Abstract
Repetitive concussions are associated with long-term cognitive dysfunction that can be attenuated by increasing the time intervals between concussions; however, biomarkers of the safest rest interval between injuries remain undefined. We hypothesize that deranged cerebral blood flow (CBF) is a candidate biomarker for vulnerability to repetitive concussions. Using a mouse model of human concussion, we examined the effect of single and repetitive concussions on cognition and on an index of CBF (CBFi) measured with diffuse correlation spectroscopy. After a single mild concussion, CBFi was reduced by 35±4% at 4 hours (P<0.01 versus baseline) and returned to preinjury levels by 24 hours. After five concussions spaced 1 day apart, CBFi was also reduced from preinjury levels 4 hours after each concussion but had returned to preinjury levels by 72 hours after the final concussion. Interestingly, in this repetitive concussion model, lower CBFi values measured both preinjury and 4 hours after the third concussion were associated with worse performance on the Morris water maze assessed 72 hours after the final concussion. We conclude that low CBFi measured either before or early on in the evolution of injury caused by repetitive concussions could be a useful predictor of cognitive outcome.
Keywords: animal models, brain trauma, optical imaging
Introduction
Sport-related concussions are a form of mild traumatic brain injury (TBI) that produce neurologic manifestations in the absence of macroscopic structural brain damage.1 Repetitive concussions may be associated with increased risk of dementia and development of neurodegenerative diseases, including chronic traumatic encephalopathy.2, 3, 4 Animal studies show the dramatic consequences of repetitive TBI on histopathology and neurologic function;5, 6, 7, 8, 9, 10, 11, 12 however, injury mechanisms associated with repetitive concussion remain largely unknown, and physiologic biomarkers are incompletely characterized.13, 14, 15 A physiologic biomarker that predicts neurologic outcome after concussions would be useful as an objective means of managing return-to-play guidelines rather than relying solely on symptom reporting, which can be unreliable,16, 17 and it could be an important component of personalized medicine applicable to all athletes.
One physiologic biomarker that has been used to study the effects of concussions on brain function is cerebral blood flow (CBF). Studies in humans who have sustained one or more concussions show decreased CBF as well as deranged cerebrovascular reactivity in acute and chronic periods after injury.15, 18, 19, 20, 21, 22, 23, 24, 25 Although these studies show promise that CBF derangements may be useful to diagnose concussion or to prognosticate neurologic outcome, patient heterogeneity with respect to age/gender, concussion mechanism, time of CBF assessment, prior history of concussions, and lack of baseline data make interpretation of existing human studies problematic.15, 22, 26
To assess the possible contribution of CBF changes to cognitive outcome after repetitive concussions in a controlled study, we used a murine repetitive closed head injury (CHI) model that is relevant to human sport-related concussion5 in that it features head impact followed by unrestricted rotational acceleration. This model results in long-term cognitive deficits in the absence of macroscopic and microscopic brain injury.9 Cerebral blood flow was assessed with a relatively new, noninvasive optical method known as diffuse correlation spectroscopy (DCS).27, 28 Diffuse correlation spectroscopy uses near-infrared light to quantify an index of average CBF of the microvasculature (CBFi) that is highly correlated with absolute CBF.29, 30, 31 It offers advantages over other small animal CBF modalities in that measurements can be performed without resection of the scalp or thinning of the skull, unlike laser Doppler flowmetry or laser speckle contrast imaging.32 Further, DCS measurements can be made relatively quickly (<2 minutes), unlike magnetic resonance imaging, thus limiting anesthesia exposure and readily enabling multiple CBFi measurement time points in a given animal.
Using DCS and our established mouse CHI repetitive concussion model, we tested three hypotheses relating derangements in CBFi to CHI and cognitive outcome: (1) Repetitive (but not single) CHI induces long-lasting decreases in CBFi; (2) CBFi decreases as a function of the number of CHIs; and (3) Decreased CBFi postCHI is predictive of functional outcome.
Materials and methods
Adult (3 months old) male C57/BL6 mice were used in all experiments. Food and water were provided ad libitum. All protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and followed the NIH Guidelines for the Care and Use of Laboratory Animals. Mice were housed in a pathogen-free facility with a 12-hour light/dark cycle. Experimenters performing behavioral testing were blinded to the treatment group. All experiments complied with the ARRIVE guidelines for how to report animal experiments.
