 1989. Antigen receptor tail clue. Nature 338: 383–384. [PubMed] [Google Scholar]
- 4.Reth M., Wienands J. 1997. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15: 453–479. [DOI] [PubMed] [Google Scholar]
- 5.Kurosaki T. 2002. Regulation of B-cell signal transduction by adaptor proteins. Nat. Rev. Immunol. 2: 354–363. [DOI] [PubMed] [Google Scholar]
- 6.Dal Porto J. M., Gauld S. B., Merrell K. T., Mills D., Pugh-Bernard A. E., Cambier J. 2004. B cell antigen receptor signaling 101. Mol. Immunol. 41: 599–613. [DOI] [PubMed] [Google Scholar]
- 7.Scharenberg A. M., Humphries L. A., Rawlings D. J. 2007. Calcium signalling and cell-fate choice in B cells. Nat. Rev. Immunol. 7: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson G. L., Lapadat R. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912. [DOI] [PubMed] [Google Scholar]
- 9.Rajewsky K. 1996. Clonal selection and learning in the antibody system. Nature 381: 751–758. [DOI] [PubMed] [Google Scholar]
- 10.Manz R. A., Hauser A. E., Hiepe F., Radbruch A. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23: 367–386. [DOI] [PubMed] [Google Scholar]
- 11.Winstedt L., Järnum S., Nordahl E. A., Olsson A., Runström A., Bockermann R., Karlsson C., Malmström J., Palmgren G. S., Malmqvist U., et al. 2015. Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS - a novel therapeutic opportunity. PLoS One 10: e0132011 10.1371/journal.pone.0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings J. M., Tucker D. K., Kottke M. D., Saito M., Delva E., Hanakawa Y., Amagai M., Kowalczyk A. P. 2011. Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can be reversed by expression of exogenous Dsg3. J. Invest. Dermatol. 131: 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilden D., Devlin M., Wroblewska Z. 1978. A technique for the elution of cell-surface antibody from human brain tissue. Ann. Neurol. 3: 403–405. [DOI] [PubMed] [Google Scholar]
- 14.Jahnmatz M., Kesa G., Netterlid E., Buisman A. M., Thorstensson R., Ahlborg N. 2013. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J. Immunol. Methods 391: 50–59. [DOI] [PubMed] [Google Scholar]
- 15.Wenig K., Chatwell L., von Pawel-Rammingen U., Björck L., Huber R., Sondermann P. 2004. Structure of the streptococcal endopeptidase IdeS, a cysteine proteinase with strict specificity for IgG. Proc. Natl. Acad. Sci. USA 101: 17371–17376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincents B., von Pawel-Rammingen U., Björck L., Abrahamson M. 2004. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry 43: 15540–15549. [DOI] [PubMed] [Google Scholar]
- 17.Johansson B. P., Shannon O., Björck L. 2008. IdeS: a bacterial proteolytic enzyme with therapeutic potential. PLoS One 3: e1692 10.1371/journal.pone.0001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandakumar K. S., Johansson B. P., Björck L., Holmdahl R. 2007. Blocking of experimental arthritis by cleavage of IgG antibodies in vivo. Arthritis Rheum. 56: 3253–3260. [DOI] [PubMed] [Google Scholar]
- 19.Tradtrantip L., Asavapanumas N., Verkman A. S. 2013. Therapeutic cleavage of anti-aquaporin-4 autoantibody in neuromyelitis optica by an IgG-selective proteinase. Mol. Pharmacol. 83: 1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R., Otten M. A., Hellmark T., Collin M., Björck L., Zhao M. H., Daha M. R., Segelmark M. 2010. Successful treatment of experimental glomerulonephritis with IdeS and EndoS, IgG-degrading streptococcal enzymes. Nephrol. Dial. Transplant. 25: 2479–2486. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia A. 1990. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol. 8: 773–793. [DOI] [PubMed] [Google Scholar]
- 22.Avalos A. M., Ploegh H. L. 2014. Early BCR events and antigen capture, processing, and loading on MHC Class II on B cells. Front. Immunol. 5: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tangye S. G., Tarlinton D. M. 2009. Memory B cells: effectors of long-lived immune responses. Eur. J. Immunol. 39: 2065–2075. [DOI] [PubMed] [Google Scholar]
- 24.Dobbs A. K., Yang T., Farmer D., Kager L., Parolini O., Conley M. E. 2007. Cutting edge: a hypomorphic mutation in Igbeta (CD79b) in a patient with immunodeficiency and a leaky defect in B cell development. J. Immunol. 179: 2055–2059. [DOI] [PubMed] [Google Scholar]
- 25.Hardy I. R., Anceriz N., Rousseau F., Seefeldt M. B., Hatterer E., Irla M., Buatois V., Chatel L. E., Getahun A., Fletcher A., et al. 2014. Anti-CD79 antibody induces B cell anergy that protects against autoimmunity. J. Immunol. 192: 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]