Abstract
This article considers the use and modality of iron therapy to treat iron deficiency in patients with heart failure, an aspect of care which has received relatively little attention compared with the wider topic of anaemia management. Iron deficiency affects up to 50% of heart failure patients, and is associated with poor quality of life, impaired exercise tolerance, and mortality independent of haematopoietic effects in this patient population. The European Society of Cardiology Guidelines for heart failure 2012 recommend a diagnostic work-up for iron deficiency in patients with suspected heart failure. Iron absorption from oral iron preparations is generally poor, with slow and often inefficient iron repletion; moreover, up to 60% of patients experience gastrointestinal side effects. These problems may be exacerbated in heart failure due to decreased gastrointestinal absorption and poor compliance due to pill burden. Evidence for clinical benefits using oral iron is lacking. I.v. iron sucrose has consistently been shown to improve exercise capacity, cardiac function, symptom severity, and quality of life. Similar findings were observed recently for i.v. ferric carboxymaltose in patients with systolic heart failure and impaired LVEF in the double-blind, placebo-controlled FAIR-HF and CONFIRM-HF trials. I.v. iron therapy may be better tolerated than oral iron, although confirmation in longer clinical trials is awaited. Routine diagnosis and management of iron deficiency in patients with symptomatic heart failure regardless of anaemia status is advisable, and, based on current evidence, prompt intervention using i.v. iron therapy should now be considered.
Keywords: Iron deficiency, Iron, Heart failure, Anaemia, Intravenous, Oral, Quality of life
Iron deficiency: a common co-morbidity in heart failure
Iron deficiency is one of the most prevalent co-morbid conditions in chronic heart failure. In the absence of any iron treatment, it is estimated that up to 50% of patients with heart failure have low levels of available iron.1 The prevalence of iron deficiency is higher in the more advanced stages of heart failure (NYHA class III and IV), in females, and in patients with high levels of inflammatory markers (such as C-reactive protein) as well as increased levels of NT-proBNP,2,3 but, even in patients at lower risk such as those with NYHA class I or II, the prevalence remains >30%.1,2
These estimates include both absolute iron deficiency (in which iron stores are low) and functional iron deficiency (in which iron supply is inadequate to meet the demand for erythropoiesis and other cellular functions despite normal or abundant body iron stores). The two states have different aetiologies. Various pathological changes in chronic heart failure predispose patients to absolute iron deficiency, which is usually defined as ferritin <100 ng/mL.4–6 Poor nutrition is frequent, and patients with concomitant renal disease may be advised to follow a low-protein (and thus low-iron) diet.7 Low intake of iron can be compounded by physiological and functional changes to the intestinal wall which may impair iron uptake from the gut, including the presence of mucosal oedema, and reduced gastrointestinal blood flow8 (Table 1). Patients with heart failure are also at risk of iron loss secondary to gastritis or ulceration caused by concomitant pharmacotherapy, and from proteinuria arising from chronic renal disease.9,10 Lastly, administration of antiplatelet drugs or anticoagulation agents increases susceptibility to bleeding, with consequent blood loss.11 Functional iron deficiency, in contrast, arises from the generalized inflammatory state which characterizes heart failure. Increased levels of proinflammatory cytokines such as interleukin-6 (IL-6) enhance production and release of hepcidin, an acute phase protein synthesized in the liver. Hepcidin regulates the release of stored iron from enterocytes and hepatocytes by causing degradation of the iron transporter protein, ferroportin. As a result, high levels of hepcidin can ‘trap’ iron in storage cells.6,12,13 In a recent study published by Jankowska et al., the early stages of systolic heart failure were characterized by high levels of circulating hepcidin.14 However, hepcidin levels declined as the severity of heart failure progressed, and as iron deficiency developed. Moreover, low hepcidin was independently associated with unfavourable outcome.14 The authors hypothesized that the pathophysiological mechanisms underlying iron deficiency in heart failure are markedly different from those in chronic inflammatory diseases, a possibility that requires further evidence to be confirmed. Functional iron deficiency is usually defined in heart failure as ferritin <300 ng/mL with transferrin saturation (TSAT) <20%.
Table 1.
| Cause | Mechanism | Comment |
|---|---|---|
| Reduced iron intake | Low protein diet Anorexia | Low protein diets may be recommended for concomitant renal disease |
| Impaired intestinal absorption | Mucosal oedema | Can alter intestinal epithelial permeability |
| Decreased gastric emptying Modified intestinal motility | Contributing factors include overactivation of the sympathetic nervous system, concomitant drugs, co-morbid diabetic gastroparesis | |
| Reduced intestinal villus blood flow and/or mesenteric and portal blood flow | Restricts passive diffusion from intestinal tissue to blood | |
| Disrupted iron uptake process | Impaired expression of iron transporters observed in the duodenum | |
| Gastrointestinal tract damage | Gastritis | Iron loss through gastrointestinal bleeding |
| Ulcers | ||
| Uraemia | Loss of iron in protein | Proteinuria associated with concomitant chronic renal failure |
| Medication | Antiplatelet drugs, e.g. aspirin | Can contribute to gastrointestinal blood loss |
| Anticoagulants | ||
| Erythropoiesis-stimulating agents | Provoke iron deficiency through enhanced demand for erythropoiesis | |
| Venepuncture | Frequent venepuncture for blood tests | |
| Chronic inflammation | Impaired release of iron from storage cells (functional iron deficiency) | Inflammatory cytokines stimulate increased hepcidin production and release, which inhibits transport of iron out of macrophages and hepatocytes by blocking export via ferroportin |
While iron deficiency is usually identified in the clinic based on levels of ferritin and TSAT, due to their ease of use and wide availability, it should be noted that either inflammation or oxidative stress may artificially increase ferritin concentrations, regardless of actual iron status. In contrast to other clinical settings, the ferritin threshold defining deficient iron storage is increased to 100 ng/mL in heart failure patients to take account of the chronic inflammatory state. The recent FAIR-HF15 and CONFIRM-HF16 trials achieved beneficial results when ferritin and TSAT were used to define inclusion criteria and thresholds for maintenance treatment, and the European Society of Cardiology (ESC) Guidelines for heart failure 2012 included cut-off values for iron deficiency based on the FAIR-HF study.17 Nevertheless, diagnosis based on ferritin and TSAT may be inadequate in advanced disease18 or acute heart failure,4,19 when ferritin becomes an unreliable marker. Iron deficiency can therefore be underdiagnosed in these types of patients. Measurement of soluble transferrin receptor, hepcidin, or reticulocyte haemoglobin (Hb) content may offer a more sensitive assessment of iron status and response to therapy,4 and these techniques may become more widely adopted in the future.
Iron deficiency is closely associated with low Hb levels in patients with heart failure.3,20 A large population-based study in Canada has suggested that anaemia of chronic disease predominates, followed by iron deficiency anaemia,21 but the two types both primarily arise from insufficient iron available for haematopoiesis in the erythrocytes.
The impact of iron deficiency in heart failure: a co-morbidity independent of anaemia
Conventionally, attention has focused on the effect of inadequate iron supply on erythropoiesis, and the resulting risk of anaemia. Awareness is growing, however, of the many haematopoietic functions of iron. Iron is an essential cofactor for haem and non-haem proteins involved in cellular activities such as oxygen storage (as a component of myoglobin), generation of cellular energy (as part of oxidative enzymes and mitochondrial respiratory chain proteins) in skeletal muscle and myocardiocytes, and the synthesis and degradation of lipids, carbohydrates, and nucleic acids.22
In patients with heart failure, it has been shown that the myocardium has a reduced iron content,23 and it is possible that myocardial iron depletion plays a role in deteriorating systolic function. Several observational studies have assessed the association between iron deficiency and outcomes in patients with heart failure (Table 2).1–3,21,24–27 Regardless of whether or not patients had anaemia, or the severity of heart failure, the presence of iron deficiency showed a significant association with mortality after adjustment for confounding factors in most studies.1,2,24,25 Only in one analysis, a community-based study in the USA, was no correlation found, but in this analysis disease severity (measured with NYHA functional class and NT-proBNP levels) was not assessed.3 Overall, the increase in mortality risk appears to be in the order of 40–50%.1,2
Table 2.