Closed Head Injury Model
A weight drop CHI model was used to produce concussive TBI as previously reported.9 In this model, a single CHI does not produce structural or cognitive deficits, whereas five-hit daily repetitive CHI (5HD rCHI) results in permanent cognitive deficits without overt structural brain injury, edema, blood–brain barrier damage, acute cell death, or chronic brain tissue loss.9
Mice were anesthetized using 4.5% isoflurane in a 70:30 mixture of nitrous oxide (N2O) and oxygen (O2) for 45 seconds. This anesthetic regimen maintains blood pressure and blood gases within the normal range for mice.33 Anesthetized mice were placed on a delicate task wiper (Kimwipe, Kimberly-Clark, Irving, TX, USA), grasped by the tail, and a 54-g metal bolt was dropped 38 inches through a guide tube onto the dorsal aspect of the skull between the coronal and lambdoid sutures. At impact, the mouse head and bolt readily penetrated the Kimwipe, allowing for acceleration of the head in the anterior-posterior plane. Control mice were age-matched, sham-injured mice that underwent anesthesia for the same duration but were not subjected to concussive injury. All mice were recovered in room air. Mice were randomly selected to be subjected to one or five sham injuries (sham groups) or one or five closed head injuries (CHI groups) either once (single hit model), or once per day for five consecutive days (5HD model).
Assessment of Cerebral Blood Flow Index Using Diffuse Correlation Spectroscopy
Measurements of CBF were made using DCS. Diffuse correlation spectroscopy uses near-infrared light to noninvasively probe dynamic optical properties of cortical microvaculature. It measures temporal fluctuations in reflected near-infrared light intensity on the tissue surface, which are primarily caused by moving red blood cells. A temporal intensity autocorrelation function is used to quantify the intensity fluctuations and the autocorrelation function is fit to simple models to derive a tissue CBFi (mm2/second).27, 28 Previous studies in both animals and humans have shown that absolute CBFi, as well as relative changes in CBFi over time, measured with DCS is strongly correlated with CBF measured by other techniques, including fluorescent microspheres, Xenon-CT, laser Doppler flowmetry, bolus tracking time domain near-infrared spectroscopy, arterial spin-labeled and phase encoded velocity mapping magnetic resonance imaging, and transcranial Doppler ultrasound.30
To quantify CBFi, anesthesia was induced in mice using 4.5% isoflurane in a 70:30 N2O:O2 mixture for 45 seconds. Isoflurane concentration was then reduced to 1.5%, and mice were placed supine and allowed to stabilize for 1 minute. The DCS measurements were made by gently holding the DCS sensor over each hemisphere for 5 seconds of 1 Hz acquisition of intensity autocorrelation functions. Data were acquired over each hemisphere in sequence; measurements were repeated up to three times per hemisphere account for local inhomogeneities under the optical sensor. Depilatory cream was used to remove hair on the scalp 1 day before the start of the experiment to improve the signal-to-noise ratio in DCS data.
For the single CHI model, DCS data were obtained at baseline immediately before CHI, as well as at 4 and 24 hours after CHI or sham injury. For the 5HD rCHI model, DCS data were obtained at the following time points: baseline before injury (d1), four hours after the third injury (d3), four hours after the final injury (d5), 72 hours after the final injury (d8), and 2.5 weeks after the final injury (d22, arrows in Figure 1). Total measurement time on each day for a given mouse did not exceed 2 minutes. Diffuse correlation spectroscopy measurement time points were chosen to best capture the evolution of CBF changes after CHI while minimizing the cumulative isoflurane exposure to the animal.
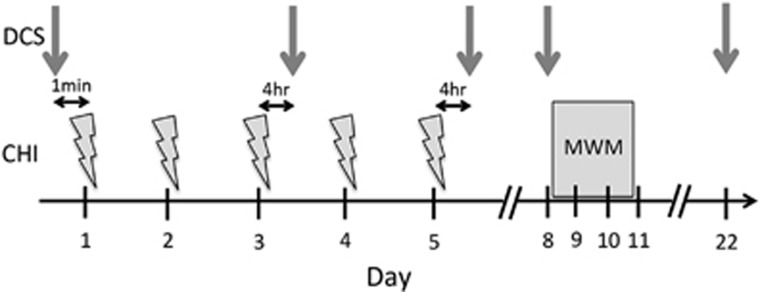
Figure 1.
Study protocol for repetitive closed head injury (CHI). Mice were subjected to repetitive closed head injuries (CHI, indicated by lightning bolts) once daily for 5 days. Diffuse correlation spectroscopy (DCS) measurements of cerebral blood flow index (indicated by gray arrows) were made at baseline immediately before the first CHI (d1), 4 hours after the third CHI (d3), 4 hours after the fifth CHI (d5), as well as 72 hours and 22 days after the final hit (d8 and d22, respectively). Cognitive deficits were assessed on days 8 to 11 using the Morris water maze (MWM).
