Abstract
Dietary glycemic modulation through high-fat, low-carbohydrate diets, which induce a state of systemic ketosis and alter systemic metabolic signaling, have been incorporated into the clinical management of patients with neurological disease for more than a century. Mounting preclinical evidence supports the antitumor, proapoptotic, and antiangiogenic effects of disrupting glycolytic metabolism through dietary intervention. In recent years, interest in incorporating such novel therapeutic strategies in neuro-oncology has increased. To date, 3 published studies incorporating novel dietary therapies in oncology have been reported, including one phase I study in neuro-oncology, and have set the stage for further study in this field. In this article, we review the biochemical pathways, preclinical data, and early clinical translation of dietary interventions that modulate systemic glycolytic metabolism in the management of primary malignant brain tumors. We introduce the modified Atkins diet (MAD), a novel dietary alternative to the classic ketogenic diet, and discuss the critical issues facing future study.
Keywords: cancer metabolism, glioma, ketogenic diet, modified Atkins diet, seizure
Malignant gliomas (MGs) are the most common primary CNS tumors. Despite the introduction of combination chemoradiotherapy with temozolomide in 2005, treatment options remain limited, and median survival is only 12–15 months for patients newly diagnosed with glioblastoma (GBM).1 While in general, tumorigenesis is thought to involve genetic, epigenetic, and microenvironmental changes that vary widely between tumor types, the preferential metabolism of glucose through aerobic glycolysis (ie, Warburg effect) is common.2,3 Recent advances in the understanding of the biochemical pathways underlying this metabolic phenotype may provide novel therapeutic targets for intervention.
Biochemical Pathways Implicated in the Glycolytic State
Unlike normal glia, glioma cells have both a high glycolytic rate and are dependent on glucose for energy metabolism.3 Metabolism of glucose is 3-times greater in glioma cell lines as compared with normal glia.4 Gliomas have been shown to poorly metabolize ketone bodies for energy,5–8 and withdrawal of glucose has induced apoptosis at rates dramatically higher than in normal human astrocytes.9 It appears that this metabolic “addiction” to glucose is in part due to alterations in mitochondrial structure and function that may result from genetic, epigenetic, and enzymatic alterations within cancer cells.10–12 Glucose not only serves to fuel tumor cell aerobic glycolysis but also results in a proglutamatergic state that may drive peritumoral glutamate excitotoxicity and subsequent tumor invasion into surrounding healthy brain.13,14
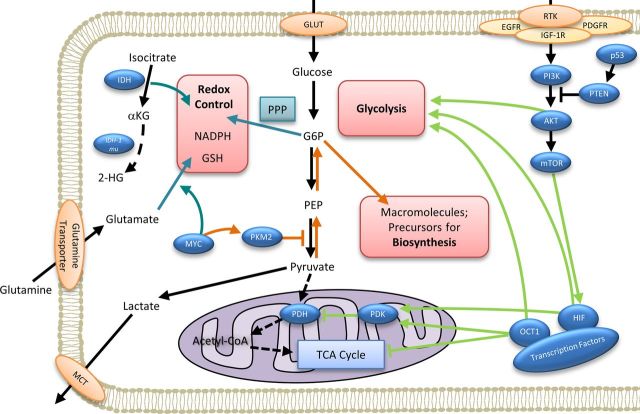
Current research suggests that this preferential metabolism of glucose through glycolysis may arise as a selective advantage in the hypoxic conditions experienced during early tumor development.15 Compared with oxidative phosphorylation, glycolysis not only provides a significantly higher rate of ATP production but also provides an effective mechanism for (i) shunting carbon toward biosynthetic pathways necessary to drive cellular proliferation, and (ii) generating the cellular redox potential necessary to scavenge excess reactive oxygen species (ROS) and ensure cancer cell viability. This cellular reprogramming of glucose metabolism to fuel tumor cell growth is largely thought to be driven by the Akt/ phosphoinositide 3-kinase (PI3 K)/mammalian target of rapamycin (mTOR) pathway, which is commonly activated in gliomas (Fig. 1).16 Pathway activation begins with membrane-associated receptor tyrosine kinases such as the insulin-like growth factor receptor (IGFR), epithelial growth factor receptor (EGFR), or platelet derived growth factor receptors (PDGFR) which are frequently overexpressed in gliomas.17 Stimulation of these receptors results in dimerization, transphosphorylation, and activation of PI3 K. Downstream effects include activation of Akt and mTOR, which activates transcription factors including hypoxia-inducible factor 1 (HIF1). HIF1 stimulates expression of glucose transporters on the cell surface, thereby increasing cellular glucose influx,18–20 and shifts metabolic pathways towards glycolysis through activation of inhibitory mitochondrial pyruvate dehydrogenase kinases (PDKs).21
Fig. 1.