Observational studies of clinical outcomes according to presence or absence of iron deficiency in patients with heart failure
| Parameter | Study design | n | Population | Definition of iron deficiency | Findings |
|---|---|---|---|---|---|
| Mortality | Prospective, two-centre2 | 546 | LVEF ≤45%, NYHA class I–IV | Ferritin <100 ng/mL or 100–300 ng/mL with TSAT <20% | Iron deficiency significantly related to mortality (HR 1.74, 95% CI 1.30–2.33, P < 0.001) on multivariate analysis |
| Pooled cohort analysis (three countries)1 | 1506 | NYHA class I–IV, reduced or preserved LVEF | Ferritin <100 ng/mL or 100–299 ng/mL with TSAT <20% | Iron deficiency significantly related to mortality (HR 1.42, 95% CI 1.14–1.77, P = 0.002) on multivariate analysis | |
| Retrospective, single-centre24 | 274 | LVEF ≤45%, NYHA class I–IV | Progression of iron deficiency was defined as increasing red cell distribution width with decreasing mean cell volume | Progression of iron deficiency significantly related to mortality (HR 2.78, 95% CI 1.64–4.73, P < 0.001) | |
| Community-based survey3 | 574 | Self-reported, community-dwelling heart failure patients | Ferritin <100 ng/mL or 100–299 ng/mL with TSAT <20% | No significant association between iron deficiency and all-cause or cardiovascular mortality on multivariate analysis | |
| Prospective, two-centre25 | 157 | LVEF ≤45%, NYHA class I–IV | TSAT <20% | Iron deficiency anaemia is associated with two-fold greater risk for death than iron deficiency without anaemia | |
| Patients with iron deficiency anaemia have a four-fold greater risk for death than iron-replete patients with or without anaemia | |||||
| Exercise capacity | Prospective, two-centre26 | 155 | NYHA class I–IV | Ferritin <100 ng/mL or 100–300 ng/mL with TSAT <20% | Significant association between iron deficiency and (i) reduced peak oxygen consumption (VO2) (P < 0.05), (ii) higher ventilatory response to exercise (VE–VCO2 slope), on multivariate analysis |
| Prospective, two-centre25 | 27 | LVEF ≤45%, NYHA I–IV | TSAT <20% | Iron deficiency was significantly associated with reduced peak oxygen consumption (VO2) (P = 0.03) | |
| Prospective, single-centre27 | 26 | NYHA II–III, preserved ejection fraction | Ferritin <100 ng/mL or 100–299 ng/mL with TSAT <20% | No significant association between iron deficiency and exercise capacity parameters | |
| Quality of life | Post-hoc analysis of singe-centre study data20 | 552 | NYHA III–IV | Ferritin <100 ng/mL or <800 ng/mL with TSAT <20% | Significantly worse health-related quality of life associated with iron deficiency on multivariate analysis based on MLHFQ overall score (P = 0.008) and physical dimension score (P = 0.002) |
| Pooled cohort analysis (three countries)28 | 1278 | NYHA I–IV, mean ejection fraction 38% | Ferritin <100 ng/mL or 100–299 ng/mL with TSAT <20% | Multivariate analysis showed that iron deficiency, but not anaemia, was associated with impaired health-related quality of life based on MLHFQ (P = 0.016) |
CI, confidence interval; HR, hazard ratio; MLHFQ, Minnesota Living with Heart Failure Questionnaire; TSAT, transferrin saturation.
Animal and human studies have suggested a strong causal relationship between all levels of iron deficiency—even in the absence of anaemia—and the extent of physical activity.29 Data from iron-deficient anaemic rats have shown a substantial improvement in walking ability within 15–18 h of iron administration despite unaltered Hb levels.30,31 In heart failure, there is evidence that iron deficiency, and not anaemia, is an independent predictor of exercise intolerance in patients with heart failure. In a prospective study, peak oxygen consumption (VO2) and the ventilatory response to exercise (VE–VCO2 slope) were measured in 155 patients with stable heart failure (NYHA class I–IV) and mean LVEF of 26%.26 Iron deficiency was defined as ferritin <100 ng/mL, or 100–300 ng/mL with TSAT <20%. The results consistently indicated a significantly reduced exercise tolerance in patients with iron deficiency vs. patients with adequate iron, a difference that was maintained after adjustment for demographic and clinical variables.26 These data confirmed findings from a previous prospective study in 27 heart failure patients in whom iron deficiency was defined as TSAT <20%.32 The negative influence of iron deficiency on exercise tolerance can be linked to the non-haematopoietic effects of iron, especially if one considers its role in mitochondrial function in cells with high energy demand, such as cardiomyocytes and skeletal myocytes.4,23 Mitochondria are the key sites of cellular iron processing, where synthesis of iron–sulfur (Fe/S) clusters and haem takes place, but are also the location for generation of reactive oxygen species (ROS). The activity of mitochondrial Fe/S cluster proteins is reduced in failing hearts, an effect attributed to the lack of an Fe/S cluster centre.33,34 On the other hand, clinical data and evidence from animal models with specific genetic defects have suggested that myocardial iron overload35 or accumulation of iron in the mitochondria can induce or exacerbate cardiomyopathy,34 presumably as a consequence of ROS-dependent mechanisms. The question of whether iron accumulates in the mitochondria of failing hearts due to a shared aetiology, such as hypertension or myocardial infarction, has not been examined.
In a post-hoc analysis of a cohort of >500 chronic heart failure patients, iron deficiency was associated with lower quality of life than in those patients with normal iron levels, regardless of anaemia status. In multivariate analysis, the effect on quality of life appeared to be due to physical factors; a role for erythropoiesis was excluded.20
The pathophysiological impact of iron deficiency in heart failure patients with preserved ejection fraction (HFpEF) is not yet fully elucidated. An observational study of 751 patients with heart failure which included a subpopulation of patients with HFpEF (defined as LVEF >50%) found that functional iron deficiency (TSAT <20%) occurred with a similar frequency in patients with or without HFpEF.36 One well-designed study of 26 patients with HFpEF observed no significant association between iron deficiency (defined as ferritin <100 ng/mL or 100–299 ng/mL with TSAT <20%) and exercise capacity.27 However, multivariate analysis of data from an epidemiological multicentre study of 1278 patients with heart failure showed an association between iron deficiency and health-related quality of life which was independent of whether LVEF was preserved or reduced.28 Further studies with larger populations are needed to determine whether iron therapy can improve symptoms and cardiac function in HFpEF patients.
Characteristics of iron therapies
Oral iron
Oral iron therapy is relatively inexpensive and widely used, but the absorption of iron is low. It also means that iron uptake can be inadequate to meet enhanced demand during treatment with an erythropoiesis-stimulating agent (ESA), limiting the haematopoietic response.37 Oral iron is most frequently given in the form of ferrous (Fe[II]) salts, such as ferrous sulfate.
Oxidation of Fe(II) to Fe(III) can provoke oxidative damage at the mucosal boundary, resulting in local toxicity which leads to gastrointestinal side effects in up to 60% of patients.38 These include complications such as constipation, dyspepsia, nausea, diarrhoea, and heartburn, which are particularly frequent with ferrous sulfate preparations and often lead to poor compliance. Many constituents of food and some medications interact with ferrous salts, such that iron uptake is best when taken on an empty stomach, but gastrointestinal side effects are often improved by concomitant food, so there can be a difficult balance between maximizing uptake and minimizing gastrointestinal side effects.
Oxidative stress, with the potential for adverse consequences, has been observed in animal studies during treatment with ferrous salts, especially at high doses. Evidence suggests that this may include disruption of cell membranes, lipid peroxidation, DNA strand breakage, and immunological disturbances.39 Moreover, ferrous salts have been found to increase the severity of experimental colitis and affect the intestinal microbiomass due to redox reactions which lead to the production of ROS and mediate intestinal damage in rodents,40 with evidence of increased prevalence of intestinal adenomas.41 Long-term iron overload via dietary iron in rats induces necrosis in multiple organs, an effect attributed to cellular iron accumulation and consequent oxidative stress.42 The effects of oxidative stress can be avoided by use of the oral preparation iron[III]–polymaltose complex, which includes a polymaltose shell for stability so that iron is released in a controlled manner and non-transferrin-bound iron (NTBI) does not increase.43 The structure of the iron[III]–polymaltose complex also minimizes the interactions with food or concomitant medications that can affect uptake of iron from ferrous salts.43 Clinically, data from patients with inflammatory bowel disease have demonstrated a significant increase in lipid peroxidation following ferrous sulfate treatment for 14 days compared with iron[III]–polymaltose complex.44
As a consequence of the adverse events associated with oral iron therapy, it is considered unsuitable for patients who have defective gastrointestinal absorption, those who cannot tolerate the required oral dose, or individuals who show poor compliance.39 Oral iron is also unlikely to be adequate if a rapid effect is required, or if the rate of chronic iron loss exceeds the replenishment rate that is possible with oral administration.39
Intravenous iron preparations
Intravenous (i.v.) iron preparations bypass the absorption difficulties associated with oral iron, even in the presence of elevated hepcidin levels.43,45,46 A series of i.v. iron complexes has been developed which enclose an iron-containing core within a carbohydrate shell, stabilizing the structure and controlling the release of iron.43 The first complex, iron sucrose, was followed by low and high molecular weight iron dextran products and ferric gluconate. More recently, ferric carboxymaltose, iron isomaltoside 1000, and ferumoxytol have become available. These are more thermodynamically stable, with a low reduction potential, and permit administration of a much higher dose of iron in a single administration without formation of large amounts of NTBI.47
Among i.v. preparations, the less stable formulations, such as iron gluconate, are more prone to reduction, with greater risk of oxidative stress, so that the maximum single dose is smaller. Experimental evidence has shown significant differences between the effects of different i.v. iron preparations. Increased levels of markers for oxidative stress and inflammation have been observed in animals treated with sodium ferric gluconate, iron dextran, ferumoxytol, or iron isomaltoside 1000 compared with ferric carboxymaltose or iron sucrose, changes that were accompanied by hypotension and impaired liver and kidney function.47–50 However, it should be emphasized that most non-clinical in vivo studies have been conducted with iron doses that are significantly higher than those used in clinical practice. Animal data showing that ferric carboxymaltose may have a favourable safety profile in terms of oxidative stress compared with iron dextran and ferric gluconate await confirmation. Clinical data are limited, and are largely restricted to patients receiving haemodialysis. Two recent studies which used standard doses of iron sucrose reported a transient (1–2 h) increase in NTBI and oxidative stress, accompanied by a short-lived increase in lipid peroxidation.51,52 These results remain unconfirmed, and interpretation is complicated by the complex effects of chronic haemodialysis on oxidative stress, inflammation, and endothelial dysfunction.
