Abstract
Background
Hypothalamic–pituitary–adrenal (HPA) axis activity is known to be altered following events such as childhood abuse. However, despite potential adverse consequences for the offspring of women who have experienced abuse, very little is known about altered HPA axis activity during pregnancy.
Methods
During pregnancy, 180 women from diverse racial/ethnic backgrounds reported on their exposure to emotional, physical and/or sexual abuse before the age of 11, and general post-traumatic stress symptoms (ie, not limited to childhood years or abuse experiences). Around delivery, they provided hair samples for the assessment of cortisol levels during pregnancy. Hair cortisol was assessed for each pregnancy trimester. The effect of childhood abuse on hair cortisol was assessed using mixed-effects analyses of covariance models allowing for within-subject correlated observations, and were first performed in the entire sample and subsequently stratified by race/ethnicity.
Results
Controlling for post-traumatic stress symptoms, hair cortisol levels varied by history of child abuse, F(2,166)=3.66, p=0.028. Childhood physical and/or sexual abuse was associated with greater hair cortisol levels, t(166)=2.65, p=0.009, compared with no history of abuse. Because childhood rates of abuse and hair cortisol levels varied by race/ethnicity, analyses were stratified by race/ethnicity. The associations between history of abuse and cortisol levels were only significant among black women, F(2,23)=5.37, p=0.012.
Conclusions
Childhood abuse, especially physical and/or sexual abuse, is associated with differences in cortisol production during pregnancy, particularly among black women. Future research should investigate how these differences impact physical and mental health outcomes among offspring of affected women.
Long-lasting effects of child abuse on the hypothalamic–pituitary–adrenal (HPA) axis have been documented.1 For example, child abuse is linked to glucocorticoid resistance and greater corticotropin-releasing factor activity in later life.2 Recent studies have begun to document the importance of considering effects of child abuse on HPA axis functioning in pregnant women3 given the importance of maternal cortisol production, a hormonal end product of the HPA axis that is released from the adrenal cortex, for healthy fetal development.
Disrupted maternal HPA axis functioning has been implicated in numerous adverse outcomes among mothers and offspring. For example, higher absolute cortisol levels at awakening and throughout the day have been associated with preterm birth.4,5 A disrupted maternal HPA axis during pregnancy has also been linked to long-term disruptions in HPA axis functioning among offspring,6,7 potentially increasing lifetime risk for psychopathology, as well as poorer offspring cognitive and behavioural development8,9 and childhood wheezing.10
Biologically, pregnancy is a unique time for HPA axis functioning, with prior studies demonstrating increases in cortisol production as pregnancy progresses.11,12 Notably, these studies have been conducted primarily in Caucasian samples and have not accounted for potential long-term effects that childhood maltreatment may have on dysregulation of the HPA axis that may extend into pregnancy. Recent studies link child abuse to increased salivary cortisol awakening response over the second and third trimesters.3,13 Specifically, pregnant women who experienced sexual abuse showed greater morning cortisol responses compared to women who experienced non-sexual abuse or no abuse,13 and prior day stress influenced the subsequent morning cortisol response only among women who had experienced sexual abuse.3
Assessment of cortisol concentrations in hair has recently emerged as a novel approach for measuring long-term cortisol exposure. Hair cortisol represents an integrated marker of HPA axis activity, reflects cortisol levels over weeks to months based on a hair growth rate of approximately 1 cm/month,14 and can be used to create a retrospective calendar of cortisol production over the course of pregnancy.11,12 Unlike measures of serum and salivary cortisol levels, which require sampling across multiple time points, resulting in increased participant burden and are influenced by more acute stressors,15 a single hair sample provides an integrated measure of chronic cortisol production. Thus, the use of hair cortisol in larger scale epidemiological studies may be more informative about effects of childhood abuse on naturally occurring, integrated, cortisol levels over a particular time interval.
Examining these associations in ethnically diverse samples is important. Racial/ethnic minorities are more likely to report childhood abuse compared to non-Hispanic white individuals,16 as are individuals from lower socioeconomic backgrounds,17 yet these groups have been under-represented in the existing literature. In addition, investigating associations between traumatic events and HPA axis functioning independent of post-traumatic stress disorder (PTSD) status is important,18,19 as only a small percentage of individuals exposed to trauma develop PTSD, and data indicate that exposure to trauma, particularly early and severe exposure such as child abuse, may disrupt HPA axis functioning regardless of the presence of psychopathology.20–22
The aims of the present study were to investigate associations of childhood abuse with an integrated measure of cortisol production (in scalp hair) in a sample of pregnant women from diverse racial/ethnic backgrounds while controlling for PTSD symptoms.
