Abstract
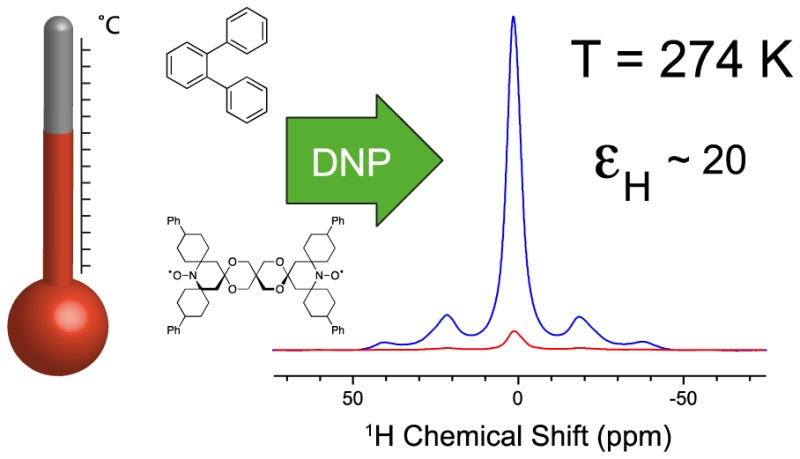
Efficient dynamic nuclear polarization (DNP) in solids, which enables very high sensitivity NMR experiments, is currently limited to temperatures of around 100 K and below. Here we show how by choosing an adequate solvent, 1H cross effect DNP enhancements of over 80 can be obtained at 240 K. To achieve this we use the biradical TEKPol dissolved in a glassy phase of ortho-terphenyl (OTP). We study the solvent DNP enhancement of both TEKPol and BDPA in OTP in the range from 100 to 300 K at 9.4 and 18.8 T. Surprisingly, we find that the DNP enhancement decreases only relatively slowly for temperatures below the glass transition of OTP (Tg = 243 K), and 1H enhancements around 15–20 at ambient temperature can be observed. We use this to monitor molecular dynamic transitions in the pharmaceutically relevant solids Ambroxol and Ibuprofen.
Dynamic nuclear polarization (DNP) has emerged as a powerful method to enhance sensitivity in solid-state magic angle spinning (MAS) NMR.1−3 DNP works by transferring the large polarization of unpaired electron spins to nuclei. Typically MAS DNP experiments are currently performed at temperatures around 100 K, with the substrate formulated in a glass-forming matrix in the presence of a carefully chosen paramagnetic polarizing agent. Today’s most efficient polarizing agents for MAS DNP are usually nitroxide biradicals4 bearing two TEMPO moieties tethered with various linkers.4−9 A variety of monoradicals have also been used.10−13 The nature of the polarizing agent is crucial to the success of the DNP experiment, since it determines the efficiency of the polarization transfer process from electron to nucleus.
Experiments conducted with the most efficient biradicals clearly show that the DNP enhancement strongly increases as temperature is lowered.8,9,14 This is because reduced temperatures increase electron and nuclear spin relaxation times, which enables significant saturation of the electronic transitions with available microwave powers,9,10,14 and accumulation of nonequilibrium nuclear spin polarization.8,9,14 Nevertheless, the need for low temperatures is a major limitation for many potential MAS DNP applications, and most notably for the study of dynamics, or the investigation of solid proteins15,16 (solid-state NMR spectra of proteins generally show a dramatic loss of resolution at ∼100 K due to the freezing of the hydration shell and side-chain motions that produces a heterogeneous distribution of conformers15−20). The development of efficient high-field MAS DNP closer possible to ambient temperatures is thus one of the major current challenges in the field. Photoexcited triplet states,21,22 or time domain DNP,23 have been applied at room-temperature at low magnetic field (0.3–0.4 T), but these technologies are currently not available for high fields. Solution DNP24 at 9.4 T has also been demonstrated at ambient temperature,25 but does not transfer to solid-state MAS NMR.