A custom-built DCS instrument was employed that used an 852-nm long-coherence length laser (CrystaLaser, Reno, NV, USA), a photon-counting avalanche photodiode (Perkin-Elmer, Quebec, Canada), and a hardware autocorrelator board (www.correlator.com, NJ). A single 6-mm source-detector pair embedded in a rigid black sensor was used. Given the relatively short distance to the brain in the mouse (approximately 0.5 mm), this separation was sufficient to probe cortical brain tissue assuming a mean photon depth sensitivity of approximately one-third to one-half the distance between source and detector.34 Each intensity autocorrelation function was fit to the semi-infinite homogenous solution of the correlation diffusion equation,27 assuming a fixed value for the absorption and reduced scattering coefficients at 852 nm of 0.1 and 4.1/cm, respectively.
The DCS data were rejected according to the following objective criteria. First, intensity autocorrelation curves were discarded if the tail of the curve differed from 1 by more than 0.005, indicating movement of the sensor during the 1-second averaging time. Second, after removing any outliers in CBFi within a given repetition (i.e., any values that fell within ±1.5 times the standard deviation of the mean CBFi value across the 5 seconds of 1 Hz acquisition), data sets with a coefficient of variation (≡standard deviation/mean) of CBFi greater than 20% were discarded, again indicative of sensor movement. Finally, only measurement sessions for which the average hemispheric CBFi across multiple repetitions had a coefficient of variation of less than 20% were included.
Assessment of Blood Pressure and Blood Gases
To assess the effects of 5HD rCHI on systemic physiology, an arterial line was placed 4 hours after the fifth sham or CHI in a subset of animals (n=8/group). The right femoral artery was catheterized with heparinized PE10 tubing under isoflurane anesthesia (2.5% induction, 1% maintenance, in 70:30 N2O:O2 mixture). Rectal temperature was maintained at 37°C using a thermostatically controlled heating pad (Harvard Apparatus, Holliston, MA, USA). After femoral arterial cannulation, the animal was allowed to stabilize for 2 minutes. After stabilization, continuous mean arterial pressure (mm Hg) was recorded (PowerLab, ADInstruments, Colorado Springs, CO, USA), and an arterial blood sample was drawn to assess pH, partial pressure of oxygen (pO2, mmHg), partial pressure of carbon dioxide (pCO2, mmHg), hemoglobin concentration (Hgb, g/dL), and hematocrit (Hct, %) at the same time that DCS measurements were made.
Assessment of Cognitive Function Using the Morris Water Maze
Cognitive function was assessed using a version of the Morris water maze (MWM, Figure 1) as previously described.33 Mice were tested beginning 3 days after the fifth rCHI, or beginning 24 hours after a single CHI. Briefly, a circular tank of 83 cm in diameter and 60 cm deep was filled with water to a depth of 29 cm. The walls of the tanks and the room in which the tanks were housed contained highly visible cues. Water temperature was maintained at 25°C. During hidden trials, a clear, Plexiglas platform (10 cm in diameter) was submerged 0.5 cm below the surface of the water in the southwest quadrant 15 cm from the wall. Mice were randomized to one of four starting locations (north, south, east, or west) and were given 90 seconds to find the location of the hidden platform, mount the platform, and remain on it for 5 to 10 seconds. Mice were then placed under a heat lamp. The time until the mouse mounted the platform (escape latency) was measured and recorded (AnyMaze 8.42, Stoelting, Wood Dale, IL, USA). Mice that failed to mount the platform within 90 seconds were placed on the platform by the experimenter, and allowed to remain for 5 to 10 seconds. For visible platform trials, the height of the platform was raised 0.5 cm above the water level; mice were given 90 seconds to mount the platform, and remain on it for 5 to 10 seconds. For probe trials, the platform was removed and the time spent in the target quadrant (total 60 seconds swim time) was recorded. Mice were subjected to three hidden trials on the first day of testing, two hidden trials on the second day of testing, followed by a probe trial at either 1 or 24 hours after the completion of hidden trials. Two sets of visible platform trials were performed on the third day of testing.
Statistical Analyses
Data are presented as mean±standard error of the mean. Unpaired t-tests were used to test for differences between sham injured and CHI groups in systemic parameters as well as CBFi. The Bonferroni method was used to adjust P values for multiple comparisons, as appropriate. For the MWM cognitive testing, hidden and visible platform trials of the MWM were analyzed by repeated measures analysis of variance (group × time; GraphPad Prism 6, La Jolla, CA, USA). Multiple linear regression was used to investigate the relationship between CBFi measured at each time point in the rCHI model and an animal's subsequent average performance across all five hidden trials of the MWM (group × CBFi). Reducing the five hidden trials to a single value by averaging was motivated by a canonical correlation analysis. Multiple regression and canonical correlation analyses were performed using R 2.11.35 For all analyses, P<0.05 was considered as significant.
