Abstract
Background
1.7% of children taking medication on an outpatient basis in Germany have at least one adverse drug reaction (ADR). The corresponding figure for hospitalized children is estimated at 10%.
Method
This review is based on pertinent literature retrieved by a selective search in PubMed.
Results
According to reports submitted to the Drug Commission of the German Medical Association (Arzneimittelkommission der deutschen Ärzteschaft, AkdÄ), serious ADRs can arise, for example, after the administration of dimenhydrinate, α -adrenergic nose drops, enemas containing phosphate, ACE inhibitors, angiotensin-2-receptor antagonists (sartans), and methylphenidate. The causes of ADRs include overdoses, drug administration despite contraindications, and inadequate monitoring of long-term treatment. Errors can also be made in communication, labeling, and drug administration. The risk of ADRs is especially high in off-label use. Computerized physician order entry systems, individual packaging and labeling of single doses, and the use of bar codes for patient and drug identification can help prevent such errors.
Conclusion
The process of drug administration should be optimized through suitable interventions and electronic support, with due consideration of local circumstances. Clinical trials on children should be encouraged as a means of improving drug safety, and additional financial incentives should be created for trials concerning drugs that are off-patent. Physicians and pharmacists should take care to report adverse reactions as they are required to do by professional code, particularly in the case of new drugs, off-label use, or medication errors. A recognized national standard for dosing that can be implemented in computerized physician order entry systems is needed so that evidence-based pediatric dosages can be calculated.
Proving the safety and efficacy of medicinal products and identifying their correct dosage are the responsibility of drug regulating authorities. After marketing authorization has been granted, data on rare adverse reactions and regarding the safety of long-term use are collected in the context of pharmacovigilance (Box). Children are at higher risk from medications because drugs are often used without license and without tested dosages in the individual age groups (off-label use).
Box. Important definitions (from [39]).
-
Drug safety
The totality of measures taken to monitor the safety of a medicinal product continuously and systematically, with the aim of discovering adverse reactions when used in accordance with their intended purpose, to evaluate and understand these adverse reactions and to be able to take relevant measures to minimize risk. The insights gained into the safety of a drug constitute an important contribution to the continuous updating of the licensing status of medicines (ensuring the safety of the medicinal product)
-
Medication Safety
The totality of measures to ensure an optimized medication process with the aim of reducing medication errors and thereby avoidable risks to patients during drug treatment (ensuring quality and safety of the medication process)
-
Adverse drug reactions (ADR) (=side effects)
Harmful and unintended reactions to a medicine that is licensed for use in humans; distinction needs to be made between use in accordance with their intended purpose, as a result of medication error, and as a result of misuse or professional exposure.
-
Medication errors
Deviation from the medication process that is optimal for the patient, which results, or may result, in fundamentally avoidable harm to the patient.
Medication errors can occur at every step of the medication process and can be caused by all those involved in the medication process, especially by doctors, pharmacists, or other healthcare professionals, as well as by patients, their relatives, or third parties.
In the past, children were harmed by medicinal products disproportionately often, and subsequent to various catastrophic incidents, laws and regulations were passed in order to improve drug safety (e1, e2). The predecessor of the US Food and Drug Administration (FDA) was founded in 1906 because—among other reasons—children had been harmed by hazardous substances such as morphine, as manufacturers of medicinal products had previously not been required to list ingredients or warn against misuse on the labels (1). After the thalidomide catastrophe, Germany’s Medicinal Products Act provided in 1976 for compulsory testing for efficacy and safety of drugs before they were granted marketing authorization (e3). Children were often excluded from clinical studies because of ethical considerations.
The 2002 EU initiative Better Medicines for Children was the first to systematically tackle this discrimination of children in Europe. Changes to laws made studies of drugs in children easier and even demanded these explicitly, but at the same time, children were protected from pointless studies by tight restrictions (12th Amendment to the Medicinal Products Act 2004, EU regulation on medicinal products for pediatric use 2007). For the purposes of developing new drug treatments for disorders that may affect not only adults but also children, and for the respective extensions to indications, a pediatric investigation plan (PIP) is mandatory, without which a drug cannot be licensed, not even for adults. Many new medications are therefore licensed for use in children in certain age groups.
What remains unanswered is the question of licensing approval and safety of medicinal products whose patent has expired. The financial incentive for optional special licensing of such medications (pediatric use marketing authorization, PUMA) in the form of a 10-year period of exclusive marketing was obviously insufficient, since only two such licenses have been granted thus far (e4).
The institution of the Pharmacovigilance Risk Assessment Committee (PRAC) in the European Medicines Agency (EMA) assessed risks and benefits of medications that had already been on the market for a lengthy period, with the result that the use of metoclopramide and codeine was clearly restricted in children (e5– e7).
The described measures have improved drug safety. However, the continuing high proportion of off-label medication use in children still poses an increased risk for adverse drug reactions, including medication errors (2, 3). For this reason, the need for improved drug safety and medication safety in pediatric and adolescent medicine is particularly great.
This study aims to explain the practical problems of drug safety in children in Germany (and Europe) and to show possible approaches for a solution.
