Abstract
Oxidative stress is a major brain injury mechanism after ischemic stroke. 12/15-lipoxygenase (12/15-LOX) is a key mediator of oxidative stress, contributing to neuronal cell death and vascular leakage. Nonetheless, the mechanism leading to its upregulation is currently unknown. We show here that Signal Transducers and Activators of Transcription (STATs), specifically STAT6 and possibly STAT1, increase transcription of 12/15-LOX in neuronal cells. Both p-STAT6 and -1 bound to specific STAT binding sites in the mouse 12/15-LOX promoter. Small interfering RNA (siRNA) knockdown showed STAT6 to be the dominant regulator, reducing 12/15-LOX promoter activation and cell death in oxidatively stressed HT22 cells. STAT6 siRNA efficiently prevented the increase of 12/15-LOX in murine primary neurons, both after induction of oxidative stress and after oxygen-glucose deprivation. Early activation of STAT6 and STAT1 in mice was consistent with a role in regulating 12/15-LOX in focal ischemia. Brains of human stroke patients showed increased p-STAT6 and p-STAT1 in the peri-infarct region, along with 12/15-LOX and markers of apoptosis. These results link STAT6 and STAT1 to the 12/15-LOX damage pathway and suggest disregulation of STAT-dependent transcription as injury mechanism in stroke. Selectively targeting STATs may thus be a novel therapeutic approach to reducing brain injury after a stroke.
Keywords: ischemia, lipoxygenase, neuron, STAT, transcription factor
Introduction
In the healthy brain, an intricate network of pro- and antioxidative proteins and peptides maintains a redox balance inside the cell, preventing cellular injury but allowing for redox-dependent signaling events. After an ischemic stroke this redox network breaks down. The ensuing oxidative stress leads to neuronal death and various forms of cerebral pathology including neurovascular injury, blood–brain barrier disruption, edema, and hemorrhage.1, 2 Components of the redox network include the antioxidant glutathione, which decreases after ischemia, while pro-oxidative enzymes including those of the arachidonic acid cascade are upregulated. In particular, 12/15-lipoxygenase (12/15-LOX) is elevated in both mouse and human brain after an ischemic stroke,3 and contributes to delayed cell death in the area surrounding the core infarct.4, 5, 6 Inhibition of 12/15-LOX reduces infarct size, edema, and blood–brain barrier leakage, and is being investigated as a novel approach to stroke therapy.3, 5, 6, 7, 8
One of the key regulators of this redox homeostatic network, along with the hypoxia-inducible factors, is the Signal Transducers and Activators of Transcription (STAT) family of proteins.9 Phosphorylation at specific tyrosine residues and subsequent dimerization is required for STAT activation; the dimers then translocate to the nucleus, where they bind to their target gene's promoter region.10 STAT-dependent gene regulation has been implicated in a number of pathologies, including several forms of cancer and polycystic kidney disease.11, 12 In the ischemic mouse brain after experimental stroke, loss of STAT3 has been shown to downregulate protective manganese-dependent superoxide dismutase,2, 13 and STAT3 is generally seen as neuroprotective. Conversely, STAT1 is activated and contributes to ischemic injury.14 For STAT6, the situation is less clear. While changes in STAT6 phosphorylation after ischemia in rodents have been documented (see discussion for details),15 possible functional consequences were not investigated in those studies.
Surprisingly, although the elevation of 12/15-LOX levels in neurons and ischemic cortex is a critical factor contributing to neuronal death and brain injury after cerebral stroke, its transcriptional regulation in the brain has not been elucidated. We investigated here a possible regulation of 12/15-LOX through STATs, with a specific focus on STATs 1 and 6. Our findings here show that predominantly STAT6, but possibly also STAT1 contributes to the upregulation of 12/15-LOX in oxidatively stressed neuronal cells, and also coincide with increased 12/15-LOX in the infarcted human brain in vivo.
Materials and methods
Human Brain Tissue Samples
Samples were taken from two patients with ischemic stroke, who were previously described in.3 Briefly, patient I was a 77-year-old female who suffered from an ischemic stroke, with hemorrhagic transformation after thrombolytic treatment. The second patient (patient II) was a 59-year-old male with a history of hypertension and diabetes mellitus type II. He suffered from an acute ischemic stroke due to severe right carotid stenosis (atherothrombotic stroke). The patient did not receive tissue plasminogen activator, and upon autopsy showed no signs of neurodegenerative disease. Samples for immunohistochemistry were immediately fixed with 4% paraformaldehyde and kept at −80°C until use. Samples for western blotting were immediately frozen, then homogenized in cell Lysis buffer and stored at −80°C until use. The study was approved by the Ethics Committee of the Hospital Vall d'Hebron [PR(HG)85/04]. Informed consent was acquired from relatives before the autopsy.
Animals
All experiments with animals were performed in accordance with National Institutes of Health guidelines, the Arrive guidelines, and were approved by the Massachusetts General Hospital Institutional Animal Care and Use of Laboratory Animals Committee. C57Bl6 male mice (25 to 30 g, 12 weeks old) for mouse transient focal ischemia and C57Bl6 female mice (14 days timed pregnant) for mouse primary cortical neurons cultures were purchased from Charles River Laboratories (Wilmington, MA, USA).
Focal Cerebral Ischemia
Male mice (25 to 30 g) were subjected to 45 minutes of transient focal cerebral ischemia and reperfusion. The mice were anesthetized with 1.5% isoflurane in 30% oxygen and 70% nitrous oxide using a face mask. Rectal temperature was controlled at 37°C with a feedback heating pad. A Silicon rubber-coated monofilament (Doccol, Sharon, MA, USA) was introduced into the right common carotid artery. Filament size was 6-0, diameter was 0.09 to 0.11 mm, length was 20 mm. After 45 minutes of middle cerebral artery occlusion, blood flow was restored by withdrawal of the nylon suture. To confirm adequate induction of focal ischemia and successful reperfusion, regional blood flow was measured before, during, and after middle cerebral artery occlusion by Laser-Doppler flowmetry (3 mm lateral to bregma). Their relative cerebral blood flow (% of basal) was reduced to around 20% of the baseline, and restored to near baseline after removal of the filament in all mice we used for this study. Sham-operated mice did not undergo the filament insertion into the middle cerebral artery. The total number of animals used for this study was five mice for sham and nine mice for middle cerebral artery occlusion group.
Primary Cortical Neuron Culture
The protocol followed was adapted from a standard procedure described previously18, 19 with minor modifications. Cortical neurons were prepared from brains of C57Bl6 mouse embryos prepared on embryonic day 15, plated on coated dishes with poly-d-lysine, and cultured in DMEM (Invitrogen, San Diego, CA, USA) containing 10% FBS, penicillin (50 U/mL), and streptomycin (50 μg/mL). The next day, they were either used in the glutamate-induced oxidative stress model, or switched to neurobasal medium and matured in vitro (see below).
Glutamate-Induced Oxidative Stress Model In Vitro
Glutathione depletion was induced in mouse primary cortical neurons or in HT22 cells 1 day after seeding by treatment with 10 mmol/L glutamate, and oxidative glutamate toxicity in cells was measured by lactate dehydrogenase (LDH) release to detect cell death.18, 20
Oxygen-Glucose Deprivation and Reoxygenation
Neurons were cultured in a 12-well plate (Greiner Bio-One, Monroe, NC, USA) in Neurobasal medium with 2% B27 supplement (Invitrogen) until 7 days after seeding. The neurons were then transfected with 10 nmol/L small interfering RNA (siRNA) for 12 hours, followed by 2 hours of oxygen-glucose deprivation (OGD) and 12 hours of reoxygenation (OGD/R). For this purpose, the medium was replaced with DMEM without glucose and other supplements, and the cells were placed in a gas-tight humidified chamber (Heidolph, incubator 1000, Brinkmann Instruments, Westbury, NY, USA) at 37°C, which contained an anaerobic gas mixture (90% N2, 5% H2, and 5% CO2). After 2 hours of OGD, the cell medium was changed back to Neurobasal medium with glucose and 2% B27 and cells were reoxygenated for 12 hours. For each of three experiments, all treatment conditions were performed in duplicate in a single 12-well plate, and cells were imaged with equal settings for fluorescence detection. For measurement of signal intensity in each image, the NIH ImageJ program was used, and fluorescence intensity was normalized to the control value. Separate cohorts of cells were used for measurement of cell death using the LDH assay.
