Abstract
Agonists for TLR9 and Stimulator of IFN Gene (STING) act as vaccine adjuvants that induce type-1 immune responses. However, currently available CpG oligodeoxynucleotide (ODN) (K-type) induces IFNs only weakly and STING ligands rather induce type-2 immune responses, limiting their potential therapeutic applications. Here, we show a potent synergism between TLR9 and STING agonists. Together, they make an effective type-1 adjuvant and an anticancer agent. The synergistic effect between CpG ODN (K3) and STING-ligand cyclic GMP–AMP (cGAMP), culminating in NK cell IFN-γ (type-II IFN) production, is due to the concurrent effects of IL-12 and type-I IFNs, which are differentially regulated by IRF3/7, STING, and MyD88. The combination of CpG ODN with cGAMP is a potent type-1 adjuvant, capable of inducing strong Th1-type responses, as demonstrated by enhanced antigen-specific IgG2c and IFN-γ production, as well as cytotoxic CD8+ T-cell responses. In our murine tumor models, intratumoral injection of CpG ODN and cGAMP together reduced tumor size significantly compared with the singular treatments, acting as an antigen-free anticancer agent. Thus, the combination of CpG ODN and a STING ligand may offer therapeutic application as a potent type-II IFN inducer.
Keywords: Adjuvant, cGAMP, CpG ODN, IFN-γ, STING, TLR
Introduction
Pathogen-derived factors, such as LPS or unmethylated CpG DNA (CpG), stimulate innate immune cells to produce cytokines, such as IL-12 and type-I or type-II IFNs, which help generate Th1-type responses and cellular immunity 1,2. IL-12 acts on naïve CD4+ T cells to drive Th1 development and IFN-γ production 3,4. IFN-γ-producing Th1 cells, in turn, are the main players in the induction of type-1 immunity, which is distinguished by high phagocytic activity 5,6. Moreover, Th1 cells play key roles in the generation of antitumor immunity, helping with proper activation and effector functions of CTL, including IFN-γ production 7,8. Thus, agents that can induce strong Th1-type responses, CTL, and NK cells 9 are urgently needed, as they may play critical roles in developing efficient vaccine adjuvants or immunotherapeutic agents against intracellular pathogens or cancer.
CpG oligodeoxynucleotides (ODNs) are synthetic single-stranded DNAs containing unmethylated CpG motifs with imm-unostimulatory properties due to their resemblance to bacterial genomes, and are recognized by TLR9 in certain types of innate immune cells 10,11. Upon ligand binding, TLR9 signals through the adaptor molecule MyD88, leading to production of IRF7-dependent type-I IFNs and NF-κB-dependent cytokines 12. Additionally, in vivo, CpG ODNs have been reported to induce Th1-type responses because of the types of cytokines that are induced by CpG ODNs in APCs 12. Among the different types of CpG ODNs, D-type CpG ODNs strongly induce both type-I and type-II IFNs, but are not capable of inducing B-cell activation 12,13. K-type CpG ODNs (K3 CpG) strongly induce B-cell activation, resulting in IL-6 and antibody production, while they only weakly induce type-I and type-II IFNs 12,13. However, since D-type CpG ODNs form aggregates, only K3 CpG is available for clinical use 12,13.
Along with microbial DNA, host DNA can also become a danger signal, specifically if it inappropriately locates in the cytosol, thereby leading to production of IFNs and proinflammatory cytokines 14,15. One recently identified cytosolic DNA sensor is cyclic GMP–AMP (cGAMP) synthase, which catalyzes production of a noncanonical cyclic dinucleotide cGAMP (2′3′cGAMP), containing noncanonical 2′,5′ and 3′,5′ linkages between its purine nucleosides 16. Canonical cGAMP (3′3′) is synthesized within bacteria and differs from mammalian 2′3′cGAMP in that GMP and AMP nucleosides are joined by bis-(3′,5′) linkages 17,18.
In addition to cGAMP, c-di-AMP and c-di-GMP, which are cyclic dinucleotides of bacterial origin, are ligands for the adaptor molecule Stimulator of IFN Gene (STING) that signals through the TBK1-IRF3 axis to induce type-I IFN production and NF-κB-mediated cytokine production 19,20. Recent studies have shown that these cyclic dinucleotides function as potent vaccine adjuvants due to their ability to enhance antigen-specific T-cell and humoral immune responses 21. Nevertheless, our group previously demonstrated that a STING ligand, DMXAA, induces type-2 immune responses unexpectedly 22 via STING-IRF3-mediated production of type-I IFNs. As type-2 immune responses often fail to induce type-1 immune responses, the clinical usefulness of STING ligands, including cyclic dinucleotides, was debatable. For example, the most common adjuvant, aluminum salt (alum), lacks the ability to induce cell-mediated immunity, which is considered protective in cases of intracellular pathogen-derived diseases or cancer 23. To overcome this limitation, alum has been combined with many different kinds of adjuvants, including monophosphoryl lipid A 24 and CpG ODN 25.
Based on the evidence described above, we tried to overcome the issues that K3 CpG and cGAMP possess individually by combining K3 CpG and 3′3′cGAMP. We investigated the immunological characteristics, potency as a vaccine adjuvant and potential as an antitumor immunotherapeutic of this combination, as well as its mechanisms of action in vitro and in vivo. In vitro, the effect of combined K3 CpG and cGAMP was analyzed using human and mouse PBMCs (mPBMCs). Additionally, the effect of this combination was analyzed in vivo via an immunization model by measuring the induction of antigen-specific T- and B-cell responses after combination immunization. Finally, we evaluated the ability of combined K3 CpG and cGAMP to suppress tumor growth in a mouse tumor model. Our results suggest that the combination of K3 CpG and cGAMP makes a potent type-1 adjuvant and a promising immunotherapeutic agent for cancer.