METHODS
Participants
Analyses included women from the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, a prospective pregnancy cohort designed to examine the role of prenatal stress on stress responses and respiratory health in children. PRISM originally recruited N=275 women at M=26.9 (SD=8.1) weeks gestation from the Beth Israel Deaconess Medical Center and the East Boston neighbourhood Health Center in Boston, Massachusetts, USA, between March 2011 and August 2012. Eligibility criteria included being ≥18 years of age, free of chronic illness, fluent in English or Spanish, and not having endorsed drinking more than seven alcoholic drinks per week prior to pregnancy or any alcohol after pregnancy recognition, given potential links to increased risk for child health problems.23 Supplemental funding allowed for hair collection starting a short time into the recruitment phase. Two hundred and twenty-five of the 275 women originally recruited were approached to participate in hair collection, and all consented. Of these, 28 women were excluded from the current analyses due to shift work (n=17), oral steroid use in the past 6 months (n=5), or multigestational pregnancy (n=6) as these factors influence cortisol production.24 To examine relations among childhood abuse history and hair cortisol across racial/ethnic groups, those identifying as multiracial/ethnic (n=17) were also excluded. Women not included in these analyses did not differ significantly from those included based on mean age, body mass index (BMI), smoking or education level. This study was approved by the relevant institutions’ human studies ethics committees, and women provided written informed consent in their preferred language.
Measures
Childhood abuse
Women’s experiences of childhood abuse were assessed within 2 weeks of enrolment using items from the Childhood Trauma Questionnaire (CTQ)—short form,25 which inquires about experiences up to age 11 years. The CTQ has demonstrated good test–retest reliability and validity, including among racially/ethnically mixed community samples.26–28 Two items were used to assess emotional abuse, three items to assess physical abuse, and two items to assess sexual abuse; see online supplementary table 1 for the full list of items. Items were answered on a five-point scale including 0 (never), 1 (rarely), 2 (sometimes), 3 (often) and 4 (very often).
Childhood abuse was categorised as present if participants reported experiencing any item in the respective subscale sometimes (2) to very often (4). Women were categorised as having experienced ‘Any Abuse’ if they rated at least one item as 2 or higher, and by type of abuse experienced. Because only six women reported sexual abuse and five of them also reported physical abuse, physical and sexual abuse were combined into one category, ‘physical/sexual abuse’. The majority of women (80.0%) categorised as having experienced physical/sexual abuse also reported emotional abuse. Women who reported emotional abuse in the absence of physical or sexual abuse were categorised as ‘emotional abuse only’.
Hair cortisol
Near the time of delivery, a hair strand roughly 3 mm in diameter was cut with scissors as close to the scalp as possible from the posterior vertex, the suggested standard position for hair cortisol collection, given that this region has the most uniform rate of growth, lowest interindividual variability, and lowest proportion of resting phase in the hair follicle.29 Hair samples were cut into three 3 cm segments, length permitting, with each 3 cm segment corresponding to one trimester based on a rate of hair growth of approximately 1 cm/month.12 The 3 cm segment closest to the scalp reflects cortisol levels during the third trimester, and the next two 3 cm segments reflect second and first trimester levels, respectively. Owing to varied hair lengths, we were able to assess cortisol levels in all three trimesters in 100 (55.6%) women; the second and third trimesters in 65 (36.1%) women; and third trimester in 15 (8.3%) women.
Hair was stored in manila envelopes at room temperature away from direct sunlight until shipment for analysis (Kirschbaum laboratory, Dresden, Germany) using a published protocol.30 Briefly, the hair was washed in isopropanol, and cortisol was extracted from 7.5 mg of whole non-pulverised hair using methanol in the presence of internal standards. Samples were centrifuged at 15 200g, and the supernatant was collected; alcohol was evaporated under a stream of nitrogen and reconstituted with double-distilled water and then injected into a Shimadzu HPLC-tandem mass spectrometry system (Shimadzu, Canby, Oregon, USA) coupled to an AB Sciex API 5000 Turbo-ion-spray triple quadrupole tandem mass spectrometer (AB Sciex, Foster City, California, USA) with purification by on-line solid-phase extraction.31 Lower limits of quantification were 0.1 pg/mg; interassay and intra-assay variabilities were 3.7–8.8%.