It is challenging to compare accurately the risk of hypersensitivity reactions between different i.v. iron preparations since events are very rare and reporting can be poor. I.v. iron complexes that contain dextran ligands can cause dextran-induced anaphylactic reactions. These are rare, particularly when using low molecular weight dextran preparations,53,54 but are serious events and have led to recommendations that i.v. iron dextran should be avoided.55 Sodium ferric gluconate, iron sucrose, and ferric carboxymaltose do not contain dextran ligands, although hypersensitivity reactions unrelated to dextran ligands are still possible.
Iron is essential for mounting an effective immune response,56 but it is also biologically plausible that excess iron can impair normal neutrophil and T-cell functioning, with an associated risk of increased microbial growth.57 The clinical evidence is largely based on observational studies on haemodialysis patients, which have yielded conflicting results, but taking a cautious approach the current Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in Chronic Kidney Disease advises avoiding i.v. iron therapy in patients with active systemic infections.58 Conversely, withholding iron therapy with consequent iron deficiency may increase the risk of infections.57
Randomized controlled trials are needed to evaluate all important safety endpoints, including infection. Ongoing monitoring of newer i.v. agents such as ferric carboxymaltose, ferumoxytol, and iron isomaltoside 1000 is essential to establish an extensive evidence base similar to that which already exists for earlier preparations such as iron sucrose. Additional clinical trials are being undertaken to help provide such data, in response to requests from regulatory authorities.
Management of iron deficiency in heart failure
The recent ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 recognize iron deficiency as a common co-morbidity in heart failure, independent of anaemia, which can contribute to muscle dysfunction and cause anaemia.17 The guidelines recommend that assessment of all patients with suspected heart failure should include an evaluation of iron status to detect iron deficiency using ferritin and TSAT levels, but this is not yet regarded as standard practice.59 A suggested algorithm for the diagnosis of iron deficiency in patients with heart failure is shown in Figure 1, based on typical thresholds for ferritin and TSAT in this setting.15,17,60
Figure 1.
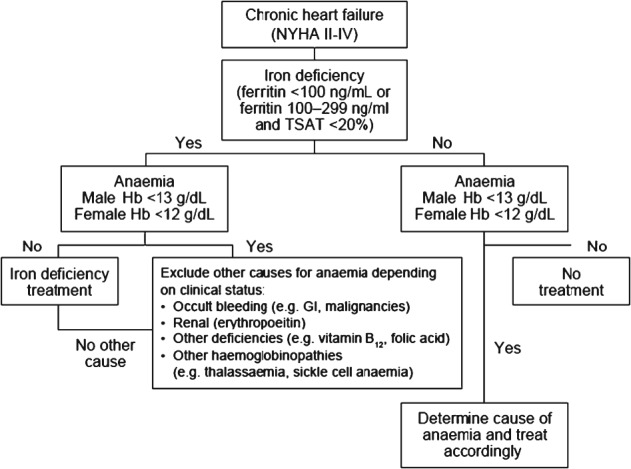
Suggested algorithm for diagnosis of iron deficiency in patients with heart failure.54–59 TSAT, transferrin saturation.
Iron therapy should be considered in all patients with heart failure who have iron deficiency regardless of whether anaemia is present or not. If ESA therapy is indicated for the treatment of anaemia—predominantly in patients with renal dysfunction—concomitant iron therapy may be necessary to meet the enhanced demand for iron during ESA-stimulated erythropoiesis.5 The majority of heart failure patients receive oral iron therapy, with only ∼1 in 10 iron-treated patients being given i.v. therapy.61
Certain characteristics of patients with heart failure which can limit intestinal absorption of oral iron preparations can be particularly pronounced and therefore i.v. iron is the preferred option. First, patients with heart failure are usually receiving multiple medications for heart failure and frequently for one or more co-morbid conditions. Thus, adhering to an oral iron dosing regimen (typically three times daily) may be challenging. Secondly, various factors can contribute to abnormal intestinal morphology and function in heart failure and therefore inhibit iron absorption, such as oedema of the absorptive mucosa.62 Sandek et al. compared bowel wall thickness in 22 patients with heart failure and observed significantly greater thickness compared with controls, accompanied by significant changes in permeability.63 In a subsequent study, the same team showed that intestinal blood flow is reduced by 30–43% in heart failure patients vs. controls,64 an effect which would impair oral iron uptake from the gut. Other changes can include overactivation of the sympathetic nervous system, the effect of concomitant medications such as H2 blockers, and complications of co-morbid conditions such as diabetes-related gastroparesis (Table 2). Thirdly, where hepcidin levels are raised, expression of the iron transport protein ferroportin in enterocytes is suppressed such that release of absorbed iron into the bloodstream is restricted.65 While more evidence is required, it appears that increased levels of circulating hepcidin characterize early stages of heart failure, although iron deficiency becomes more prevalent in more severe stages of the disease.14 This apparent paradox remains unexplained, but hepcidin levels appear to be elevated in most patients with heart failure.14 Additionally, even after iron is absorbed, elevated levels of hepcidin inhibit ferroportin-mediated transport of stored iron out of enterocytes and macrophages, causing iron sequestration46 and reducing the amount of iron available for haematopoiesis and other cellular functions. The high intracellular iron levels achieved by i.v. administration, however, can overcome this ‘hepcidin block’ by stimulating overexpression of ferroportin to release iron for transport in the plasma bound to transferrin14,66 (Figure 2).
Figure 2.
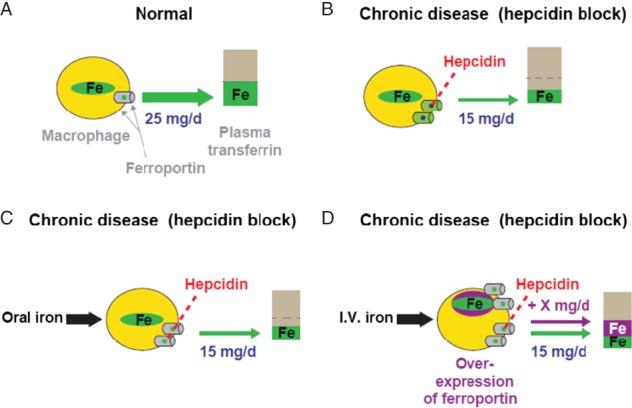
Effect of oral or i.v. iron therapy on ‘hepcidin block’ of iron release from macrophages. (A) Under normal circumstances, ∼25 mg of stored iron per day is transported out of macrophages to plasma transferrin by the iron transporter protein ferroportin. (B) In chronic disease, elevated levels of hepcidin cause degradation of ferroportin, restricting ferroportin-mediated transport to ∼15 mg iron/day, (C) The rate of iron absorption from iron therapy is inadequate to influence this ‘hepcidin block’. (D) I.v. iron therapy results in high intracellular iron levels which overcome the ‘hepcidin block’ by stimulating overexpression of ferroportin (modified from Aapro et al.46).
Iron therapy dosing
The median required dose of iron to achieve iron repletion in iron-deficient patients with heart failure is 1000 mg.67 In patients with iron deficiency anaemia, the rate of iron bioavailability from a therapeutic dose of oral 100 mg ferrous sulfate, the most commonly used oral iron preparation, and iron[III]–polymaltose complex, is ∼10%.68,69 Given that a typical dose of ferrous sulfate is 100–200 mg iron/day, in the ‘best case’ scenario, a patient who tolerates treatment of 200 mg/day might absorb only 20 mg of iron and thus require 50 days to correct the iron deficit. In a ‘worst case’ scenario, given that patients with chronic heart failure may have as little as half the usual rate of active carrier-mediated intestinal absorption capacity,62 a patient who can tolerate up to 100 mg iron/day might absorb only 5% (5 mg). It would thus require 200 days to replenish iron stores. In practice, correction of iron deficiency for heart failure patients will often take longer due to non-adherence or interruption due to gastrointestinal side effects, or missed doses due to the high pill burden. Therefore, replenishment of iron stores with oral iron can be expected to require >6 months. In contrast, one or two doses of i.v. iron therapy can achieve rapid repletion (Table 3). I.v. administration, however, places additional demands on the treatment centre (Table 3).