Results
Combination of K3 CpG and cGAMP potently induces IFN-γ in human PBMCs (hPBMCs)
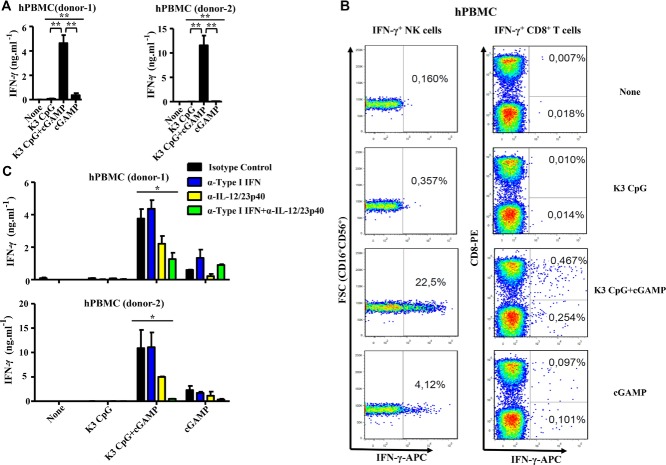
K3 CpG is a humanized K-type (also known as B) CpG ODN that has been reported to induce type-1 immune responses, yet only weakly induces IFNs 13,26. On the other hand, while cGAMP can induce robust type-I IFNs and acts as an adjuvant 21, other STING ligands were reported to induce type-2 immune responses 22. To overcome these known limits of K3 CpG and cGAMP, we examined the immunostimulatory properties of a combination of K3 CpG and the canonical 3′3′cGAMP in vitro in hPBMCs. After screening many cytokines using multiple hPBMCs to find interactions between TLR9- and STING-mediated signaling pathways (data not shown), we found that our combination displays potent synergism in the induction of IFN-γ, approximately 10- to 90-fold more than stimulation with K3 CpG or cGAMP alone (Fig. 1A).
Figure 1.
K3 CpG and cGAMP (TLR9 and STING agonists, respectively) synergistically induce innate IFN-γ production by human NK cells. (A) hPBMCs from two healthy donors were incubated with K3 CpG (10 μg/mL), cGAMP (10 μM), or K3 CpG (10 μg/mL) + cGAMP (10 μM) for 24 h and the supernatant IFN-γ concentrations were measured by ELISA. Data are representative of at least two independent experiments, and are shown as the mean + SD of duplicates from one experiment, representative of at least two performed. *p < 0.05; **p < 0.01 (one-way ANOVA with Bonferroni's multiple comparison test). (B) hPBMCs from three healthy donors were stimulated with K3 CpG, cGAMP, or K3 CpG + cGAMP for 16 h, with the last 4 h in the presence of Brefeldin A. After stimulation, cells were analyzed by flow cytometry for the detection of IFN-γ-producing cells. The percentage of IFN-γ-producing CD3+CD8+ T cells, CD3+CD8− T cells (including CD4+ T cells), and CD3−CD56+CD16+ NK cells are indicated in the quadrants. Data from one donor, which is representative of three donors, is shown. (C) hPBMCs from two healthy donors were treated with 5 μg/mL of isotype control, type-I IFN neutralizing, IL-12/23p40 neutralizing, or type-I IFN + IL-12/23p40 neutralizing antibodies 30 min prior to 24 h of stimulation with K3 CpG, cGAMP, or K3 CpG + cGAMP. IFN-γ production was measured by ELISA. Data are representative of at least two independent experiments, and are shown as the mean + SD of duplicates from one experiment, representative of at least two performed. *p < 0.05; **p < 0.01 (one-way ANOVA with Bonferroni's multiple comparison test).
Next, to identify the major IFN-γ-producing cell type in hPBMCs, we performed intracellular staining of IFN-γ in hPBMCs stimulated with K3 CpG, cGAMP, or the combination (gating strategy is shown in Supporting Information Fig. 2). Our results indicate that CD3−CD56+CD16+ NK cells are the major producers of synergistic IFN-γ among the hPBMCs in response to the combination stimulation, while CD8+ T cells and other cells produced a minimal amount of IFN-γ (Fig. 1B).
Type-I IFNs and IL-12 are capable of activating NK cells for IFN-γ production in addition to inducing type-1 immune responses 27,28. Therefore, we next examined the role of IL-12 and type-I IFNs in the combination-induced innate IFN-γ production in hPBMCs. Treatment with IL-12 neutralizing antibody partially reduced the synergistic IFN-γ induction by the combination stimulation (Fig. 1C). Although treatment with type-I IFN neutralizing antibody did not have any effect on the combination-induced IFN-γ production, neutralizing both type-I IFNs and IL-12 at the same time further reduced the synergistic IFN-γ production (Fig. 1C). These results suggest that IL-12 works in coordination with type-I IFNs for the synergistic production of IFN-γ by hPBMCs. Taken together, the results above indicate that, when combined, K3 CpG and cGAMP can be potent NK activators, leading to the production of large amounts of IFN-γ through mechanisms partially dependent on IL-12 and type-I IFNs.
Cellular and intracellular mechanisms of the synergistic IFN-γ induction by K3 CpG and cGAMP in mice
To examine the synergism between our TLR9 and STING agonists for early (innate) IFN-γ induction in mice, we stimulated mPBMCs in vitro with K3 CpG, cGAMP, or the combination. Large amounts of IFN-γ production were observed in a synergistic manner similar to what we observed in hPBMCs (Fig. 2A). Since IRF3 and IRF7 are the necessary downstream molecules for cGAMP- and CpG-mediated type-I IFN induction, respectively 17,29, we examined the roles of IRF3 and IRF7 in the synergistic IFN-γ production using mPBMCs derived from either mice deficient for both IRF3 and IRF7 (double knockout, DKO). The synergistic IFN-γ production was abrogated in the IRF3/7 DKO mPBMCs (Fig. 2A).
Figure 2.