Small Interfering RNA Transfection
The siRNA probes targeted to mouse STAT1 or 6 and mouse 12/15-LOX were purchased from Qiagen (Valencia, CA, USA). The target sequences for the mouse-specific STAT1 siRNA mixture were as follows: ATGCATCTTACTGAAGGTGAA (SI02710729), ATGAGTTGGTTTAATATATAT (SI02735054), CCAATGCTCTATCAAACTATA (SI00183547), and TCCTATTATTATTTAATATAA (SI02668862). The target sequences for the mouse-specific STAT6 siRNA mixture were as follows: CCAGAAGATCTTCAACGACAA (SI00183596), CACAGGAGAGATCATGAACAA (SI026688869), CTCGAATGTGATACAACTGTA (SI00183575), and CTGGAGAAGCCCAGAAACAAA (SI00183589). The target sequences for the mouse-specific 12/15-LOX siRNA mixture were as follows: AAGCTTCTAGTTCCTCACCTA (SI00896644), CTGGCAAGTCATGAATCGGTA (SI04421158), AAGCCTTAATAGAGTCTAATA (SI00896630), and ACCGTTATTAACTTCCCTAAA (SI00896637). Nontargeting, scrambled siRNA (SI03650318) was used as a control in all siRNA transfection experiments. Primary cortical neurons grown on 24-well plates (3 × 105 cells/well) or 6-well plates (2 × 106 cells/plate) previously coated with poly-d-lysine were transfected for 24 hours with 10 nmol/L siRNA per well using HiPerFect Transfection Reagent (Qiagen) according to the manufacturer's guidelines.
Immunofluorescent Staining
The primary cortical neurons subjected to OGD/R were fixed with 4% formaldehyde in PBS and then were incubated with 0.1% Triton X-100 for 15 minutes at room temperature. The cells were washed two times with Tris-buffered saline and blocked with PBS containing 3% bovine serum albumin (BSA) for 1 hour at room temperature, and incubated overnight at 4°C with primary antibodies used at 1:50 dilution in PBS containing 0.1% Tween and 0.3% BSA. Sheep polyclonal antiserum to 15-LOX-1 (kindly provided by Dr J Cornicelli, Pfizer) and rabbit polyclonal STAT6 (sc-621, Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. Colocalization studies were performed with both primary antibodies incubated simultaneously in the same well. The cells were washed with PBS containing 0.1% Tween and incubated at room temperature for 1 hour with the following secondary antibodies (1:100 dilution; Alexa fluor 488 affini pure anti-sheep IgG; Rhodamine (TRITC)-conjugated donkey anti-rabbit IgG, Jackson ImmunoResearch, West Grove, PA, USA) in PBS containing 0.1% Tween and 0.3% BSA. Cells were imaged using a Nikon Eclipse Ti-S fluorescence microscope or a Zeiss LSM5 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Cell Death Assay
Cell viability was quantified by a standard measurement of LDH release using LDH kit (Roche Diagnostics, Indianapolis, IN, USA). The general procedure was followed using the manufacturer's guidelines.
Immunohistochemistry
Human brain tissue samples for immunohistochemistry were taken from the peri-infarct cortex on the ipsilateral side as well as the corresponding contralateral cortex, and immediately fixed with 4% paraformaldehyde and kept at −80°C until use. Frozen sections (20 μm thick) were prepared with 0.1% Triton X-100 for 15 minutes at room temperature and blocked with PBS containing 3% BSA for 1 hour at room temperature, and incubated overnight at 4°C with primary antibodies used at 1:50 dilution in PBS containing 0.1% Tween and 0.3% BSA. 12/15-LOX is a rabbit polyclonal antibody directed against the C terminus of mouse and human 12/15-LOX, and affinity purified against the same peptide used for immunization, characterized in Pekcec et al19 and Yigitkanli et al;3 MDA2 is a mouse monoclonal antibody directed against malondialdehyde-modified lysine residues (a kind gift from Dr J Witztum, University of California, San Diego).21 All other antibodies (mouse monoclonal p-STAT1(Y701), sc-8394; rabbit polyclonal p-STAT6 (Y641), sc-11762-R; goat polyclonal apoptosis-inducing factor (AIF), sc-9416) used were from Santa Cruz Biotechnology. Colocalization studies were performed with both primary antibodies incubated simultaneously in the same section. The sections were washed with PBS containing 0.1% Tween and incubated at room temperature for 1 hour with the following secondary antibodies (1:100 dilution; Fluorescein (FITC)-conjugated donkey anti-mouse IgG; Rhodamine (TRITC)-conjugated donkey anti-rabbit IgG, and Fluorescein (FITC)-conjugated donkey anti-goat IgG, Jackson ImmunoResearch) in PBS containing 0.1% Tween and 0.3% BSA. Brain sections were imaged using a Nikon Eclipse Ti-S fluorescence microscope or a Zeiss LSM5 confocal microscope (Carl Zeiss).
Western Blot Analysis
Whole-cell protein extractions were obtained from the cerebral cortex of ipsilateral hemisphere or from the primary cerebral cortical neurons. Equal amount of protein sample was run on a SDS gel, subsequently transferred onto a nitrocellulose membrane, and immunoblotted. The primary antibodies used were monoclonal or polyclonal antibodies against p-STAT1 (Y701); sc-8394, p STAT6 (Y641); sc-111762-R, STAT1; sc-464, and STAT6; sc-621 (1:1,000; Santa Cruz Biotechnology), β-actin (1:5,000; Sigma-Aldrich, St. Louis, MO, USA), and 12-LOX (1:1,000; ab23678, Abcam, Cambridge, MA, USA). The signal was then detected with horseradish peroxidase-conjugated IgG using an enhanced chemiluminescent substrate, ECL kit (Thermo Scientific, Rockford, IL, USA).
Real Time RT-PCR Analysis
Total RNA was isolated from the ipsilateral hemisphere with the RNeasy Plus Mini Kit (Qiagen). To generate cDNA, M-MLV reverse transcriptase and random primers (Invitrogen) were used. For real-time RT-PCR analysis, Fast SYBR Green master mix (Applied Biosystem, Foster City, CA, USA) was used. The following different set of primer sequences (5'-3') were used: mouse 12/15-LOX; CTT CCT TCT GGA TGG GAT CA and GGT GGG GTA GAC CCA GTT TT; CCT GGT TCT GCA ACT GGA TT and AGT TCC TCC TCC CTG TGG TT; CGA AAT CGC TGG TCT ACA GG and CGT GGT TGA AGA CTC TCA AGG; CAG GGA TCG GAG TAC ACG TT and GAT TGT GCC ATC CTT CCA GT; CAC TGC GCA GCA CTC TTC CAT CC and CAC CAT AAC AGC CTG GCG TCT GC. As a control, mouse GAPDH was used with the following primer sequences (5'-3'): AAG GTC ATC CCA GAG CTG AA and ATG TAG GCC ATG AGG TCC AC. The mixtures were subjected to real-time RT-PCR on an Applied Biosystem 7000 Real-Time PCR System.