Table 3.
| Characteristics | Oral iron | I.v. iron |
|---|---|---|
| Intestinal absorption | Relatively low | Parenteral administration |
| Impaired by concomitant food (depending on formulation) | ||
| Impaired by concomitant medication including phosphate binders and gastrointestinal medications that reduce acidity, e.g. omeprazole | ||
| Impaired export of iron from enterocytes into the bloodstream due to elevated hepcidin levels in chronic inflammatory conditions, e.g. heart failure | ||
| May be limited by the physiological changes in the intestinal tract typical of heart failure patients (e.g. oedema, local ischaemia, poor blood perfusion) | ||
| Iron bioavailability | May be inadequate during ESA therapy (accelerated erythropoiesis) | Generally high |
| Gastrointestinal adverse events | Affect 20–30% of patients, e.g. constipation, dyspepsia, bloating, nausea, diarrhoea, heartburn. Most frequent with ferrous sulfate | Less frequent due to i.v. administration. To be confirmed with larger controlled trials with extended follow-up (>1 year) |
| Hypersensitivity reactions | Not applicable | Risk of (very rare) anaphylaxis with dextran-containing formulations |
| Risk of (very rare) hypersensitivity reactions | ||
| Safety data to be confirmed with larger controlled trials with extended follow-up (>1 year) | ||
| Oxidative stress | High doses can saturate the iron transport system if the iron is rapidly released (e.g. ferrous sulfate) resulting in NTBI in the plasma | Mainly occurs with less stable preparations, e.g. sodium ferric gluconate or high-dose iron sucrose. To be confirmed with larger controlled trials with extended follow-up (>1 year) |
| Risk of infection | Unknown (lack of rigorous assessment) | An association between increased risk for infection and i.v. iron therapy is biologically plausible, but evidence is conflicting. To be confirmed with larger controlled trials with extended follow-up (>1 year) |
| Compliance | High pill burden (typically three tablets/day) | Administered by health professional |
| Affected by gastrointestinal intolerance | ||
| Convenience for patients | Administered at home | Requires clinic visits |
| Dose | Typically 100–200 mg iron/day | Up to 1000 mg iron in a single injectiona or up to 20 mg iron/kg body weight infusionb,c |
| Cost | Inexpensive | More expensive per dose but fewer doses required |
| Staffing | None | Administration staff must be trained to evaluate and manage possible anaphylactic reactions |
| Observation time | None | Patient should be observed for at least 30 min following each injection |
| Equipment | None | Administration should take place in an environment where full resuscitation facilities can be assured |
ESA, erythropoiesis-stimulating agent; NTBI, non-transferrin-bound iron.
Ferric carboxymaltose (maximum 15 mg iron/kg body weight).
Ferric carboxymaltose, iron dextran (Cosmofer®).
See reference 29 for dosing recommendations for different i.v. iron preparations.
Oral iron therapy in heart failure: the clinical evidence
Despite the fact that oral iron is still often the iron therapy of choice for many cardiologists, the evidence base related to oral iron therapy in heart failure is limited. No randomized trials have compared oral iron vs. no iron therapy in the absence of ESA therapy. In randomized trials of ESA therapy with oral iron vs. oral iron alone, mean iron parameters and Hb level, symptom severity, and exercise tolerance remained unaltered in the oral iron treatment groups72–75 (Table 4). In the largest of these, 319 patients with symptomatic heat failure and Hb in the range 9–12.5 g/dL were randomized to darbepoetin or placebo, both with daily oral iron therapy.73 At the end of the 27-week study period, mean values for Hb were almost unchanged compared with baseline in the patients receiving only oral iron (11.6 g/dL vs. 11.3 g/dL at baseline), with little change in ferritin and TSAT levels. Mean NYHA class was 2.54 compared with 2.67 at baseline, while maximum exercise duration decreased from 409 s to 363 s during the study.73
Table 4.
Overview of randomized trials of oral iron with erythropoiesis-stimulating agent therapy or with placebo in patients with heart failure
| Study | Design and duration | Population | Oral iron | ESA/placebo treatment groups | n | Outcomes for oral iron alone (placebo group) at end of study | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom severity/quality of life | NYHA class | Exercise capacity | Hb | Ferritin | TSAT | ||||||
| Ghali et al., 200873 | Randomized, double-blind, 52 weeks | HF ≥3 months, LVEF ≤40%, Hb 9–12.5 g/dL, TSAT ≥15% | Daily until ferritin >800 ng/mL (type/dose not specified) | Darbepoetin alpha 0.75 µg/kg every 2 weeks | 162 | 71% reported improvement on PGA | Minor improvement [mean (SE) 0.13 (0.04)] | No change vs. baseline | No change vs. baseline | NA | NA |
| Placebo | 157 | ||||||||||
| Kourea et al., 200875 | Randomized, single-blind, 12 weeks | NYHA class II–III, LVEF <40%, Hb <12.5 g/dL, SCr <2.5 mg/dL | Ferrous sulfate 250 mg b.i.d. | Darbepoetin alpha 1.5 µg/kg every 20 days | 21 | No change vs. baseline | NA | Significant deterioration vs. baseline (P = 0.044) | No change vs. baseline | NA | NA |
| Placebo | 20 | ||||||||||
| Van Veldhuisen et al., 200774 | Randomized, double-blind, 26 weeks | HF ≥3 months, LVEF ≤40%, Hb 9–12.5 g/dL, TSAT ≥15% | 200 mg iron/day (type not specified) | Darbepoetin alpha 0.75 µg/kg every 2 weeks | 56 | Minor improvement [mean (SE) 4.9 (2.1)] on KCCQa | Minor improvement [mean (SE) 0.23 (0.08)]a | No relevant change vs. baselinea | No relevant change vs. baselinea | No change vs. baselinea | No relevant change vs. baselinea |
| Darbepoetin alpha 50 µg every 2 weeks | 54 | ||||||||||
| Placebo | 55 | ||||||||||
| Palazzuoli A et al., 200672 | Randomized, double-blind, 12 weeks | NYHA class III-IV, LVEF <35%, Hb <11 g/dL, mild renal dysfunction | Ferrous gluconate 300 mg/day | Epoetin beta 6000 IU twice weekly | 20 | NA | No change vs. baseline | No change vs. baseline | No change vs. baseline | NA | NA |
| Placebo | 20 | ||||||||||
ESA, erythropoiesis-stimulating agent; Hb, haemoglobin; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; NA, not assessed; PGA, Patient Global Assessment; SCr, serum creatinine; SE, standard error; TSAT, transferrin saturation.
No statistical comparison provided for final data vs. baseline data within the placebo group.
The placebo-controlled IRONOUT study (NCT02188784) is currently underway to assess whether treatment with oral iron polysaccharide therapy can improve functional capacity in iron-deficient patients with heart failure.
Intravenous iron therapy in heart failure: the clinical evidence
Single-arm studies
Three single-arm trials have assessed the effect of i.v. iron without ESA therapy on Hb level and iron indices in patients with heart failure, using iron sucrose or iron dextran preparations.76–78 The entry criteria for the three trials varied in terms of severity of heart failure, the presence or absence of anaemia and iron deficiency, and duration of follow-up (12–26 weeks), but in each case there was a significant increase in mean values for Hb, ferritin, and TSAT from baseline. Upon treatment with i.v. iron, various cardiac endpoints also improved significantly, including NYHA functional class,76,78 LVEF,77 and exercise tolerance.76,78 These results are consistent with findings from single-blinded or double-blinded randomized trials of monotherapy with i.v. iron in heart failure patients with iron deficiency, in which ferric carboxymaltose15 or iron sucrose32,79 were administered (Table 5).
Table 5.