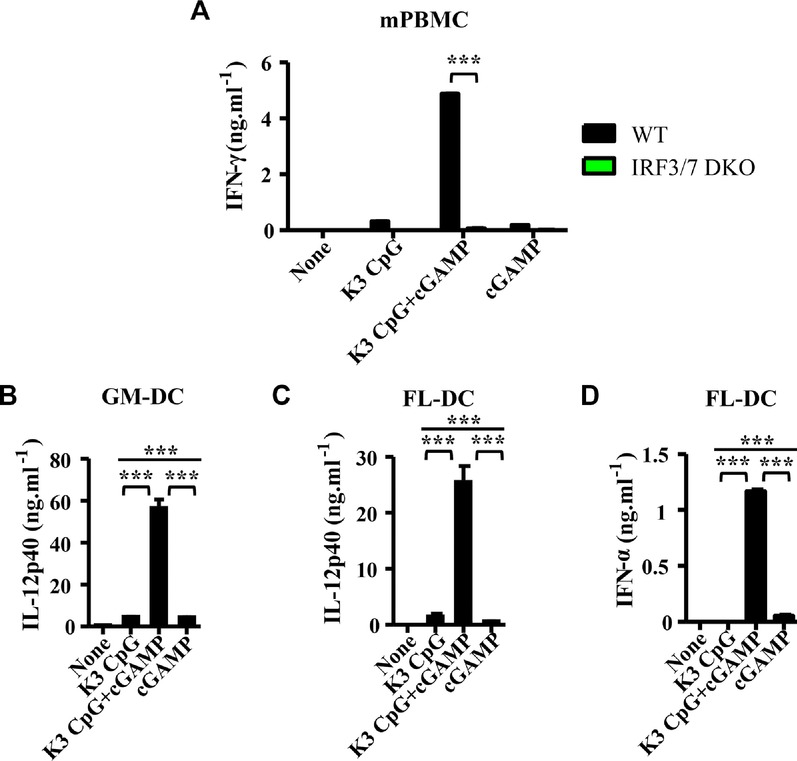
Combination of K3 CpG and cGAMP causes synergistic induction of innate IFN-γ in mPBMCs in an IRF3/7-dependent manner and production of IFN-α and IL-12 by DCs. (A) mPBMCs from WT and IRF3/7 DKO mice were stimulated with K3 CpG, cGAMP, or K3 CpG + cGAMP for 24 h and IFN-γ production was measured by ELISA. Data are representative of two independent experiments, and are shown as the mean + SEM of duplicates from one experiment, representative of two performed. ***p < 0.001 (Student's t-test); (B) GM-DCs were stimulated with K3 CpG, cGAMP, or K3 CpG + cGAMP for 24 h, and IL-12p40 production was measured by ELISA. (C and D) FL-DCs were stimulated with K3 CpG, cGAMP, or K3 CpG + cGAMP for 24 h, and (C) IL-12p40 and (D) IFN-α production were measured by ELISA. (B to D) Data are representative of two independent experiments and are shown as the mean + SD of duplicates from one experiment, representative of two performed. ***p < 0.001 (one-way ANOVA with Bonferroni's multiple comparison test).
As IL-12 and type-I IFNs are responsible for the synergistic IFN-γ production in hPBMCs (Fig. 1C), we further examined the ability of combined K3 CpG and cGAMP to activate dendritic cells (DC) that can produce IL-12 and/or type-I IFNs. When we incubated GM-CSF-derived DCs (GM-DCs) and Flt3L-derived DCs (FL-DCs) with K3 CpG, cGAMP, or the combination, we found a similar synergy to the one we observed in mPBMCs (Fig. 2B to D). The combination of K3 CpG and cGAMP induced significantly higher IL-12p40 production by both GM-DCs (Fig. 2B) and FL-DCs (Fig. 2C), and significantly higher IFN-α production by FL-DCs (Fig. 2D) than the amounts induced by singular stimulations. This suggests a potential role for IL-12 and type-I IFNs in the synergistic IFN-γ induction by our combination. Together these results demonstrate that the synergy between K3 CpG and cGAMP that potently induces IFN-γ in hPBMCs was reproduced in mice. The mechanisms for this synergism involve IRF3/7-mediated intracellular signaling, and the synergy induces type-I IFNs by plasmacytoid DCs (pDCs) as well as IL-12 production by both conventional DCs and pDCs.
TLR9/STING agonists induce type-1 immunity, CD8+ T cells, and suppress type-2 immunity
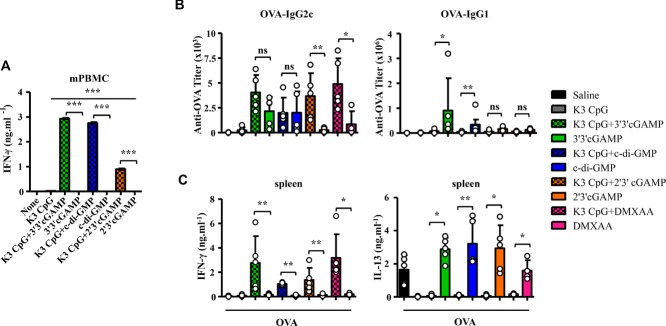
Given the presence of different kinds of agonistic STING ligands, c-di-GMP, the mammalian 2′3′cGAMP and DMXAA, which was reported to induce type-2 immune responses 18,19,22, we next examined the ability of K3 CpG to synergize with these other STING ligands. mPBMCs stimulated with not only 3′3′cGAMP, but also 2′3′cGAMP and c-di-GMP synergized with K3 CpG to induce innate IFN-γ production (Fig. 3A).
Figure 3.
Combinations of TLR9 and STING agonists are potent type-1 adjuvants that also suppress type-2 immune responses in vivo. (A) mPBMCs were stimulated with K3 CpG (10 μg/mL), STING agonists (10 μM), or K3 CpG + STING agonists for 24 h and IFN-γ production was measured by ELISA. Data are representative of two independent experiments, and are shown as the mean + SEM of duplicates from one experiment, representative of two performed.*p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA with Bonferroni's multiple comparison test). (B and C) Mice (n ≥ 4) were immunized i.m. with OVA (10 μg) with or without K3 CpG (10 μg), 3′3′/2′3′cGAMP (1 μg), c-di-GMP (1 μg), DMXAA (50 μg), or K3 + 3′3′/ 2′3′cGAMP/c-di-GMP/ DMXAA at days 0 and 10. (B) On day 17, OVA-specific serum IgG1 and IgG2c were measured by ELISA. (C) Spleen cells were stimulated with OVA (10 μg/mL) protein for 48 h. Production of IFN-γ and IL-13 were measured by ELISA. (B and C) Each symbol represents an individual mouse. Data are representative of two independent experiments and are shown as the mean + SD of biological replicates from one experiment, representative of two performed. *p < 0.05; **p < 0.01 (Mann–Whitney U-test).
To evaluate the adjuvant properties of these combinations in vivo, we immunized mice with the OVA protein and K3 CpG, STING agonists, or combinations of K3 CpG and STING agonists twice, at days 0 and 10. At day 17, antigen-specific antibody responses and spleen cell responses were examined. All mouse groups adjuvanted with STING agonists, such as cGAMP, c-di-GMP, and DMXAA, but not those adjuvanted with the TLR9 agonist, K3 CpG, had type-2 immune responses characterized by a high titer of serum anti-OVA IgG1 (Fig. 3B), and OVA-specific IL-13 production by splenocytes (Fig. 3C). By sharp contrast, the addition of K3 CpG converted all of the type-2 immune responses induced by STING agonists into type-1 immune responses, characterized by strong induction of OVA-specific serum IgG2c and splenocyte IFN-γ, while shutting down OVA-specific IgG1 and IL-13 production (Fig. 3B and C). We also observed synergistic induction of IFN-γ by OVA-specific CD8+ T cells (Supporting Information Fig 1A). Furthermore, our in vivo CTL cytotoxicity assay (gating strategy is shown in Supporting Information Fig. 3) revealed that compared to the PBS, K3 CpG, or cGAMP immunization groups, combination of K3 CpG and cGAMP could induce strong antigen-specific CD8+ CTL cytotoxicity (Supporting Information Fig. 1B) These results suggest that combinations of TLR9 and STING agonists result in potent type-1 adjuvants, capable of inducing robust CD8+ T-cell responses, in addition to the induction of synergistic adaptive IFN-γ production in the antigen-stimulated spleen cells of the combination-immunized mice, and of suppressing the type-2 immune responses that are induced by STING ligands.