Chromatin Immunoprecipitation Assay
Chromatin isolation and chromatin immunoprecipitation (ChIP) assay were performed according to the manufacturer's guidelines using a EZ-ZymeTM Chromatin prep kit and a EZ-ChIPTM kit (Upstate, Temecula, CA, USA). Briefly, mouse primary cerebral cortical neurons (9.5 × 106 cells/dish) were seeded on 10-cm cell culture dish pre-coated with poly-d-lysine. In all, 10 mmol/L of glutamate was treated for 3 hours, and then cells were fixed with 1% formaldehyde. Each soluble chromatin was isolated and digested using EZ-Zyme lysis buffer and EZ-Zyme enzymatic cocktail after fixation. The diluted chromatin solution with ChIP dilution buffer was precleared, and then the precleared chromatin solution was divided and used in immunoprecipitation assays with phospho-STAT1 or 6 antibodies. After multiple washing, the antibody–protein–DNA complex was eluted. After reversal crosslink incubation, protein and RNA were removed by proteinase K and RNase A. Purified DNA was used as a template for PCR with primers specific for several putative STAT-binding sites on the mouse 12/15-LOX promoter. The sequences of the PCR primers used are F1 forward, 5'-GCA CCT ACT CTG GTA CTG-3', R1 reverse, 5'-CTT GCT ACA CAC TCT TGC C-3' F2 forward, 5'-GGC AAG AGT GTG TAG CAA G-3', R2 reverse, 5'-TTA CTC CAG CTC GAA CTG-3' F3 forward, 5'-GGC AGT TCG AGC TGG AGT-3', R3 reverse, 5'-GGG CTC TCC GTG AGT AGT AAG-3' F4 forward, 5'-CTT ACT ACT CAC GGA GAG CCC-3', R4 reverse, 5'-GGC TTT ACT GGA GAG ACA GAC-3' F5 forward, 5'-GTC TGT CTC TCC AGT AAA GCC-3', R5 reverse, 5'-CCC TTG ATT TCT CTC CCT ATG C-3'.
Transient Transfection and Luciferase Activity Assay
HT22 cells were cultured in 24-well plates (2.5 × 104 cells/well) and transfected with 15-LOX-1-luciferase reporter DNA using Lipofectamine (Invitrogen). The luciferase reporter DNA construct containing 15-LOX-1 promoter region was kindly gifted from Dr Liu at Department of medicine, Karolinska University Hospital Solna and Karolinska Institutet, Stockholm, Sweden.22 A 1,081-bp fragment of the 15-LOX-1 promoter region containing several STAT binding sites was ligated into pGL3-basic (Promega, Madison, WI, USA). Two hundred fifty nanograms of pGL3-15-LOX-1 promoter reporter DNA were used per well. After 24 hours of incubation, the cells were treated with 10 nmol/L of siRNA for STAT1 or 6 or non-targeting siRNA per well for 24 hours. Cells were treated with 10 mmol/L of glutamate for 24 hours and subsequently analyzed for luciferase activity. A Luciferase Assay System (Promega) was used to detect luciferase activity from cell lysates, according to the manufacturer's guidelines.
Statistical Analysis
Data are presented as mean±s.e.m. Statistical significance was determined using Student's t-test when comparing two groups, or one-way ANOVA followed by post hoc testing, as indicated. Differences were considered as statistically significant at P<0.05.
Results
Phosphorylated STAT1 and STAT6 Are Recruited onto the Mouse 12/15-Lipoxygenase Promoter in Response to Glutamate-Induced Oxidative Stress
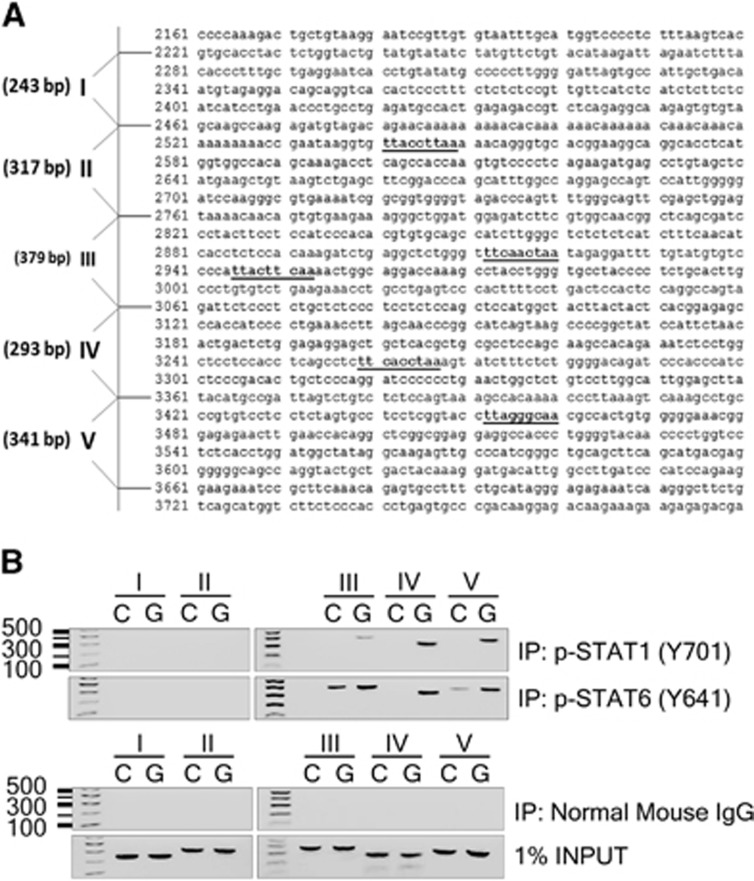
Previous studies have shown a crucial role for 12/15-LOX in cell death caused by glutamate-induced oxidative stress in immature primary neurons.20, 23, 24 In this model, freshly isolated primary neurons, which do not express functional glutamate receptors and are thus not subject to excitotoxicity, are treated with millimolar levels of glutamate. This leads to glutathione depletion,25 followed by lipoxygenase activation and death of the cell.23 To clarify whether STAT1 or STAT6 has a role as transcriptional regulator of 12/15-LOX gene expression, we examined recruitment of STAT1 and STAT6 onto the mouse 12/15-LOX promoter using a ChIP assay in mouse primary cortical neurons. The 12/15-LOX promoter in mouse has not been published to date. We identified a putative promoter region by its homology with the human 12/15-LOX promoter, and found several canonical p-STAT binding motifs (TTCNNNGAA or TTNNNNNAA). The mouse 12/15-LOX promoter was then divided into five consecutive domains (Figure 1A), and probed with the corresponding primer sets to perform the ChIP assay. Binding of p-STAT1 and p-STAT6 was detected using phosphorylation-specific antibodies for STAT6 (Tyrosine-641) and STAT1 (Tyrosine-701), in homogenates from control- or glutamate-treated primary neurons. After 3 hours of glutamate challenge, p-STAT1 and p-STAT6 bound strongly to domains III, IV, and V. The signal in control-treated cells was considerably lower, supporting a role for p-STAT1 and p-STAT6 in regulating 12/15-LOX under glutamate-induced oxidative stress (Figure 1B). Binding to domains I and II was not detected, despite the presence of a putative STAT binding site in the second domain. Incubation with non-specific mouse IgG abolished the signal, while PCR analysis with 1% of input DNA confirmed equal loading in control and glutamate-treated samples.
Figure 1.
STAT1 and STAT6 as transcriptional regulators of 12/15-lipoxygenase (12/15-LOX) under oxidative stress. (A) The putative mouse 12/15-LOX promoter region features several canonical STAT binding sites. (B) Chromatin immunoprecipitation (ChIP) analysis in five consecutive domains of the mouse 12/15-LOX promoter showed enhanced binding of p-STAT1 and p-STAT6 to domains III, IV, and V in glutamate-stressed mouse primary cortical neurons (G), compared with control conditions (C). Bottom panels, negative control with mouse IgG, and positive loading controls. IP, immunoprecipitation; STAT, Signal Transducers and Activators of Transcription.