Method
We conducted a selective literature search in PubMed for German-language or English-language articles for the publication period up to April 2015, using the following search terms: “medication error”, “adverse drug event”, “adverse drug reaction”, “off label”, “physician order entry”, “cdss”, unit dose”, or “clinical pharmacist”, in combination with “pediatrics” or “children”.
Problems arising from using medicines
Rates of adverse drug reactions
Different analyses have shown that the incidence of adverse drug reactions in children during inpatient stays in the hospital is 9.53% (95% confidence interval 6.81% to 12.26%) and 10.9% (4.8% to 17.0%). Antibiotics, asthma medications, antiepileptic drugs, and cardiac drugs are the most frequent causes in absolute terms (4– 6).
The pooled incidence of adverse drug reactions as a reason for inpatient admission is 2.9% (2.6% to 3.1%). A study from the UK (ADRIC) showed that 22.1% (17% to 28%) of the events had been avoidable and therefore had to be considered to be medication errors (8, e8).
Medication errors with the potential for harming patients were three times as common in a pediatric hospital compared with a hospital for adults (29 versus 9.1 per 1000 treatment days) (9, e9, e10).
The representative study on the health of children and adolescents in Germany (KiGGS, the German Health Interview and Examination Survey for Children and Adolescents) showed that in the outpatient setting, 50.8% of children and adolescents had been treated with medicinal products within seven days preceding the survey (10). This included nutritional supplements, such as vitamins or trace elements, however. This also explains the low prevalence of adverse drug reactions, of 1.7%. The largest prevalence of adverse drug reactions was reported for patients with attention deficit-hyperactivity disorder (ADHD) who were treated with methylphenidate (11.9%). In patients who took four medicines or more, the prevalence was increased by a factor of 7 compared with patients taking only one drug. This means that polypharmacy and drug interactions (eTable) constitute a known and relevant risk for increased adverse drug reactions in pediatrics as well as in adult medicine (11, 12).
eTable. Selected drug interactions of relevance to the pediatric setting (modified from [11]).
| Drug 1 | Drug 2 | Interaction |
|---|---|---|
| ACE inhibitor | Spironolactone | Increase in serum potassium concentration owing to additive effects on renal reabsorption |
| ACE inhibitor | Allopurinol | Increased risk of life-threatening skin reactions |
| Azathioprine/mercaptopurine | Allopurinol | Inhibition of xanthine oxidase by allopurinol inhibits the breakdown of azathioprine, dosage reduction required |
| Beta blocker | Insulin | Risk of hypoglycemia, as warning signs of hypoglycemia are lessened |
| Beta blocker | Beta mimetics, e.g. salbutamol | Mutual inhibition of effect |
| Carbamazepine, oxcarbazepine | Antiepileptic drugs e.g. lamotrigine, valproate | Increased breakdown of antiepileptic drugs owing to enzyme induction |
| Ciclosporin | Rifampicin | Drop in ciclosporin concentration owing to enzyme induction (CYP3A4) |
| Ciclosporin | Clarithromycin | Rise in ciclosporin concentration owing to enzyme induction (CYP3A4) |
| Lamotrigine | Oral contraceptives | Induction of lamotrigine breakdown |
| Ciprofloxacine/ofloxacine | Theophylline | Rise in plasma concentration of theophylline owing to enzyme induction (CYP1A2) |
| Meropenem | Valproate | Drop in plasma concentration of valproate |
| Phenobarbital | Antiepileptic drugs e.g. carbamazepine, lamotrigine | Increased breakdown of antiepileptic drugs owing to enzyme induction |
| Proton pump inhibitors e.g. omeprazole | Propranolol | Reduced resorption of propranolol |
| Combination of several QT interval prolonging substances, such as macrolide antibiotics (e.g. clarithromycin), quinolone antibiotics (e.g. ciprofloxacin), azole antibiotics (e.g. fluconazole), ondansetron | Additive effects on QT interval | |
ACE, angiotensin converting enzyme
Poor rate of spontaneous reporting of adverse drug reactions
Particularly rare adverse reactions were not captured in the licensing studies. For this reason, the fact that, in Germany, the authorities receive notably fewer spontaneous reports of adverse reactions in patients younger than 20 (13, e11) than in other European countries, is a serious matter. The reasons for such underreporting include
A lack of awareness of the problem
A lack of time
The fear of potential legal consequences in adverse drug reactions as a result of off-label uses and medication errors.
By extending the term “adverse drug reactions” to include events associated with improper use of medications (Box), all medication errors that may have caused harm to a patient should be reported. In order to increase the reporting/notification rate, an online portal was set up according to the new EU pharmacovigilance regulation (2010), which affected patients and their parents can use to make reports to the independent federal higher authorities (14). By August 2015, all package leaflets containing information for users and prescribing information had to include a note on the options for and importance of the notification/report. Medicines with insufficient data on long-term use, with a conditional license, or with a new active substance are additionally marked with a black triangle, in order to draw attention to the importance of reporting adverse reactions. Independently, doctors and pharmacists are obliged by their respective professional codes to report adverse reactions to their drug commissions.