Molecular mechanisms underlying the oncogenic metabolic phenotype. Oncogenic pathway alterations driving the Warburg effect involve (i) activation of the PI3 K/Akt/mTOR pathway driving a glycolytic state (light green arrows); (ii) pyruvate kinase induced negative regulation of glycolysis, which provides glycolytic intermediates necessary to facilitate macromolecular biosynthesis (orange arrows); and (iii) shunting of glucose through the pentose phosphate pathway to build redox potential (dark green arrows). In the first of these major metabolic alterations (light green arrows), PI3 K activates Akt, which stimulates aerobic glycolysis and enhances transcription factor expression including hypoxia-inducible factor 1 (HIF1) through the action of the mammalian target of rapamycin (mTOR). Expression of transcription factors including HIF1 and Oct 1 further drives a proglycolytic state by enhancing the activity of mitochondrial pyruvate dehydrogenase kinases, which shunt pyruvate back towards glycolysis and away from mitochondrial oxidative phosphorylation. In the second of these major metabolic alterations (orange arrows), C-myc-induced expression of cytosolic dimeric pyruvate kinase M2 functionally negatively regulates glycolysis, shunting free carbons back toward macromolecular biosynthesis, which supports cell proliferation. In the third major metabolic alteration, PKM2-mediated regulation of glycolysis also shunts glycolytic intermediates toward alternative carbon-consuming pathways (dark green arrows) including the pentose phosphate and hexosamine pathways, which generate reducing power in the form of NADPH. C–myc-driven conversion of glutamate to glutathione (GSH) and isocitrate dehydrogenase (IDH) mediated conversion of isocitrate to alpha-ketoglutarate (dark green arrows) also resulting in additional reducing power in the form of NADPH and GSH necessary to scavenge excess reactive oxygen species generation. Abbreviations: αKG, alpha-ketoglutarate; 2-HG, 2-hydroxyglutarate; AMPK, AMP-activated protein kinase; CHO, carbohydrate; EGFR, epidermal growth factor receptor; G6P, glucose-6-phosphate; GLUT, glucose transporter; GSH, glutathione; HIF, hypoxia-inducible factor; IDH, isocitrate dehydrogenase; IDH-mu, IDH-mutant; IGF-R1, insulin-like growth factor-1 receptor; MCT, monocarboxylic acid transporter; mTOR, mammalian target of rapamycin; NADPH, nicotinamide adenine dinucleotide phosphate; Oct 1, Octamer transcription factor 1; PI3 K, phosphoinositide 3-kinase; P53, tumor protein 53; PDGFR, platelet-derived growth factor receptor; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PEP, phosphoenolpyruvic acid; PKB, protein kinase B, also known as Akt; PKM2, pyruvate kinase-M2 isoenzyme; PPP, pentose phosphate pathway; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase; TCA, tricarboxylic acid.
Recent research suggests that cytosolic pyruvate kinases appear to provide additional regulation of this metabolic phenotype. The dimeric pyruvate kinase M2 isoenzyme (PKM2) is frequently overexpressed in tumors including gliomas and functionally serves as a negative regulator of glycolysis, resulting in the accumulation of glycolytic intermediates.22 These intermediary substrates are shunted towards macromolecular biosynthesis and subsidiary pathways of carbon catabolism, which serve to generate cellular reducing power in the form of NADPH. This tightly regulated control of redox potential ensures cancer cell viability within a relative promutagenic environment that may further drive oncogenesis.22
Therapeutic Interventions Targetting Glycemic Modulation
Preclinical studies have shown the antitumor, proapoptotic, and antiangiogenic effects of interrupting these glycolytic pathways in cancer cell lines including gliomas;23,24 however, no drugs are currently available that can be translated into clinical use now. Alternative therapeutic strategies for targeting glycolytic metabolism in gliomas do exist and include dietary glycemic modulation.
Dietary therapies that modulate glycemic homeostasis and result in a state of systemic ketosis have been termed ketogenic diets (KDs). KDs are high-fat, low-carbohydrate diets that alter host metabolic signaling by reducing serum glucose, driving ketone body production, and decreasing circulating insulin-like growth factor-1 (IGF-1). Such metabolic alterations have been shown in preclinical studies to have anticonvulsant and antitumor effects.25,26 A mouse epilepsy model study suggested that inhibition of mTOR signaling may underlie the antiepileptic properties of a KD; however, this has not been confirmed, and the exact mechanism of a KD has not yet been resolved.26 Studies of KDs in malignant glioma xenograft mouse models have demonstrated lowering of plasma glucose levels, reduction in tumor growth by 35%–65%, reduced microvessel density, and improved survival.25,27–29 Importantly, these studies have shown that inducing ketosis in the absence of calorie restriction does not appear to have this same effect on tumor volume and enhanced survival.27 Subsequent studies have also reported antiangiogenic,24 radiosensitizing,30 and chemotherapy augmenting31,32 effects of the diet, which appear to be linked to calorie restriction. Recent animal studies have also investigated the impact of intermittent fasting as a potential alternative mechanism for regulating cell signaling through these metabolic pathways.33 In these studies, intermittent fasting was found to augment the effects of chemotherapy, enhance response to radiation, and delay tumor progression more effectively than long-term calorie restriction without substantial weight loss.33,34 In fact, studies have suggested that such an intervention may actually be protective to normal cells, a process that in part may depend on circulating levels of glucose and IGF-1.33,34
Clinically, KDs have been safely employed in the management of patients with medically refractory epilepsy since around the 1920s.35,36 Over this time, several standard therapeutic regimens have been developed and can be characterized by the degree of carbohydrate, calorie, and fluid restriction and by the need for inpatient admission (Table 1).37–39 Of these, the classic KD was the first to be studied and is characterized by a 4:1 ratio of fat to carbohydrate and protein intake (in grams) with ∼ 90% of calories provided by fat. Patients have traditionally been admitted for initial inpatient fast; calories and fluid intake are strictly monitored and restricted to ensure adequate systemic ketosis as measured by urinary ketones.38 To date, the conventional KD has been most widely integrated into the clinical management of children with medically refractory epilepsy.40 In these patients, tolerability and efficacy have been high.37,38,40,41 Attempts to translate this into adults, however, have been met with some challenge. While efficacy has been similar to children in those able to remain on the diet, compliance with dietary restriction, burden of weighing food, and impact on daily living has been difficult to maintain for adults.39,42 In an effort to provide a more tolerable alternative, several KD variants have been developed including (i) lower-ratio KDs (primarily 3:1 ratio), (ii) medium-chain triglyceride (MCT) oil diets in which some of the long-chain fatty acids are replaced by MCTs, and (iii) the low glycemic index diet that limits consumption of high glycemic index foods.43,44 While easier to tolerate, ketosis can be harder to achieve on these diets, and prospective studies are ongoing to address antiepileptic efficacy.37
Table 1.