STAT1 and STAT6 Activate the 12/15-Lipoxygenase Promoter and Contribute to Cell Death in Murine HT22 Neuronal Cells
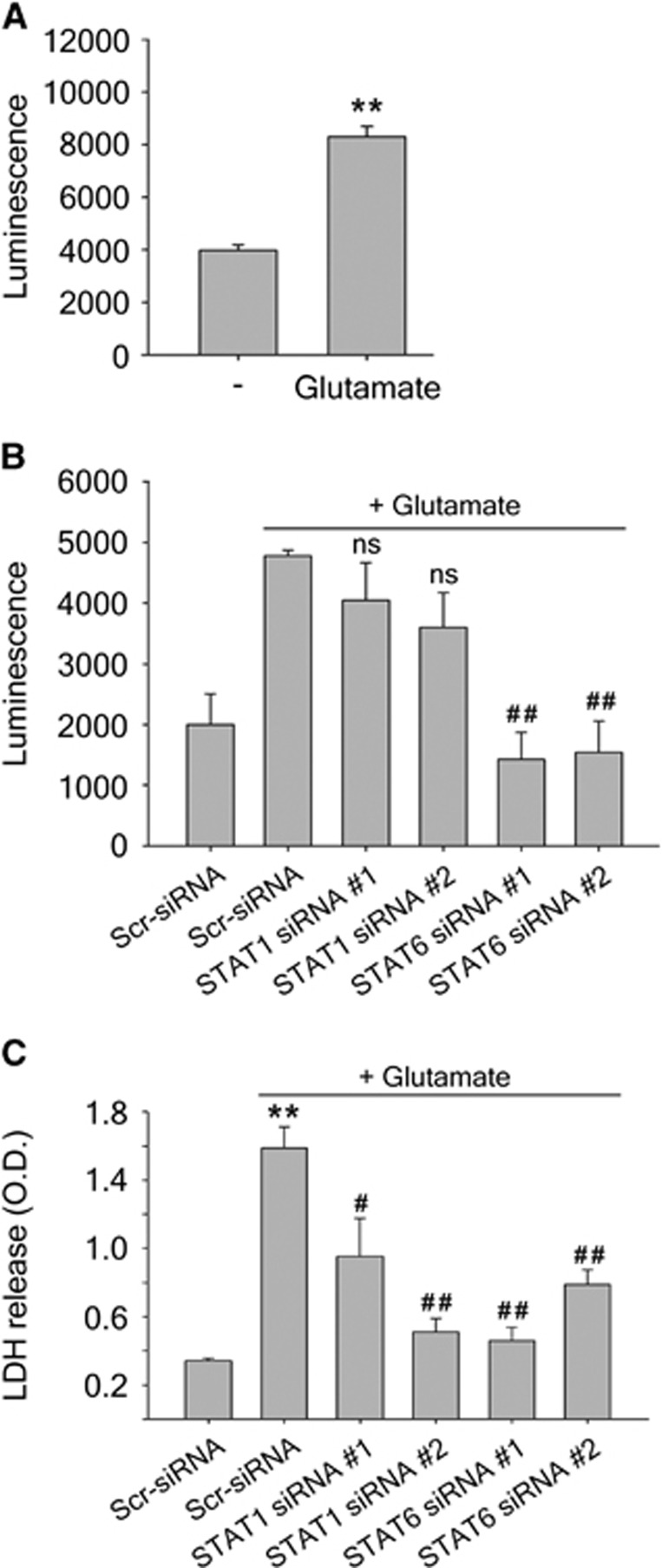
To confirm the upregulation of 12/15-LOX under glutamate oxidative stress, we performed a luciferase assay in the mouse hippocampal cell line HT22, which is also known to undergo 12/15-LOX dependent oxidative glutamate toxicity.23, 26, 27 Twenty-four hours after transfection with the pGL3-15-LOX-1 luciferase DNA construct containing the human 12/15-LOX promoter (a kind gift from Dr C Liu, Karolinska Institute22), glutamate treatment for 24 hours led to an increase in luciferase luminescence, suggesting promoter activation (Figure 2A). We then used siRNAs directed toward STAT1 and STAT6 to investigate STAT involvement in 12/15-LOX upregulation. Transfection of HT22 cells for 24 hours with two different STAT1 siRNAs attenuated the increase of luciferase signal seen after glutamate treatment, although the reduction was not statistically significant (Figure 2B). STAT6 siRNA was more effective, leading to a significant decrease in luciferase signal. Finally, we determined the effect of the STAT-specific siRNAs on the survival of HT22 cells treated with glutamate by measuring cytosolic LDH released into the medium. Glutamate treatment led to massive release of LDH, signifying cell death, and this was reduced by siRNAs to either STAT1 or STAT6 (Figure 2C). Taken together these results show that targeting both STAT1 and STAT6 may be neuroprotective, although knockdown of STAT6 appears to overall have the greater effect.
Figure 2.
(A and B) Transcriptional activation of 12/15-lipoxygenase (12/15-LOX) after glutamate treatment determined by luciferase assay in transfected HT22 cells. (A) Glutamate treatment enhances luciferase activity, indicating 12/15-LOX promoter activation; **P<0.01 versus control (n=3 in control, n=5 in glutamate). (B) Transcriptional activation of 12/15-LOX is blocked by knockdown of STAT6, and marginally reduced by STAT1 small interfering RNA (siRNA). ns, non-significant; ##P<0.01 versus scrambled siRNA with glutamate (n=4 per group, n=2 in scrambled siRNA with glutamate). (C) Knockdown of STAT1 or STAT6 reduces neuronal cell death under glutamate-induced oxidative stress. Transfection with siRNAs targeting either STAT1 or STAT6 24 hours before treatment with 10 mmol/L glutamate protected neuronal HT22 cells, as indicated by reduced release of lactate dehydrogenase (LDH) from the cells (n=4 per group). **P<0.01 versus control cells transfected with non-targeting scrambled siRNA; #P<0.05; ##P<0.01 versus glutamate-treated cells transfected with non-targeting scrambled siRNA. Data are mean±s.e.m. One-way ANOVA with Bonferroni's multiple comparison test (A and C) or with Dunnett's multiple comparison test (B) was used to compare groups. STAT, Signal Transducers and Activators of Transcription.