In a very positive step, the EMA and the supreme federal authorities have made their databases on adverse drug reactions available online. Recently received reports of medication harms and deaths in children give cause for alarm. Even for medications that have been established for many years and do not require a prescription—such as first generation H1 antihistamines, phosphate-containing enemas, or decongestant nasal drops—not paying attention to the dosage information and contraindications can have severe consequences (Table 1) (15, 16, e12, e13).
Table 1. Serious adverse drug reactions in children and adolescents (selected reports/notifications to AkdÄ).
| Substance | Sequelae | Cause |
|---|---|---|
| Dimenhydrinate | Deaths in infants/toddlers | Licensed dose exceeded |
| α -adrenergic nasal drops | Coma in neonates | Dose exceeded/incorrect concentration administered |
| Phosphate-containing enemas | Deaths in infants/toddlers | Contraindication in children not heeded <6 years |
| ACE inhibitors, sartans | Intrauterine damage | Contraindication in pregnant women not heeded |
| Methylphenidate | Terminal liver failure | Insufficient monitoring in long-term treatment |
AkdÄ, Drug Commission of the German Medical Association (Arzneimittelkommission der Ärzteschaft); ACE, angiotensin converting enzyme
Pharmacological specifics
Ultimately the medication errors mentioned in this study were due to insufficient attention given to pharmacokinetic and pharmacodynamic particularities in pediatric patients. Children are not small adults but are subject to continuous growth and adaptation processes that require continual adaptation of dosages. Earlier studies provided detailed explanations of this subject (17– 19).
The linear extrapolation of adult dosages cannot replace studies of pharmacokinetics/pharmacodynamics (PK/PD) in pediatric patients. Conversions based on body weight can lead to overdoses especially in overweight adolescents, whereas toddlers may receive dosages that are too low. By contrast, when using calculations based on body surface area, dosages in toddlers may be too high (20). For this reason, dosing recommendations that are based on body weight or surface area cannot be universally valid for neonates through to adolescents (21). This fact is often not heeded, even for licensed dosages and in guidelines (22).
Off-label use
The KiGGS study found that 30% of medicines taken had been used off-label (23). This was particularly so for cardiovascular disorders or for topical applications (23, 24). Altogether the prevalence of off-label uses increases in line with patients’ decreasing age and is higher in the inpatient setting—and especially in intensive care units. Studies in neonatal intensive care units have shown prevalence rates of more than 90% in some cases (25, e14). The risk of experiencing an adverse drug reaction during inpatient treatment is increased by a factor of 2.25 (1.95 to 2.50) when off-label drugs are used (8, e15).
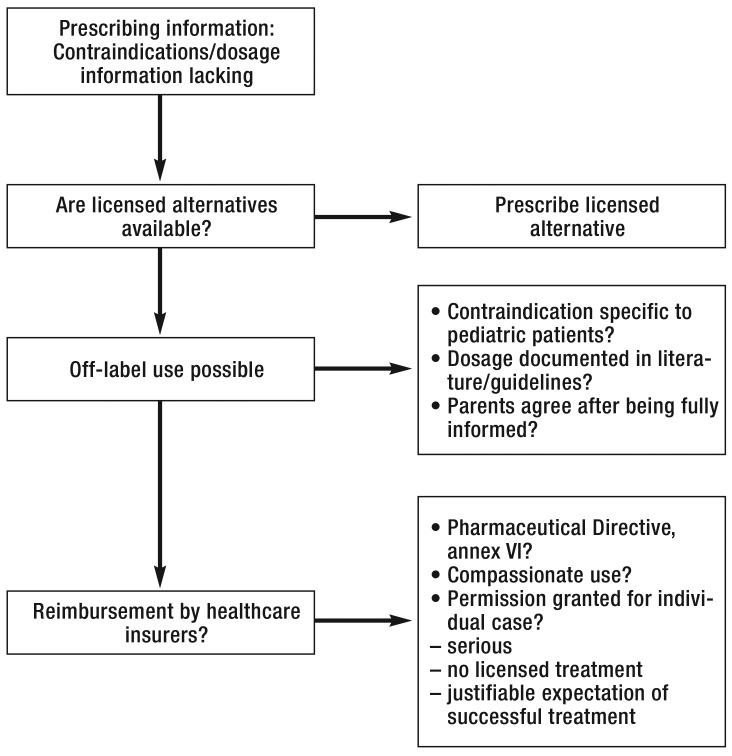
Where licensed alternative treatments are lacking, however, the risks and benefits of off-label use have to be assessed, and patients’ parents have to be informed accordingly (eFigure). Another consideration before prescribing expensive medicines is that statutory health insurers may not reimburse the costs for off-label treatments in certain circumstances (26). On the other hand, denying a treatment that is not licensed but is state of the art in scientific terms may have legal consequences (e16). A detailed overview of this topic is given by Rojahn and Stute (26).
eFigure.
Decision pathways in off-label use in children and adolescents
Often, the absence of dosage units or age-related contraindications in prescribing information is based on nothing more than lack of experience owing to lacking clinical studies of usage in a particular age group; in case of the correct dosage, no increased potential for adverse drug reactions in pediatric patients is to be expected in this scenario. Specific side effects in children—such as growth delays after glucocorticoids or dental discoloration due to tetracyclines—are the exception rather than the rule. It should be borne in mind, however, that certain additives in ready-prepared medicines—for example, preserving agents—may be unsuitable for children (27).