Ketogenic diets and the therapeutic experience in patients with medically refractory epilepsy
| Diet Name | Glycemic Intervention ([fat]:[carb + protein] ratio) | Calorie and Fluid Restriction | Inpatient Admission Required | Tolerabilitya (%, range) | Efficacyb (%, range) |
|---|---|---|---|---|---|
| PEDIATRICS37,38,40–42,45,46 | |||||
| Conventional KD | 4:1 ratio | Yes | Yes | 83.5% (42–100) | 78.0% (35–85) |
| Conventional KD variants | 3:1 ratio or MCT | Variable | Variable | 81.0% (78–83) | 55.1% (50–64) |
| MAD | 10–20 g net CHO/day | No | No | 68.6% (60–80) | 50.0% (50) |
| Adults39,48,49 | |||||
| Conventional KD | 4:1 ratio | Yes | Yes | 47.0% (22–64) | 41.7% (22–55) |
| Conventional KD variants | 3:1 ratio or MCT | Variable | Variable | 54.2% (33–75) | 27.5% (13–42) |
| MAD | 10–20 g CHO/day | No | No | 59.4% (38–78) | 29.4% (13–57) |
Abbreviations: CHO, carbohydrate; KD, ketogenic diet; MAD, modified Atkins diet; MCT, monocarboxylic acid transporter.
Ketogenic diets employed and studied in the management of children and adults with medically refractory epilepsy include the classic KDs, KD variants, and the modified Atkins diet (MAD). These diets employ varying degrees of carbohydrate, calorie, and fluid restriction that are defined by the ratio of [fat]:[carbohydrate + protein] by weight or the degree of carbohydrate restriction combined with increased fat intake. The major structured dietary therapies include those like the classic KD, which employ strict carbohydrate, calorie, and fluid restriction and require inpatient admission but are more difficult to employ in adults and those like the MAD which employ strict carbohydrate restriction and monitor other dietary parameters but do not require hospitalization and are better tolerated in adults.
aDefined as the percent of patients remaining on the diet at predefined study endpoint or final patient follow-up.
bDefined as the percent of patients with >50% seizure reduction of those remaining on diet at predefined study endpoint or final follow up.
Another alternative, the modified Atkins diet (MAD), is a low-carbohydrate diet that induces ketosis without restricting total calories, fluid intake, or protein consumption and does not require inpatient admission. Patients are prescribed no more than 20 grams of net (subtracting fiber) carbohydrates per day. Studies in adults with intractable epilepsy have demonstrated favorable tolerability, ease of administration, efficacy in seizure reduction, and practicality for adults with medically refractory epilepsy.47–49 Long term responses have not differed significantly among different ketogenic dietary therapies,50,51 and reduction in seizures has been similar following transition from the MAD to KD.52 As such, the MAD has emerged as a safe, effective, and feasible alternative to the classic KD in adults with medically refractory epilepsy.
Dietary Glycemic Modulation in Neuro-oncology: Challenges to the Classic Ketogenic Diet
In light of the expanding literature on the metabolic implications of KDs, interest in adopting KDs in oncology has increased. To date, 2 prospective studies have investigated the safety and feasibility of KDs for patients with advanced systemic solid tumors (Table 2).53,54 Tolerability has ranged from 50% at 4 weeks in 10 participants undergoing a carbohydrate-restricted diet (total carbohydrate intake targeted at <5% of total energy)53 to 31% at 3 months in 15 participants undergoing a 70 g/day carbohydrate-restricted diet.54 Side effects have included fatigue, constipation, leg cramps, and hunger, with reported weight loss ranging from 2–3 kg. These studies were not powered to determine efficacy, although best responses were reported, with partial response observed in 1 participant and stable disease observed in 10 participants.
Table 2.
Prospective studies of ketogenic diets in oncology
| Trial | Carbohydrate Restriction | Timeline of Assessment | Tolerability (% on diet) | Weight Loss (mean, kg) | Response |
|---|---|---|---|---|---|
| Schmidt et al54 | 70 g/day | 3 months | 31% (of 16) | 2 kg | 5 SD, 6 PD, 4 Unknown |
| Fine et al53 (RECHARGE) | CHO restriction to 5% of total energy | 1 month | 50% (of 10) | 3 kg | 5 SD, 1 PR, 4 PD |
| Rieger et al55 (ERGO) | 60 g/day | ∼5 weeks | 85% (of 20) | 2.2% loss | Median OS 32 weeks |
Abbreviations: CHO, carbohydrate; g, grams; OS, overall survival; PD, progressive disease; PR, partial response; SD, Stable disease.
In glioma, one prospective human study, the ERGO trial (NCT00575146), enrolled 20 participants with recurrent GBM and prescribed a 60 g/day carbohydrate-restricted KD without calorie restriction (Table 2).55 Feasibility, the primary outcome measure, was defined as the percent of participants remaining on the diet until disease progression, although participants were permitted to continue the diet until second progression and combine it with cytoactive treatments at progression. Three participants discontinued the diet, all reportedly due to its negative impact on quality of life. The remaining 85% of participants continued for a median of 36 days, with stable ketonuria reported in 73% of them. A small but significant weight loss of 2.2% was observed. Median progression-free survival was 5 weeks (range: 3–13 weeks), and the investigators concluded that additional investigations into alternative combinations were needed.
In summary, these existing prospective studies have incorporated widely varying degrees of glycemic modulation, limited calorie restriction, varying durations of follow up time, and differing response criteria. Thus, variable tolerability has been observed and conclusions regarding the efficacy of such interventions are limited. Based on the well-defined clinical experience in adult patients with medically refractory epilepsy, it is most likely that a classic, calorie-restricted KD will not be feasible for adult oncology patients. Alternative methods are necessary. Based on the growing literature supporting the MAD as a safe, feasible, and efficacious alternative to the classic KD in patients with medically refractory epilepsy, the MAD may also provide an appealing alternative for glycemic modulation in neuro-oncology.
Johns Hopkins Institutional Experience with a Modified Atkins Diet in Glioma
The comprehensive Johns Hopkins Adult Epilepsy Diet Center (AEDC) was previously established to manage children and adults with medically refractory epilepsy and has robustly implemented the MAD in the clinical management of these patients. Over the past several years, patients with glioma and epilepsy have been managed in the AEDC. Herein, we review our experience.