Synergistic induction of IFN-γ depends on IRF3/7, STING, MyD88, IL-12, and type-I IFN signaling
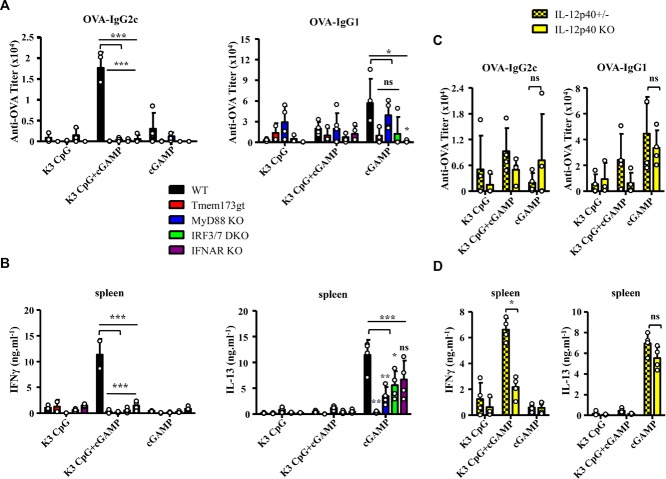
We showed in mPBMCs that synergistic production of innate IFN-γ was completely dependent on IRF3 and IRF7, which are required for the induction of type-I IFNs by cGAMP and K3 CpG, respectively. Since cGAMP is a ligand for STING, and K3 CpG is a ligand for TLR9 that signals via the adapter molecule MyD88, we evaluated the involvement of IRF3/7, MyD88, STING, and type-I IFNs in the combination-induced synergistic production of antigen-specific IFN-γ, using IRF3/7 DKO, IFN-α/β receptor (IFNAR) KO, MyD88 KO, and STING mutant mice. Combination-induced antigen-specific IgG2c in the sera and IFN-γ production by spleen were significantly decreased in the STING mutant, IRF3/7 DKO, MyD88 KO, and IFNAR KO mice, compared with the WT mice (Fig. 4A and B).
Figure 4.
The synergistic effect of the combination of K3 CpG and cGAMP on antigen-specific IFN-γ induction is dependent on IRF3/7, STING, MyD88, IL-12, and type-I IFN signaling. (A) WT, Tmem173gt, IRF3/7 DKO, MyD88 KO, and IFNAR KO C57BL/6J mice (n ≥ 3) were immunized with OVA and K3 CpG, cGAMP, or K3 + cGAMP at days 0 and 10, via the i.m. route. On day 17, OVA-specific serum IgG2c and IgG1 were measured by ELISA. Each symbols represent an individual mouse and data are representative of two independent experiments and are shown as the mean + SD of biological replicates from one experiment, representative of two performed. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA with Bonferroni's multiple comparison test). (B) Spleen cells from immunized mice were stimulated with OVA for 48 h. Production of IFN-γ and IL-13 were measured by ELISA. Data are representative of two independent experiments and are shown as the mean + SD of biological replicates from one experiment, representative of two performed. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA with Bonferroni's multiple comparison test). (C) IL-12p40 +/− and −/− C57BL/6J mice were immunized with OVA and K3 CpG, cGAMP, or K3 CpG + cGAMP at days 0 and 10, via the i.m. route. On day 17, OVA-specific serum IgG2c and IgG1 were measured by ELISA. Data are representative of two independent experiments and are shown as the mean + SD of biological replicates from one experiment, representative of two performed. *p < 0.05 (Mann–Whitney U-test). (D) Spleen cells were stimulated with OVA protein for 48 h. Production of IFN-γ was measured by ELISA. Data are representative of two independent experiments and are shown as the mean + SD of biological replicates from one experiment, representative of two performed. *p < 0.05 (Mann–Whitney U-test).
Our in vitro studies in mouse and hPBMCs also showed that IL-12 contributes to synergistic induction of innate IFN-γ. Therefore, we next investigated the involvement of IL-12 by using IL-12p40+/− and IL-12p40−/− mice. We found that IL-12p40 was required for the synergistic induction of antigen-specific IFN-γ, but not for the induction of IgG2c antibody responses (Fig. 4C and D). Overall our results suggest that the combination of K3 CpG and cGAMP is a potent type-1 adjuvant, synergistically inducing the production of antigen-specific IFN-γ in an IRF3/7, STING, MyD88, IL-12, and type-I IFN signaling-dependent manner.
Combination of K3 CpG and cGAMP efficiently suppresses tumor growth in a murine model
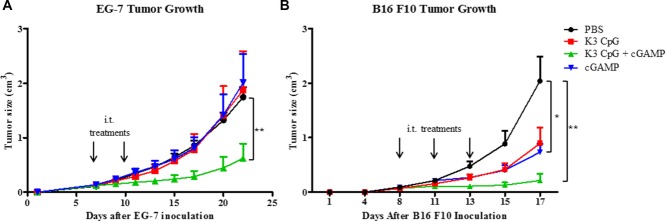
Because Th1 and CD8+ T-cell responses are important for the generation of antitumor immunity, we investigated the immunotherapeutic potential of the K3 CpG and cGAMP combination in a mouse tumor model. We inoculated mice with OVA-expressing EG-7 lymphoma cells by s.c. injection. On days 7 and 10, mice were given intratumor injections of PBS, K3 CpG (10 μg), cGAMP (10 μg), or K3 CpG and cGAMP. Combination treatment significantly suppressed the tumor growth compared with PBS, K3 CpG, or cGAMP treatments (Fig. 5A), suggesting that our combination can work as an antigen-free immunotherapeutic agent for cancer. In addition, the antitumor effect of the combination, in the EG-7 tumor model, was dependent on the CD8+ T-cell activity, rather than the NK-cell activity, as the combination failed to suppress the tumor growth in the RAG2 KO mice (Supporting information Fig. 4B), and significantly higher amounts of IFN-γ were produced only by the OVA-specific CD8+ T cells of the mice that were treated with the combination (Supporting Information Fig. 4A).