In some preparations, however, it is not clear whether they have marketing authorization for a particular age group or indication or not. The wording in the prescribing information is often unclear; ages are not given as numerals, and the term “children” often not only refers to 2–11 year olds, as per the ICH definition (Table 2) (28).
Table 2. Age groups according to ICH guideline (40).
| Preterm newborn infants | |
| Term newborn infants | 0–27 days |
| Infants and toddlers | 28 days–23 months |
| Children | 2–11 years |
| Adolescents | 12–16/18 years |
Prescribing information commonly provides a further classification into infants (28 days to 11 months), toddlers (12 –23 months), preschool children (2–5 years) and school children (6–11 years).
ICH, The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
Additionally, child-appropriate forms of medication are often lacking and available preparations have to be split, ground up, or diluted in deviation from the instructions for use, or pharmacists have to prepare a special formulation (29).
For new substances, the requirement is—in addition to studies in pediatric patients—the development of an age-appropriate form of the medicine. Because of the lack of success of the PUMA concept so far, however, in the medium term established medications will have to be used off-label and often in non-evidence based dosages (e17). The legislator ought to act on this.
The data of the KiGGS study show that dosages outside of the license resulted in off-label use in even more cases than the absence of a license (23). Especially underdosing was widespread, apparently also due to patients’ own parents’ unauthorized adjustment of dosages. Especially for antibiotics (21.3% of cases received doses that were too low) this increases the risk of antimicrobial resistance developing. Parents should urgently be educated about this association (23).
Improving drug safety
Reducing medication errors by using eHealth
The largest error source in the overall medication process on pediatric wards is the doctor’s prescription, at 77.8% (9, e9). The primary objective is therefore to improve the quality of prescribing in a formal and substantial sense. This entails selecting an appropriate substance in an age-appropriate form of medicine and at a correct dosage, being watchful with regard to drug interactions, and to pass on information to all additional participants in the medication process (nursing staff, pharmacy, patients, parents) (30).
Studies have shown that the use of electronic systems improves the quality of prescribing and reduces medication errors in pediatric patients (Table 3) (31– 34, e18– e20). Study results relating to electronic prescribing systems (computerized physician order entry, CPOE) have been heterogeneous and range from a reduction in the rate of errors of 99% to an increase of 14% (34).
Table 3. Pediatric studies included in a recent Cochrane review (33), on CPOE, unit-dose systems with barcode scanners, and pharmacists on wards.
| Study | Design | Study setting | Endpoint | Result | ||
|---|---|---|---|---|---|---|
| Electronic prescribing systems | Intervention | Control | ||||
| King 2003 (e19) | Retrospective before/after study | I: 2 general wards C: 1 general ward, 2 surgical ward 36103 patients in total, USA | Medication errors per 1000 patient days | Before: 4.48 After: 3.13 | Before: 4.80 After: 5.19 | p < 0.001 |
| Walsh 2008 (e20) | Before/after study | I: General wards, surgical ward, PICU, NICU C: none Total sample of 627 patients, USA | Serious medication errors that reached the patient, per 1000 patient days | Before: 23.1 After: 20.6 | - | IRR (95% CI 0.69 to 1.78) |
| Unit-dose system with barcode scanner (BCMA) | Intervention | Control | ||||
| Moriss 2009 (36) | Prospective before/after study | I: NICU C: not reported 958 patients in total, USA | Adverse drug reactions that are affected by the intervention as expected, per 1000 doses | Before: 0.86 After: 0.43 | - | p = 0.008 |
| Pharmacist on ward | Intervention | Controlphar | ||||
| Kaushal 2008 (38) | Prospective before/after study | I: General ward (part time), surgical ward (part time), PICU (full time) C: general ward, surgical ward, cardiac-intensive ward 4863 patients in total, USA | Serious medication errors per 1000 patient days | General wards | ||
| Before: 8 After: 9 | Before: 7 After: 8 | p = 0.78 | ||||
| Surgical wards | ||||||
| Before: 7After: 9 | Before: 8 After: 10 | p = 0.89 | ||||
| PICU | Cardiac-intensive | |||||
| Before: 29 After: 6 | Before: 20 After: 30 | p < 0.01 | ||||
| Zhang 2012 (e28) | Randomized controlled study | Patients with neurological disorders, respiratory disorders, or digestive disorders, 150 patients in total, China | Hospital stay in days | 7.33 ± 3.52 | 9.06 ± 5.47 | p = 0.02 |
CPOE, computerized physician order entry; I, intervention; C, control; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; IRR, incidence rate ratio; 95% CI, 95% confidence interval
The American Academy of Pediatrics in a position paper recommends the implementation of a CPOE system that uses clinical decision support (CDS) in order to warn of risks—such as interactions or drug allergies—that enables calculating dosages, and which should be integrated into an electronic patient record (31). The integrated dosage calculation tool is of great importance in the pediatric setting because dangerous calculation errors—often by the power of 10, for example—can be reduced (35, e21, e22).