Results
One hundred eighteen (50 rCHI and 36 sham; 16 single CHI and 16 sham) adult male mice were used for this study. Twelve of the fifty rCHI mice were euthanized before the end of the studies, typically after the fourth or fifth CHI or before completion of MWM testing, due to significant motor deficits (n=11), which may occur with inadvertent cerebellar damage from posterior cranial impacts, or due to evidence of prolonged tonic seizures after regaining consciousness (n=1). All other mice survived the experimental period.
Baseline CBFi ranged from 1.4 to 3.8 × 10−5 mm2/s across all mice, with an average value of 2.5±0.1 × 10−5 mm2/s. No differences in CBFi were observed between right and left hemispheres or between sham and injured groups at baseline (not shown). The average intra-hemispheric coefficient of variation (ratio of standard deviation to mean CBFi across multiple measurement repetitions) across all animals and all measurements was 4.8±0.1%.
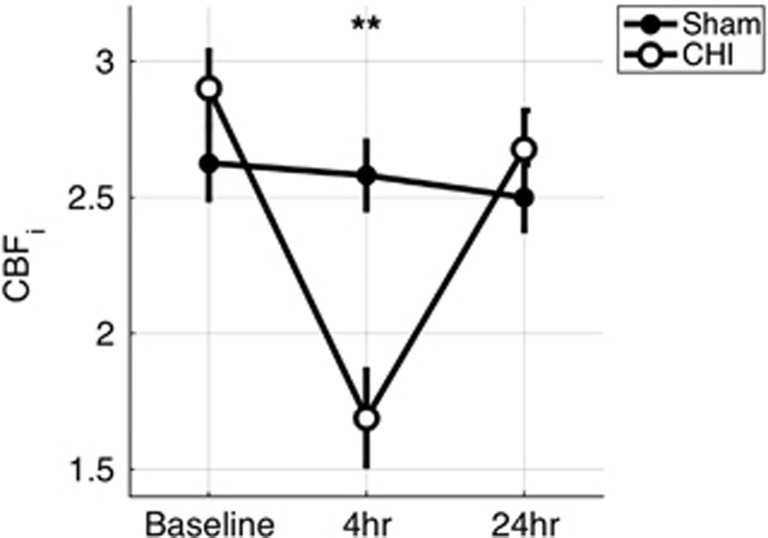
Four hours after a single CHI, CBFi was significantly decreased from baseline levels by 42±6% and was significantly lower than that of sham-injured mice (N=8/8, P<0.01, Figure 2). Twenty-four hours later, average CBFi was no longer significantly different from baseline or from sham-injured levels (both P>0.05).
Figure 2.
Cerebral blood flow index after single closed head injury (CHI). Mean (s.e.) CBFi (in units of 10−5 mm2/s) for sham (filled circle, n=8) and single CHI (open circle, n=8) mice at baseline, 4 and 24 hours after CHI. **P<0.01, unpaired t-test.
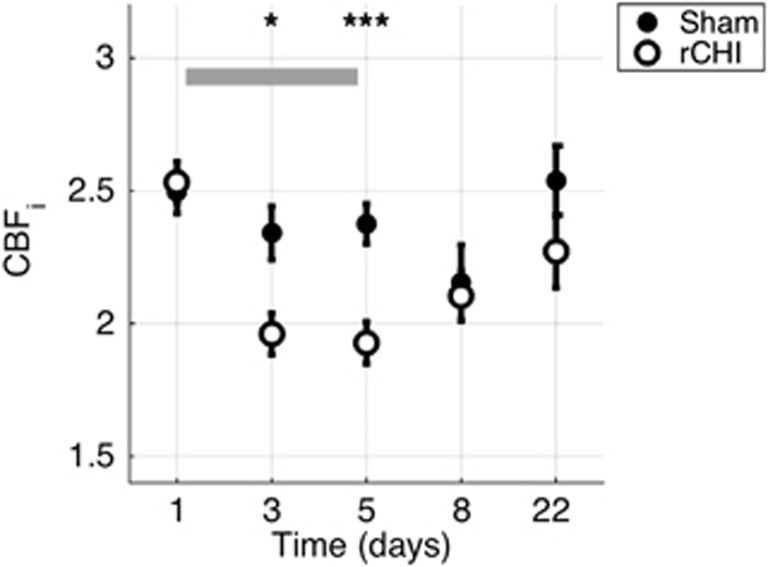
Five-hit daily rCHI resulted in similar decreases in CBF (Figure 3). By 4 hours after the third concussion, the average CBFi in the CHI group was 18±3% lower than baseline levels (P<0.001) and was significantly lower than that of the sham-injured group (P=0.018). Four hours after the fifth injury, average CBFi in the rCHI group was significantly decreased compared with both baseline (by 21±4%) and with sham-injured groups (P<0.001). However, by 72 hours after the fifth CHI (d8), CBFi had returned to baseline levels and it remained there at d22. Right and left hemisphere CBFi did not differ significantly at any time point in either group (not shown).