Covariates
At enrolment, participants self-reported their age, education level, number of children and race/ethnicity. Prenatal BMI was computed as kg/m2 based on self-reported height and pre-pregnancy weight. Internal validation data comparing self-reported height and weight with measured values among women recruited into a sociodemographically similar Boston birth cohort during early pregnancy (<10 weeks gestation) suggest good agreement between self-reported and measured data across all levels of height and weight.10
To assess the effects of childhood abuse independent of PTSD symptoms, all analyses controlled for PTSD symptoms. Within 2 weeks of enrolment, PTSD symptomatology (re-experiencing, avoidance, numbing, hyperarousal) was assessed using the 17-item Posttraumatic Stress Disorder Checklist—Civilian Version,32 which has demonstrated good test–retest reliability and convergent validity with other PTSD scales and clinical diagnostic interviews.33 During pregnancy, participants indicated how much they had been bothered by each symptom since the occurrence of the most stressful event experienced during their lifetime (ie, not limited to childhood years or to abuse experiences). Responses on a five-point scale ranging from 1 (not all) to 5 (extremely) were summed, with possible scores ranging from 17 to 85.
On the day of hair collection, participants reported on their natural hair colour, frequency of shampooing, whether their hair had recently been coloured or chemically straightened, and whether they had used any hair products (gels, oils, sprays, etc). Participants also indicated whether they had used inhaled corticosteroids (ICS) in the past year.34
Analyses
Hair cortisol values were log-transformed to reduce skewness. Mixed-effects analyses of covariance models allowing for within-subject correlated observations and assumed independence across participants were used to examine the effects of childhood abuse on hair cortisol levels throughout pregnancy. Analyses were first performed in the entire sample and then stratified by race/ethnicity. All analyses controlled for maternal age, education, ICS use, BMI, and PTSD symptoms, and in non-stratified analyses, race/ethnicity. A first-order autoregressive correlation pattern was assumed for the within-subject observations —that is, observations from adjacent trimesters were assumed to be more highly correlated than between the first and third trimesters. Residual analyses were used to verify the assumptions of the model. The 2 degree of freedom (df) test of any association between history of child abuse and hair cortisol levels during pregnancy was the primary test. The corresponding single df tests were used to specify the association. Race/ethnicity was coded to compare black and Hispanic women with white women. Racial/ethnic group differences were analysed using one-way analyses of variance with three levels (white, black, Hispanic) and, if significant, were followed by Hochberg’s GT2 for post hoc comparison of the group means or by Pearson χ2 tests. Analyses were performed using SAS software, V.9.3 and SPSS V.20 (IBM, New York, New York, USA). For all analyses, p<0.05 was considered statistically significant.
RESULTS
Cohort description
Women were primarily minorities, 18.9% black and 45.6% Hispanic (table 1). Minority participants were significantly younger and had a higher pre-pregnancy BMI compared to white participants; black (26.4%) and Hispanic (63.4%) women were more likely to have ≤ a high school education compared to white women (3.2%).
Table 1.