Figure 5.
The combination of K3 CpG and cGAMP efficiently suppresses tumors in the EG-7 and B16 F10 mouse tumor models. (A) Mice were injected with 1 × 106 EG-7 lymphoma cells (in 100 μL of PBS) s.c. on day 0. On days 7 and 10, mice were given intratumor injections of PBS (n = 8), K3 CpG (n = 8), cGAMP (n = 8), or K3 CpG + cGAMP (n = 9), and were monitored for tumor growth for 22 days. (B) Mice were injected with 0.5 × 106 B16 F10 cells (in 100 μL of PBS) s.c. on day 0. On days 8, 11, and 13, mice were given intratumor injections of PBS (n = 8), K3 CpG (n = 8), cGAMP (n = 8), or K3 CpG + cGAMP (n = 8), and mice were monitored for tumor growth for 17 days. Data are representative of two independent experiments and are shown as the mean + SEM of biological replicates from one experiment, representative of two performed. *p < 0.05; **p < 0.01 (Mann–Whitney U-test).
To investigate the antitumor effect of our combination in a tumor model that does not express an artificial antigen, such as OVA, we inoculated mice with B16 F10 melanoma cells that were shown to rely on NK cells for clearance 30 by s.c. injection. On days 8, 11, and 13, mice were given intratumor injections of PBS, K3 CpG (10 μg), cGAMP (10 μg), or K3 CpG and cGAMP. Although cGAMP showed a significant antitumor effect compared to the PBS treatment group, antitumor effect of the combination was the strongest among all groups (Fig. 5B).
Discussion
Efficient vaccines against intracellular pathogens or cancer require adjuvants that induce type-1 immune responses. Cyclic dinucleotides, such as cGAMP and c-di-GMP, have attracted attention as potential vaccine adjuvants because they directly bind to the transmembrane molecule STING and activate the TBK1-IRF3-dependent signaling pathway to induce type-I IFNs 31. However, evidence that STING agonists induce type-2 immune responses 22, rather than protective type-1 immune responses, suggests that their potential therapeutic applications are limited. In this study, we solve this issue by combining STING-agonists with K3 CpG, a TLR9 ligand. This combination synergistically enhances innate and adaptive IFN-γ production. It acts as a potent type-1 adjuvant, strongly inducing antibody responses, and CD4+ Th1 and CD8+ T cells, and as an antitumor agent that can efficiently suppress tumor growth in mouse tumor models of lymphoma and melanoma.
The current study demonstrates that the combination of K3 CpG and cGAMP synergistically induces innate IFN-γ production in both human and mPBMCs (Figs.1 and2), suggesting that this phenomenon is conserved between human and mouse. Importantly, combination stimulation does not affect cell viability (Supporting Information Fig. 5), which may affect cytokine production. Our in vitro results also demonstrate that the mechanisms of action involve IL-12 and type-I IFNs. Specifically, during the synergism between K3 CpG and cGAMP, type-I IFNs were dispensable since the loss of their effect can be compensated by the increased production of IL-12 (Fig. 1C and Supporting Information Fig. 6B). A previous report suggested that type-I IFNs and IL-12 can synergistically induce IFN-γ production by CD4+ T cells after Listeria monocytogenes infection. They showed that the synergy was significantly decreased in the absence of both cytokines, but partially decreased in the absence of either one of the cytokines, which is consistent with our results 32. Moreover, we found that, similar to the synergy observed in PBMCs, our combination can synergistically induce IL-12p40 production in GM-DCs and FL-DCs (Fig. 2C and D), suggesting a potential role for conventional and plasmacytoid DCs in the combination-induced synergy. A similar IL-12 synergy was reported by Krummen et al. by the combination of TLR ligands, CpG and Poly I:C, in BM-derived DCs that required the combination of MyD88- and TRIF-dependent signaling pathways 33. Our results also demonstrate that the combination of molecules activating MyD88-dependent (TLR9) and independent (STING) signaling pathways results in a robust immunostimulatory agent, suggesting that such combinations might be useful for immunotherapeutic applications.
According to our findings, NK cells are the major IFN-γ-producing cells in the hPBMC culture following combination stimulation (Fig. 1B). On the other hand, previous reports have shown that although NK cells express low levels of TLR9, cells that respond to CpG stimulation are the TLR9-expressing pDCs and B cells in hPBMCs 34. Also, IL-12 and type-I IFNs have been reported to regulate IFN-γ production and cytotoxicity in NK cells 28,35. Given those reports and our in vitro data, our proposed mechanism for the synergistic induction of innate IFN-γ is that mainly pDCs may respond to K3 CpG, while, together with pDCs, other cells, such as conventional DCs or macrophages, may respond to cGAMP to produce high amounts of type-I IFNs and IL-12, which then synergize to induce IFN-γ production in NK cells, by signaling through IL-12 and type-I IFN receptors (Supporting Information Fig. 6A).
The first report about the adjuvant effect of 2′3′cGAMP showed that i.m. cGAMP immunization can induce antigen-specific B- and T-cell responses in a STING-dependent manner 21. Our in vivo immunization studies using 3′3′cGAMP are also consistent with the previous reports; it induces strong antigen-specific B- and T-cell responses in a STING-dependent manner (Fig. 4A and B). We also showed that 3′3′cGAMP is a type-2 adjuvant that can induce not only IgG1, but also IgG2c antibody responses and Th2-type cytokine responses in spleen cells (Fig. 3B and C). Although type-2 adjuvants do not usually induce the production of Th1-like Ig isotype (IgG2c), cGAMP can do so, probably due to its ability to induce type-I IFNs, since type-I IFNs induce IgG2c antibody responses 36. Moreover, we found that distinct mechanisms were involved in the induction of B- and T-cell responses by cGAMP, in which cGAMP-induced antibody responses, but not Th2 responses, were dependent on type-I IFN signaling (Fig. 4B). In addition, because cGAMP is known to signal only through the STING-IRF3 axis to induce type-I IFN production 17, we expected to observe the loss of antibody and T-cell responses in IRF3/7 DKO mice. However, while cGAMP-induced antibody responses were slightly reduced in the IRF3/7 DKO mice, cGAMP-induced T-cell responses were partially dependent on IRF3/7 and, surprisingly, on MyD88, although such effects were completely dependent on STING (Fig. 4A and B). Therefore, we are further investigating the possibility that in addition to the STING-IRF3 pathway, cGAMP might activate an unknown signaling pathway that involves the adapter molecule MyD88.