Figure 3.
Cerebral blood flow index (CBFi) after repetitive closed head injury (rCHI). Mean (s.e.) CBFi (in units of 10−5 mm2/s) as a function of time for sham (filled circle, n=36) and rCHI (open circle, n=50) mice. The gray-shaded rectangle indicates the time period in which rCHI occurred. *P<0.05, ***P<0.001, unpaired t-test.
To rule out an effect of physiologic variables on the observed decreases in CBFi measured 4 hours after the fifth concussion, systemic blood pressure and arterial blood gases were measured at the same time as CBFi in a subset of rCHI animals (n=8/group). No differences in mean arterial blood pressure, pH, partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), hemoglobin (Hgb), or hematocrit (Hct) were observed between groups (Table 1), and all values fell within the normal range expected for adult mice.
Table 1. Mean (s.e.) systemic physiologic parameters measured 4 hours after the fifth and final closed head injury for sham (n=8) and rCHI (n=8) mice.
| Sham | rCHI | |
|---|---|---|
| pH | 7.45 (0.02) | 7.45 (0.02) |
| pCO2 (mm Hg) | 28 (1) | 29 (2) |
| pO2 (mm Hg) | 137 (12) | 138 (14) |
| MABP (mm Hg) | 107 (2) | 105 (4) |
| Hgb (g/dL) | 12.0 (0.6) | 11.9 (0.3) |
| Hct (%) | 33.3 (3.2) | 32.4 (1.4) |
Hct, hematocrit; Hgb, hemoglobin; MABP, mean arterial blood pressure; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; rCHI, repetitive closed head injury.
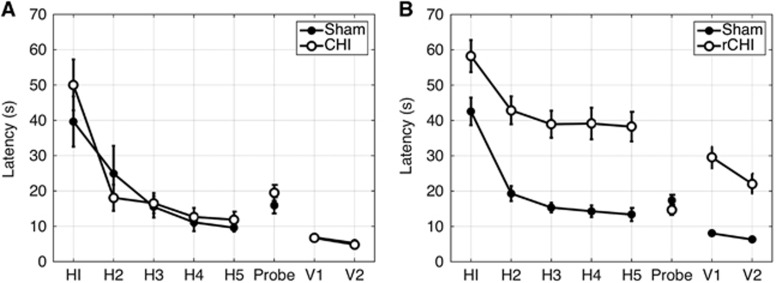
A total of 16 single CHI/sham animals (n=8 CHI and n=8 sham) and 57 rCHI/sham animals (n=31 rCHI and n=26 sham) underwent cognitive function testing using the MWM (Figure 4). Of these, three rCHI mice were excluded because of failure to complete testing due to severe motor deficits. As previously reported, a single CHI did not lead to cognitive deficits in the CHI group as compared with the sham group. However, repeated measures ANOVA revealed significant cognitive deficits in the rCHI group as compared with sham in both hidden and visible platform trials (P<0.001 for group and for time in each comparison, P>0.05 for group × time) (Figure 4). No differences between rCHI and sham groups were observed in probe trial performance. Swim speeds during hidden platform and probe trials were not significantly different between groups, although during visible trials rCHI mice swam significantly slower than their respective sham groups (see Supplementary Figure 1).
Figure 4.
Results of Morris water maze trials. Mean (s.e.) latency on five hidden, one probe, and two visible platform trials for (A) single closed head injury (CHI) (open circles, n=8) and sham (filled circles, n=8) animals and (B) repetitive CHI (rCHI, open circles, n=28) and sham (filled circles, n=26) mice. rCHI mice performed significantly worse than sham mice on both hidden and visible trials (P<0.001 for group, repeated measures analysis of variance (ANOVA)).
We assessed the relationship between group and d3 CBFi using multiple linear regression with group, d3 CBFi, and the interaction between group and d3 CBFi as regressors, and with subsequent mean hidden trial latency as the response. We observed a significant negative relationship within the rCHI group (slope=−17±5, P=0.003), but not within the sham group (slope=−3±6, P=0.65). Thus, on average for rCHI mice, for each 1 × 10−5 mm2/s decrease in d3 CBFi we estimate a 17-second increase in mean hidden trial latency. This negative relationship between d3 CBFi and MWM performance was significantly different from the null relationship observed in the sham group (P=0.017). Interestingly, similar trends were seen between baseline CBFi and subsequent MWM hidden trial performance after rCHI, with estimated slopes of −11±4 (P=0.003) and −2±5 (P=0.73) for rCHI and sham groups, respectively. Similar significant relationships were not seen between d5, d8, or d22 CBFi and MWM performance (hidden trials). We note that baseline CBFi was significantly correlated with d3 CBFi for the rCHI mice (r=0.46, P=0.02) but not for the sham mice (r=0.16, P=0.51). Furthermore, we were not able to investigate the relationship between CBFi in the single CHI model and MWM performance because the animals that were measured with DCS in this cohort did not undergo MWM testing.