Prescriptions can be sent electronically to the hospital pharmacy. The ward can then be supplied with individual doses that are packaged and labeled individually for each patient (unit-dose system). Barcode scanners and patient bracelets can additionally be used to check whether the packaged medicine is given to the right patient. Such add-ons to the CPOE system can additionally help reduce medication errors with regard to the application of the medication (Table 3) (36, e23). On the downside, prescribing by using electronic systems takes longer and incurs costs for software and hardware.
Modules that identify adverse drug reactions by analyzing laboratory results, diagnoses, or entered free text will in future be able to make a valuable contribution to pharmacovigilance but these still require notable improvement (6, 37, e24).
The standardized medication plan of the Action Plan on Medication Safety (Aktionsplan Arzneimitteltherapiesicherheit) and the Drug Commission of the German Medical Association (Arzneimittelkommission der Ärzteschaft, AkdÄ) includes a barcode that makes it possible to enter relevant information into electronic prescribing systems and thus improve the exchanges between hospitals, physicians in private practice, and pharmacies. Its benefits are currently being evaluated in model projects, but the perspective is not specifically on pediatric patients. In the long term, this function might be integrated in the electronic health insurance card.
Access to evidence based information needs to be optimized
Electronic prescribing systems provide an opportunity to undertake complex dosage calculations, in which—in addition to age, weight, or body surface area—further parameters can be included, such as a patient’s renal function status. One problem that urgently requires a solution in this setting is the fact that relevant evidence-based data sets for such calculations are lacking. Such data sets would have to be structured in a certain way in order to be able to assign dosages for different indications to unequivocally defined patient groups by using numerals. It is extremely laborious to extract such data from prescribing information or guidelines, and the information contained in these is often unsatisfactory. The aim should be a database that makes available evidence-based dosage data sets for import into CPOE systems. In Germany, standard reference material with evidence-based dosages for children—such as for example the British National Formulary (BNF) for Children—is lacking.
Freely accessible information on licensed dosages from current prescribing information is included in “ZAK—Zugelassene Arzneimittel für Kinder” [licensed medicines for children] (zak-kinderarzneimittel.de). We would point out, however, that several pharmaceutical manufacturers are not participating in this initiative.
Additional measures
Integrating pharmacists in pediatric wards effects a reduction in medication errors and, first and foremost, has a positive effect on the preparation and administration of medicines—steps in the medication process that are affected by electronic systems to a negligible extent only (Table 3) (9, 38, e25– e29).
Several studies have shown the positive effect of providing education/training to staff and/or patients or their parents. Depending on the individual circumstances and the kind of education/training, the rate of medication errors fell by 49–87% as a result. Training seems particularly indicated in processes that are prone to errors—for example, administration of medicines through a tube or the use of inhaled drugs (e30, e31). Hospital standards in written form and simple aids, such as checklists, or the pediatric emergency ruler can also make an important contribution towards medication safety (33, 34, e32).
A critical incident reporting system (CIRS) is recommended in pediatric hospitals and can help avoid repetitions of errors by means of countermeasures extracted from the system (e33). Table 4 shows a summary of discussed error sources and possible approaches to solutions.
Table 4. Error sources and measure to improve drug/medication safety.
| Error source | Solution approach |
|---|---|
| Particular characteristics of pediatric pharmacology | Teaching clinical pediatric pharmacology, dosage tables/database |
| Off-label use | Good quality dose-effect studies for medications out of patent in order to identify the age-appropriate dosage |
| Child-appropriate medication | Awareness of licensing status/off-label use, developing suitable forms of medicines |
| Incorrect dosage calculation | Electronic drug safety testing, dosage database |
| Contraindications/ interactions | Electronic prescribing system with decision support |
| Errors in transit | Complete, written, better, electronic ordering |
| Incorrect use/ incorrect labeling/ patients mixed up | Education/training, checks (as in transfusion), pharmacist on the ward, barcode labels |
Conclusion
In recent years, important and correct decisions have been made at the legal level with regard to drug safety in children. The widespread and risk-ridden off-label use was identified as the main problem, but problems persist with regard to initiating studies with established medications that are out of patent. Providing public funds or other incentives would be helpful, for example, in dose finding studies (PK/PD studies) in individual age groups. Spontaneous reports/notifications of adverse drug reactions are important with a view to identifying rare adverse reactions and long-term harms. These should be undertaken especially for preparations marked with a black triangle, after off-label use, or in case of medication errors.
Hospitals need to be aware that problems need to be solved and improved in all areas of the medication process, and the question arises which measures are required for this. Such measures will have to be dovetailed during implementation, and local conditions need to be considered, as otherwise new error sources may develop. Electronic solutions are expensive, but the counterargument is that medication errors can cause not only risks for patients but also incur high costs for extended inpatient stays.
In order to enable evidence-based dosage calculations, a recognized national standard volume is required, as is its implementation into relevant data sets for electronic prescribing systems.
Key Messages.
Because of continuous physiological changes, children are more vulnerable to adverse drug reactions than adults; a scenario in which the high rate of off-label uses poses a substantial additional risk.
Spontaneous reports/notifications of suspected cases of adverse drug reactions are rare, especially after off-label use and medication errors.