Here we show how by using ortho-terphenyl (OTP) as a polarizing medium it is possible to achieve DNP MAS enhancements of over 80 at 240 K and of about 15–20 at ambient temperature at 9.4 and 18.8 T. We then show that temperature-dependent DNP enhanced NMR can be applied to study dynamic transitions in powdered organic compounds of pharmaceutical relevance.
We note that early low field MAS DNP experiments were often done at room temperature.26,27 In the very first DNP experiments at 5 T Becerra et al. observed enhancements of ∼10 via the solid-effect at room temperature using BDPA (α,γ-bisdiphenylene-β-phenylallyl) as a polarization source and polystyrene as the glass matrix.28 Following this pioneering study, attention quickly shifted to experiments at 100 K or lower due to the dramatically higher enhancements observed at low temperature.10,29 Only very recently has attention shifted back to attempts to obtain efficient MAS DNP at higher temperatures.9,15 As an example we recently showed that the biradical TEKPol, which was designed to have long electron relaxation times, dissolved in tetrachloroethane (TCE), yielded enhancements of ∼200 around 100 K, and that enhancements above 10 were still possible at 200 K.9
To further increase the enhancements above 200 K, and inspired by extensive EPR literature,30−32 here we study two polarizing agents, BDPA and TEKPol, using ortho-terphenyl (OTP) as the glass-forming medium, which forms homogeneous glass phases at higher temperatures than TCE. OTP is well-known as a glass former in EPR.30−32 Recently it was also demonstrated that highly deuterated OTP is a good medium for MAS DNP experiments at ∼80 K with TEMPOL or bis-TEMPO terephthalate radicals.33 OTP has a melting point of 330 K (57 °C), and by rapid cooling from the melt it forms a metastable amorphous phase at room temperature, which will slowly recrystallize unless it is frozen below the glass transition temperature Tg = 243 K (−30 °C).31,34 The relatively high glass transition temperature of OTP makes it a good candidate for DNP experiments above 200 K. The radicals used here were chosen because they are among the best DNP polarizing agents, they are soluble in organic phases, and they provide the possibility to test the DNP enhancements with the three main DNP mechanisms at high-field in solids.
Samples were prepared by dissolving the radicals in partially deuterated OTP (95% OTP-d14 and 5% OTP) as described in the SI. The sample was then melted inside the rotor and rapidly frozen to ∼100 K inside the spectrometer. The effective sample temperature was determined by measuring the 79Br T1 of a small amount of KBr added to the rotor.35
The 1H DNP field profile for BDPA in OTP recorded at 9.4 T and 112 K is reported in Figure S1. One can clearly distinguish the maxima in enhancement peaks due to solid-effect (SE) and Overhauser effect (OE). Both the SE and OE lead to substantial proton DNP enhancements of 140 and 80, respectively, at 9.4 T. In the same experiment performed using BDPA in TCE at 9.4 T, both the OE and SE enhancements were reduced to around 4–5, confirming that the glass-forming matrix has a major impact in the DNP efficiency.
Figure 1 shows the 1H SE and OE DNP enhancements of bulk OTP in the range from about 100 to 300 K, both at 9.4 and 18.8 T. Interestingly, for OE DNP, enhancement progressively decreases as the temperature increases with enhancements around 15 at 290 K. At 9.4 T the enhancement decreases only slowly (∼20%) between 100 K and Tg, with a value of 60 obtained at Tg. Above the glass transition temperature the enhancement decreases rapidly in the metastable phase. At 18.8 T, the OE DNP enhancement (around 80) at low temperature is comparable to that at 9.4 T, and it is the largest DNP enhancement observed so far at 18.8 T. However, it drops more rapidly than at 9.4 T with increasing temperature, to about 30 at Tg and 20 at 280 K. In Figure 1A we also report the behavior of the SE DNP peak of BDPA/OTP at 9.4 T. At low temperature the enhancement is about 140, indicating a very efficient SE mechanism not far below the CE observed with the best dinitroxide biradicals in glycerol/water or TCE at the same field and temperature.8,9 The enhancement then decreases to around 60 at Tg. Above Tg the enhancement rapidly decreases. The SE is about twice as efficient as the OE at 9.4 T at 117 K, but the OE appears less sensitive to temperature, such that they are comparable at Tg. Together with the DNP enhancement, we also measured the 1H DNP build-up time (TB) (Figure S5), that for the OE we found to be equivalent to the 1H longitudinal relaxation time (T1), as previously reported13 (details in SI).