Sample descriptives
| All women (N=180)
|
White (n=64)
|
Hispanic (n=82)
|
Black (n=34)
|
p Value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | M±SD | n (%) | M±SD | n (%) | M±SD | n (%) | M±SD | ||
| Age (years) | 31.01±5.41 | 33.40±4.0 | 29.93±5.65 | 29.52±5.85 | <0.001 | ||||
| BMI (kg/m2) | 25.81±5.38 | 24.17±4.44 | 26.35±5.50 | 27.58±6.0 | 0.005 | ||||
| Highest education level | <0.001 | ||||||||
| <High school | 45 (25.0) | 1 (1.6) | 38 (46.3) | 6 (17.6) | |||||
| High school | 18 (10.0) | 1 (1.6) | 14 (17.1) | 3 (8.8) | |||||
| Some college | 34 (18.9) | 6 (9.4) | 15 (18.3) | 13 (38.2) | |||||
| Undergraduate | 38 (21.1) | 21 (32.8) | 11 (13.4) | 6 (17.6) | |||||
| Graduate | 44 (24.4) | 35 (54.7) | 4 (4.9) | 5 (14.7) | |||||
| Parity† | 0.094 | ||||||||
| 0 | 41 (22.8) | 21 (32.8) | 12 (14.6) | 8 (23.5) | |||||
| 1 | 65 (36.1) | 23 (35.9) | 30 (36.6) | 12 (35.3) | |||||
| 2 | 74 (41.1) | 20 (31.3) | 40 (48.8) | 14 (41.2) | |||||
| ICS use in past 12 months | 4 (2.2) | 2 (3.1) | 0 (0) | 2 (5.9) | ns | ||||
| Hair-related variables | |||||||||
| Shampooing | 0.017 | ||||||||
| <5 times/week | 122 (67.8) | 33 (51.6) | 60 (73.2) | 29 (85.3) | |||||
| 5 or 6 times/week | 16 (8.9) | 7 (10.9) | 6 (7.3) | 3 (8.8) | |||||
| Daily | 37 (20.6) | 21 (32.8) | 15 (18.3) | 1 (2.9) | |||||
| >1/day | 1 (0.6) | 1 (1.6) | 0 (0) | 0 (0) | |||||
| Colouration | 86 (47.8) | 30 (46.9) | 47 (57.3) | 9 (26.5) | 0.012 | ||||
| Chemical straightening | 20 (11.1) | 2 (3.1) | 3 (3.7) | 15 (44.1) | <0.001 | ||||
| Hair products used | 48 (26.7) | 16 (25.0) | 25 (30.5) | 7 (20.6) | ns | ||||
| PCL-C | 26.60±14.98 | 23.20±10.12 | 28.32±17.23 | 28.91±16.10 | 0.075 | ||||
| Hair sample availability by trimester | 0.030 | ||||||||
| T1, 2 and 3 | 100 (55.6) | 32 (50.0) | 52 (63.4) | 16 (47.1) | |||||
| T2 and 3 only | 65 (36.1) | 27 (42.2) | 27 (32.9) | 11 (32.4) | |||||
| T3 only | 15 (8.3) | 5 (7.8) | 3 (3.7) | 7 (20.6) | |||||
| Hair cortisol (pg/mg; log) | |||||||||
| T1 | 1.19±0.88 | 0.84±0.74 | 1.28±0.84 | 1.58±1.05 | 0.011 | ||||
| T2 | 1.18±0.81 | 0.89±0.76 | 1.27±0.75 | 1.56±0.87 | 0.001 | ||||
| T3 | 1.26±0.74 | 0.97±0.72 | 1.35±0.68 | 1.61±0.72 | <0.001 | ||||
Differences by race/ethnicity were tested using one-way analyses of variance and Pearson χ2 tests.
Parity reflects the number of previous children.
BMI, body mass index; ICS, inhaled corticosteroids; PCL-C, Posttraumatic Stress Disorder Checklist—Civilian Version.
Childhood abuse and hair cortisol during pregnancy
The correlation across trimester hair cortisol levels was calculated as r=0.93 for adjacent trimester values and r=0.86 for observations between the first and third trimesters. These high correlation values indicate that women with higher cortisol levels from any trimester tend to have higher values at all trimesters; conversely, women with lower cortisol levels from any trimester tend to have lower values at all trimesters.
Hair cortisol levels varied by category of child abuse, F(2,166)=3.66, p=0.028. Specifically, women who experienced physical/sexual abuse during childhood had higher hair cortisol levels than women who did not experience childhood abuse, t(166)=2.65, p=0.009. No difference was observed between women who reported only emotional abuse and women who did not experience childhood abuse, t(166)=0.56, p>0.50.
Role of race/ethnicity
Hair cortisol levels varied significantly by race/ethnicity during the first, F(2,97)=4.74, p=0.011, second, F(2,162)=7.97, p=0.001 and third, F(2,177)=10.43, p<0.001, trimesters. Black women had significantly higher levels of hair cortisol than white (p=0.015, 0.001 and <0.001 for first, second and third trimesters, respectively) but not Hispanic women (all p>0.10). Compared with white women, Hispanic women had significantly higher hair cortisol levels during the second and third trimesters (p=0.014 and 0.004 for second and third trimesters, respectively; figure 1).
Figure 1.