Overview of randomized trials of intravenous iron in patients with heart failure
| Study | Design and duration | Population | I.v. iron regimen/comparator(s) | n | Outcomes at end of study | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom severity/quality of life | NYHA class | Exercise capacity | Hb | Ferritin | TSAT | |||||
| Ponikoswki et al., 2014 (CONFIRM-HF)16 | Double-blind, randomized, 52 weeks | NYHA class II–III, LVEF ≤45%, ferritin <100 ng/mL or 100–300 ng/mL with TSAT <20%, Hb <15 g/dL | Ferric carboxymaltose 500–2000 mg iron in therapy phase (baseline and week 6); 500 mg iron as maintenance (weeks 12, 24, 36) if iron deficiency still present | 152 | Improved vs. placebo (P = 0.001)a | Improved vs. placebo (P < 0.001) | Improved vs. placebo (P = 0.002)c | Increased vs. placebo (P < 0.001) | Increased vs. placebo (P < 0.001) | Increased vs. placebo (P < 0.001) |
| Reduced hospitalization for worsening heart failure (P = 0.009) | ||||||||||
| Placebo | 152 | |||||||||
| Anker et al., 2009 (FAIR-HF)15 | Double-blind, randomized, 24 weeks | NYHA class II (LVEF ≤40%) or III (LVEF ≤45%), ferritin <100 ng/mL or 100–299 ng/mL with TSAT <20%, Hb 9.5–13.5 g/dL | Ferric carboxymaltose 200 mg iron/week until iron repletiond | 304 | Improved vs. placebo (P < 0.001)a,e | OR for improvement by one class vs. placebo: 2.40; 95% CI 1.55 (P < 0.001) | Greater improvement from baseline vs. placebo (P < 0.001)c | No difference (P = 0.21) | Increased vs. placebo (P < 0.001) | Increased vs. placebo (P < 0.001) |
| Placebo | 155 | |||||||||
| Okonko et al., 200832 | Observer-blinded, randomized, 18 weeks | Ferritin <100 ng/mL or 100–300 ng/mL with TSAT <20%, Hb <14.5 g/dL | Iron sucrose 200 mg iron/weekf | 24 | Improved vs. controls (P < 0.002)a | Improved vs. controls (P = 0.03) | Trend to improvement vs. controls (P = 0.08) | No difference (P > 0.2) | Increased vs. controls (P < 0.001) | Increased vs. controls (P < 0.001) |
| No treatment | 11 | |||||||||
| Toblli et al., 200779 | Double-blind, randomized, 26 weeks | LVEF ≤35%, Hb <12.5 g/dL, ferritin <100 ng/mL, TSAT <20%, b <12.5 g/dL (men), <11.5 g/dL (women) | Iron sucrose 200 mg iron/week for 5 weeks | 20 | Improved vs. controls (P < 0.01)g | Improved vs. controls (P < 0.01) | Improved vs. placebo (P < 0.01)c | Increased vs. placebo (P < 0.01) | Increased vs. placebo (P < 0.01) | Increased vs. placebo (P < 0.01) |
| Placebo | 20 | |||||||||
CI, confidence interval; Hb, haemoglobin; OR, odds ratio; TSAT, transferrin saturation.
Patient Global Assessment scale.
Significant improvement on Kansas City Cardiomyopathy questionnaire (P < 0.05 at weeks 12, 36, and 52) and European Quality of Life-5 Dimensions (EQ-5D) Visual Analogue Scale (significant difference only at week 36 (P = 0.002).
Six-minute walk test scale.
Weekly dosing until iron repletion was complete (correction phase) then every 4 weeks to week 24.
Kansas City Cardiomyopathy questionnaire and EQ-5D Visual Analogue Scale.
Until ferritin was >500 ng/mL, then 200 mg iron/month thereafter.
Minnesota Living with Heart Failure Questionnaire.
Randomized trials
Two randomized studies which used iron sucrose therapy demonstrated improved exercise capacity, cardiac function, symptom severity, and quality of life32,79 compared with placebo. Okonko et al. showed that the benefits of i.v. iron were seen in patients with or without anaemia at baseline, but were greater in anaemic individuals.32 In a second trial, 40 patients with anaemia, iron deficiency, and LVEF <35% were randomized to placebo or i.v. iron sucrose therapy for 5 weeks in a 6-month, double-blind study79 then followed to 5 years.80 At 6 months, the number of hospitalizations was significantly lower in the patients who had received i.v. iron therapy compared with the placebo-treated group {0 events vs. 5 events (P < 0.01); relative risk 2.33 for hospitalization with placebo vs. i.v. iron [95% confidence interval (CI) 1.59–3.42]}.79 Hospitalization remained significantly less frequent with i.v. iron vs. placebo at 1 year (10% vs. 50%) and 5 years (20% vs. 85%) (both P < 0.01) and, notably, mortality was significantly lower at 5 years (20% vs. 55%, P < 0.05).80 These findings require confirmation.
FAIR-HF was a randomized, double-blind, placebo-controlled, 24-week multicentre study of 459 patients with heart failure (NYHA class II–III) and iron deficiency, with or without anaemia.15 Patients were enrolled if their Hb level was between 9.5 and 13.5 g/dL, ferritin was <100 ng/mL (or <300 ng/mL and TSAT <20%), and if LVEF was ≤40% in NYHA class II or LVEF ≤45% in NYHA III. Ferric carboxymaltose was administered i.v. at a dose of 200 mg of iron, which was selected based on previous trials of iron sucrose,32,76,79 although single doses up to 1000 mg of iron are now more standard.60 The primary endpoints were quality of life and symptoms, as assessed by the Patient Global Assessment score, and NYHA class, both at week 24. Both endpoints were significantly improved vs. the placebo arm already at week 4 and then throughout the whole study duration, e.g. by the end of the 24-week study period. On the Patient Global Assessment score, 50% of patients reported ‘much’ or ‘moderate’ improvement in the ferric carboxymaltose group compared with 27% in the placebo group [odds ratio (OR) for being in a better rank 2.51; 95% CI 1.75–3.61; P < 0.001). By week 24, the NYHA class was I or II in 47% of patients in the ferric carboxymaltose group vs. 30% of patients in the placebo arm (OR for improvement by one class, 2.40; 95% CI, 1.55–3.71). The improvement in the 6-min walk test (6MWT) by week 24 was also greater in the treatment arm (39 m vs. 9 m with placebo, P < 0.001). Notably, the between-group differences were significant as early as week 4 and were also maintained throughout the study period for all secondary endpoints. The effects of ferric carboxymaltose were observed regardless of baseline NYHA class, Hb level, LVEF, or the presence or absence of anaemia.15,67 At week 24, the rates of death, adverse events, and serious adverse events were similar in the two treatment groups, with a favourable trend for i.v. iron, and no severe allergic reactions occurred.
When data from the FAIR-HF trial were analysed in terms of cost-effectiveness from a UK perspective, the cost of purchasing and administrating ferric carboxymaltose (€604 per patient for the 24-week study) was partly offset by lower costs for hospital treatment compared with the placebo arm (€249 vs. €687).81 The incremental cost-effectiveness ratio (€4414) for ferric carboxymaltose vs. placebo in terms of each quality-adjusted life year (QALY) gained was far below the cost-effectiveness threshold regarded as acceptable, i.e. treatment was considered to be cost-effective. For comparison, treatment with ACE agents, aldosterone receptor blockers, or beta-blockers in patients with heart failure is estimated to have an incremental cost-effectiveness ratio in the range of €2715–7689 per QALY gained.82
Recently, the results of the CONFIRM-HF study have been reported.16,83 The study population comprised patients with NYHA class II–III with LVEF ≤45%, iron deficiency (defined as ferritin <100 ng/mL or 100–300 ng/mL if TSAT <20%), and Hb <15 g/dL with no immediate need for transfusion. CONFIRM-HF used the objective primary endpoint of the 6MWT, with a 12-month study period compared with 6 months in FAIR-HF. During the therapy phase, ferric carboxymaltose was administered as bolus injections equivalent to 500 or 1000 mg iron over 1 min, with a total dose of 500–2000 mg of iron dosed at baseline and (if necessary) at week 6, based on the patient’s weight and Hb value at screening. In the maintenance phase, a dose of 500 mg of iron could be given at weeks 12, 24, and 36 if iron deficiency was still present. As the primary endpoint, changes from baseline of the 6MWT distance at week 24 showed significant improvement in the ferric carboxymaltose treatment group vs. the placebo arm: the mean (SD) difference was 33 (11) m (P = 0.002) and it was sustained until study end, at week 52 (p < 0.001). The treatment effect was observed in all subpopulations examined at week 24, including stratification by NYHA class, LVEF, ferritin at baseline, and Hb at baseline. The secondary endpoints of NHYA class, Patient Global Assessment, quality of life, and Fatigue Score all showed a significant improvement with ferric carboxymaltose vs. placebo from week 24 onwards.16 Notably, the risk of hospitalization due to worsening heart failure was also reduced in the ferric carboxymaltose group vs. placebo-treated patients (hazard ratio 0.39; 95% CI 0.19–0.82; P = 0.009). The incidence of deaths and adverse events was comparable in both treatment arms. The findings of CONFIRM-HF are thus compatible with those of FAIR-HF, but with patients followed to 1 year and using higher single doses (up to 1000 mg of iron). It is striking that the benefits of i.v. iron were achieved with 75% of patients requiring no more than two injections of ferric carboxymaltose.
Further randomized controlled studies of i.v. iron are underway to evaluate the effect of ferric carboxymaltose [including EFFECT-HF (NCT01394562), ICHF (NCT01837082), FAIR-HF-HFpEF, and PRACTICEASIAHF (NCT01922479)], sodium ferric gluconate (NCT01925703), and iron sucrose (NCT00384657) in patients with iron deficiency and heart failure. One area of particular interest is patients with HFpEF and iron deficiency anaemia, in whom the limited available evidence does not suggest that either ESA or oral iron treatment improves functional capacity or quality of life.84,85 Robust data on the effect of i.v. iron therapy to treat iron deficiency in patients with HFpEF are lacking, an issue that will be addressed in the ongoing FAIR-HF-HfpEF study which includes only patients with LVEF >45%.