Figure 1.
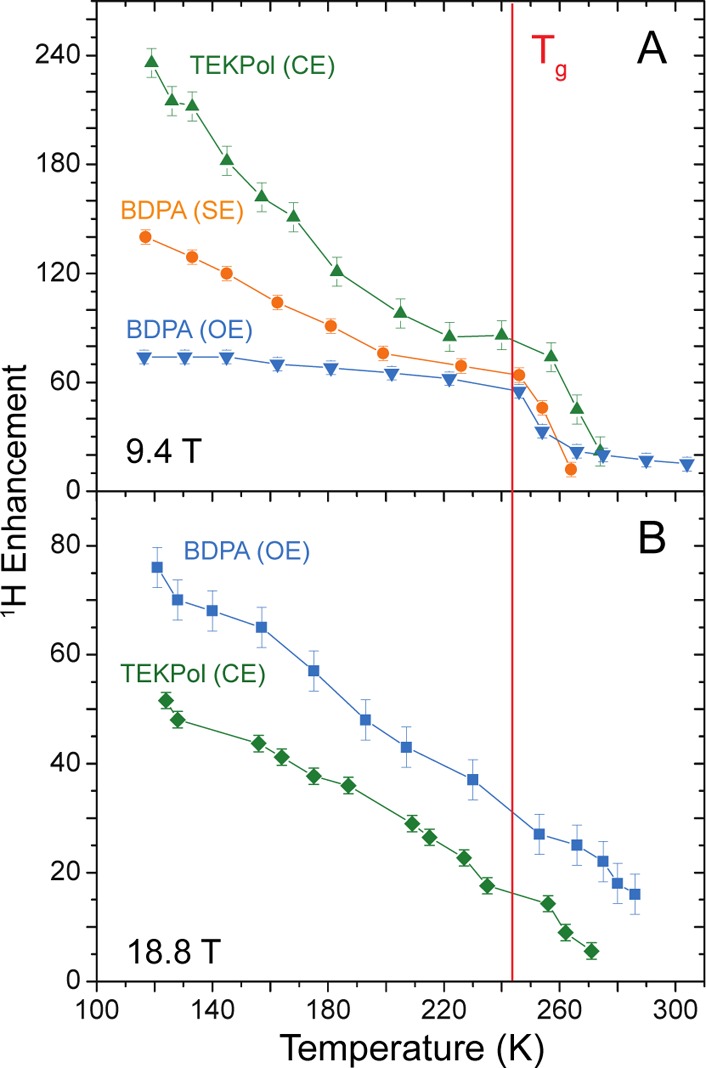
1H DNP enhancements as a function of temperature for 32 mM BDPA and 16 mM TEKPol in 95% OTP-d14 at 9.4 T (A) and 18.8 T (B). The red line indicates Tg. Numerical values are given in SI for all the points.
The recently introduced dinitroxide biradical TEKPol is one of the most efficient CE polarizing agents, providing enhancements between 150 and 500, depending on sample formulation, at 9.4 T and ∼100 K.36,37Figure 1 shows the CE DNP enhancement for 16 mM TEKPol in 95% deuterated OTP. At 120 K and 9.4 T the bulk 1H DNP enhancement in OTP is 240, significantly higher than that obtained in TCE in analogous conditions (see Figure S5), indicating that the OTP phase also has a beneficial impact on CE DNP. With increasing temperature the enhancement decreases but is still around 80 at the glass transition temperature. Above Tg the enhancement rapidly decreases, but remains ∼20 up to 273 K. The trend measured at 18.8 T for the TEKPol/OTP combination (Figure 1B) shows a strong enhancement (51) at low temperature, ∼15 at Tg and ∼7 at 273 K.