Discussion
Prior studies in both animals and humans have showed that single mild TBI produces persistent decreases in CBF that recover with time in some but not all cohorts.18, 20, 24, 36 The present investigation expands upon prior work in several ways. First, we apply a novel, noninvasive optical modality, namely, DCS, which can be used repeatedly in a single animal, thus enabling longitudinal CBF measurements. Second, we explore the cumulative effects of multiple mild TBIs on cerebral hemodynamics. Third, we investigate whether CBF is a potentially relevant biomarker of cognitive outcome. The results have translational implications for athletes, soldiers, and others at significant risk for repetitive TBI.
We found that a single CHI produced a robust decrease in CBFi at 4 hours that resolved by 24 hours, (Figure 2) and that rCHI produced similar decreases in CBFi that were not cumulative and that resolved within 72 hours (Figure 3). Decreased CBFi 4 hours after the fifth CHI was not due to altered arterial blood pressure, blood gases, or body temperature (Table 1), suggesting a role for intrinsic brain mechanisms. The mechanisms underlying reduced CBFi are unknown but may include deranged cerebral autoregulation, flow/metabolism coupling, neurogenic regulation of vascular tone, endothelium, astrocyte contributions, and microvascular communication. Intracranial hypertension would be expected to reduce CBFi, although this model does not produce cerebral edema or macroscopic hemorrhage, even after 5 daily injuries.9 Alternatively, decreases in CBFi could be due to endothelial dysfunction and/or loss of endogenous nitric oxide production, leading to impaired arteriolar function.37 A third possibility is aberrant constriction of perivascular pericytes due to oxidative/nitrosative stress, which could reduce blood flow at the capillary level.38 A fourth possibility is that cerebral oxygen and/or glucose metabolism may be depressed or that neurovascular coupling is impaired,39 driving the observed reduction in CBFi. Further studies are needed to distinguish among these possible mechanisms.
Although literature reports suggest that regional CBF may be significantly reduced in patients with mild TBI in the chronic, postinjury period,18, 19, 21, 24 we observed no long-term (i.e., d22) decreases in cortical CBFi after rCHI. In fact, CBF recovered to baseline levels by 72 hours after five CHIs. Nonetheless, one limitation of DCS is the inability to interrogate brain structures below the cortex, and it is possible that CBF may be altered in brain structures concomitant with injury in these regions, as CBF derangements and damage to the neural microstructure have been reported in subcortical brain regions in humans with mild TBI.15, 40
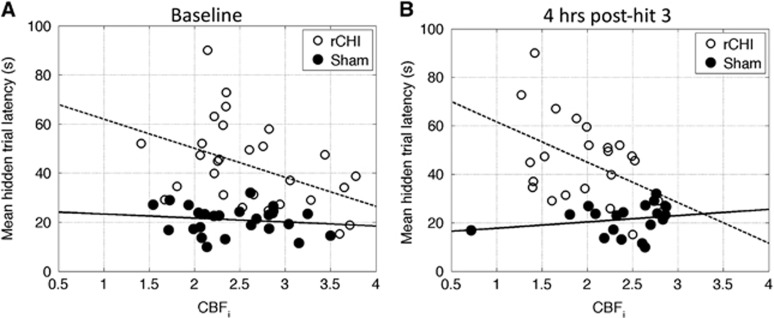
Given that the rCHI model used herein produces MWM deficits that persist for up to 1 year9 and that we observed a return in CBFi to baseline levels by 72 hours after injury, it appears that cognitive deficits are not due to chronic changes in cortical hemodynamics. However, we did find that baseline CBFi (before any CHI), as well as the extent of decreased CBFi observed 4 hours after the third hit, was associated with subsequent cognitive deficits after rCHI, i.e., the lower the CBFi, the longer the latency in MWM hidden trials measured 72 hours after the final CHI (Figure 5). These trends were not seen in the sham group. This observation suggests that decreases in baseline CBFi may be associated with an increased risk of cognitive deficits after repetitive concussions. If this finding translates to humans, then CBFi might prove useful in identifying athletes and soldiers at an increased risk for cognitive deficits after multiple concussions. Furthermore, CBFi measured early after concussion might be a physiologic biomarker of the brain's vulnerability to subsequent concussion, and, by extension, it may suggest that an athlete or soldier should not return to play/battlefield if CBFi is decreased after CHI. We note that by 4 hours after the fifth injury, CBFi was no longer associated with cognitive outcome, and this lack of association between CBFi and outcome persisted at 72 hours and 2 weeks after rCHI. Thus, CBFi measured either before concussion or early on in the evolution of injury caused by repetitive concussions could be a useful predictor of cognitive outcome; however, there exists a time in the injury progression in which CBFi ceases to be prognostic. More work is needed to clarify factors that complicate the prognostic value of hemodynamic perturbations after a larger number of concussions.