Meta-analyses
Two meta-analyses have evaluated the use of i.v. iron in iron-deficient patients with heart failure (NYHA class II–IV).86,87 The first of these, by Avni et al., included only those trials of i.v. iron therapy alone, involving a total of 594 heart failure patients with or without anaemia.86 The results show a trend towards better NYHA class and improved quality of life following i.v. iron therapy, with significant improvements in the number of hospitalizations, the 6MWT, and LVEF. There was no increase in adverse events vs. controls, and no increase in serious adverse events including infections, neurological or cardiac events. The second analysis, which also included trials of concomitant ESA therapy with i.v. iron vs. no treatment (n = 631), demonstrated a significant reduction in NYHA class, hospitalization, and LVEF in the iron-treated patients.87 Neither meta-analysis showed a mortality benefit, possibly due to inadequate sample sizes, but a clinically relevant advantage for i.v. iron in terms of symptoms, functional capacity, and hospitalization seems conclusive. However, it should be borne in mind that these studies provided safety data for no more than a 6-month period.
Suggested treatment algorithm
Based on data from the FAIR-HF trial,15 the CONFIRM-HF study,16 the ESC Guidelines,17 the summary of product characteristics for ferric carboxymaltose,60 and the study design of the EFFECT-HF trial, a suggested treatment algorithm for iron deficiency in patients with heart failure is shown in Figure 3.
Figure 3.
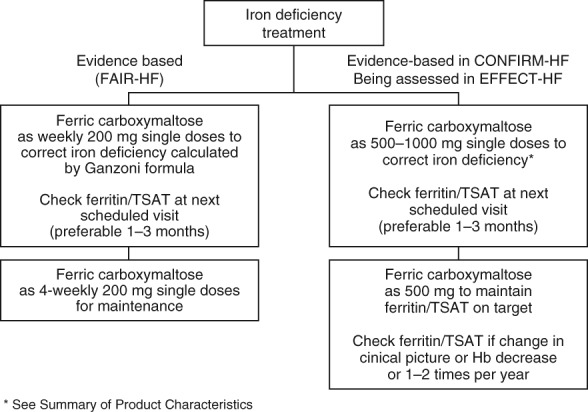
Suggested algorithm for treatment of iron deficiency in patients with heart failure.15–17,59,60,88 TSAT, transferrin saturation.
Comparative studies of oral vs. intravenous iron in heart failure
To date, only one pilot study (IRON-HF) has compared the efficacy and safety of oral vs. i.v. iron in patients with heart failure.89 This was a double-blind trial of 23 patients with heart failure (NYHA class II–IV with EF <40%) with anaemia (Hb 9–12 g/dL), ferritin <500 ng/mL, and TSAT <20%. Patients were randomized to iron sucrose (at a dose of 200 mg iron/week for 5 weeks), oral ferrous sulfate (200 mg of iron three times a day) for 8 weeks, or placebo. Follow-up data were only available in 18 patients. The primary endpoint was the effect of iron supplementation alone (i.v. or oral) on VO2 over a 3-month follow-up period. Results showed that mean (SD) ferritin increased in both the i.v. iron (from 167 ± 149 ng/mL to 293 ± 270 ng/mL) and the oral iron group (115 ± 141 ng/mL to 218 ± 189 ng/mL). However, the increase in iron availability, as measured by TSAT, was less pronounced in the oral iron group vs. i.v. iron. This could explain why a mean increase in peak VO2 (mean 3.5 mL/kg/min) was observed in the i.v. iron group and not in the oral iron group (mean −0.86 mL/kg/min), a difference which was considered as clinically relevant by the authors.
An increase in Hb from baseline was also observed in all groups; however, it should be recognized that mean ferritin was relatively high at baseline (132 ng/mL compared with 53 ng/mL in the i.v. iron arm of the FAIR-HF study). It should be noted that this was a small cohort study which resulted in no significant difference between groups.89 A definitive comparison of i.v. vs. oral iron therapy in patients with heart failure is still pending.
Evidence from other clinical settings
The most extensive evidence base comparing different routes of administration for iron therapy is in the field of chronic kidney disease. The most recent meta-analysis of randomized controlled trials included 28 studies involving 2098 dialysis-dependent and non-dialysis-dependent patients.90 Levels of Hb, ferritin, and TSAT were all significantly increased by i.v. iron vs. oral iron, and there was a significant reduction in requirement in ESA dose in dialysis-dependent patients treated with i.v. iron. The scale of the mean between-group differences was clinically relevant: 0.90 g/dL for Hb, 243 ng/mL for ferritin, and 10.2% for TSAT. This is consistent with recent randomized trials using i.v. ferumoxytol in patients with chronic kidney disease with or without a requirement for haemodialysis91,92 and using i.v. ferric carboxymaltose in patients with non-dialysis-dependent chronic kidney disease,93,94 each of which showed that the haematopoietic response was significantly greater with i.v. iron vs. oral iron therapy.
In post-partum iron deficiency anaemia, numerous randomized trials have compared the efficacy and safety of i.v. vs. oral iron.95 These have consistently shown a more rapid improvement in iron stores and Hb levels with i.v. preparations, accompanied by improved fatigue and quality of life vs. oral iron therapy, with improved compliance. Using non-dextran i.v. iron preparations, safety was good, with fewer gastrointestinal adverse events than oral iron.95 Similarly, in chemotherapy-induced anaemia, a recent meta-analysis of 11 randomized trials of i.v. vs. oral iron therapy concluded that i.v. iron was associated with an improved haematopoietic response with no safety concerns, and that the requirement for blood transfusion was lower than with oral iron.96 Lastly, several randomized trials testing the effect of i.v. vs. oral iron therapy in patients with iron deficiency anaemia associated with inflammatory bowel disease have shown a faster and more prolonged haematopoietic response with i.v. therapy, leading to recommendations from the ‘Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases’ that i.v. should be the preferred route for administration.97 One recent large randomized trial of 338 patients with inflammatory bowel disease and iron deficiency anaemia (Hb <12 g/dL and TSAT <20%) showed a trend to improved efficacy with oral iron (iron sulfate 200 mg/day) vs. i.v. iron isomaltoside 1000 in terms of the primary efficacy endpoint of change in Hb from baseline to week 8 (P = 0.09), but patients with known intolerance to oral iron were excluded and i.v. iron dosing may have been inadequate; therefore; further trial data are awaited.98
Across a range of clinical settings, randomized trials have shown that oral iron therapy does not replenish iron stores, including non-dialysis-dependent chronic kidney disease,91–93 inflammatory bowel disease,98,99 heavy uterine bleeding,100 and post-partum anaemia.101–103
Conclusion
Iron deficiency is widespread in patients with heart failure. Currently, however, it is not universally monitored or managed despite convincing evidence that iron deficiency is a risk factor for poor outcome independent of anaemia status. Iron is poorly absorbed from oral preparations, compounded by chronic inflammation and other contributing factors in patients with heart failure, which delays or prohibits iron replenishment. Evidence for a clinical benefit using oral iron preparations in heart failure is lacking. Clinical studies have instead focused on the use of i.v. iron, which bypasses the problem of gastrointestinal absorption and poor compliance. Early studies using iron sucrose consistently showed that correction of iron deficiency improved exercise capacity, cardiac function, symptom severity, and quality of life.32,76,77,79 These studies were recently confirmed in the large 6-month FAIR-HF study, where functional capacity, symptoms, and quality of life rapidly increased in patients with heart failure of NYHA class II–III and impaired LVEF15 in comparison with placebo treatment. Longer term data were provided by the CONFIRM-HF trial, in which symptomatic, iron-deficient patients with heart failure showed a sustained improvement in functional capacity, symptoms, and quality of life, and reduced risk of hospitalization due to worsening heart failure over 1 year of i.v. iron therapy.16 The ESC Guidelines point out that ferric carboxymaltose may be considered for treatment of these patients, although the effect in patients with preserved LVEF remains unknown.17 It should also be borne in mind that assessment of the hard endpoint of mortality is not feasible within the population sizes possible in controlled studies, and that a full assessment of safety would require more extended follow-up. The structures of new i.v. preparations permit far larger doses of iron to be administered safely in a single visit. Routine diagnosis and management of iron deficiency in patients with symptomatic heart failure, regardless of anaemia status, and prompt intervention using i.v. iron therapy should now be considered, pending the outcome of larger ongoing randomized controlled trials and longer term safety data.
Funding
An initial draft of this article was developed by a freelance medical writer with funding from Vifor Pharma, based on an agreed outline approved by the authors. Following revisions, the final draft was approved for submission by the authors. The authors received no honoraria in relation to the current article, which was written in accordance with ICJME Guidelines.
Conflicts of interest: T.M. has received honoraria from Vifor Pharma. I.C.M. has received speakers’ fees, honoraria, and consultancy fees from several manufacturers of ESAs and i.v. iron, including Affymax, AMAG, Amgen, Ortho Biotech, Pharmacosmos, Hoffmann-La Roche, Takeda, and Vifor Pharma.