In summary, TEKPol CE is the most efficient at 9.4 T, while at 18.8 T, BDPA OE is better. However, even at 18.8 T CE DNP with TEKPol offers the advantage of a shorter TB (Figure S5), which enables more rapid repetition and better overall or absolute sensitivity.38 This remains valid when taking into account paramagnetic bleaching/depolarization effects38,39 in the TEKPol/OTP and BDPA/OTP samples at 8.0 kHz MAS. In the absence of microwaves at 9.4 T and 106 K, we observed a signal reduction of about 50% (θ = 0.5) for BDPA and 70% (θ = 0.3) for TEKPol/OTP.
The high temperature DNP enhancements reported here may not be limited to OTP, and it is likely that other glass formers may provide analogous results. Figure S2 shows preliminary results for the OE DNP enhancement at 18.8 T for BDPA in deuterated polystyrene (Tg ≈ 373 K). The enhancement was 27 at 115 K and decreases with temperature. At 150 K the enhancement was ∼15 and was then essentially temperature independent to room temperature. As mentioned above, Becerra et al. observed room temperature SE enhancements of ∼10 for BDPA in polystyrene at 5 T.28
Previous EPR and NMR studies revealed that the conformational dynamics of glassy OTP progressively increase close to Tg, and above the glass transition temperature translational motions become possible.31,32,34 This increased mobility can shorten electron and nuclear relaxation times that might explain the rapid decay of the DNP enhancement in the metastable OTP phase above Tg. Note that above Tg OTP slowly recrystallizes and radical aggregation might also contribute to the reduced enhancement. We observe that the BDPA OE DNP at 273 K is stable for several hours, while at 300 K the enhancement decays in a couple of hours.
We note that in all the cases above, the nuclear spin relaxation of the protons in the rigid OTP glass phase is strongly correlated to the DNP enhancements (see Figure S5). For the SE curve, the enhancement is found to be essentially linear in TB, in line with expectations from several models12,40−42 as detailed in SI. Figure S3 shows the normalized enhancements vs temperature for all three DNP mechanisms in these radicals. CE DNP is the most efficient mechanism, but decreases faster with temperature than the OE and SE. This is probably because CE is sensitive to shortening of the electron relaxation times6,9 and to the 1H T1 of the sample.43 Interestingly, for the OE at 9.4 T, εOE decreases less rapidly than εCE and εSE especially below Tg. The same trend of the OE is not so evident at 18.8 T (see discussion in SI). Since OE in insulators is for the moment known only in BDPA derivatives, we cannot affirm that this OE behavior is general or specific to BDPA. A full theoretical and quantitative description of the observations here requires a more detailed study including high field, variable temperature EPR data.