Although K3 CpG was reported as an adjuvant capable of inducing type-1 immune responses 37, we found that K3 CpG by itself was a weak type-1 adjuvant, as it failed to induce antigen-specific antibody or T-cell responses at levels comparable with the cGAMP or combination immunization groups (Fig. 3B and C). Interestingly, the combination of a weak type-1 adjuvant, K3 CpG, with a type-2 adjuvant, cGAMP, resulted in a strong type-1 adjuvant that induced synergistic antigen-specific IFN-γ production and strong Th1-like antibody and CD8+ T-cell responses (Fig. 3 and Supporting Information Fig. 1). Our findings are also consistent with a previous study showing that the combination of CpG and IFA, a type-2 adjuvant, induces type-1 immune responses while suppressing type-2 immune responses 37. Importantly, in addition to the induction of potent type-1 immune responses by our combination, we showed that it can also suppress the type-2 responses that are induced by cGAMP that is important for increased safety as dominant type-2 responses have been reported to cause a number of chronic diseases, such as allergy 5,38,39. Our results are also consistent with the findings of Lin et al., in that production of IgG2c was enhanced while the production of IgG1 was suppressed by CpG 40. Furthermore, the synergistic effect of our combination on antigen-specific IFN-γ induction is dependent on IRF3 and IRF7 (Fig. 4A and B), indicating that type-I IFNs may also play an important role in this synergy. This idea is further supported by the complete abolishment of synergy that we observed in IFNAR KO mice (Fig. 4A and B). Moreover, because MyD88 is a downstream signaling molecule of TLR9 and cGAMP is a ligand of STING, we found that the type-1 immunity-inducing effect of the combination is dependent on both MyD88 and STING, as expected (Fig. 4A and B). On the other hand, we showed that IL-12p40 is required for the synergistic induction of Th1-type cytokine responses, but not for the induction of IgG2c antibody responses (Fig. 4C and D). Because IL-12 is important for Th1-cell development and IFN-γ production 3,4, it is reasonable to observe IL-12 dependency in the Th1-type cytokine responses. A possible explanation for the IL-12-independent IgG2c induction by our combination could be that production of type-I IFNs in the KO mice might be compensating for the absence of IL-12. Previous reports showed that type-I IFNs can induce IgG2c antibody responses in a T-cell-independent manner 36, while IL-12 induces IgG2c antibody responses by inducing IFN-γ production from T or NK cells 41. In addition, use of anti-IL-12/23p40 neutralizing antibodies in our in vitro studies and IL-12p40 mice in the in vivo studies cannot rule out the possible involvement of IL-23 in the mechanisms of innate or adaptive IFN-γ synergy, as IL-23 signaling, which was shown to affect NK-cell activation and T-cell responses 42,43, will be defective in both experimental designs. Our studies regarding this issue showed that although no antigen-specific IL-17 was detected in the spleen cell cultures of the immunized mice as an indirect indicator of in vivo IL-23 induction, and no IL-23 was induced in mPBMCs by combination stimulation, IL-23 is induced in the FL-DCs only by combination stimulation, but not by cGAMP or K3 CpG stimulations (data not shown), suggesting a possible role for IL-23 in the mechanisms of innate or adaptive IFN-γ synergy, which needs further investigation.
Finally, we found the K3 CpG and cGAMP combination has a strong antitumor effect, as only treatment with the combination could efficiently suppressed tumor growth in the EG-7 mouse tumor model (Fig. 5A). Because our in vivo results show that the combination induces strong CD8+ T-cell responses (Supporting Information Fig. 1A and B), and the antitumor effect of the combination is lost in the RAG2 KO mice (Supporting Information Fig. 4B), which lacks CD8+ T cells, we concluded that the antitumor effect of our combination is due to the induction of robust CD8+ cytotoxic T-cell activation. Our hypothesis is supported by a previous report showing that vaccination with OVA-conjugated CpG ODN also has potent antitumor effects, which are dependent on CD8+ T cells 44. Moreover, since we identified NK cells as the main players in the IFN-γ synergy in our in vitro hPBMC studies, we also investigated the antitumor effect of our combination in the B16 F10 mouse melanoma tumor model, which relies on NK cells for clearance 30 and does not express artificial antigens. Although cGAMP, itself, could significantly suppress the tumor growth compared to the control group, combination had the strongest antitumor effect, resulting in almost complete tumor elimination (Fig. 5B). Thus, our combination is a strong antitumor agent, capable of suppressing tumors that relies not only on CD8+ T cells, but also on NK cells for clearance. Furthermore, the advantage of our combination therapy over previously reported CpG-based antitumor agents, such as OVA-conjugated CpG ODN 44 or nanoparticle-conjugated CpG ODN 45, is that it does not require a chemical conjugation between K3 CpG and cGAMP. Additionally, unlike those systems, our approach does not require the injection or conjugation of a tumor antigen. It works as an antigen-free antitumor agent rather than a preventive vaccine.
In conclusion, our study suggests that combination of TLR9 and STING agonists is an advantageous type-1 adjuvant for vaccines requiring strong cellular immune responses, and a promising antitumor agent that can also stimulate human NK cells for synergistic IFN-γ production. Thus, our results provide insight into the mechanisms of the combined action of TLR9 and STING signaling pathways, which potentially promote the immunotherapeutic and adjuvant properties of our combination.
Materials and methods
Mice
Seven- to ten-week-old female C57BL/6J mice were purchased from CLEA Japan, Inc. (Osaka, Japan). MyD88 KO mice were purchased from Oriental BioService, Inc. (Kyoto, Japan). IL-12p40 KO and STING mutant mice (Tmem173gt), which have a loss-of-function mutation at the ligand-binding site of STING 46, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). IRF3/7 DKO mice were generated from IRF3 KO 22 and IRF7 KO mice, the latter of which was provided by the RIKEN BRC (Ibaraki, Japan) via the National Bio-Resource Project of the MEXT, Japan 47. IFNAR2 KO mice were obtained from B&K Universal. All of the animal experiments were conducted according to the guidelines of the Animal Care and Use Committee of RIMD and IFReC of Osaka University, and the use of animals was approved by Osaka University.
