Abstract
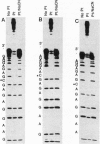
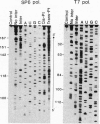
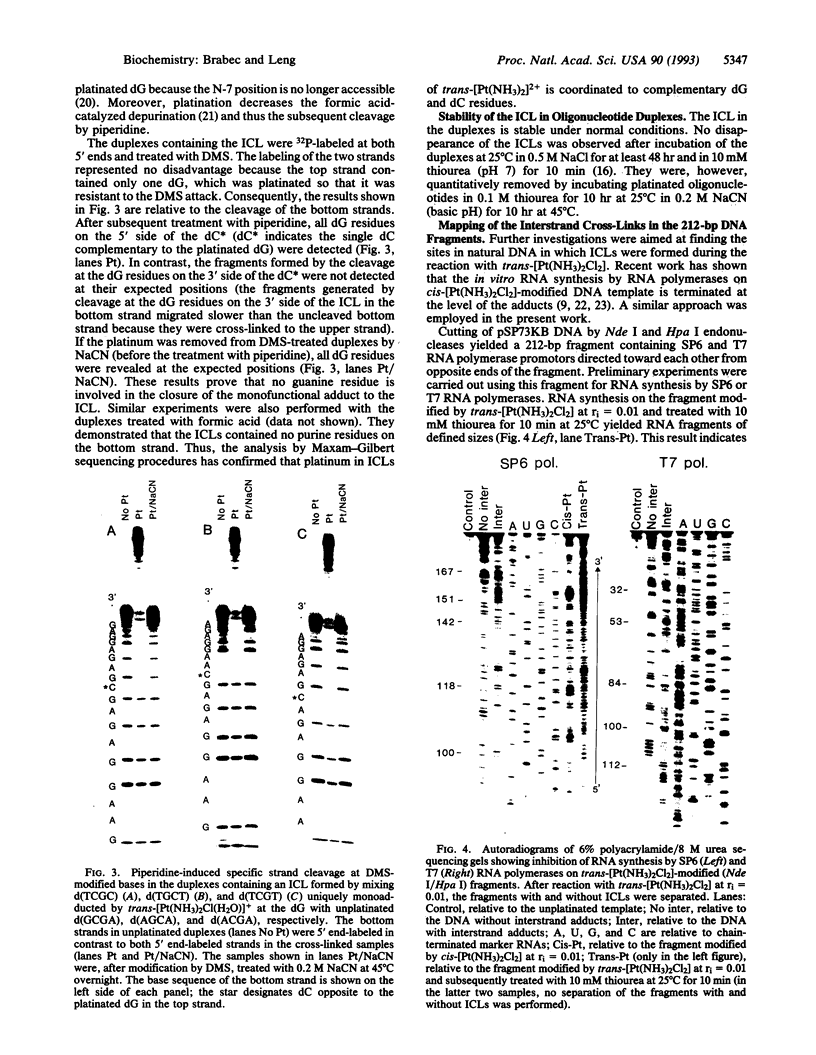
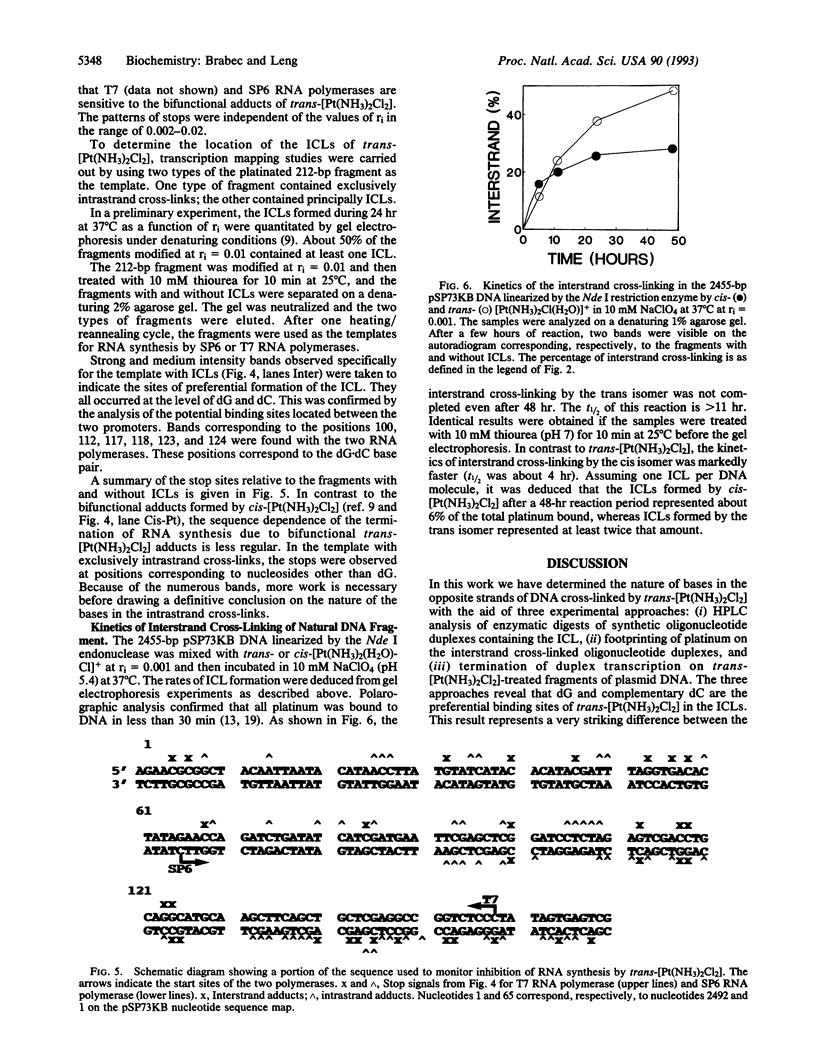
Bases in the opposite strands of DNA cross-linked by clinically ineffective trans-diamminedichloroplatinum(II) (trans-[Pt(NH3)2Cl2]) have been identified by means of three experimental approaches. These include HPLC analysis of enzymatic digests of synthetic oligonucleotide duplexes containing the interstrand cross-link, footprinting experiments on the interstrand cross-linked oligonucleotide duplexes, and termination of the duplex transcription on trans-[Pt(NH3)2Cl2]-treated fragments of plasmid DNA. The results reveal that deoxyguanine and complementary deoxycytosine residues are preferential binding sites of trans-[Pt(NH3)2Cl2] in the interstrand adducts. The interstrand cross-linking reaction was studied by means of gel electrophoresis for the cis and trans isomers. The rate of formation of interstrand cross-links was lower for the trans isomer; however, trans-[Pt(NH3)2Cl2] formed about twice the amount of interstrand cross-links as compared with the cis isomer after 48 hr. The present results are suggested to be relevant to differences in clinical activity of the two platinum(II) isomers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod V. D., Kramer F. R. Transcription from bacteriophage T7 and SP6 RNA polymerase promoters in the presence of 3'-deoxyribonucleoside 5'-triphosphate chain terminators. Biochemistry. 1985 Oct 8;24(21):5716–5723. doi: 10.1021/bi00342a005. [DOI] [PubMed] [Google Scholar]
- Brabec V., Reedijk J., Leng M. Sequence-dependent distortions induced in DNA by monofunctional platinum(II) binding. Biochemistry. 1992 Dec 15;31(49):12397–12402. doi: 10.1021/bi00164a014. [DOI] [PubMed] [Google Scholar]
- Comess K. M., Costello C. E., Lippard S. J. Identification and characterization of a novel linkage isomerization in the reaction of trans-diamminedichloroplatinum(II) with 5'-d(TCTACGCGTTCT). Biochemistry. 1990 Feb 27;29(8):2102–2110. doi: 10.1021/bi00460a020. [DOI] [PubMed] [Google Scholar]
- Corda Y., Anin M. F., Leng M., Job D. RNA polymerases react differently at d(ApG) and d(GpG) adducts in DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1992 Feb 25;31(7):1904–1908. doi: 10.1021/bi00122a002. [DOI] [PubMed] [Google Scholar]