Figure 5.
Cerebral blood flow index (CBFi) relationship with outcome. CBFi (in units of 10−5 mm2/s) measured at baseline (A) and 4 hours after the third closed head injury (CHI) (B) in a five-hit daily repetitive CHI (rCHI) model versus average hidden trial latency across five hidden trials for sham (closed circles) and rCHI mice (open circles). The dotted and solid lines are trend lines computed from linear models for rCHI and sham mice, respectively.
One caveat of the current study is that we did not test whether a return of CBFi to baseline after the third injury marks a safe interval to resume concussions. Future studies will investigate whether timing the fourth and fifth injuries when CBFi has returned to baseline improves outcome over mice that get a fourth and fifth concussion regardless of CBFi levels. In addition, CBFi measurements are performed under 1.5% isoflurane; thus, the observed decreases in CBFi compared with sham after single and repetitive CHI could be confounded by failure of the injured cerebral vasculature to vasodilate in response to isoflurane. Further experiments using alternative anesthetics that have less effect on cerebral hemodynamics, as well as experiments to quantify cerebrovascular reactivity to blood pressure or inhaled carbon dioxide are needed to resolve these questions. Finally, CBFi measured with DCS is susceptible to errors arising from the assumption of a fixed value for the tissue reduced scattering coefficient, μs'(λ). We assumed a fixed value of μs'(λ) for all mice across all time points. This assumption is justified in part because our cohort was relatively homogeneous, and because mice do not develop edema or hemorrhage (which would change the density of scatterers and thus μs'(λ)) after single or repetitive CHI9 using this model. Still, future experiments would benefit from the concurrent use of a time or frequency-domain near infrared spectroscopy device to quantify μs'(λ) for each animal at each measurement time point and to input these measurements into the intensity autocorrelation function fit for CBFi.28
In conclusion, CHIs sustained by mice result in transient decreases in cortical CBF. Data from this investigation suggest that decreased cortical CBFi may be a marker of an acute vulnerable period after concussive brain injury. This result is an important step forward in the field, as current return-to-play/battlefield guidelines incorporate symptom resolution as major criteria,1 despite the lack of proof of validity of this approach.15 Furthermore, baseline CBFi in the uninjured may be used to identify those at an increased risk for cognitive deficits after CHI. Additional physiologic biomarkers as well as further studies in both animals and humans are needed to better delineate the brain's absolute vulnerable period to repetitive injury to objectively and accurately determine safe rest intervals between concussions.
Acknowledgments
The authors would like to thank the Harvard Catalyst for help with statistical analysis, Dr Ivy Lin for help with optical probe construction, and Dr Stefan Carp for assistance with DCS acquisition software.
Author contributions
EB, MW, JG, and BM were involved in study design. EB, BM, JG, HS, LM, and MW were involved in data acquisition. EB, MW, and MV were involved in data analysis. EB, CA, WM, MF, and MW were involved in data interpretation. EB, MV, WM, MF, and MW were involved in manuscript preparation.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by National Football League Players Association Grant to Harvard College and PO1NS 55104 06 (MJW); National Institute of Health award R01-EB001954 (MAF); the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers; and MGH ECOR Fund for Medical Discovery (EMB).
MAF holds patents on the technology used in this article.