Reagents
The 2′3′ and 3′3′ cGAMPs were purchased from Invivogen (San Diego, CA, USA). DMXAA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 5% NaHCO3. Yamasa (Chiba, Japan) kindly donated c-di-GMP. OVA was purchased from Kanto Chemical (Osaka, Japan) and the endotoxin levels were determined by Toxicolor® (Seikagaku Corp., Tokyo, Japan) as less than 1 EU/mg. K3 CpG ODN was synthesized by GeneDesign as previously described 48. CFSE was purchased from Life Technologies (Carlsbad, CA, USA).
Immunizations and spleen cell cultures
After anesthetization, C57BL/6J mice were i.m. immunized with OVA (10 μg), or OVA and K3 CpG (10 μg), DMXAA (50 μg), c-di-GMP (1 μg), 2′3′ or 3′3′ cGAMP (1 μg), or K3 CpG + 2′3′/3′3′cGAMP/c-di-GMP/DMXAA at days 0 and 10. On day 17, OVA-specific serum IgG1 and IgG2c were measured by ELISA as previously described 49. The secondary antibodies used in IgG2c and IgG1 ELISAs were horseradish peroxidase conjugated goat anti-mouse IgG2c and IgG1 (Bethyl Laboratories, Montgomery, TX). On day 17, spleen cells were collected and single cell suspensions were prepared using a gentleMACS dissociator (Miltenyi Biotech, Gladbach, Germany). After red blood cell lysis using Tris-NH4Cl buffer, cells were cultured in RPMI (containing 1% penicillin/streptomycin and 10% fetal calf serum [FCS)] and stimulated with total OVA (10 μg/mL) or OVA peptides (10 μg/mL) that are specific for MHC class I (OVA 257) or MHC class II (OVA 323) for 48 h. Production of IFN-γ and IL-13 were measured by ELISA.
hPBMC isolation and stimulation
All hPBMC experiments were conducted following approval from the Institutional Review Board of the National Institute of Biomedical Innovation. hPBMCs were isolated from the blood of healthy volunteer blood donors using human lymphocyte separation medium (IBL, Japan), and 1 × 106 cells were cultured in RPMI. PBMCs were stimulated with K3 CpG (10 μg/mL), cGAMP (10 μM), or K3 CpG + cGAMP for 24 h and production of IFN-γ and IL-12 were measured by ELISA.
For in vitro neutralization experiments, hPBMCs that were cultured as described above were subjected to IL-12/23p40 (clone: C8.6, BioLegend, San Diego, CA, USA), type-I IFN (clone: MMHAR-2, PBL Interferon Source, Piscataway, NJ, USA), or both IL-12/23p40 and type-I IFN neutralizing antibody treatments (5 μg/mL) 30 min before 24 h of stimulation.
mPBMC and DC cultures
mPBMCs were isolated from C57BL/6J mice using mouse lymphocyte separation medium (IBL, Japan), and 0.5 × 106 cells were cultured in RPMI. GM-DC cultures were prepared by flushing BM cells from the tibia and femurs of C57BL/6J mice and culturing these cells for 7 days in the presence of 20 ng/mL of GM-CSF (PeproTech, Rocky Hill, NJ, USA). GM-DCs were cultured in RPMI, containing 1% penicillin/streptomycin and 20% FCS. FL-DC cultures were prepared from BM cells of C57BL/6J mice that were cultured for 7 days in the presence of 100 ng/mL of human Flt3L (PeproTech). FL-DCs were cultured in RPMI, containing 1% penicillin/streptomycin and 10% FCS.
In vitro cytotoxicity assay
Splenocytes were isolated from C57BL/6J mice and 1 × 106 cells were cultured in RPMI in 96-well round-bottom plates for 24 h with the stimulants. After the stimulation, in order to prepare a positive control, Triton X-100 was added into the nonstimulated cells that were incubated at 37°C for 15 min. After centrifugation, supernatants of the cells were mixed with the substrate mix and incubated for 15 min at room temperature. ODs at 490 nm were measured and the percent cytotoxicity was calculated according to the instructions of the Non-Radioactive Cytotoxicity Assay Kit (Promega, WI, USA).
Cytokine measurement
Mouse IL-12p40, mouse IL-13, human IFN-γ, and human IL-12 levels were measured using ELISA kits from R&D Systems (Minneapolis, MN, USA). Mouse IFN-γ levels were determined using an ELISA kit from BioLegend.
Staining for intracellular cytokine and cell surface molecules
hPBMCs were stimulated with K3 CpG (10 μg/mL), cGAMP (10 μM), or K3 CpG + cGAMP for 16 h, with the last 4 h being in the presence of Brefeldin A. After the stimulation, cells were harvested and stained for surface molecules with CD16-PerCP-Cy5.5 (BD Biosciences, Franklin Lake, NJ), CD56-BV421 (BioLegend), CD3-FITC (BD Biosciences), and CD8-PE (Miltenyi Biotech) antibodies. Fixed and permeabilized cells were stained with IFN-γ-allophycocyanin (BioLegend) for the detection of intracellular IFN-γ and analyzed using the BD FACSCANTO II flow cytometer.
In vivo CTL cytotoxicity assay
Six-week-old C57BL/6J mice were immunized with OVA (10 μg) only, or OVA and either K3 CpG (10 μg), cGAMP (1 μg), or K3 + cGAMP once, via the i.m. route. On day 7, splenocytes from the naïve C57BL/6J mice were labeled with 2 or 0.2 μM of CFSE for 10 min at 37°C. The cells, which were labeled with 2 μM of CFSE, were subjected to peptide pulsing by incubating them with the OVA257 (10 μg/mL) for 90 min at 37°C. Then, the cells were washed, and equal numbers from each cells were transferred to the immunized mice via the i.v. route. Splenocytes were isolated, and upon staining with the LIVE/DEAD® Fixable Near-IR Dead Cell Stain (Invitrogen, Carlsbad, CA, USA), CFSE-labeled cells were analyzed by flow cytometry 24 h after the transfer.
Tumor cells and treatment
EG-7-OVA thymoma cells were purchased from American Type Culture Collection (VA, USA) and cultured in RPMI. A total of 1 × 106 cells were s.c. injected to the back of mice on day 0. On days 7 and 10, mice were given intratumor injections of PBS (50 μL), K3 CpG (10 μg), cGAMP (10 μg), or K3 CpG + cGAMP, and mice were monitored for tumor growth for 22 days.