STAT1 and STAT6 Activation Contribute to Increased Levels of 12/15-Lipoxygenase Protein in Oxidatively Stressed Primary Neurons
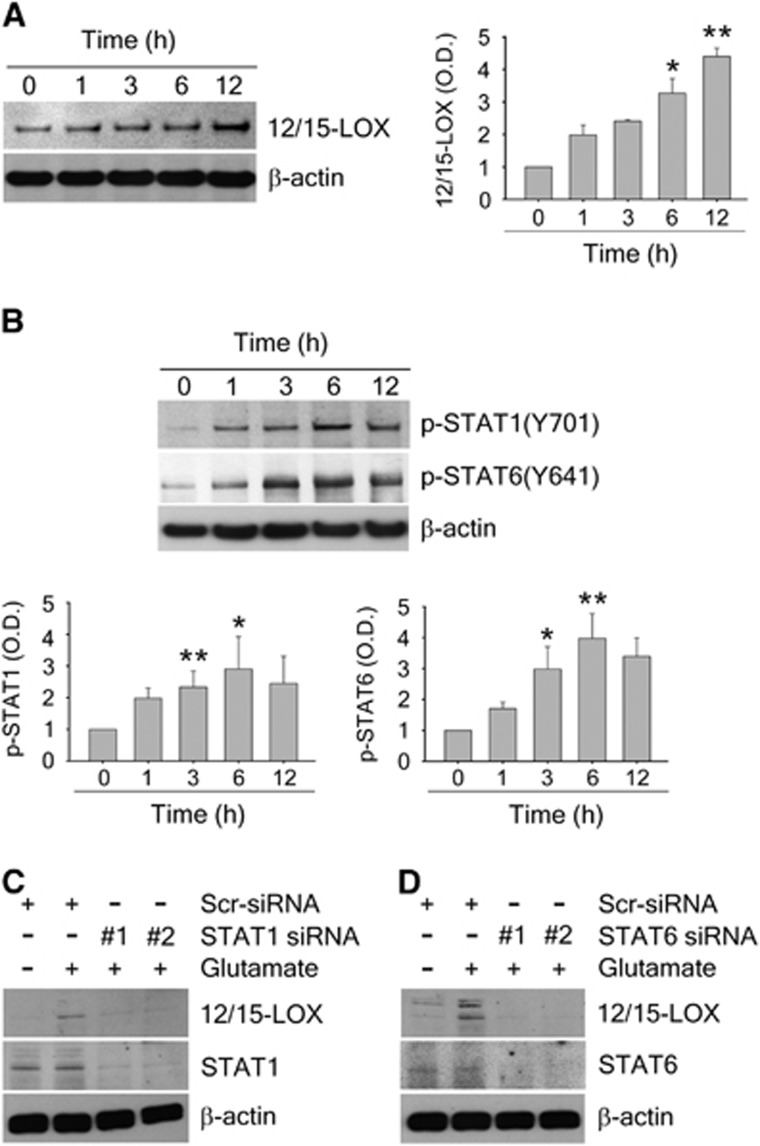
To investigate the effect of STAT activation on 12/15-LOX expression on the protein level, we compared changes in expression over time after glutamate challenge by western blotting. We observed a gradual increase over time for 12/15-LOX (Figure 3A), which was preceded by increases in phosphorylation of STAT1 and STAT6 (Figure 3B), consistent with a role for these STATs in upregulating 12/15-LOX. To more directly gauge these effects, we used STAT1- and STAT6-specific siRNAs. These largely prevented the glutamate-induced increase of 12/15-LOX (Figures 3C and 3D).
Figure 3.
12/15-lipoxygenase (12/15-LOX) upregulation via STAT1 and STAT6 in cortical neurons treated with glutamate. (A) Time-dependent increase of 12/15-LOX protein in primary cortical neurons after treatment with 10 mmol/L glutamate for the time indicated. *P<0.05 (6 hours); **P<0.01 (12 hours) versus control (0 hour) (n=2 per group). (B) Early phosphorylation of STAT1 and STAT6 at 3 hours after glutamate addition. *P<0.05; **P<0.01 versus control (n=3 per group). Data are mean±s.e.m. One-way ANOVA with Bonferroni's multiple comparison test (A) or with Dunnett's multiple comparison test (B) was used to compare groups. (C and D) STAT knockdown reduces protein levels of 12/15-LOX in glutamate-stressed primary neurons. STAT, Signal Transducers and Activators of Transcription.
STAT6 Regulates Increased 12/15-Lipoxygenase After Oxygen-Glucose Deprivation and Reoxygenation in Primary Neurons
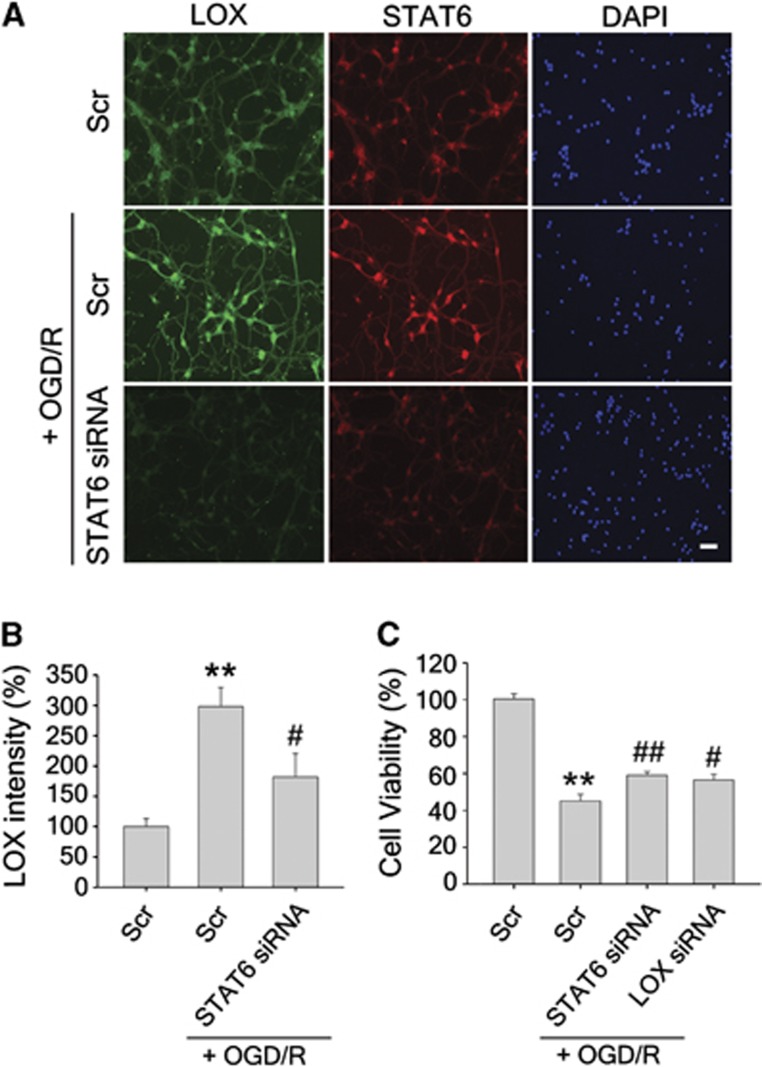
A frequently used model of in vitro ischemia is OGD, followed by reoxygenation. To determine whether STAT-dependent regulation of 12/15-LOX occurs during OGD/R, we subjected mouse primary neurons to 2 hours of OGD and 12 hours of reoxygenation. While immunofluorescence showed only little 12/15-LOX staining in normoxic neurons transfected with a scrambled control siRNA, levels of 12/15-LOX were higher after OGD/R (Figure 4, left top and middle panels). In cells pretreated with STAT6-specific siRNA, the signal for STAT6 was clearly diminished, indicating efficient knockdown. These cells also showed reduced 12/15-LOX staining, confirming that increased 12/15-LOX depends on changes in STAT6. In these experiments, STAT1 knockdown was less effective, showing a clear reduction of 12/15-LOX in only one of three experiments (results not shown). Quantitative evaluation of the immunofluorescent images from three independent experiments confirmed both the increase of 12/15-LOX after OGD/R and the significant reduction in 12/15-LOX signal when STAT6 is knocked down (Figure 4B). To determine whether STAT6 knockdown affects viability after OGD/R in these cells, we treated a separate cohort of cells, and measured survival as LDH content in cell lysates, normalized to control-treated cells (Figure 4C).
Figure 4.