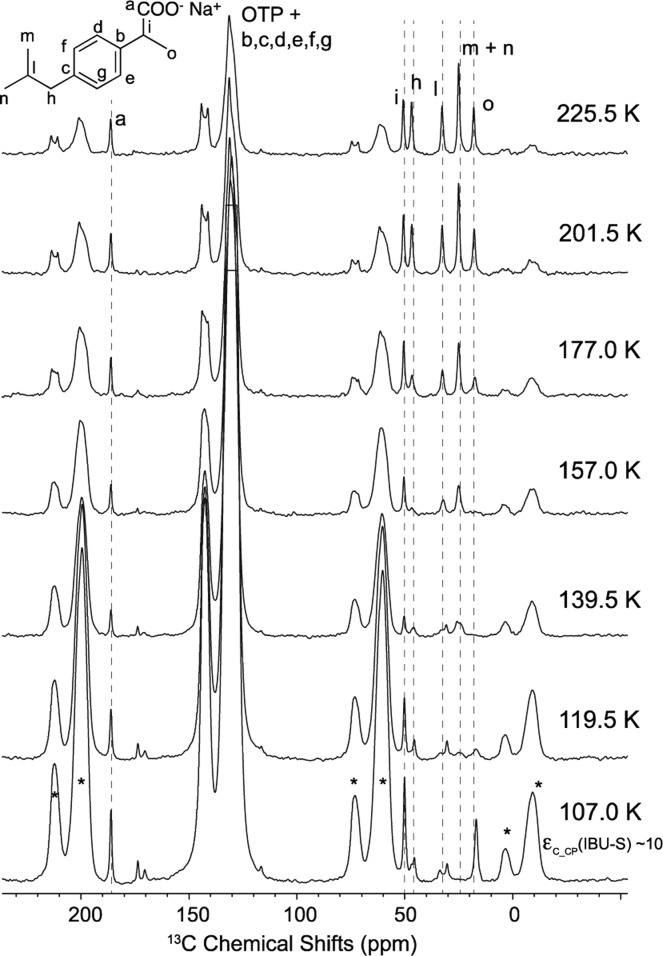
To demonstrate that the potential for DNP over larger temperature ranges than previously possible and that enhancements are not limited to the solvent itself, we applied the OTP/TEKPol medium to the characterization of microcrystalline ambroxol hydrochloride, a commercial mucolytic drug, and ibuprofen, the common analgesic. It has previously been shown that microparticulate powders can be polarized at ∼100 K by spin diffusion from DNP active media.44−47 Microcrystalline ambroxol hydrochloride was thus impregnated with a molten solution of 16 mM TEKPol in OTP (see SI). Ambroxol is not soluble in OTP, and Figure S4 shows that the high DNP polarization obtained in the OTP glass matrix is successfully relayed into the impregnated microcrystalline powder47 over temperatures from 113 to 236 K. At 113 K enhancements of over 50 are observed for the ambroxol peaks and a value of 8 is observed for the drug at 236 K (see the SI). In Figure 2 the same approach was applied to monitor dynamics inside the microcrystalline phase of the hydrated sodium salt of ibuprofen (IBU-S) between 107 and 225 K. IBU-S undergoes several conformational dynamic processes in the crystal that can be characterized by spectral changes in variable temperature solid-state NMR.20 Despite the dynamics, enhancements of around 10 are obtained, and the spectral changes are clearly observed in the DNP enhanced spectra, which thus allow these processes to be monitored with unprecedented sensitivity and without any significant loss in resolution (details in SI). These demonstrations open up many possibilities, including, e.g., measuring dynamics with natural abundance 2H spectra.48
Figure 2.
DNP enhanced (9.4 T) 13C CP MAS spectra of microcrystalline IBU-S impregnated with 16 mM TEKPol in OTP (9.4 T, MAS = 7.0 kHz, 30 s of recycle delay). All the spectra were acquired with microwave irradiation and 128 scans. Resonance assignment was taken from ref (20). Stars denote sidebands.
In conclusion, we have shown that efficient DNP radicals such as BDPA and TEKPol dissolved in OTP yield enhancements at 243 K (the Tg for OTP) of about 60–80 and that we can even obtain enhancements of ∼20 in the metastable phase around room temperature at 9.4 and 18.8 T. We have also shown that this medium can be used to enhance the NMR spectra of microcrystalline pharmaceutical compounds over a large range of temperatures, which enables monitoring of dynamical processes with DNP NMR.
Acknowledgments
We thank Lénaïc Leroux for technical help. Financial support is acknowledged from ERC Advanced Grant No. 320860 and EQUIPEX contract ANR-10-EQPX-47-01.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b08423.