Clinical studies in children and appropriate forms of medication are needed and should be financially supported in the case of effective substances that are out of patent.
eHealth systems, such as electronic prescribing systems with decision support, make an important contribution towards increasing medication safety in the pediatric setting, as long as they meet the specific requirements of these patients.
A database for evidence-based dosages is urgently needed, especially for substances that are used off-label.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
Prof Rascher has received funding (third party funding) for commissioned clinical studies and for running an infectious disease epidemiological GLP laboratory supported by Pfizer Vaccines Research, US. He has also received study funding (third party funding) from Novartis Pharma and Vertex Pharmaceuticals. In 2013 he was involved as principal investigator of clinical studies sponsored by Alexion, Novartis, and Shire.
The remaining authors declare that no conflict of interest exists.
References
- 1.Borchers AT, Hagie F, Keen CL, Gershwin ME. The history and contemporary challenges of the US Food and Drug Administration. Clin Ther. 2007;29:1–16. doi: 10.1016/j.clinthera.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Wong IC, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf. 2004;27:661–670. doi: 10.2165/00002018-200427090-00004. [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency. European Medicines Agency pre-authorisation evaluation of medicines for human use. London: EMA; 2004. Evidence of harm from off-label or unlicensed medicines in children. EMEA/126327/2004. [Google Scholar]
- 4.Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52:77–83. doi: 10.1046/j.0306-5251.2001.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clavenna A, Bonati M. Adverse drug reactions in childhood: a review of prospective studies and safety alerts. Arch Dis Child. 2009;94:724–728. doi: 10.1136/adc.2008.154377. [DOI] [PubMed] [Google Scholar]
- 6.Haffner S, von Laue N, Wirth S, Thurmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf. 2005;28:453–464. doi: 10.2165/00002018-200528050-00008. [DOI] [PubMed] [Google Scholar]
- 7.Smyth RM, Gargon E, Kirkham J, et al. Adverse drug reactions in children—a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth RL, Peak M, Turner MA, et al. ADRIC: Adverse drug reactions in children—a programme of research using mixed methods. Programme Grants Appl Res. 2014 [PubMed] [Google Scholar]
- 9.Fortescue EB, Kaushal R, Landrigan CP, et al. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;111:722–729. doi: 10.1542/peds.111.4.722. [DOI] [PubMed] [Google Scholar]
- 10.Knopf H, Du Y. Perceived adverse drug reactions among non-institutionalized children and adolescents in Germany. Br J Clin Pharmacol. 2010;70:409–417. doi: 10.1111/j.1365-2125.2010.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135:e99–e108. doi: 10.1542/peds.2014-2015. [DOI] [PubMed] [Google Scholar]
- 12.Feudtner C, Dai D, Hexem KR, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med. 2012;166:9–16. doi: 10.1001/archpediatrics.2011.161. [DOI] [PubMed] [Google Scholar]
- 13.Seyberth HW. Mitteilungen von unerwünschten Arzneimittelwirkungen (UAW) bei Kindern. Monatsschr Kinderheilkd. 2008;156:63–66. [Google Scholar]
- 14.European Parliament and Council. Regulation amending pharmacovigilance of medicinal products for human use (EU) No 1235/2010. OJEU. 2010;53:1–16. [Google Scholar]
- 15.Rascher W. Verordnungsfreie Arzneimittel mit Todesfolge. Monatsschr Kinderheilkd. 2013;161:941–942. [Google Scholar]
- 16.Seyberth HW. Arzneimittel(-un-)sicherheit bei verschreibungsfreien Arzneimitteln. Monatsschr Kinderheilkd. 2013;161:535–536. [Google Scholar]
- 17.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 18.de Wildt SN, Tibboel D, Leeder JS. Drug metabolism for the paediatrician. Arch Dis Child. 2014;99:1137–1142. doi: 10.1136/archdischild-2013-305212. [DOI] [PubMed] [Google Scholar]
- 19.Seyberth HW. Physiologische Besonderheiten des kindlichen Organismus. Monatsschr Kinderheilkd. 2008;156:261–267. [Google Scholar]
- 20.Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child. 2013;98:737–744. doi: 10.1136/archdischild-2013-303720. [DOI] [PubMed] [Google Scholar]
- 21.Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 22.Seyberth HW. Probleme der Arzneimittelanwendung bei Kindern. Dtsch Arztebl Int. 2009;106:23–24. [Google Scholar]
- 23.Knopf H, Wolf IK, Sarganas G, Zhuang W, Rascher W, Neubert A. Off-label medicine use in children and adolescents: results of a population-based study in Germany. BMC Public Health. 2013;13 doi: 10.1186/1471-2458-13-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bücheler R, Meisner C, Kalchthaler B, et al. „Off-label“ Verschreibung von Arzneimitteln in der ambulanten Versorgung von Kindern und Jugendlichen. Dtsch Med Wochenschr. 2002;127:2551–2557. doi: 10.1055/s-2002-35819. [DOI] [PubMed] [Google Scholar]