References
- Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. 2011;4:599–606. doi: 10.1161/CIRCHEARTFAILURE.111.960906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–829. doi: 10.1093/eurheartj/ehs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister O, Evéquoz D, Mach F, Moschovitis G, Samiie K, Waeber G. Should anaemia and iron deficiency be treated in patients with chronic heart failure? Cardiovasc Med. 2012;15:109–115. [Google Scholar]
- van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–493. doi: 10.1038/nrcardio.2011.77. [DOI] [PubMed] [Google Scholar]
- Schena FP. Management of patients with chronic kidney disease. Intern Emerg Med. 2011;6(Suppl 1):77–83. doi: 10.1007/s11739-011-0688-2. [DOI] [PubMed] [Google Scholar]
- Sica DA. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail. 2003;9:287–292. doi: 10.1111/j.1527-5299.2003.02399.x. [DOI] [PubMed] [Google Scholar]
- Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonko DO, Anker SD. Anemia in chronic heart failure: pathogenetic mechanisms. J Card Fail. 2004;10(1 Suppl):S5–S9. doi: 10.1016/j.cardfail.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Scharf RE. Management of bleeding in patients using antithrombotic agents: prediction, prevention, protection and problem-oriented intervention. Hamostaseologie. 2009;29:388–398. [PubMed] [Google Scholar]
- von Haehling S, Anker MS, Jankowska EA, Ponikowski P, Anker SD. Anemia in chronic heart failure: Can we treat? What to treat? Heart Fail Rev. 2012;17:203–210. doi: 10.1007/s10741-011-9283-x. [DOI] [PubMed] [Google Scholar]
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J. 2012;34:827–834. doi: 10.1093/eurheartj/ehs377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAIR-HF Trial Investigators. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- for the CONFIRM-HF Investigators. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu385. pii:ehu385 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- Nanas JN, Matsouka C, Karageorgopolous D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleśkowska-Florek W, Zymliński R, Biegus J, Siwołowski P, Banasiak W, Anker SD, Filippatos G, Cleland JG, Ponikowski P. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35:2468–2476. doi: 10.1093/eurheartj/ehu235. [DOI] [PubMed] [Google Scholar]
- Comin-Colet J, Enjuanes C, González G, Torrens A, Cladellas M, Meroño O, Ribas N, Ruiz S, Gómez M, Verdú JM, Bruguera J. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15:1164–1172. doi: 10.1093/eurjhf/hft083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz JA, McAlister FA, Armstrong FW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- Arora NP, Ghali JK. Iron deficiency anemia in heart failure. Heart Fail Rev. 2012;18:485–501. doi: 10.1007/s10741-012-9342-y. [DOI] [PubMed] [Google Scholar]
- Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure: implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–480. doi: 10.1016/j.jacc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Aung N, Ling HZ, Cheng AS, Aggarwal S, Flint J, Mendonca M, Rashid M, Kang S, Weissert S, Coats CJ, Richards T, Thomas M, Woldman S, Okonko DO. Expansion of the red cell distribution width and evolving iron deficiency as predictors of poor outcome in chronic heart failure. Int J Cardiol. 2013;168:1997–2002. doi: 10.1016/j.ijcard.2012.12.091. [DOI] [PubMed] [Google Scholar]
- Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Cardiac Fail. 2011;17:899–906. doi: 10.1016/j.cardfail.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kasner M, Aleksandrov AS, Westermann D, Lassner D, Gross M, von Haehling S, Anker SD, Schultheiss HP, Tschöpe C. Functional iron deficiency and diastolic function in heart failure with preserved left ejection fraction. Int J Cardiol. 2013;168:4652–4657. doi: 10.1016/j.ijcard.2013.07.185. [DOI] [PubMed] [Google Scholar]
- Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der Meer P, Jankowska EA, Comín-Colet J. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174:268–275. doi: 10.1016/j.ijcard.2014.03.169. [DOI] [PubMed] [Google Scholar]
- Haas JD, Brownlie T., 4th Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–690S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- Willis WT, Gohil K, Brooks GA, Dallman PR. Iron deficiency: improved exercise performance within 15 h of iron treatment in rats. J Nutr. 1990;120:909–916. doi: 10.1093/jn/120.8.909. [DOI] [PubMed] [Google Scholar]
- Finch CA, Miller LR, Inamdar AR, Person R, Seiler K, Mackler B. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J Clin Invest. 1976;58:447–453. doi: 10.1172/JCI108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Guzmán Mentesana G, Báez AL, Lo Presti MS, Domínguez R, Córdoba R, Bazán C, Strauss M, Fretes R, Rivarola HW, Paglini-Oliva P. Functional and structural alterations of cardiac and skeletal muscle mitochondria in heart failure patients. Arch Med Res. 2014;45:237–246. doi: 10.1016/j.arcmed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CF, Lian WS. Prooxidant mechanisms in iron overload cardiomyopathy. Biomed Res Int. 2013;2013:740573. doi: 10.1155/2013/740573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo JT, Yeo PS, Ching-Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL. Chan M, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail. 2014;16:1125–1132. doi: 10.1002/ejhf.161. M Y. [DOI] [PubMed] [Google Scholar]
- Macdougall AC. Strategies for iron supplementation: oral versus intravenous. Kidney Int. 1999;55(Suppl 69):S61–S66. doi: 10.1046/j.1523-1755.1999.055suppl.69061.x. [DOI] [PubMed] [Google Scholar]
- Anker SD, von Haehling S. Iron Deficiency and Anemia in Heart Failure. 2nd ed. Bremen, Germany: UNI-MED Verlag; 2012. [Google Scholar]
- Crichton RR, Danielson BG, Geisser P. Iron Therapy with Special Emphasis on Intravenous Administration. 4th ed. Bremen, Germany: UNI-MED Verlag AG; 2008. [Google Scholar]
- Toblli JE, Cao G, Olivieri L, Angerosa M. Comparative study of gastrointestinal tract and liver toxicity of ferrous sulfate, iron amino chelate and iron polymaltose complex in normal rats. Pharmacology. 2008;82:127–137. doi: 10.1159/000142728. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Staun M, Bhandari S, Muñoz M. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis. 2013;7:429–440. doi: 10.1016/j.crohns.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Britton RS, Ramm GA, Olynyk J, Singh R, O’Neill R, Bacon BR. Pathophysiology of iron toxicity. Adv Exp Med Biol. 1994;356:239–253. doi: 10.1007/978-1-4615-2554-7_26. [DOI] [PubMed] [Google Scholar]
- Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12–33. doi: 10.3390/pharmaceutics3010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen K, Ulvik RJ, Grimstad T, Berstad A, Berge RK, Hausken T. Effects of ferrous sulphate and non-ionic iron–polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:831–838. doi: 10.1111/j.1365-2036.2005.02652.x. [DOI] [PubMed] [Google Scholar]
- Auerbach M. Clinical experience with intravenous iron. Transfus Altern Transfus Med. 2007;9(Suppl 2):26–30. [Google Scholar]
- Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23:1954–1962. doi: 10.1093/annonc/mds112. [DOI] [PubMed] [Google Scholar]
- Geisser P, Baer M, Schaub E. Structure/histoxicity relationship of parenteral iron preparations. Arzneimittelforschung. 1992;42:1439–1452. [PubMed] [Google Scholar]
- Toblli JE, Cao G, Oliveri L, Angerosa M. Assessment of the oxidative stress induced by intravenous ferumoxytol, ferric carboxymaltose, iron sucrose and iron dextran in a nonclinical model. Arzneimittelforschung. 2011;61:399–410. doi: 10.1055/s-0031-1296218. [DOI] [PubMed] [Google Scholar]
- Toblli JE, Cao G, Oliveri L, Angerosa M. Evaluation of toxicity and oxidative stress induced by intravenous isomaltoside 1000 in a nonclinical model. Arzneimittelforschung. 2011;61:553–565. doi: 10.1055/s-0031-1300553. [DOI] [PubMed] [Google Scholar]
- Toblli JE, Cao G, Olivieri L, Angerosa M. Comparison of the renal, cardiovascular and hepatic toxicity data of original intravenous iron compounds. Nephrol Dial Transplant. 2010;25:3631–3640. doi: 10.1093/ndt/gfq260. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez N, Echeverria A, Sanchez-Ibarrola A, Páramo JA, Coma-Canella I. Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrology (Carlton) 2010;15:178–183. doi: 10.1111/j.1440-1797.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- Rangel EB, Espósito BP, Carneiro FD, Mallet AC, Matos AC, Andreoli MC, Guimarães-Souza NK, Santos BF. Labile plasma iron generation after intravenous iron is time-dependent and transitory in patients undergoing chronic hemodialysis. Ther Apheresis Dial. 2010;14:186–192. doi: 10.1111/j.1744-9987.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm. 2012;69:310–320. doi: 10.2146/ajhp110262. [DOI] [PubMed] [Google Scholar]
- Hayat A. Safety issues with intravenous iron products in the management of anemia in chronic kidney disease. Clin Med Res. 2008;6:93–102. doi: 10.3121/cmr.2008.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. 3.2. Using iron agents. Am J Kidney Dis. 2006;47(Suppl 3):S58–S70. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2 Suppl 2):568S–580S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- Ishida JH, Johansen KL. Iron and infection in hemodialysis patients. Semin Dial. 2014;27:26–36. doi: 10.1111/sdi.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;4:279–335. [Google Scholar]
- Silverberg SD, Schwartz D, Schwartz I, Ben AE. The missed opportunities to diagnose and treat iron deficiency in patients hospitalized with heart failure. Int J Cardiol. 2013;168:2164–2166. doi: 10.1016/j.ijcard.2013.01.249. [DOI] [PubMed] [Google Scholar]
- Ferinject® Summary of Product Characteristics. Vifor International Inc., St Gallen, Switzerland.