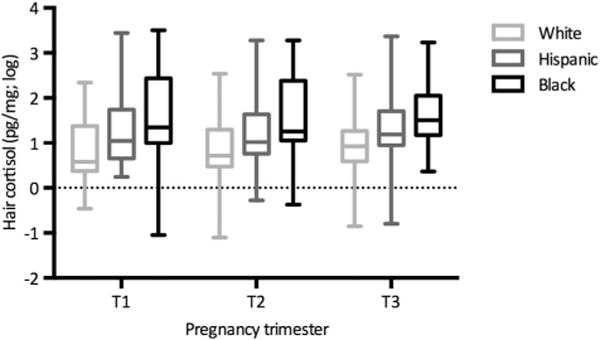
Maternal prenatal hair cortisol by pregnancy trimester and race/ethnicity. Average maternal hair cortisol is represented by race/ethnicity for each of the three pregnancy trimesters. Hair cortisol levels varied by race/ethnicity during the first F(2,97)=4.74, p=0.011, second F(2,162)=7.97, p=0.001, and third F(2,177)=10.43, p<0.001, trimesters. Black women had higher levels of hair cortisol than white (p=0.015, 0.001 and <0.001 for first, second and third trimesters, respectively), but not Hispanic women (all p>0.10). Hispanic women had significantly higher hair cortisol levels during the second and third trimesters (p=0.067, 0.014, and =0.004 for first, second and third trimesters, respectively) than white women. Hair cortisol rose significantly over the course of pregnancy among white women (p<0.0001), but not among Hispanic (p=0.12) and black (p=0.83) women. T1=first trimester; T2=second trimester; T3=third trimester.
There was also a significant association between race/ethnicity and childhood abuse history, including any abuse, χ2(2)=9.77, p=0.008, emotional abuse (regardless of the presence of other types of abuse), χ2(2)=7.24, p=0.027, physical abuse, χ2(2) =11.36, p=0.003, and sexual abuse, χ2(2)=10.25, p=0.006. Specifically, black women were more likely to report a history of any abuse compared with white or Hispanic women (32.4%, 11.1% and 10.9%, respectively); black women were also more likely to report having experienced each of the three types of abuse when considered separately, compared with Hispanic and white women (figure 2).
Figure 2.
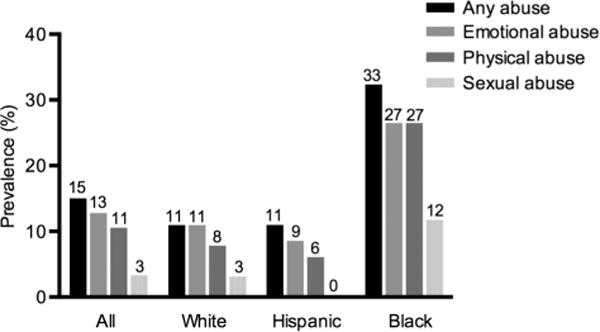
Prevalence of childhood abuse up to age 11 years. Per cent prevalence of all three types of abuse considered individually, and of the presence of any abuse by race/ethnicity. History of Childhood abuse varied by race/ethnicity (any abuse, χ2(2)=9.77, p=0.008; emotional abuse (regardless of the presence of other abuse types), χ2(2)=7.24, p=0.027; physical abuse, χ2(2)=11.36, p=0.003; sexual abuse, χ2(2) =10.25, p=0.006). Black women were more likely to report a history of any abuse and each of the three types of abuse considered individually. Note: Abuse categories are not mutually exclusive.
Given the observed associations of race/ethnicity with history of abuse and hair cortisol levels, analyses were repeated, stratified by race/ethnicity. In these analyses, the association between childhood abuse and hair cortisol levels was only evident among black women, F(2,23)=5.37, p=0.012, not among white F(2,54)=0.56, p>0.50, or Hispanic F(2,72)=0.59, p>0.50 women. In these stratified analyses, parity significantly predicted hair cortisol among Hispanic women, F(2,72)=3.19, p=0.047, such that hair cortisol was higher among women experiencing their first pregnancy t(1,72)=2.52, p=0.014; other covariates were not significant.