From January 2012 through April 2014, patients with glioma and resultant seizure disorders were prospectively observed and their data reviewed retrospectively to evaluate the safety and clinical impact of a MAD. Institutional Review Board approval was obtained and all patients were consented. Patients were excluded from starting diet therapy if there was a history of contraindicated metabolic disorder, significant atherosclerotic disease, acute pancreatitis, or cardiac or renal dysfunction. The MAD protocol is well established and consists of a 20g net carbohydrate (total carbohydrate grams minus fiber grams) per day restriction. During this interval, 7 participants were prescribed the MAD after initial visit to the AEDC, and 1 participant continued a 15 g/day diet begun 5 months before enrollment. High-fat foods were encouraged. Total daily calories and carbohydrate-free fluids were allowed ad libitum. Multivitamin, vitamin D, and calcium supplementation were prescribed. Baseline laboratory assessment included complete blood cell count (CBC), comprehensive metabolic panel (CMP), fasting lipid panel (FLP), urine calcium, creatinine, and human chorionic gonadotropin level (for women). Repeat CBC, CMP, and FLP were performed at 3 and 6 months. Baseline and follow-up imaging were collected as available. Participants were instructed to record daily seizure frequency and weekly weights on a monthly calendar. Urine ketones were initially followed biweekly and then at least weekly after one month. Guidelines for implementation of such a diet have been previously reported, standardized, and generalized in patients with medically refractory epilepsy.48,49 Baseline and follow-up laboratory and clinical data were compared using Student t tests (P < .05).
Eight participants with glioma were treated with MAD during the specified interval (Table 3). Six were male (75%), mean age was 41.5 ± 10.0 years, and mean diet duration was 13.2 ± 8.0 months (range: 2–24 months). Mean baseline weight was 78.8 ± 18.3 kg, and body mass index (BMI) was 25.7 ± 3.5.
Table 3.
Demographic, oncologic, and epileptic characteristics
| Patient | Sex | Age at Diet Initiation (years) | Carb Limit (grams/day) | Tumor Type | Tumor Location | Prior Tumor Treatment | Active Chemo During MAD | Seizure Type | Prior AED (no.) | Active AEDs at Initiation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 30 | 20 | AA | L parietal | Sx, XRT, TMZ | None | CPS | 1 | LEV |
| 2 | Female | 54 | 20 | Sec GBM | L parietal | Sx, XRT, TMZ | TMZ, Bev | CPS | 5 | LEV, PHT, LMT, KLO |
| 3 | Male | 48 | 20 | LGO | L frontal | Sx | None | SPS, CPS | 3 | LEV |
| 4 | Male | 37 | 15 | Rec GBM | L temporal | Sx, XRT, TMZ, Bev, ICT-107, Veliparib | Bev, LBH589 | GTC | 1 | LEV |
| 5 | Female | 38 | 20 | LGO | L temporal | Sx | None | GTC | 1 | None |
| 6 | Male | 28 | 20 | LGO | L temporal | Sx, TMZ | TMZ | GTC, SPS | 6 | PHT, TPM |
| 7 | Female | 54 | 20 | LGA | L parietal | Sx, XRT, TMZ | None | CPS, GTC | 1 | LEV |
| 8 | Female | 43 | 20 | GBM | L frontal | Sx, XRT, TMZ | None | CPS, GTC | 5 | LCM, VPA, LEV |
Abbreviations: AA, anaplastic astrocytoma; AED, antiepileptic drug; BEV, bevacizumab; CPS, complex partial seizures; CZ, clonazepam; GBM, glioblastoma multiforme; GTC, generalized tonic-clinic seizures); LCM, lacosamide; LEV, levetiracetam; LGA, low-grade astrocytoma; LGO, low-grade oligodendroglioma; LMT, lamictal; no., number; PHT, phenytoin; SPS, simple partial seizures; Sx, surgery; TMZ, temozolomide; TPM, topamax; VPA, valproic acid; XRT, radiation therapy.
At initiation, all participants had a diagnosis of seizure disorder including simple partial seizures (n = 2), complex partial seizures (n = 5), and generalized tonic-clonic seizures (n = 5). Average seizure duration ranged from 1–10 years with mean baseline frequency of 2.14 ± 4.0 seizures per month. On average, participants had previously tried 2.9 ± 2.2 antiepileptic drugs without achieving seizure control. Active AEDs at initiation ranged from 0–4 (one participantt elected to use MAD as an alternative to AED although counseled that MAD is typically used as adjunctive treatment).
Tumor types included low-grade oligodendroglioma (n = 3), low-grade astrocytoma (n = 1), anaplastic astrocytoma (AA, n = 1), and GBM (n = 3). Of those with GBM, one participant enrolled after initial concurrent chemoradiation, another enrolled at second recurrence, and the last enrolled after secondary progression from AA. Tumor location was frontal (n = 2), temporal (n = 3), or parietal (n = 3) and left sided in all participants. Those with GBM and AA had previously undergone surgery and radiation. Prior chemotherapy included temozolomide (TMZ, n = 6), bevacizumab (BEV, n = 1), and clinical trial therapies (n = 1). After diet initiation, 2 participants were treated with TMZ (one with TMZ and BEV), and the participant with recurrent GBM began clinical trial with BEV and LBH589 (NCT00859222).
At 13.2 months of follow-up (Table 4), 7 of 8 participants remained on MAD; one stopped at 3 months after MAD did not provide adequate seizure control. All participants are living and survival rates for those with progressive GBM are comparable to expected survival: 13 months for the participant with secondary GBM compared with 7.8 months expected56 and 17 months for the patient with recurrent GBM compared with 7.4 months expected.57 At analysis, average weight loss was 3.4 ± 6.5 kg. No participant was underweight (BMI range: 20.3–30). During the entire study observation period, a total of 3 participants were on glucocorticoids. At diet initiation, one participant was on dexamethasone (DEX, 2 mg), and one was on hydrocortisone (dexamethasone equivalent dose = 0.56 mg). One tapered off and did not resume glucocorticoids. The second was on a stable DEX dose (2 mg/day) throughout the study. An additional participant was started on DEX (4 mg) after initiating the diet. Mean seizure frequency was 1.25 ± 3.3 per month at 3 months and 0.4 ± 0.2 at 6 months. Active AEDs did not significantly change. At a mean follow up time of 13.2 months, five participants (63%) experienced at least a 50% reduction in seizure frequency. Four were seizure free. Laboratory values were unchanged at 6 months (Supplementary Table S1); lipid values were previously published for a subset of patients.58
Table 4.