Materials and Methods and supplementary discussions (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ni Q. Z.; Daviso E.; Can T. V.; Markhasin E.; Jawla S. K.; Swager T. M.; Temkin R. J.; Herzfeld J.; Griffin R. G. Acc. Chem. Res. 2013, 46, 1933–1941 10.1021/ar300348n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. J.; Zagdoun A.; Lelli M.; Lesage A.; Copéret C.; Emsley L. Acc. Chem. Res. 2013, 46, 1942–1951 10.1021/ar300322x. [DOI] [PubMed] [Google Scholar]
- Griffin R. G.; Prisner T. F. Phys. Chem. Chem. Phys. 2010, 12, 5737. 10.1039/c0cp90019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C.; Hu K.-N.; Joo C.-G.; Swager T. M.; Griffin R. G. J. Am. Chem. Soc. 2006, 128, 11385–11390 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- Matsuki Y.; Maly T.; Ouari O.; Karoui H.; Le Moigne F.; Rizzato E.; Lyubenova S.; Herzfeld J.; Prisner T.; Tordo P.; Griffin R. G. Angew. Chem., Int. Ed. 2009, 48, 4996–5000 10.1002/anie.200805940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagdoun A.; Casano G.; Ouari O.; Lapadula G.; Rossini A. J.; Lelli M.; Baffert M.; Gajan D.; Veyre L.; Maas W. E.; Rosay M.; Weber R. T.; Thieuleux C.; Copéret C.; Lesage A.; Tordo P.; Emsley L. J. Am. Chem. Soc. 2012, 134, 2284–2291 10.1021/ja210177v. [DOI] [PubMed] [Google Scholar]
- Kiesewetter M. K.; Corzilius B.; Smith A. A.; Griffin R. G.; Swager T. M. J. Am. Chem. Soc. 2012, 134, 4537–4540 10.1021/ja212054e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvée C.; Rosay M.; Casano G.; Aussenac F.; Weber R. T.; Ouari O.; Tordo P. Angew. Chem., Int. Ed. 2013, 52, 10858–10861 10.1002/anie.201304657. [DOI] [PubMed] [Google Scholar]
- Zagdoun A.; Casano G.; Ouari O.; Schwarzwälder M.; Rossini A. J.; Aussenac F.; Yulikov M.; Jeschke G.; Copéret C.; Lesage A.; Tordo P.; Emsley L. J. Am. Chem. Soc. 2013, 135, 12790–12797 10.1021/ja405813t. [DOI] [PubMed] [Google Scholar]
- Hall D. A.; Maus D. C.; Gerfen G. J.; Inati S. J.; Becerra L. R.; Dahlquist F. W.; Griffin R. G. Science 1997, 276, 930–932 10.1126/science.276.5314.930. [DOI] [PubMed] [Google Scholar]
- Lumata L.; Ratnakar S. J.; Jindal A.; Merritt M.; Comment A.; Malloy C.; Sherry A. D.; Kovacs Z. Chem. - Eur. J. 2011, 17, 10825–10827 10.1002/chem.201102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzilius B.; Smith A. A.; Griffin R. G. J. Chem. Phys. 2012, 137, 054201. 10.1063/1.4738761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can T. V.; Caporini M. A.; Mentink-Vigier F.; Corzilius B.; Walish J. J.; Rosay M.; Maas W. E.; Baldus M.; Vega S.; Swager T. M.; Griffin R. G. J. Chem. Phys. 2014, 141, 064202. 10.1063/1.4891866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosay M.; Tometich L.; Pawsey S.; Bader R.; Schauwecker R.; Blank M.; Borchard P. M.; Cauffman S. R.; Felch K. L.; Weber R. T.; Temkin R. J.; Griffin R. G.; Maas W. E. Phys. Chem. Chem. Phys. 2010, 12, 5850. 10.1039/c003685b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbey Ü.; Linden A. H.; Oschkinat H. Appl. Magn. Reson. 2012, 43, 81. 10.1007/s00723-012-0357-2. [DOI] [Google Scholar]