Sensitivity analyses
When analyses were repeated among the 100 women who had hair cortisol measures available in all three trimesters, findings were substantively unchanged.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the influence of childhood abuse on an integrated measure of cortisol production, cortisol levels measured in hair, among pregnant women from racially/ethnically diverse backgrounds. Women experiencing physical and/or sexual abuse in childhood had higher levels of hair cortisol than women who had not experienced abuse. Our data provide tentative support for the hypothesis that physical and/or sexual abuse alters HPA axis functioning into adulthood and pregnancy, as reflected in hair cortisol levels, and are in line with previous findings linking childhood sexual abuse (but not non-sexual forms of abuse) to increased maternal salivary cortisol levels during pregnancy.3,13
These findings also underscore the need for considering race/ethnicity when studying associations between child abuse and HPA axis functioning. Here, black women were more likely to report childhood abuse compared with white and Hispanic women, consistent with prior research,16 and also showed evidence of higher cortisol levels throughout pregnancy. In analyses stratified by race/ethnicity, a significant association between childhood physical/sexual abuse and hair cortisol was observed among black women only. Furthermore, similar to existing studies examining cortisol levels across pregnancy in primarily Caucasian samples11,12 we saw an increase in hair cortisol levels over pregnancy in white women. By contrast, we found that both Hispanic and black women had higher cortisol levels compared with white women throughout pregnancy, and did not show a significant increase over pregnancy. This finding warrants replication, as it will be important to see if this pattern holds true in future studies including ethnic minorities.
Our findings suggest that differences in hair cortisol levels by history of abuse were not solely the result of psychopathology (ie, PTSD symptoms) experienced as a result of abuse or other traumatic events, as the associations between child abuse and hair cortisol levels were significant while controlling for PTSD symptoms. These findings corroborate other studies reporting associations between experiencing potentially traumatic events and cortisol levels (in hair and in serum), irrespective of post-traumatic stress symptoms.22,35
Altered maternal HPA axis functioning during pregnancy as a consequence of childhood abuse experiences may adversely affect maternal and offspring outcomes in a number of ways. First, the HPA axis interacts with other important biological processes, such as inflammation. Greater inflammation, in turn, has been linked to increased rates of preterm births, especially among black women.4,36 Second, a disrupted maternal HPA axis has been linked to disrupted HPA axis functioning among offspring,6,7 increased rates of childhood wheezing10 and poorer cognitive and behavioural development,8,9 increasing risk for several developmental problems.
The present study has a number of strengths, including the assessment of cortisol levels in hair as an integrated measure of HPA axis functioning, the focus on pregnant women, and the inclusion of women from varying racial/ethnic backgrounds exposed to differential rates of childhood abuse. There were also limitations. First, ratings of childhood abuse were based on participant recall. However, the CTQ is a well-validated instrument for the assessment of childhood abuse, and has previously been shown to have good reliability among community samples.28 The present study focused on childhood abuse only, that is, abuse that occurred before the age of 11, and did not include information on participants’ exposure to abuse during the teenage and adult years or to exposure to any other types of trauma. Thus, the noted associations between history of childhood abuse and cortisol levels may be attributable to other trauma exposures or stressors that covary with exposures to childhood abuse. Alternative explanations, such as exposure to discrimination, which has previously been linked to increased HPA axis activity among African-Americans,37 and other psychosocial risk factors should be considered in the future. Second, as is commonly found in the literature,38 there was considerable overlap between the different types of abuse. Thus, separating out the effects of different abuse types on cortisol levels was not possible. Third, differences in rates of hair growth among individuals from different racial/ethnic backgrounds are not well understood.39 Nonetheless, existing studies report only small differences by race/ethnicity, and between individuals from similar racial/ethnic backgrounds in the rate of hair growth.39,40
In conclusion, this study suggests that exposure to childhood abuse is associated with changes in HPA axis regulation extending into pregnancy. Among pregnant women, having experienced physical or sexual abuse in childhood was associated with higher levels of hair cortisol. These associations were particularly strong among black women, who also reported higher levels of childhood abuse compared with white and Hispanic women. Future studies should compare women from different racial/ethnic backgrounds with comparable levels of trauma exposure to determine whether the differences in cortisol levels found here can be explained by different exposure levels or physiological mechanisms that vary between racial/ethnic groups. Given the potential negative consequences on health of offspring, the pathways through which psychosocial factors lead to disrupted HPA axis functioning during pregnancy need to be better understood.
Supplementary Material
What is already known on this subject.
Childhood abuse is known to have adverse effects on long-term health. How these adverse physiological effects manifest later in life during pregnancy is poorly understood.
What this study adds.
This study shows that women exposed to physical and/or sexual abuse during childhood have greater levels of hair cortisol than women without a history of childhood abuse, and that this is especially true for black women. Future research should focus on the offspring of women who were abused during childhood, and further investigate racial/ethnic differences in prevalence of abuse and the physiological consequences of abuse.