Clinical changes observed during dietary intervention
| Follow-up Characteristic | Overall (n = 8) | P value |
|---|---|---|
| Duration of diet, months, mean (std) | 13.17 (8.0) | |
| Weight | .48 | |
| Baseline weight, kg, mean (std) | 78.8 (18.3) | |
| Follow up weight, kg, mean (std) | 72.6 (15.0) | |
| Weight change, kg, mean (std) | −3.4 (6.5) | |
| Body mass index | .40 | |
| Baseline BMI, mean (std) | 25.7 (3.5) | |
| Follow up BMI, mean (std) | 24.2 (3.2) | |
| BMI change (reduction), mean (std) | 0.95 (1.9) | |
| Seizure frequency | ||
| Baseline seizure frequency, mean per week (std) | 0.54 (1.0) | |
| 3-month seizure frequency, mean per week (std) | 0.31 (0.83) | .62 |
| 6-month seizure frequency, mean per week (std) | 0.10 (0.04) | .27 |
| Seizure reduction at follow Up | NA | |
| 50% seizure reduction (n, %) | 5 (63%) | |
| Seizure free (n) | 4 |
Abbreviations: BMI, body mass index; kg., kilogram; std., standard deviation.
Seizure reduction at follow-up includes the number and percent of patients with a 50% reduction in seizure frequency or who were seizure free at the time of data analysis (mean follow up 13.2 ± 8.0 months).
In summary, in this series of participants with glioma and seizures treated with a 20 g/day carbohydrate-restricted MAD, the diet was well tolerated without significant toxicity and, although not statistically significant, resulted in improvement in seizure control. All participants are living, and survival rates for those with GBM are comparable with expected median survival for their respective stages of disease. Of the 8 participants treated in this study, only one discontinued the diet (due to lack of antiseizure efficacy), and the 7 (88%) remaining participants continued the diet for 2–24 months. In comparison, Kossoff et al evaluated 30 adult patients with intractable epilepsy who were treated with a 15 g/day MAD; 26 (87%) remained on the diet at 1 month, 20 (67%) at 3 months, and 14 (47%) completed all prescribed 6 months.48 Of those who stopped the diet prior to 6 months, 9 (56%) did so due to lack of efficacy, 6 (38%) due to feasibility, and 1 did not start after enrollment. Cervenka et al. evaluated 25 adult patients with intractable epilepsy who were treated with a 20 g/day MAD.49 Of the 22 who actually initiated the diet, 21 (95%) remained on the diet at 1 month, and 14 (64%) at 3 months. While prospective data are necessary to determine the feasibility and tolerability of the MAD in patients with glioma, these data suggest that the MAD may provide a safe, tolerable, and feasible intervention to explore as a potential method of dietary glycemic modulation and adjunct to standard chemoradiation in glioma. Such an approach also provides an opportunity to pursue novel therapeutic combinations incorporating dietary metabolic regulation with conventional cytoactive treatments and to explore longer-term maintenance approaches that avoid the myelosuppressive or emetogenic side effects of prolonged chemotherapy.
Future Directions in Advancing Dietary Glycemic Modulation in Glioma
Moving forward, clinical studies will need to address 4 key issues currently facing the translation of dietary glycemic modulation in neuro-oncology: (i) the lack of a defined nutritional goal or prescribed “dose” of dietary glycemic modulation, (ii) concerns regarding associated weight loss in a potentially “at risk” population, (iii) the need to define optimal endpoints for response and efficacy with such metabolic interventions, and (iv) the safety and efficacy of combination strategies incorporating glycemic modulation into other cytoactive treatment regimens.
Defining a Dose of Dietary Glycemic Modulation
As with any therapeutic intervention, defining the optimal dose necessary to achieve clinical and biological activity as well as to ensure patient safety and tolerability is important. In a dietary intervention that targets glycemic modulation, dosing considerations include precise definition of daily calorie, carbohydrate (total or net), protein, and fat intake as well as the frequency and duration of these interventions. At present, 6 studies utilizing KDs in patients with glioma are recruiting participants (Table 5). These studies vary in terms of the prescribed dietary intervention, the duration of the study, and the means of calorie restriction. Given the recent preclinical data suggesting that periods of intermittent fasting may provide superior anticancer activity to long-term calorie restriction without substantial weight loss,33,34 intermittent fasting is potentially an attractive adjunct. Thus in glioma, a diet that combines the tolerability and defined dosing strategy of the MAD with the caloric impact of intermittent fasting may prove optimal.
Table 5.
Active studies evaluating ketogenic diets in glioma
| Disease State | Sample Size | Dietary Intervention | Duration of Study | Primary Outcome | Study Status | |
|---|---|---|---|---|---|---|
| University of
Michigan NCT01535911 |
Recurrent GBM | 12 | Total calories 20–25/kg/day | 6 weeks ± 6 weeks if response | Tumor response (PET-CT) | Recruiting |
| Tel-Aviv Sourasky Med
Ctr NCT01092247 |
Recurrent HGG | Unknown | Individualized to patient (details not specified) | 1 year | “Tumor progression and patient longevity” | Unknown |
| Mid-Atlantic Epilepsy and Sleep
Ctr/Univ of Pittsburgh NCT01865162 |
Recurrent GBM | 6 | Classic KD (4:1 ratio) + 1600 kcal restriction | 6 months | Safety | Recruiting |
| Johann Wolfgang Goethe
University Hospitals NCT01754350 |
Recurrent GBM or gliosarcoma | 2-arm: intervention vs standard diet | KD (<60 g/day CHO, 21–23 kcal/kg/day) + intermittent fasting | 6 months | 6 month PFS | Recruiting |
| St. Joseph's Hospital and Medical
Center, Phx NCT02046187 |
Newly Diagnosed GBM | Unknown | Classic KD (4:1 ratio) | 1 year | Tolerance and compliance (self-report) | Recruiting |
| Johns Hopkins
Hospital NCT02286167 |
Newly diagnosed GBM | 25 | MAD + intermittent fasting | 8 weeks | Feasibility, biologic activity | Recruiting |
Abbreviations: GBM, glioblastoma, HGG, high-grade glioma; KD, ketogenic diet (reference: www.clinicaltrials.gov. Last accessed: November 17, 2014.); PET-CT, Computed tomographic positron emission tomography.