- Siemer A. B.; Huang K.-Y.; McDermott A. E. PLoS One 2012, 7, e47242. 10.1371/journal.pone.0047242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koers E. J.; van der Cruijsen E. A. W.; Rosay M.; Weingarth M.; Prokofyev A.; Sauvée C.; Ouari O.; van der Zwan J.; Pongs O.; Tordo P.; Maas W. E.; Baldus M. J. Biomol. NMR 2014, 60, 157–168 10.1007/s10858-014-9865-8. [DOI] [PubMed] [Google Scholar]
- Lewandowski J. R.; Halse M. E.; Blackledge M.; Emsley L. Science 2015, 348, 578–581 10.1126/science.aaa6111. [DOI] [PubMed] [Google Scholar]
- Barnes A. B.; Corzilius B.; Mak-Jurkauskas M. L.; Andreas L. B.; Bajaj V. S.; Matsuki Y.; Belenky M. L.; Lugtenburg J.; Sirigiri J. R.; Temkin R. J.; Herzfeld J.; Griffin R. G. Phys. Chem. Chem. Phys. 2010, 12, 5861. 10.1039/c003763j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concistrè M.; Carignani E.; Borsacchi S.; Johannessen O. G.; Mennucci B.; Yang Y.; Geppi M.; Levitt M. H. J. Phys. Chem. Lett. 2014, 5, 512. 10.1021/jz4026276. [DOI] [PubMed] [Google Scholar]
- Henstra A.; Lin T. S.; Schmidt J.; Wenckebach W. T. Chem. Phys. Lett. 1990, 165, 6–10 10.1016/0009-2614(90)87002-9. [DOI] [Google Scholar]
- Tateishi K.; Negoro M.; Nishida S.; Kagawa A.; Morita Y.; Kitagawa M. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 7527–7530 10.1073/pnas.1315778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can T. V.; Walish J. J.; Swager T. M.; Griffin R. G. J. Chem. Phys. 2015, 143, 054201. 10.1063/1.4927087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger C.; Bennati M.; Vieth H. M.; Luchinat C.; Parigi G.; Höfer P.; Engelke F.; Glaser S. J.; Denysenkov V.; Prisner T. F. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 64, 4–28 10.1016/j.pnmrs.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Jakdetchai O.; Denysenkov V.; Becker-Baldus J.; Dutagaci B.; Prisner T. F.; Glaubitz C. J. Am. Chem. Soc. 2014, 136, 15533–15536 10.1021/ja509799s. [DOI] [PubMed] [Google Scholar]
- Wind R. A.; Duijvestijn M. J.; Van Der Lugt C.; Manenschijn A.; Vriend J. Prog. Nucl. Magn. Reson. Spectrosc. 1985, 17, 33–67 10.1016/0079-6565(85)80005-4. [DOI] [Google Scholar]
- Afeworki M.; McKay R. A.; Schaefer J. Macromolecules 1992, 25, 4084–4091 10.1021/ma00042a006. [DOI] [Google Scholar]
- Becerra L. R.; Gerfen G. J.; Temkin R. J.; Singel D. J.; Griffin R. G. Phys. Rev. Lett. 1993, 71, 3561–3564 10.1103/PhysRevLett.71.3561. [DOI] [PubMed] [Google Scholar]
- Gerfen G. J.; Becerra L. R.; Hall D. A.; Griffin R. G.; Temkin R. J.; Singel D. J. J. Chem. Phys. 1995, 102, 9494–9497 10.1063/1.468818. [DOI] [Google Scholar]
- Earle K. A.; Moscicki J. K.; Polimeno A.; Freed J. H. J. Chem. Phys. 1997, 106, 9996. 10.1063/1.474114. [DOI] [Google Scholar]