Supplementary Material
References
- 1McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvorak J, Echemendia RJ et al. Consensus statement on concussion in sport—the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Clin J Sport Med 2013; 23: 89–117. [DOI] [PubMed] [Google Scholar]
- 2Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013; 9: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012; 79: 1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Angoa-Perez M, Kane MJ, Briggs DI, Herrera-Mundo N, Viano DC, Kuhn DM. Animal models of sports-related head injury: bridging the gap between preclinical research and clinical reality. J Neurochem 2014; 129: 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A et al. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J Neurotrauma 2002; 19: 427–438. [DOI] [PubMed] [Google Scholar]
- 7Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J et al. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery 2005; 56: 364–374. [DOI] [PubMed] [Google Scholar]
- 8Mannix R, Meehan WP, Mandeville J, Grant PE, Gray T, Berglass J et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol 2013; 74: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Meehan WP, 3rd, Zhang J, Mannix R, Whalen MJ. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 2012; 71: 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 2011; 70: 551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S et al. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci 2002; 22: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Weber JT. Experimental models of repetitive brain injuries. Prog Brain Res 2007; 161: 253–261. [DOI] [PubMed] [Google Scholar]
- 13Jeter CB, Hergenroeder GW, Hylin MJ, Redell JB, Moore AN, Dash PK. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 2012; 30: 657–670. [DOI] [PubMed] [Google Scholar]
- 14Dimou S, Lagopoulos J. Toward objective markers of concussion in sport: a review of white matter and neurometabolic changes in the brain after sports-related concussion. J Neurotrauma 2014; 31: 413–424. [DOI] [PubMed] [Google Scholar]
- 15Mayer AR, Bellgowan PS, Hanlon FM. Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci Biobehav Rev 2014; 49: 8–18. [DOI] [PubMed] [Google Scholar]
- 16Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol 2006; 13: 166–174. [DOI] [PubMed] [Google Scholar]
- 17McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players - Implications for prevention. Clin J Sport Med 2004; 14: 13–17. [DOI] [PubMed] [Google Scholar]
- 18Agrawal D, Gowda NK, Bal CS, Pant M, Mahapatra AK. Is medial temporal injury responsible for pediatric postconcussion syndrome? A prospective controlled study with single-photon emission computerized tomography. J Neurosurg 2005; 102: 167–171. [DOI] [PubMed] [Google Scholar]
- 19Bonne O, Gilboa A, Louzoun Y, Kempf-Sherf O, Katz M, Fishman Y et al. Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res 2003; 124: 141–152. [DOI] [PubMed] [Google Scholar]
- 20Ge Y, Patel MB, Chen Q, Grossman EJ, Zhang K, Miles L et al. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3 T. Brain Inj 2009; 23: 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Hofman PA, Stapert SZ, van Kroonenburgh MJ, Jolles J, de Kruijk J, Wilmink JT. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. AJNR Am J Neuroradiol 2001; 22: 441–449. [PMC free article] [PubMed] [Google Scholar]
- 22Slobounov S, Gay M, Johnson B, Zhang K. Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav 2012; 6: 224–243. [DOI] [PubMed] [Google Scholar]
- 23Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 2012; 129: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Liu W, Wang B, Wolfowitz R, Yeh PH, Nathan DE, Graner J et al. Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI. NMR Biomed 2013; 26: 651–663. [DOI] [PubMed] [Google Scholar]
- 25Metting Z, Rodiger LA, Stewart RE, Oudkerk M, De Keyser J, van der Naalt J. Perfusion computed tomography in the acute phase of mild head injury: regional dysfunction and prognostic value. Ann Neurol 2009; 66: 809–816. [DOI] [PubMed] [Google Scholar]
- 26Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 2012; 59: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Boas D, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am 1997; A 14: 192–215. [Google Scholar]
- 28Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Rep Prog Phys 2010; 73: 076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Diop M, Verdecchia K, Lee TY, St Lawrence K. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed Opt Express 2011; 2: 2068–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Durduran T, Yodh AG. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014; 85: 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab 2014; 34: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Buckley EM, Parthasarathy AB, Yodh AG, Franceschini MA. Diffuse correlation spectroscopy for measurements of cerebral blood flow: future prospects. Neurophotonics 2014; 1: 011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Khuman J, Meehan WP, 3rd, Zhu X, Qiu J, Hoffmann U, Zhang J et al. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab 2011; 31: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Patterson MS, Andersson-Engels S, Wilson BC, Osei EK. Absorption spectroscopy in tissue-simulating materials: a theoretical and experimental study of photon paths. Appl Opt 1995; 34: 22–30. [DOI] [PubMed] [Google Scholar]
- 35R Development Core Team R: A Language and Environment for Statistical Computing. In: R Foundation for Statistical Computing 2011.
- 36Henninger N, Sicard KM, Li Z, Kulkarni P, Dutzmann S, Urbanek C et al. Differential recovery of behavioral status and brain function assessed with functional magnetic resonance imaging after mild traumatic brain injury in the rat. Crit Care Med 2007; 35: 2607–2614. [DOI] [PubMed] [Google Scholar]
- 37Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT. The role of the nitric oxide pathway in brain injury and its treatment - From bench to bedside. Exp Neurol 2015; 263C: 235–243. [DOI] [PubMed] [Google Scholar]
- 38Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 39Tan CO, Meehan WP, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 2014; 83: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med 2011; 30: 33–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.