Strategies for objectively measuring compliance with prescribed diets are also necessary to study feasibility, evaluate tolerability, and ensure generalizability of results. Patient-reported dietary intake through diet records has been well studied and reported to be reliable and valid in nutrition literature.59,60 However, the subjective nature of self-reported intake presents challenges to ensuring accurate and reliable data.61–64 Dietary biomarkers (eg, caffeine metabolites for caffeine intake, urine proline betaine for citrus, total resveratrol metabolites for wine) are being evaluated and may aid in evaluating compliance; however, current markers are insufficient to monitor intake comprehensively, and further development is necessary.65 Serum and urine ketone measurements have been utilized in patients with medically refractory epilepsy with success and have provided a reliable assessment of ketogenic dietary compliance. However, their application in oncology has not been studied, and in fact preclinical studies suggest that they may be less reliable in this setting,27 particularly with the concomitant use of corticosteroids.
Mitigating Weight Loss Concerns
Importantly, given concerns of cancer-related cachexia and the importance of maintaining adequate nutrition and caloric intake in advanced cancer patients, care must be taken in applying therapeutics that could negatively impact optimal nutrition. In most studies of KDs, weight loss has been observed though not inherently prescribed. It is not clear whether this observation has resulted from self-selected dietary changes, increased physical activity, or another underlying mechanism. Existing preclinical data suggest that calorie restriction is a critical component to dietary efficacy in oncology27; however, recent reports on intermittent fasting suggest that alternative methods for deriving this metabolic “switch” may exist. Limited clinical data do suggest that intermittent fasting may safely augment the effects of dietary intervention in patients with refractory seizures.66 There is growing interest in supplementing dietary interventions with neutraceuticals, ketone preparations, and other supplements that may also promote alterations in the metabolic phenotype and prevent weight loss; however, further study is required, and initial steps to define precise dosing strategies remain imperative.23
Defining Endpoints for Efficacy
Despite years of research in patients with medically refractory epilepsy, many questions about the cerebral biologic activity of glucose modulation through KDs exist. The current studies of KDs in oncology have added only limited additional data on potential biomarkers of cerebral activity. While systemic markers (eg, fasting glucose or serum ketones) have provided some evidence of systemic biologic activity, particularly in epilepsy, they do not appear to be reliable biomarkers of antitumor response.27
Positron emission tomography (PET) has been included in the published studies on systemic malignancy and KDs and is included in at least one currently enrolling study in glioma (NCT01535911); however, given the poor sensitivity and specificity of 18F-FDG PET in glioma and substantial cerebral background signal, alternative radiographic markers of response will be necessary.67 Recent studies have highlighted the potential utility of alternative PET radiotracers including 11C-methionine, 18F-FDOPA, and recently l-(5-[11]C)-glutamine,68 though their utility as a marker of metabolic intervention is unclear.69 D-glucose is also being investigated as a potential infusible, biodegradable contrast agent in combination with MRI; however, further study is required before clinical application.70
Magnetic resonance spectroscopy (MRS) is a safe, feasible, and well-established noninvasive method for evaluating tissue metabolite concentrations. It has been investigated in gliomas for decades. Reliable and valid studies have established concentrations of cerebral metabolites,71 expected changes in spectral patterns over the course of therapy,72 and correlation with survival in certain populations.73 Recent work shows that emerging MRS techniques can be used to reliably evaluate metabolites that were previously difficult to resolve including glutamate (Glu), glutamine (Gln), glycine, and γ-aminobutyric acid.74–77 Intratumoral Glu concentrations measured by MRS have recently been shown to provide a robust marker of survival in 45 pediatric patients with medulloblastoma (HR 8.0, P = .0003).73 As such, MRS may offer a method of assessing metabolic response to dietary glycemic modulation.
Patient-reported outcome measures, including those assessing both health-related quality of life, and other patient-centered metrics will also be important. Nonvalidated patient-reported outcome measures have been incorporated into existing studies of KDs, but it will be important to determine the potential generalizability and psychosocial impact of such interventions. In addition, given the reported associations between hyperglycemia and infection, seizure control, and other important medical comorbidities, nononcologic covariates should also be included in future prospective studies.78–80
Combining Dietary Glycemic Modulation With Cytotoxic Therapies
Ultimately, dietary interventions that target the glycolytic dependency of gliomas may prove optimal when combined with additional therapies that target tumor hypoxia, cellular proliferation, or cell signaling pathways. Gliomas are both highly metabolically active and highly mutagenic. Preliminary studies suggest that responses may be improved when KDs are combined with radiation,81 chemotherapy,32 and even hyperbaric oxygen.23 Additionally, current strategies for developing longer-term maintenance therapy for gliomas in general are limited by myelosuppressive and emetogenic risk with chemotherapy as well as the potential for inducing new driver mutations.82 Additional preclinical work is necessary to determine the impact of KDs on tumor-initiating cells, but some evidence suggests that dietary glycemic modulation may impair growth of these tumor stem cells through reduction in circulating insulin, IGF-1, and other proliferative pathways.83
Conclusion
Knowledge of the metabolic pathways underlying glioma tumorigenesis continues to expand and offers novel therapeutic targets. Pharmacological drug development is ongoing to identify agents that can be successfully delivered to the target tissue and alter key metabolic pathways. Ketogenic diets (classic KD, MAD, and others) offer another readily available therapeutic avenue. Strategies that combine the tolerability of the MAD with intermittent fasting to restrict caloric intake may prove optimal. A systematic approach to developing a clearly defined dose, establishing measures of biology activity, and determining optimal endpoints is critical.
Funding
This study received no internal or external funding.