- 25.Kimland E, Odlind V. Off-label drug use in pediatric patients. Clin Pharmacol Ther. 2012;91:796–801. doi: 10.1038/clpt.2012.26. [DOI] [PubMed] [Google Scholar]
- 26.Rojahn J, Stute A. Off-Label-Use: Zwischen Freiheit und Pflicht. Lege artis. 2012;2:10–15. [Google Scholar]
- 27.European Medicines Agency. London: EMA 2006; Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG/194810/2005. [Google Scholar]
- 28.Schoettler P. ZAK - Zugelassene Arzneimittel für Kinder. Datenbank mit Kinderarzneimitteln. Pharm Unserer Zeit. 2009;38:58–61. doi: 10.1002/pauz.200800296. [DOI] [PubMed] [Google Scholar]
- 29.Standing JF, Tuleu C. Paediatric formulations—getting to the heart of the problem. Int J Pharm. 2005;300:56–66. doi: 10.1016/j.ijpharm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Neubert A, Wimmer S. Inhaltliche Kriterien für eine gute Verordnung bei Kindern. Ther Umsch. 2014;71:352–365. doi: 10.1024/0040-5930/a000523. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Pediatrics. Electronic prescribing in pediatrics: toward safer and more effective medication management. diatrics. 2013;131:824–826. doi: 10.1542/peds.2013-0192. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KB, Lehmann CU. Electronic prescribing in pediatrics: toward safer and more effective medication management. Pediatrics. 2013;131:e1350–e1356. doi: 10.1542/peds.2013-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maaskant JM, Vermeulen H, Apampa B, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database Syst Rev. 2015;3 doi: 10.1002/14651858.CD006208.pub3. Cd006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134:338–360. doi: 10.1542/peds.2013-3531. [DOI] [PubMed] [Google Scholar]
- 35.Doherty C, McDonnell C. Tenfold medication errors: 5 years’ experience at a university-affiliated pediatric hospital. Pediatrics. 2012;129:916–924. doi: 10.1542/peds.2011-2526. [DOI] [PubMed] [Google Scholar]
- 36.Morriss FH, Jr., Abramowitz PW, Nelson SP, et al. Effectiveness of a barcode medication administration system in reducing preventable adverse drug events in a neonatal intensive care unit: a prospective cohort study. J Pediatr. 2009;154:363–368. doi: 10.1016/j.jpeds.2008.08.025. 368. [DOI] [PubMed] [Google Scholar]
- 37.Neubert A, Dormann H, Weiss J, et al. Are computerised monitoring systems of value to improve pharmacovigilance in paediatric patients? Eur J Clin Pharmacol. 2006;62:959–965. doi: 10.1007/s00228-006-0197-9. [DOI] [PubMed] [Google Scholar]
- 38.Kaushal R, Bates DW, Abramson EL, Soukup JR, Goldmann DA. Unit-based clinical pharmacists’ prevention of serious medication errors in pediatric inpatients. Am J Health Syst Pharm. 2008;65:1254–1260. doi: 10.2146/ajhp070522. [DOI] [PubMed] [Google Scholar]
- 39.Koordinierungsgruppe zur Umsetzung und Fortschreibung des Aktionsplanes AMTS. Definitionen zu Pharmakovigilanz und Arzneimitteltherapiesicherheit (AMTS). Krankenhauspharmazie. 2014;35:425–428. [Google Scholar]
- 40.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Genf: ICH 2000. Topic E11: Note for guidance on clinical investigation of medicinal products in the pediatric population. CPMP/ICH/2711/99. [Google Scholar]
- e1.Shirkey H. Therapeutic orphans. J Pediatr. 1968;72:119–120. doi: 10.1016/s0022-3476(68)80414-7. [DOI] [PubMed] [Google Scholar]
- e2.Choonara I, Rieder M. Drug toxicity and adverse drug reactions in children—a brief historical review. Paediatr Perinatal Drug Ther. 2002;5:12–18. [Google Scholar]
- e3.Maio G. Zur Geschichte der Contergan-Katastrophe im Lichte der Arzneimittelgesetzgebung. Dtsch Med Wochenschr. 2001;126:1183–1186. doi: 10.1055/s-2001-17888. [DOI] [PubMed] [Google Scholar]
- e4.European Medicines Agency. Press release. London: EMA 2014; European Medicines Agency gives second positive opinion for a paediatric-use marketing authorisation. EMA/99224/2014. [Google Scholar]
- e5.European Medicines Agency. London: EMA; 2013. European Medicines Agency recommends changes to the use of metoclopramide. EMA/443003/2013. [Google Scholar]
- e6.European Medicines Agency. London: EMA; 2013. Restrictions on use of codeine for pain relief in children—CMDh endorses PRAC recommendation. EMA/385716/2013. [Google Scholar]
- e7.European Medicines Agency. London: EMA 2015; PRAC recommends restrictions on the use of codeine for cough and cold in children. EMA/163792/2015. [Google Scholar]
- e8.Gallagher RM, Mason JR, Bird KA, et al. Adverse drug reactions causing admission to a paediatric hospital. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- e10.Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10:199–205. doi: 10.1007/BF02600255. [DOI] [PubMed] [Google Scholar]