- Mor C, Fedele F, Vasko P, Frick M, Mitchell D. Diagnosis and treatment of iron deficiency and anemia in chronic heart failure: current practice in four European countries. Circulation. 2011;124 A14991. [Google Scholar]
- Sandek A, Doehner W, Anker SD, von Haehling S. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–85. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure. A link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64:1092–1102. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- Darshan D, Frazer DM, Anderson GJ. Molecular basis of iron-loading disorders. Expert Rev Mol Med. 2010;12:e36. doi: 10.1017/S1462399410001687. [DOI] [PubMed] [Google Scholar]
- Camaschella C, Silvestri L. New and old players in the hepcidin pathway. Haematologica. 2008;93:1441–1444. doi: 10.3324/haematol.13724. [DOI] [PubMed] [Google Scholar]
- Filippatos G, Farmakis D, Colet JC, Dickstein K, Lüscher TF, Willenheimer R, Parissis J, Gaudesius G, Mori C, von Eisenhart RB, Greenlaw N, Ford I, Ponikowski P, Anker SD. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail. 2013;15:1267–1276. doi: 10.1093/eurjhf/hft099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P, Johnson G, Wood L. Oral iron therapy in human subjects, comparative absorption between ferrous salts and iron polymaltose. J Med. 1984;15:367–377. [PubMed] [Google Scholar]
- Nielsen P, Kongi R, Buggisch P, Fischer R. Bioavailability of oral iron drugs as judged by a 59Fe-whole-body counting technique in patients with iron deficiency anaemia. Therapeutic efficacy of iron(II)–glycine sulfate. Arzneimittelforschung. 2005;55:376–381. doi: 10.1055/s-0031-1296875. [DOI] [PubMed] [Google Scholar]
- Macdougall IC, Geisser P. Use of intravenous iron supplementation in chronic kidney disease: an update. Iran J Kidney Dis. 2013;7:9–22. [PubMed] [Google Scholar]
- Macdougall IC. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: oral or intravenous? Curr Med Res Opin. 2010;26:473–482. doi: 10.1185/03007990903512461. [DOI] [PubMed] [Google Scholar]
- Palazzuoli A, Silverberg D, Iovine F, Capobianco S, Giannotti G, Calabrò A, Campagna SM, Nuti R. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006;152:e9–e15. doi: 10.1016/j.ahj.2006.08.005. 1096. [DOI] [PubMed] [Google Scholar]
- Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman ML, Sun Y, Knusel B, Armstrong P. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008;117:526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- Van Veldhuisen DJ, Dickstein K, Cohen-Solal A, Lok DJ, Wasserman SM, Baker N, Rosser D, Cleland JG, Ponikowski P. Randomized, double-blind, placebo-controlled study to evaluate the effect of two dosing regimens of darbepoetin alfa in patients with heart failure and anaemia. Eur Heart J. 2007;28:2208–2216. doi: 10.1093/eurheartj/ehm328. [DOI] [PubMed] [Google Scholar]
- Kourea K, Parissis JT, Farmakis D, Paraskevaidis I, Panou F, Filippatos G, Kremastinos DT. Effects of darbepoetin-alpha on quality of life and emotional stress in anemic patients with chronic heart failure. Eur J Cardiovasc Prevent Rehab. 2008;15:365–369. doi: 10.1097/HJR.0b013e3282f849d0. [DOI] [PubMed] [Google Scholar]
- Bolger AP, Bartlett FR, Penston HS, O’Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008;21:236–2423. [PubMed] [Google Scholar]
- Gaber R, Kotb NA, Ghazy M, Nagy HM, Salama M, Elhendy A. Tissue Doppler and strain rate imaging detect improvement of myocardial function in iron deficient patients with congestive heart failure after iron replacement therapy. Echocardiography. 2012;29:13–18. doi: 10.1111/j.1540-8175.2011.01532.x. [DOI] [PubMed] [Google Scholar]
- Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Toblli JE, Di Gennaro FP. Hospitalization and mortality in elderly cardio-renal patients with iron deficiency anemia receiving intravenous iron therapy: a five year follow-up from a pilot study. Circulation. 2012;126 A16373. [Google Scholar]
- Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, Ponikowski P, Anker SD. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR-HF trial: an analysis for the UK. Eur J Heart Fail. 2012;14:782–790. doi: 10.1093/eurjhf/hfs083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde LE, Bertoldi EG, Goldraich L, Polanczyk CA. Cost-effectiveness of heart failure therapies. Nat Rev Cardiol. 2013;10:338–354. doi: 10.1038/nrcardio.2013.60. [DOI] [PubMed] [Google Scholar]
- for the CONFIRM-HF Investigators. Ponikowski P, van Veldhuisen D, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Rationale and design of the CONFIRM-HF study: a double-blind, randomized, placebo-controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Heart Failure. 2014;1:52–58. doi: 10.1002/ehf2.12006. [DOI] [PubMed] [Google Scholar]
- Vullaganti S, Goldsmith J, Teruya S, Alvarez J, Helmke S, Maurer MS. Cardiovascular effects of hemoglobin response in patients receiving epoetin alfa and oral iron in heart failure with a preserved ejection fraction. J Geriatr Cardiol. 2014;11:100–105. doi: 10.3969/j.issn.1671-5411.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MS, Teruya S, Chakraborty B, Helmke S, Mancini D. Treating anemia in older adults with heart failure with a preserved ejection fraction with epoetin alfa: single-blind randomized clinical trial of safety and efficacy. Circ Heart Fail. 2013;6:254–263. doi: 10.1161/CIRCHEARTFAILURE.112.969717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012;14:423–429. doi: 10.1093/eurjhf/hfs017. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Schleinitz MD, Gemignani A, Wu WC. Outcomes of patients with chronic heart failure and iron deficiency treated with intravenous iron: a meta-analysis. Cardiovasc Hematol Disord Drug Targets. 2013;13:35–44. doi: 10.2174/1871529x11313010004. [DOI] [PubMed] [Google Scholar]
- The EFFECT-HF study. EFFECT-HF [ NCT01394562 http://clinicaltrials.gov/show/NCT01394562 ] (6 January 2014)
- Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168:3439–3442. doi: 10.1016/j.ijcard.2013.04.181. [DOI] [PubMed] [Google Scholar]
- Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1 doi: 10.1002/14651858.CD007857.pub2. CD007857. [DOI] [PubMed] [Google Scholar]
- Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJ. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26:1599–1607. doi: 10.1093/ndt/gfq613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall IC, Bock AH, Carrera F, Eckardt K-U, Gaillard C, Van Wyck D, Roubert B, Nolen JG, Roger SD. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29:2075–2084. doi: 10.1093/ndt/gfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafallah AA, Dennis AE. Iron deficiency anaemia in pregnancy and postpartum: pathophysiology and effect of oral versus intravenous iron therapy. J Pregnancy. 2012;2012:630519. doi: 10.1155/2012/630519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafter-Gvili A, Rozen-Zvi B, Vidal L, Leibovici L, Vansteenkiste J, Gafter U, Shpilberg O. Intravenous iron supplementation for the treatment of chemotherapy-induced anaemia—systematic review and meta-analysis of randomised controlled trials. Acta Oncol. 2013;52:18–29. doi: 10.3109/0284186X.2012.702921. [DOI] [PubMed] [Google Scholar]
- Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553. doi: 10.1002/ibd.20285. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Staun M, Tandon RK, Altorjay I, Thillainayagam AV, Gratzer C, Nijhawan S, Thomsen LL. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED) Am J Gastroenterol. 2013;108:1877–1888. doi: 10.1038/ajg.2013.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D’Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT®) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- for the Ferric Carboxymaltose Study Group. Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49:19–28. doi: 10.1111/j.1537-2995.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia. Obstet Gynecol. 2007;110:267–278. doi: 10.1097/01.AOG.0000275286.03283.18. [DOI] [PubMed] [Google Scholar]
- Breymann C, Gliga F, Bejenariu C, Strizhova N. Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. Int J Gynecol Obstet. 2008;101:67–73. doi: 10.1016/j.ijgo.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol. 2008;199:e1–e7. doi: 10.1016/j.ajog.2008.07.046. [DOI] [PubMed] [Google Scholar]