Conflict of interest statement. R.E.S.: grant from the American Academy of Neurology. M.C.C.: grants from Nutricia and NIH (NINDS R01NS075020). B.H.: grants from Johns Hopkins Institute for Clinical and Translational Research. (ICTR), funded in part by NIH grants (UL1 TR 000424-06 NCATS), NIH Roadmap for Medical Research, and Nutricia. E.H.K: grant from Nutricia; consultant to Atkins Nutritionals, Inc., Eisai, Upsher-Smith, GW Pharmaceuticals, and NeuroPace. A.L.H.: research support from NIH (NINDS), Maryland Innovation Initiative, and Johns Hopkins University School of Medicine; receives income from his clinical practice and reading EEGs (21% effort); serves as an Associate Editor for Epilepsia, and has provided expert opinion in medicolegal cases. J.B.: research support from the Cancer Therapeutic Evaluation Program of NCI; the Children's Tumor Foundation; non-salary research support from GlaxoSmithKline, Lilly, Sanofi.
Supplementary Material
References
- 1. Johnson DR, O'Neil BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. [DOI] [PubMed] [Google Scholar]
- 2. Seyfried TN, Flores RE, Poff A, et al. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. [DOI] [PubMed] [Google Scholar]
- 4. Oudard S, Arvelo F, Miccoli L, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fredericks M, Ramsey RB. 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system. J Neurochem. 1978;31:1529–1531. [DOI] [PubMed] [Google Scholar]
- 6. Tisdale MJ, Brennan RA. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983;47:293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oudard S, Arvelo F, Miccoli L, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurer GD, Brucker DP, Bahr O, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;26(11):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jelluma N, Yang X, Stokoe D, et al. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res. 2006;4(5):319–330. [DOI] [PubMed] [Google Scholar]
- 10. Arismendi-Morilo GJ, Castellano-Ramirez AV. Ultrastructural pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies. J Electron Micros (Tokyo). 2008;57(1):33–39. [DOI] [PubMed] [Google Scholar]
- 11. Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (London). 2010;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiebish MA, Han X, Cheng H, et al. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res. 2008;49(12):2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takano T, Lin JH, Arcuino G, et al. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001;7:1010–1015. [DOI] [PubMed] [Google Scholar]
- 14. de Groot J, Sontheimer H. Glutamate and the biology of gliomas. Glia. 2011;59(8):1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y, Zhou Y, Shingu T, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286(37):32843–32853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Rev Can. 2011;11:85–89. [DOI] [PubMed] [Google Scholar]
- 17. Westhoff MA, Karpel-Massler G, Bruhl O, et al. A critical evaluation of PI3 K inhibition in Glioblastoma and Neuroblastoma therapy. Mol Cell Ther. 2014;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–3899. [DOI] [PubMed] [Google Scholar]
- 19. Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13(10):444–451. [DOI] [PubMed] [Google Scholar]
- 20. Rathmell JC, Fox JC, Plas DR, et al. Akt-directed glucose metabolism can prevent Bax conformational change and promote growth factor-independent survival. Mol Cell Biol. 2003;23(20):7315–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JW, Tchernyshyov I, Semenza GL, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. [DOI] [PubMed] [Google Scholar]
- 22. Morfouace M, Lallier L, Oliver L, et al. Control of glioma cell death and differentiation by PKM2–Oct4 interaction. Cell Death Dis. 2014;5:e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poff AM, Ari C, Arnold P, et al. Ketone supplementation decreases tumor viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res. 2004;10(16):5622–5629. [DOI] [PubMed] [Google Scholar]
- 25. Zhou W, Mukherjee P, Kiebish MA, et al. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDaniel SS, Rensing NR, Thio LL, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52(3):e7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seyfried TN, Sanderson TM, El-Abbadi MM, et al. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003;89(7):1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seyfried TN, Marsh J, Shelton LM, et al. Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res. 2012;100(3):310–326. [DOI] [PubMed] [Google Scholar]
- 29. Seyfried TN, Mukherjee P. Anti-angiogenic and pro-apoptotic effects of dietary restriction in experimental brain cancer: role of glucose and ketone bodies. In: Meadows GG, ed. Integration/Interaction of Oncologic Growth. New York, NY: Kluwer Academic; 2005:258–270. [Google Scholar]
- 30. Abdelwahab MG, Fenton KE, Preul MC, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLos One. 2012;7(5):e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond). 2010;22(7):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allen BG, Bhatia SK, Buatti JM, et al. Ketogenic diets enhance oxidative stress and radiochemotherapy response in lung cancer xenografts. Clin Cancer Res. 2013;19(14):3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. [DOI] [PubMed] [Google Scholar]
- 34. Raffaghello L, Safdie F, Bianchi G, et al. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9(22):4474–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freeman JM, Freeman JB, Kelly MT. The Ketogenic Diet: A Treatment for Epilepsy, 3rd ed.New York, NY: Demos; 2000 [Google Scholar]
- 36. Baranano KW, Hartman AL. The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol. 2008;10(6):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henderson CB, Filloux FM, Alder SC, et al. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol March. 2006;21(3):193–198. [DOI] [PubMed] [Google Scholar]
- 38. Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn). 2013;19(3):756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klein P, Tyrlikova I, Mathews GC. Dietary treatment in adults with refractory epilepsy: A review. Neurology. 2014;83(21):1978–1985. [DOI] [PubMed] [Google Scholar]
- 40. Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119(3):535–543. [DOI] [PubMed] [Google Scholar]
- 41. Kossoff EH, Pyzik PL, McGrogan JR, et al. Efficacy of the ketogenic diet for infantile spasms. Pediatrics. 2002;109:780–783. [DOI] [PubMed] [Google Scholar]
- 42. Mady MA, Kossoff EH, McGregor AL, et al. The ketogenic diet: Adolescents can do it, too. Epilepsia. 2003;44:847–851. [DOI] [PubMed] [Google Scholar]
- 43. Neal EG, Chaffe H, Schwartz RH, et al. A randomized trial of classical and MCT KDs in the treatment of epilepsy. Epilepseia. 2009;50(5):1109–1117. [DOI] [PubMed] [Google Scholar]