Acknowledgments
Funding The study was supported by R21HD080359 (Wright, PI). During preparation of this manuscript, the authors were supported by R01HL095606 (Wright and Bosquet Enlow, MPI) and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital (Bosquet Enlow).
Footnotes
Contributors HMCS contributed to data analysis, manuscript drafting and review. MBE and RJW contributed to study planning, study conduct and manuscript review. TR contributed to manuscript drafting and review. CG contributed to data analysis and manuscript review.
Competing interests None declared.
Ethics approval Brigham and Women’s Hospital & Beth Israel Deaconess Medical Center.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. J R Soc Med. 2012;105:151–6. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heim C, Newport DJ, Mletzko T, et al. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Bublitz MH, Stroud LR. Maternal history of child abuse moderates the association between daily stress and diurnal cortisol in pregnancy: a pilot study. Stress. 2013;16:706–10. doi: 10.3109/10253890.2013.825768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian LM, Glaser R, Porter K, et al. Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom Med. 2013;75:658–69. doi: 10.1097/PSY.0b013e31829bbc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corwin EJ, Guo Y, Pajer K, et al. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 2013;38:1786–96. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor A, Dunn E, Kostaki A, et al. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor TG, Bergman K, Sarkar P, et al. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Dev Psychobiol. 2013;55:145–55. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–92. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 9.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wright RJ, Fisher K, Chiu YH, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–93. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Anna-Hernandez KL, Ross RG, Natvig CL, et al. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011;104:348–53. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschbaum C, Tietze A, Skoluda N, et al. Hair as a retrospective calendar of cortisol production—increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–7. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Bublitz MH, Stroud LR. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: preliminary findings. Psychoneuroendocrinology. 2012;37:1425–30. doi: 10.1016/j.psyneuen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staufenbiel SM, Penninx BW, Spijker AT, et al. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38:1220–35. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–42. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- 17.Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol. 2007;40:313–32. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- 18.de Kloet CS, Vermetten E, Heijnen CJ, et al. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–26. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Meewisse ML, Reitsma JB, de Vries GJ, et al. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–92. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 20.Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62(Suppl 17):16–22. [PubMed] [Google Scholar]
- 21.Bronner MB, Peek N, Vries M, et al. A community-based survey of posttraumatic stress disorder in the Netherlands. J Trauma Stress. 2009;22:74–8. doi: 10.1002/jts.20379. [DOI] [PubMed] [Google Scholar]
- 22.Van Voorhees EE, Dennis MF, Calhoun PS, et al. Association of DHEA, DHEAS, and cortisol with childhood trauma exposure and post-traumatic stress disorder. Int Clin Psychopharmacol. 2014;29:56–62. doi: 10.1097/YIC.0b013e328364ecd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patra J, Bakker R, Irving H, et al. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)—a systematic review and meta-analyses. BJOG. 2011;118:1411–21. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granger DA, Hibel LC, Fortunato CK, et al. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–48. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein DP, Fink L. Childhood trauma questionnaire: a retrospective self-report manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 26.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein DP, Ahluvalia T, Pogge D, et al. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Scher CD, Stein MB, Asmundson GJ, et al. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress. 2001;14:843–57. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- 29.Stalder T, Kirschbaum C. Analysis of cortisol in hair—state of the art and future directions. Brain Behav Immun. 2012;26:1019–29. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Stalder T, Kirschbaum C, Alexander N, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:2573–80. doi: 10.1210/jc.2013-1056. [DOI] [PubMed] [Google Scholar]
- 31.Gao W, Stalder T, Foley P, et al. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:1–8. doi: 10.1016/j.jchromb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Weathers FW, Huska JA, Keane TM. PCL-C for DSM-IV. Boston: National Center for PTSD-Behavioral Science Division; 1991. [Google Scholar]
- 33.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 34.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–17. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Steudte S, Kirschbaum C, Gao W, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013;74:639–46. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Coussons-Read ME, Lobel M, Carey JC, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–9. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37:107–18. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon L, Browne K, Hamilton-Giachritsis C. Patterns of risk and protective factors in the intergenerational cycle of maltreatment. J Fam Violence. 2009;24:111–22. [Google Scholar]
- 39.Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(Suppl 1):6–9. doi: 10.1111/j.1365-4632.2005.02800.x. [DOI] [PubMed] [Google Scholar]
- 40.Loussouarn G. African hair growth parameters. Br J Dermatol. 2001;145:294–7. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