- e11.Bührlen B, Reiß T, Beckmann C, Gassner UM, Gleiter CH. Stuttgart: Fraunhofer IRB Verlag; 2006. Assessment of the European community system of Pharmacovigilance. [Google Scholar]
- e12.Meyburg J, Kölker S, Hoffmann GF, Zilow EP. Koma bei Neugeborenen durch abschwellende Nasentropfen? Dtsch Arztebl. 2006;103:A3411–A3413. [Google Scholar]
- e13.Topf HG, Schwarze B, Köhler H, Neubert A, Rascher W. Schwerwiegende Nebenwirkungen durch nasales Xylometazolin. Monatsschr Kinderheilkd. 2013;161:537–542. [Google Scholar]
- e14.Magalhaes J, Rodrigues AT, Roque F, Figueiras A, Falcao A, Herdeiro MT. Use of off-label and unlicenced drugs in hospitalised paediatric patients: a systematic review. Eur J Clin Pharmacol. 2015;71:1–13. doi: 10.1007/s00228-014-1768-9. [DOI] [PubMed] [Google Scholar]
- e15.Bellis JR, Kirkham JJ, Thiesen S, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case-control study of inpatients in a pediatric hospital. BMC Med. 2013;11 doi: 10.1186/1741-7015-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Bücheler R, Meisner C, Kalchthaler B, et al. „Off-label“ Verschreibung von Arzneimitteln in der ambulanten Versorgung von Kindern und Jugendlichen. Dtsch Med Wochenschr. 2002;127:2551–2557. doi: 10.1055/s-2002-35819. [DOI] [PubMed] [Google Scholar]
- e17.European Commission. COM. 2013. Better medicines for children: From concept to reality—progress report on the paediatric regulation (EC) N°1901/2006; 443 pp. [Google Scholar]
- e18.Ammenwerth E, Schnell-Inderst P, Machan C, Siebert U. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc. 2008;15:585–600. doi: 10.1197/jamia.M2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.King WJ, Paice N, Rangrej J, Forestell GJ, Swartz R. The effect of computerized physician order entry on medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;112:506–509. doi: 10.1542/peds.112.3.506. [DOI] [PubMed] [Google Scholar]
- e20.Walsh KE, Landrigan CP, Adams WG, et al. Effect of computer order entry on prevention of serious medication errors in hospitalized children. Pediatrics. 2008;121:e421–e427. doi: 10.1542/peds.2007-0220. [DOI] [PubMed] [Google Scholar]
- e21.Crouch BI, Caravati EM, Moltz E. Tenfold therapeutic dosing errors in young children reported to US. poison control centers. Am J Health Syst Pharm. 2009;66:1292–1296. doi: 10.2146/080377. [DOI] [PubMed] [Google Scholar]
- e22.Chappell K, Newman C. Potential tenfold drug overdoses on a neonatal unit. Arch Dis Child Fetal Neonatal Ed. 2004;89:F483–F484. doi: 10.1136/adc.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Fontan JE, Maneglier V, Nguyen VX, Loirat C, Brion F. Medication errors in hospitals: computerized unit dose drug dispensing system versus ward stock distribution system. Pharm World Sci. 2003;25:112–117. doi: 10.1023/a:1024053514359. [DOI] [PubMed] [Google Scholar]
- e24.Liu Y, Lependu P, Iyer S, Shah NH. Using temporal patterns in medical records to discern adverse drug events from indications. AMIA Jt Summits Transl Sci Proc. 2012;2012:47–56. [PMC free article] [PubMed] [Google Scholar]
- e25.Wang JK, Herzog NS, Kaushal R, Park C, Mochizuki C, Weingarten SR. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics. 2007;119:e77–e85. doi: 10.1542/peds.2006-0034. [DOI] [PubMed] [Google Scholar]
- e26.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- e27.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- e28.Zhang C, Zhang L, Huang L, Luo R, Wen J. Clinical pharmacists on medical care of pediatric inpatients: a single-center randomized controlled trial. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e29.Niemann D, Bertsche A, Meyrath D, et al. A prospective three-step intervention study to prevent medication errors in drug handling in paediatric care. J Clin Nurs. 2014;24:101–114. doi: 10.1111/jocn.12592. [DOI] [PubMed] [Google Scholar]
- e30.Bertsche T, Pfaff J, Schiller P, et al. Prevention of adverse drug reactions in intensive care patients by personal intervention based on an electronic clinical decision support system. Intensive Care Med. 2010;36:665–672. doi: 10.1007/s00134-010-1778-8. [DOI] [PubMed] [Google Scholar]
- e31.Burkhart PV, Rayens MK, Bowman RK. An evaluation of children’s metered-dose inhaler technique for asthma medications. Nurs Clin North Am. 2005;40:167–182. doi: 10.1016/j.cnur.2004.08.010. [DOI] [PubMed] [Google Scholar]
- e32.Kaufmann J, Laschat M, Wappler F. Medication errors in pediatric emergencies—a systematic analysis. Dtsch Arztebl Int. 2012;109:609–616. doi: 10.3238/arztebl.2012.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e33.Frey B, Buettiker V, Hug MI, et al. Does critical incident reporting contribute to medication error prevention? Eur J Pediatr. 2002;161:594–599. doi: 10.1007/s00431-002-1055-0. [DOI] [PubMed] [Google Scholar]