Increased 12/15-lipoxygenase (12/15-LOX) in primary cortical neurons subjected to oxygen-glucose deprivation and reoxygenation (OGD/R) is attenuated by knockdown of STAT6. (A) Immunofluorescent staining shows higher levels of 12/15-LOX in neurons after 2 hours of OGD and 12 hours of reoxygenation (Scr+OGD/R, middle panel), compared with control-treated cells (Scr, top panel). Transfection with STAT6 siRNA prevented this increase (STAT6 siRNA+OGD/R, bottom panel). Scale bar=50 μm. (B) Summary graph depicting the 12/15-LOX fluorescent signal intensity level, which was significantly increased after 2 hours of OGD and 12 hours of reoxygenation, compared with control. **P<0.01 versus Scr (n=10 in Scr, n=13 in Scr+OGD/R). This 12/15-LOX signal intensity was significantly decreased by knockdown of STAT6. #P<0.05 versus Scr+OR (n=12 in STAT6 siRNA+OGD/R). Data are mean±s.e.m. One-way ANOVA with Bonferroni's multiple comparison test. (C) Knockdown of STAT6 or LOX reduces neuronal cell death after OGD/R. Transfection with siRNAs targeting either STAT6 or 12/15-LOX before treatment with 2 hours of OGD and 12 hours of reoxygenation significantly protected neurons, as indicated by increased cell viability (n=5 per group). **P<0.01 versus Scr; ##P<0.01 versus Scr+OGD/R in STAT6 siRNA+OGD/R; #P<0.05 versus Scr+OGD/R in LOX siRNA+OGD/R. Data are mean±s.e.m. One-way ANOVA with Fisher's multiple comparison test. STAT, Signal Transducer and Activators of Transcription.
Increase of 12/15-Lipoxygenase mRNA After Ischemia/Reperfusion in Mice Is Preceded by STAT Activation
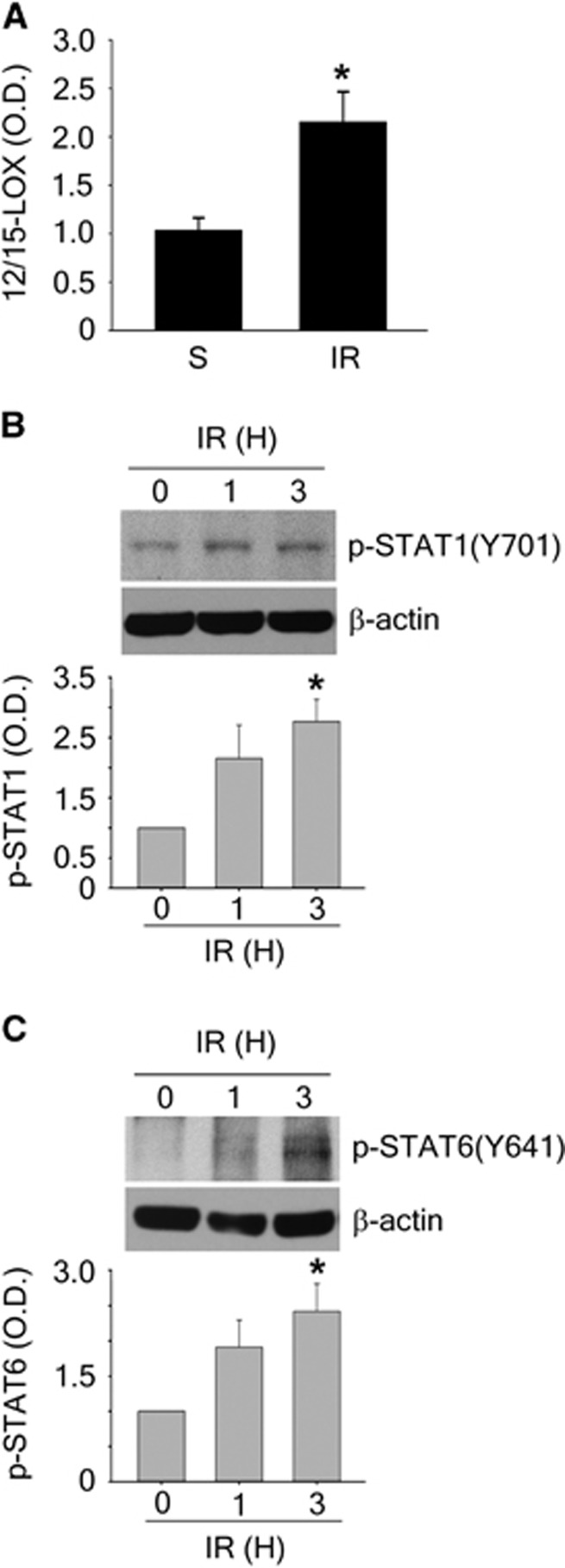
In a mouse model of experimental stroke, we previously detected a time-dependent increase of 12/15-LOX in the peri-infarct cortex over 24 hours.4 Expression of this enzyme is both transcriptionally and translationally regulated,22, 28, 29, 30, 31 so we decided to probe the mRNA levels of 12/15-LOX in ischemic mouse tissue. After transient focal ischemia with 45 minutes occlusion and 24 hours of reperfusion (Figure 5A), 12/15-LOX mRNA was significantly increased in the ischemic hemisphere as judged by real-time RT-PCR, indicating a transcriptional upregulation of 12/15-LOX in the ischemic mouse brain. Western blotting confirmed an early upregulation of p-STAT1 and p-STAT6, already at 1 and 3 hours of reperfusion after 45 minutes ischemia (Figures 5B and 5C). These findings are consistent with a role for STAT activation in regulating 12/15-LOX.
Figure 5.
12/15-lipoxygenase (12/15-LOX) mRNA is increased after ischemia-reperfusion, which is accompanied by early phosphorylation of STAT1 and STAT6. (A) In a mouse transient focal ischemia model, 12/15-LOX mRNA is increased compared with sham (S), after 45 minutes of ischemia and 24 hours of reperfusion (IR). *P<0.05 (n=6 in S, n=9 in IR). (B) STAT1 is phosphorylated at early time points after 45 minutes ischemia with 1 and 3 hours of reperfusion in the mouse cortex. *P<0.05 versus sham (n=3 per group). (C) Similarly, p-STAT6 is elevated at early time points. *P<0.05 versus sham (n=3 per group). Data are mean±s.e.m. Student's t-test (A) or one-way ANOVA with Bonferroni's multiple comparison test (B and C) was used to compare groups. STAT, Signal Transducers and Activators of Transcription.
