Abstract
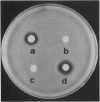
Prodrug activation via antibodies was examined by using the antibiotic chloramphenicol as a model drug. Based on the conformational change between substrate and product, this antibody-catalyzed reaction was designed to prevent product inhibition, thus enhancing turnover. Antibodies elicited against a phosphonate transition-state analogue were found to catalyze hydrolysis of a nonbioactive chloramphenicol monoester as a prodrug at a significantly higher rate above the uncatalyzed background reaction to regenerate chloramphenicol as a parent molecule. The antibody-catalyzed prodrug activation was tested by the paper-disc diffusion method using Bacillus subtilis as an indicator strain. The antibody 6D9 catalyzes the reaction with multiple turnover to generate enough chloramphenicol to inhibit bacterial growth, as indicated by a clear inhibitory zone after incubation with monoester. Using the same method, no inhibition was detected by incubation of either the monoester or the antibody alone. This result reveals that only the antibody hydrolytically activates the monoester, which can be expected to be a suitable prodrug, as it is resistant to the action of bacterial hydrolytic enzymes. The approach in this study demonstrates the use of catalytic antibody technology in medicine and may be applicable to drugs with undesirable effects, particularly in the field of cancer therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Chang C. P., Robinson R. R., Horwitz A. H. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988 May 20;240(4855):1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- HERRMANN E. C., Jr, GABLIKS J., ENGLE C., PERLMAN P. L. Agar diffusion method for detection and bioassay of antiviral antibiotics. Proc Soc Exp Biol Med. 1960 Mar;103:625–628. doi: 10.3181/00379727-103-25617. [DOI] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Imura Y., Stassen J. M., Kurokawa T., Iwasa S., Lijnen H. R., Collen D. Thrombolytic and pharmacokinetic properties of an immunoconjugate of single-chain urokinase-type plasminogen activator (u-PA) and a bispecific monoclonal antibody against fibrin and against u-PA in baboons. Blood. 1992 May 1;79(9):2322–2329. [PubMed] [Google Scholar]
- JARDETZKY O. Studies on the mechanism of action of chloramphenicol. I. The conformation of chlioramphenicol in solution. J Biol Chem. 1963 Jul;238:2498–2508. [PubMed] [Google Scholar]
- Janda K. D., Benkovic S. J., Lerner R. A. Catalytic antibodies with lipase activity and R or S substrate selectivity. Science. 1989 Apr 28;244(4903):437–440. doi: 10.1126/science.2717936. [DOI] [PubMed] [Google Scholar]
- Kleanthous C., Shaw W. V. Analysis of the mechanism of chloramphenicol acetyltransferase by steady-state kinetics. Evidence for a ternary-complex mechanism. Biochem J. 1984 Oct 1;223(1):211–220. doi: 10.1042/bj2230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen F., Kim J. B., Lindner H. R., Eshhar Z., Green B. Monoclonal immunoglobulin G augments hydrolysis of an ester of the homologous hapten: an esterase-like activity of the antibody-containing site? FEBS Lett. 1980 Mar 10;111(2):427–431. doi: 10.1016/0014-5793(80)80842-8. [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Iwasa S., Kakinuma A., Stassen J. M., Lijnen H. R., Collen D. Enhancement of clot lysis in vitro and in vivo with a bispecific monoclonal antibody directed against human fibrin and against urokinase-type plasminogen activator. Thromb Haemost. 1991 Dec 2;66(6):684–693. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J. Principles of antibody catalysis. Bioessays. 1988 Oct;9(4):107–112. doi: 10.1002/bies.950090402. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J., Schultz P. G. At the crossroads of chemistry and immunology: catalytic antibodies. Science. 1991 May 3;252(5006):659–667. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Nitahara Y., Miyamura S. Kinetic studies on enzymatic acetylation of chloramphenicol in Streptococcus faecalis. Antimicrob Agents Chemother. 1979 Dec;16(6):719–723. doi: 10.1128/aac.16.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Unowsky J. Mechanism of R factor-mediated chloramphenicol resistance. J Bacteriol. 1968 May;95(5):1976–1978. doi: 10.1128/jb.95.5.1976-1978.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- TRAUT R. R., MONRO R. E. THE PUROMYCIN REACTION AND ITS RELATION TO PROTEIN SYNTHESIS. J Mol Biol. 1964 Oct;10:63–72. doi: 10.1016/s0022-2836(64)80028-0. [DOI] [PubMed] [Google Scholar]
- Tawfik D. S., Green B. S., Chap R., Sela M., Eshhar Z. catELISA: a facile general route to catalytic antibodies. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):373–377. doi: 10.1073/pnas.90.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik D. S., Zemel R. R., Arad-Yellin R., Green B. S., Eshhar Z. Simple method for selecting catalytic monoclonal antibodies that exhibit turnover and specificity. Biochemistry. 1990 Oct 23;29(42):9916–9921. doi: 10.1021/bi00494a023. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Chemical reactivity at an antibody binding site elicited by mechanistic design of a synthetic antigen. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6736–6740. doi: 10.1073/pnas.83.18.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]