- Corda Y., Job C., Anin M. F., Leng M., Job D. Transcription by eucaryotic and procaryotic RNA polymerases of DNA modified at a d(GG) or a d(AG) site by the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1991 Jan 8;30(1):222–230. doi: 10.1021/bi00215a032. [DOI] [PubMed] [Google Scholar]
- Eastman A., Barry M. A. Interaction of trans-diamminedichloroplatinum(II) with DNA: formation of monofunctional adducts and their reaction with glutathione. Biochemistry. 1987 Jun 16;26(12):3303–3307. doi: 10.1021/bi00386a009. [DOI] [PubMed] [Google Scholar]
- Eastman A., Jennerwein M. M., Nagel D. L. Characterization of bifunctional adducts produced in DNA by trans-diamminedichloroplatinum(II). Chem Biol Interact. 1988;67(1-2):71–80. doi: 10.1016/0009-2797(88)90087-7. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Johnson N. P., Hoeschele J. D., Rahn R. O. Kinetic analysis of the in vitro binding of radioactive cis- and trans-dichlorodiammineplatinum(II) to DNA. Chem Biol Interact. 1980 May;30(2):151–169. doi: 10.1016/0009-2797(80)90122-2. [DOI] [PubMed] [Google Scholar]
- Lemaire M. A., Schwartz A., Rahmouni A. R., Leng M. Interstrand cross-links are preferentially formed at the d(GC) sites in the reaction between cis-diamminedichloroplatinum (II) and DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1982–1985. doi: 10.1073/pnas.88.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepre C. A., Chassot L., Costello C. E., Lippard S. J. Synthesis and characterization of trans-[Pt(NH3)2Cl2] adducts of d(CCTCGAGTCTCC).d(GGAGACTCGAGG). Biochemistry. 1990 Jan 23;29(3):811–823. doi: 10.1021/bi00455a031. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sip M., Schwartz A., Vovelle F., Ptak M., Leng M. Distortions induced in DNA by cis-platinum interstrand adducts. Biochemistry. 1992 Mar 10;31(9):2508–2513. doi: 10.1021/bi00124a010. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Zhen W., Link C. J., Jr, O'Connor P. M., Reed E., Parker R., Howell S. B., Bohr V. A. Increased gene-specific repair of cisplatin interstrand cross-links in cisplatin-resistant human ovarian cancer cell lines. Mol Cell Biol. 1992 Sep;12(9):3689–3698. doi: 10.1128/mcb.12.9.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]