Increased Levels of Activated p-STAT1 and p-STAT6 in Human Stroke
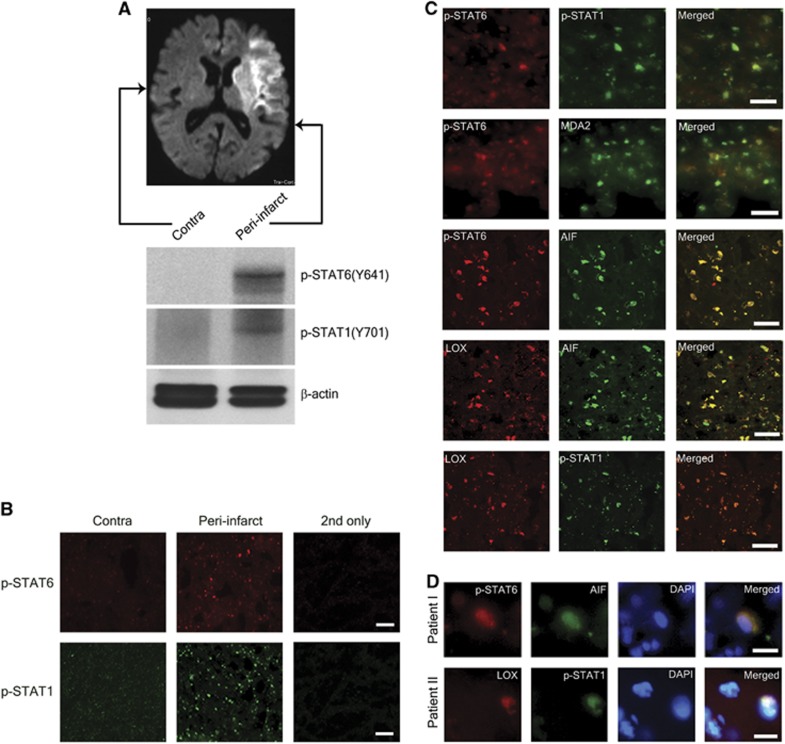
To determine the relevance of our findings for stroke in humans, we queried brain sections taken from stroke patients. As a measure of STAT activity in the brain after ischemia, we used antibodies to detect p-STAT1 and p-STAT6 in brain sections of a 77-year-old female patient with ischemic stroke.3 Western blotting showed increased levels of p-STAT1 and p-STAT6, respectively, in the sample from the peri-infarct region compared with contralateral cortex (Figure 6A). These findings, which reflect increased activity of both of these transcriptional activators, were confirmed by immunohistochemistry (Figure 6B). From previous studies in mice and humans, we know that 12/15-LOX is increased in the peri-infarct region as well, coincident with the AIF.3, 4, 5, 6 To determine a possible link to the disease state of the corresponding cells, we performed colocalization studies with sections taken from the peri-infarct cortex. Double labeling with the corresponding antibodies showed that p-STAT6 and p-STAT1 are increased in the same cells; p-STAT6 colocalizes with the oxidative stress marker MDA2, and with AIF; the same cells that show increased AIF also show high levels of 12/15-LOX; and the 12/15-LOX-positive cells are also positive for p-STAT1 (Figure 6C, top to bottom). Finally, both p-STAT6 and AIF showed partial nuclear colocalization, as shown by overlay with the DNA dye DAPI (Figure 6D, top panel). This is consistent with the transcriptional activity of STAT6, as well as the pro-apoptotic nuclear effects of AIF.32 To confirm the validity of these findings, we also investigated the brain of a second patient, a 59-year-old male. Again, we found a colocalization of 12/15-LOX with p-STAT1, also with partial overlap with the nuclear DAPI dye (Figure 6D, bottom panel). Taken together, these findings document increased levels of p-STAT6 and p-STAT1 in the ischemic peri-infarct area, which coincide with increased 12/15-LOX and the marker for non caspase-related apoptosis, AIF.
Figure 6.
STAT6 and STAT1 phosphorylation is increased in the peri-infarct cortex of a human stroke patient, and colocalizes with 12/15-lipoxygenase (12/15-LOX). (A) Western blotting of samples taken from the areas indicated in the magnetic resonance (MR) image showed that both p-STAT6 and p-STAT1 are increased in the peri-infarct cortex. (B) Immunohistochemistry similarly showed higher levels of p-STAT6 and p-STAT1 in the peri-infarct cortex. 2nd only, omitting the primary antibody abolished the signal. Scale bar=100 μm. (C) Colocalization studies indicated increased p-STAT6 and p-STAT1 in the same cells in the peri-infarct cortex that also show elevated 12/15-LOX, and the injury markers MDA2 (for oxidative stress) and AIF (for apoptosis-inducing factor). Scale bar=50 μm. (D) Overlay of the p-STAT6 signal with AIF and DAPI staining confirmed a partial nuclear localization of p-STAT6 (top panel) in the peri-infarct cortex. In a second patient, STAT1 also colocalized with 12/15-LOX, with partial nuclear localization (bottom panel). STAT, Signal Transducers and Activators of Transcription.
Discussion
Oxidative stress is a major injury mechanism in ischemic strokes, and 12/15-LOX is one of its central mediators. This study for the first time shows how 12/15-LOX is upregulated via activation of STAT6 and STAT1 in cultured neurons, and in vivo after stroke.
12/15-LOX is an enzyme with great destructive potential due to its ability to directly oxidize phospholipids and damage intracellular organelles.33, 34, 35 Consequently, it is subject to several layers of regulation including transcriptional, translational, and post-translational control mechanisms that keep the enzyme activity in check.36 After a catastrophic event such as an ischemic stroke, this regulatory network breaks down, leading to increased 12/15-LOX protein levels. The catabolic activity of 12/15-LOX is further enhanced by increases in intracellular calcium, while the major antioxidant glutathione decreases, both of which are hallmarks of ischemic injury. The 12/15-LOX protein is found mostly in neurons and endothelial cells of the peri-infarct cortex after transient focal ischemia in mice, as well as ischemic stroke in humans. Our group and others have previously shown that blocking 12/15-LOX activity is powerfully neuroprotective, qualifying 12/15-LOX as a novel drug target for stroke. An alternative to block the enzymatic activity of 12/15-LOX might be to prevent its upregulation in the first place, which could potentially be similarly neuroprotective. Identifying the mode of regulation that contributes to increased levels of 12/15-LOX in the ischemic brain appeared to be a promising first step in this direction. Transcriptional regulation of 12/15-LOX in neuronal cells and the ischemic brain has to our knowledge not been investigated to date. Our study focused on the jak/stat pathway, because STATs are involved in a variety of redox regulatory steps. STAT1 has previously been implicated in causing brain injury in a mouse model of cerebral ischemia.14 Early phosphorylation of STAT1 at Tyr701 was found 30 minutes after 2 hours transient focal ischemia in mice. STAT1 knockout mice featured reduced infarct sizes, along with reduced activation of caspase-3 and increased levels of protective phosphorylated AKT.14 In proteomics studies, STAT6 phosphorylation was also reported to be increased in mice after experimental stroke,15, 16 but with unknown consequences. In rats on the protein level, phosphorylated STAT6 was found to be increased in the penumbra compared with the infarct core, consistent with our current findings, but less so than on the contralateral side of the brain.17 STAT6 regulates 12/15-LOX in airway epithelial cells and macrophages in response to IL-4 and IL-13,22, 29, 30, 31, 37 but neural cells had not been investigated in this context. These findings suggested that STAT1 and STAT6 might be promising candidates as transcriptional regulators of 12/15-LOX.
In the brain of a human stroke patient where we had previously found increased 12/15-LOX in the peri-infarct region, we now found that both p-STAT6 and p-STAT1 were increased as well (Figures 6A and 6B), and immunohistochemistry showed 12/15-LOX colocalized with p-STAT1 in what appeared to be mostly neurons (Figure 6C). We could not test the colocalization of 12/15-LOX with p-STAT6 in this case, because both antibodies were of rabbit origin. Due to the extensive overlap of 12/15-LOX with the same markers that p-STAT6 colocalized with, we nonetheless assume a mostly overlapping expression pattern. Moreover, p-STAT6 colocalized with the injury markers MDA2 and AIF, indicating that these are damaged cells. These increases were similarly found in mice after transient focal ischemia, where both mRNA levels of 12/15-LOX (Figure 5A), and phosphorylation of STAT6 and STAT1 were increased (Figures 5B and 5C). A direct binding of p-STAT1 and p-STAT6 to distinct regions of the 12/15-LOX promoter was then shown by ChIP analysis in primary cortical neurons subjected to oxidative stress (Figure 1). This was accompanied by an increase in 12/15-LOX promoter activity, which was somewhat reduced by cotransfection with STAT1-specific siRNA, and abolished by STAT6 siRNA (Figure 2B). Overall, the p-STAT6 appears to be a much stronger regulator of 12/15-LOX activation than STAT1. Consistent with a role for these STATs as transcriptional regulators of 12/15-LOX, increased phosphorylation of STAT6 and STAT1 temporally preceded increased 12/15-LOX protein in the oxidative glutamate toxicity model (Figures 3A and 3B), and STAT1 as well as STAT6 siRNAs prevented the increased protein levels of 12/15-LOX (Figures 3C and 3D). This was confirmed for STAT6 in the commonly used model of OGD/R, where the increased immunofluorescence signal after OGD/R was attenuated by prior transfection with STAT6 siRNA, whereas the effects of STAT1 siRNA were less consistent in this model. Significant protection against cell death was also provided in the OGD/R model by STAT6 knockdown, comparable to knockdown of 12/15-LOX itself (Figure 4C). Both of these knockdowns did not provide complete protection however, suggesting other triggers of cell death besides 12/15-LOX operate in the OGD/R model. Finally, cell death in the oxidative glutamate toxicity model of oxidative stress was reduced by siRNAs directed against STAT1 or STAT6 (Figure 2C). Taken together, these results clearly show the transcriptional regulation of 12/15-LOX via STAT6. How important the role of STAT1 is in regulating 12/15-LOX remains to be established in further studies.