B16 F10 melanoma cells were purchased from RIKEN Cell Bank (Japan) and cultured in DMEM. A total of 0.5 × 106 cells were s.c. injected to the back of mice on day 0. On days 8, 11, and 13, mice were given intratumor injections of PBS (50 μL), K3 CpG (10 μg), cGAMP (10 μg), or K3 CpG + cGAMP, and mice were monitored for tumor growth for 17 days.
Statistical analysis
Mann–Whitney U-test, Student's t-test, or one-way ANOVA with Bonferroni's multiple comparison test were used for the statistical analyses (*p < 0.05; **p < 0.01; ***p < 0.001). Statistical analyses were performed using GraphPad Prism software (La Jolla, CA, USA).
Acknowledgments
We thank Dr. Cevayir Coban for her comments and critical reading of the manuscript. We also thank the staff of the Animal Resource Center for Infectious Diseases (IFReC and RIMD, Osaka University) for their support in these studies and Prof. Akira's group for providing us the use of their flow cytometer. This study was supported in part by the Adjuvant Database Grant. E.K. received a grant-in aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (grant number 24591145) and Takeda Science Foundation. E.K. was also supported by the Regional Strategy Support Program. B.T. received support in the form of a Japanese Government Scholarship from MEXT.
Glossary
- cGAMP
cyclic GMP—AMP
- FL-DC
Flt3L-derived DC
- GM-DC
GM-CSF-derived dendritic cell
- IFNAR
IFNα/β receptor
- pDC
plasmacytoid DC
- STING
Stimulator of IFN genes
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Figure S1. Combination of K3 CpGand cGAMPinduces potent CD8+T cell activity in vivo.
Figure S2. Gating strategy for intracellular IFN-γ staining experiment in hPBMCs. First, CD3+/-cells were gated from the lymphocyte gate.
Figure S3. Gating strategy for in vivo CTL assay.
Figure S4. CD8+ T cell activity, but not NK cell activity, is required for the anti-tumor effect of the combination of K3 CpG and cGAMP in the EG-7 tumor model.
Figure S5. Cytotoxicity ofthe STING ligands in the splenocytes.
Figure S6. (A)Proposed mechanisms of innate IFN-γ synergy.
Figure S7. No significant differences were observed between the PBS immunization groups of WT and STING mutant, or MyD88 KO mice.
Peer review correspondence
References
- Kawai T, Akira S. Review Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C, Macatonia SE, Tripp CS, Wolf SF, Garra AO, Murphy KM. Development of TH1 CD4+ T cells through IL-1 2 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Spellberg B, Edwards JE. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001;90509:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:1–6. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004;4:1–10. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- Barber GN. Cytoplasmic DNA innate immune pathways. Immunol. Rev. 2011;243:99–108. doi: 10.1111/j.1600-065X.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcwhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CK, Aoshi T, Jounai N, Ito J, Ohata K, Kobiyama K, Dessailly BH, et al. The chemotherapeutic agent DMXAA as a unique IRF3-dependent type-2 vaccine adjuvant. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front. Immunol. 2013;3:1–13. doi: 10.3389/fimmu.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MKL, Mckee AS, David A, Wang J, Mason R, Kappler JW. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc. Natl. Acad. Sci. USA. 2011;108:7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeratna RD, Mccluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000;18:1755–1762. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]
- Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG Motifs. J. Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- Hunter A, Gabriel KE, Radzanowski T, Neyer LE, Remington JS. Type I interferons enhance production of IFN-y by NK cells. Immunol. Lett. 1997;59:1–5. doi: 10.1016/s0165-2478(97)00091-6. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, et al. Coordinated and distinct roles for IFN-, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin—mediated natural killer cell recruitment. J. Exp. Med. 2005;202:1679–1689. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky TW, Kanne DB, Leong ML. Advances in vaccines rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines. 2013;1:131–143. doi: 10.1177/2051013613501988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and Type-I IFN synergize for IFN- production by CD4 T cells, whereas neither are required for IFN- production by CD8 T cells after Listeria monocytogenes infection. J. Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummen M, Balkow S, Shen L, Heinz S, Loquai C, Probst H-C, Grabbe S. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J. Leukoc. Biol. 2010;88:189–199. doi: 10.1189/jlb.0408228. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-g production is dependent on macrophage secretion of IL-12. Clin. Immunol. Immunopathol. 1997;84:185–193. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu BRS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KM, Jaunin F, Masouyc I, Saurat J, Hauser C. Cells mediate IL-4-dependent local tissue inflammation. J. Immunol. 1993;150:5576–5584. [PubMed] [Google Scholar]
- Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Komine O, Hamano S, et al. SOCS-3 regulates onset and maintenance of Th2- mediated allergic responses. Nat. Med. 2003;9:1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur. J. Immunol. 2004;34:1483–1487. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- Gracie JA, Bradley JA. Interleukin-12 induces interferon-γ-dependent switching of IgG alloantibody subclass. Eur. J. Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- Van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. IL-23 modulates CD56+/CD3- NK cell and CD56+/CD3 +NK-like T cell function differentially from IL-12. Int. Immunol. 2009;21:145–153. doi: 10.1093/intimm/dxn132. [DOI] [PubMed] [Google Scholar]
- Lankford CSR, Frucht DM. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 2003;73:49–56. doi: 10.1189/jlb.0602326. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Takabayashi K, Cheng P, Nguyen M, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat. Biotechnol. 2000;18:509–514. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- De Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA. 2013;110:19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Kobiyama K, Aoshi T, Narita H, Kuroda E, Hayashi M, Tetsutani K, Koyama S, et al. Nonagonistic dectin-1 ligand transforms CpG into a multitask nanoparticulate TLR9 agonist. Proc. Natl. Acad. Sci. USA. 2014;111:3086–3091. doi: 10.1073/pnas.1319268111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, et al. Article silica crystals and aluminum salts regulate the production of prostaglandin in macrophages. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Combination of K3 CpGand cGAMPinduces potent CD8+T cell activity in vivo.
Figure S2. Gating strategy for intracellular IFN-γ staining experiment in hPBMCs. First, CD3+/-cells were gated from the lymphocyte gate.
Figure S3. Gating strategy for in vivo CTL assay.
Figure S4. CD8+ T cell activity, but not NK cell activity, is required for the anti-tumor effect of the combination of K3 CpG and cGAMP in the EG-7 tumor model.
Figure S5. Cytotoxicity ofthe STING ligands in the splenocytes.
Figure S6. (A)Proposed mechanisms of innate IFN-γ synergy.
Figure S7. No significant differences were observed between the PBS immunization groups of WT and STING mutant, or MyD88 KO mice.
Peer review correspondence