- Andreozzi L.; Cianflone F.; Donati C.; Leporini D. J. Phys.: Condens. Matter 1996, 8, 3795–3809 10.1088/0953-8984/8/21/007. [DOI] [Google Scholar]
- Savitsky A.; Plato M.; Möbius K. Appl. Magn. Reson. 2010, 37, 415. 10.1007/s00723-009-0064-9. [DOI] [Google Scholar]
- Ong T.-C.; Mak-Jurkauskas M. L.; Walish J. J.; Michaelis V. K.; Corzilius B.; Smith A. A.; Clausen A. M.; Cheetham J. C.; Swager T. M.; Griffin R. G. J. Phys. Chem. B 2013, 117, 3040–3046 10.1021/jp311237d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnauss W.; Fujara F.; Hartmann K.; Sillescu H. Chem. Phys. Lett. 1990, 166, 381–384 10.1016/0009-2614(90)85047-G. [DOI] [Google Scholar]
- Thurber K. R.; Tycko R. J. Magn. Reson. 2009, 196, 84–87 10.1016/j.jmr.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki D. J.; Rossini A. J.; Purea A.; Zagdoun A.; Ouari O.; Tordo P.; Engelke F.; Lesage A.; Emsley L. J. Am. Chem. Soc. 2014, 136, 15711. 10.1021/ja5088453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli M.; Rossini A. J.; Casano G.; Ouari O.; Tordo P.; Lesage A.; Emsley L. Chem. Commun. 2014, 50, 10198–10201 10.1039/C4CC02152E. [DOI] [PubMed] [Google Scholar]
- Rossini A. J.; Zagdoun A.; Lelli M.; Gajan D.; Rascón F.; Rosay M.; Maas W. E.; Copéret C.; Lesage A.; Emsley L. Chem. Sci. 2011, 3, 108. 10.1039/C1SC00550B. [DOI] [Google Scholar]
- Thurber K. R.; Tycko R. J. Chem. Phys. 2012, 137, 084508. 10.1063/1.4747449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A.; Corzilius B.; Barnes A. B.; Maly T.; Griffin R. G. J. Chem. Phys. 2012, 136, 015101. 10.1063/1.3670019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovav Y.; Feintuch A.; Vega S. J. Magn. Reson. 2010, 207, 176–189 10.1016/j.jmr.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Hovav Y.; Feintuch A.; Vega S. J. Chem. Phys. 2011, 134, 074509. 10.1063/1.3526486. [DOI] [PubMed] [Google Scholar]
- Zagdoun A.; Rossini A. J.; Conley M. P.; Grüning W. R.; Schwarzwälder M.; Lelli M.; Franks W. T.; Oschkinat H.; Copéret C.; Emsley L.; Lesage A. Angew. Chem., Int. Ed. 2013, 52, 1222–1225 10.1002/anie.201208699. [DOI] [PubMed] [Google Scholar]
- Rossini A. J.; Zagdoun A.; Hegner F.; Schwarzwälder M.; Gajan D.; Copéret C.; Lesage A.; Emsley L. J. Am. Chem. Soc. 2012, 134, 16899. 10.1021/ja308135r. [DOI] [PubMed] [Google Scholar]
- Rossini A. J.; Widdifield C. M.; Zagdoun A.; Lelli M.; Schwarzwälder M.; Copéret C.; Lesage A.; Emsley L. J. Am. Chem. Soc. 2014, 136, 2324–2334 10.1021/ja4092038. [DOI] [PubMed] [Google Scholar]
- Takahashi H.; Hediger S.; De Paëpe G. Chem. Commun. 2013, 49, 9479. 10.1039/c3cc45195j. [DOI] [PubMed] [Google Scholar]
- Pinon A. C.; Rossini A. J.; Widdifield C. M.; Gajan D.; Emsley L. Mol. Pharmaceutics 2015, 12, 4146. 10.1021/acs.molpharmaceut.5b00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. J.; Schlagnitweit J.; Lesage A.; Emsley L. J. Magn. Reson. 2015, 259, 192–198 10.1016/j.jmr.2015.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.