While we have thus shown conclusively that 12/15-LOX is regulated by these STATs, suggesting that they may be viable targets for neuroprotection, several caveats need to be considered. (1) While we have focused here on transcriptional regulation via STAT phosphorylation, transcriptional silencers such as GATA-6 and hypermethylation of CpG islands in the 12/15-LOX promoter may also have a role in determining the amount of 12/15-LOX protein. In addition, translational control via RNA-binding proteins including hnRNP K and hnRNP E1 is also known for 12/15-LOX,28 and should be investigated. The increased 12/15-LOX mRNA levels point to a dominant effect of transcriptional events, however. (2) Both STAT1 and STAT6 will likely regulate additional genes in the ischemic brain, including the NADPH oxidases NOX1 and NOX4,38 and the cyclooxygenase COX-2.39 Furthermore, AKT phosphorylation was increased, and caspase-3 cleavage decreased in STAT1 knockouts subjected to focal ischemia,14 and the impact of 12/15-LOX on these events should be explored. However, this broad spectrum of STAT-dependent regulatory activity may also facilitate reining in several damage pathways that can otherwise contribute to ischemic oxidative stress, apoptosis, and inflammatory reactions after a stroke. (3) Because both STAT6 and STAT1 can contribute to upregulation of 12/15-LOX, both of these STATs may need to be targeted, although in our experiments STAT6 appeared to be the more crucial target. Unfortunately, very few STAT-specific inhibitors are available at present.40 (4) As always when considering acute stroke therapy, timing is a major issue. Our findings concerning early activation of STAT6 and STAT1, which confirm earlier reports, suggest that the time window after onset of stroke may be fairly limited. Nonetheless, our study highlights the need for development of more STAT-selective inhibitors, which can be used to investigate specific effects of each STAT and may lead to new approaches to stroke therapy.
Acknowledgments
The authors thank Drs C Liu, D Xu, and J Sjöberg of the Karolinska Institute, Sweden, for the human 15-LOX promoter construct. The authors thank Dr JL Witztum for a kind gift of the MDA2 antibody, and Dr J Cornicelli for the sheep antiserum to 15-LOX. Support from the National Institutes of Health (R01 NS069939 and R01 NS049430 to KvL) is gratefully acknowledged.
Author contributions
JEJ and KvL planned and directed the experiments. JEJ performed the in vitro and cell culture experiments. HK and YL performed the focal ischemia experiments. AY helped with the gene expression experiments. JM supplied the human brain sections and helped with interpretation of the results. JEJ, EHL, and KvL evaluated the results and, with input from all authors, wrote the article.
The authors declare no conflict of interest.
References
- 1Chan PH. Role of oxidants in ischemic brain damage. Stroke 1996; 27: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 2Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol 2010; 41: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol 2013; 73: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X et al. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab 2010; 30: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke 2008; 39: 2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 2006; 37: 3014–3018. [DOI] [PubMed] [Google Scholar]
- 7van Leyen K. Lipoxygenase: an emerging target for stroke therapy. CNS Neurol Disord Drug Targets 2013; 12: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C et al. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 2005; 36: 2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A et al. The role of JAK-STAT signaling within the CNS. JAKSTAt 2013; 2: e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Darnell JE, Jr. STATs and gene regulation. Science 1997; 277: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 11Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT et al. Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci USA 2011; 108: 7985–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G et al. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 2011; 108: 18067–18072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci 2009; 29: 7003–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Takagi Y, Harada J, Chiarugi A, Moskowitz MA. STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab 2002; 22: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 15Erdo F, Trapp T, Mies G, Hossmann KA. Immunohistochemical analysis of protein expression after middle cerebral artery occlusion in mice. Acta Neuropathol 2004; 107: 127–136. [DOI] [PubMed] [Google Scholar]
- 16Focking M, Besselmann M, Trapp T. Proteomics of experimental stroke in mice. Acta Neurobiol Exp 2006; 66: 273–278. [DOI] [PubMed] [Google Scholar]
- 17Jang SS, Choi JH, Im DS, Park S, Park JS, Park SM et al. The phosphorylation of STAT6 during ischemic reperfusion in rat cerebral cortex. Neuroreport 2014; 25: 18–22. [DOI] [PubMed] [Google Scholar]
- 18Ratan RR, Ryu H, Lee J, Mwidau A, Neve RL. In vitro model of oxidative stress in cortical neurons. Methods Enzymol 2002; 352: 183–190. [DOI] [PubMed] [Google Scholar]
- 19Pekcec A, Yigitkanli K, Jung JE, Pallast S, Xing C, Antipenko A et al. Following experimental stroke, the recovering brain is vulnerable to lipoxygenase-dependent semaphorin signaling. FASEB J 2013; 27: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA et al. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res 2008; 86: 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis 1990; 10: 325–335. [DOI] [PubMed] [Google Scholar]
- 22Liu C, Schain F, Han H, Xu D, Andersson-Sand H, Forsell P et al. Epigenetic and transcriptional control of the 15-lipoxygenase-1 gene in a Hodgkin lymphoma cell line. Exp Cell Res 2012; 318: 169–176. [DOI] [PubMed] [Google Scholar]
- 23Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 1997; 19: 453–463. [DOI] [PubMed] [Google Scholar]
- 24Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR et al. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem 2003; 278: 43508–43515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989; 2: 1547–1558. [DOI] [PubMed] [Google Scholar]
- 26Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem 2009; 111: 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ 2011; 18: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Ostareck DH, Ostareck-Lederer A, Wilm M, Thiele BJ, Mann M, Hentze MW. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3' end. Cell 1997; 89: 597–606. [DOI] [PubMed] [Google Scholar]
- 29Heydeck D, Thomas L, Schnurr K, Trebus F, Thierfelder WE, Ihle JN et al. Interleukin-4 and -13 induce upregulation of the murine macrophage 12/15-lipoxygenase activity: evidence for the involvement of transcription factor STAT6. Blood 1998; 92: 2503–2510. [PubMed] [Google Scholar]
- 30Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 1999; 400: 378–382. [DOI] [PubMed] [Google Scholar]
- 31Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci USA 1992; 89: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab 2004; 24: 681–692. [DOI] [PubMed] [Google Scholar]
- 33van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature 1998; 395: 392–395. [DOI] [PubMed] [Google Scholar]
- 34Kuhn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem 1990; 265: 18351–18361. [PubMed] [Google Scholar]
- 35Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 2014; 1851: 308–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Kühn H, Heydeck D, Brinckman R, Trebus F. Regulation of cellular 15-lipoxygenase activity on pretranslational, translational, and posttranslational levels. Lipids 1999; 34: S273–S279. [DOI] [PubMed] [Google Scholar]
- 37Conrad DJ, Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am J Respir Cell Mol Biol 2000; 22: 226–234. [DOI] [PubMed] [Google Scholar]
- 38Manea A, Tanase LI, Raicu M, Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 2010; 30: 105–112. [DOI] [PubMed] [Google Scholar]
- 39Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation 2005; 112: 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov 2013; 12: 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]