- 44. Pfiefer HH, Thiele EA. Low glycemic index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65(11):1810–1812. [DOI] [PubMed] [Google Scholar]
- 45. Kossoff EH, Krauss GL, McGrogan JR, et al. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61(12):1789–1791. [DOI] [PubMed] [Google Scholar]
- 46. Kossoff EH, McGrogan JR, Bluml RM, et al. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421–424. [DOI] [PubMed] [Google Scholar]
- 47. Kossoff EH, Cervenka MC, Henry BJ, et al. A decade of the modified Atkins diet (2003–13): Results, insights and future directions. Epilepsy Behav. 2013;29(3):437–442. [DOI] [PubMed] [Google Scholar]
- 48. Kossoff EH, Rowley H, Sinha SR, et al. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49:316–319. [DOI] [PubMed] [Google Scholar]
- 49. Cervenka MC, Terao NN, Bosarge JL, et al. Email management of the modified Atkins Diet for adults with epilepsy is feasible and effective. Epilepsia. 2012;53(4):728–732. [DOI] [PubMed] [Google Scholar]
- 50. Porta N, Vallee L, Boutry E, et al. Comparison of seizure reduction and serum fatty acid levels after receiving the ketogenic and modified Atkins diet. Seizure. 2009;18(5):359–364. [DOI] [PubMed] [Google Scholar]
- 51. Kossoff EH, Dorward JL, Turner Z, et al. Prospective study of the modified Atkins diet in combination with a ketogenic liquid supplement during the initial month. J Child Neurol. 2011;26(2):147–151. [DOI] [PubMed] [Google Scholar]
- 52. Kossoff EH, Bosarge JL, Miranda MJ, et al. Will seizure control improve by switching from the modified Atkins diet to the traditional ketogenic diet? Epilepsia. 2010;51(12):2496–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fine EJ, Sega-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility trial in 10 patients. Nutrition. 2012;28(10):1028–1035. [DOI] [PubMed] [Google Scholar]
- 54. Schmidt M, Pfetzer N, Schwab M, et al. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rieger J, Bahr O, Maurer GD, et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. [DOI] [PubMed] [Google Scholar]
- 57. Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28(24):3838–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cervenka MC, Patton K, Eloyan A, et al. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutr Neurosci. 2014; 10.1179/1476830514Y.0000000162. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 59. Brunner E, Stallone D, Juneja M, et al. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86:405–414. [DOI] [PubMed] [Google Scholar]
- 60. Yang YJ, Kim MK, Hwang SH, et al. Relative validities of 3-day food records and the food frequency questionnaire. Nutr Res Pract. 2010;4(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crawford PB, Obarzanek E, Morrison J, et al. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J Am Diet Assoc. 1994;94(6):626–630. [DOI] [PubMed] [Google Scholar]
- 62. Ambrosini GL, O'Sullivan TA, de Klerk NH, et al. Relative validity of adolescent dietary patterns: a comparison of a FFQ and 3-day food record. Br J Nutr. 2011;105(4):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Appannah G, Pot GK, O'Sullivan TA, et al. The reliability of an adolescent dietary pattern identified using reduced-rank regression: comparison of a FFQ and 3-day food record. Br J Nutr. 2014;112(4):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kowalkowska J, Slowinska MA, Slowinski D, et al. Comparison of a full food-frequency questionnaire with the three-day unweighted food records in young Polish adult women: implications for dietary assessment. Nutrients. 2013;5(7):2747–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hedrick VE, Dietrich AM, Estabrooks PA, et al. Dietary biomarkers: advances, limitations and future directions. Nutr J. 2012;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hartman AL, Rubenstein JE, Kossoff EH. Intermittent fasting: a “new” historical strategy for controlling seizures? Epilepsy Res. 2013;104(3):275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wong TZ, vann der Westheizen GJ, et al. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002;12(4):615–626. [DOI] [PubMed] [Google Scholar]
- 68. Qu W, Oya S, Lieberman BP, et al. Preparation and characterization of L-[5–11C]-glutamine for metabolic imaging of tumors. J Nucl Med. 2012;53(1):98–105. [DOI] [PubMed] [Google Scholar]
- 69. Chen W, Silverman DH, Delalove S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–911. [PubMed] [Google Scholar]
- 70. Chan KW, McMahon MT, Kato Y, et al. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 2012;68(6):1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kinoshita Y, Yokota A. Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed. 1997;10(1):2–12. [DOI] [PubMed] [Google Scholar]
- 72. Alexander A, Murtha A, Abdulkarim B, et al. Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin Invest Med. 2006;29(5):301–311. [PubMed] [Google Scholar]
- 73. Wilson M, Cummins CL, Macpherson L, et al. Magnetic resonance spectroscopy metabolite profiles predict survival in paediatric brain tumours. Eur J Cancer. 2013;49(2):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hourani R, Horská A, Albayram S, et al. Proton magnetic resonance spectroscopic imaging to differentiate between nonneoplastic lesions and brain tumors in children. J Magn Reson Imaging. 2006;23(2):99–107. [DOI] [PubMed] [Google Scholar]
- 75. Zhu H, Edden RA, Ouwerkerk R, et al. High resolution spectroscopic imaging of GABA at 3 Tesla. Magn Reson Med. 2011;65(3):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu H, Soher BJ, Ouwerkerk R, et al. Spin-echo magnetic resonance spectroscopic imaging at 7 T with frequency-modulated refocusing pulses. Magn Reson Med. 2013;69(5):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu H, Barker PB. MR spectroscopy and spectroscopic imaging of the brain. Methods Mol Biol. 2011;711:203–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Derr RL, Ye X, Islas MU, et al. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hong YJ, Han HS, Jeong Y, et al. Impact of hyperglycemia on survival and infection-related adverse events in patients with metastatic colorectal cancer who were receiving palliative chemotherapy. Cancer Res Treat. 2014;46(3):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang CW, Tsai JJ, Ou HY, et al. Diabetic hyperglycemia is associated with severity of epileptic seizures in adults. Epilepsy Res. 2008;79(1):71–77. [DOI] [PubMed] [Google Scholar]
- 81. Abdelwahab MG, Fenton KE, Preul MC, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7(5):e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mihavlova MM, Sabatini DM, Yilmaz OH. Dietary and metabolic control of stem cell function in physiology and cancer. Cell Stem Cell. 2014;14(3):292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.