Abstract
A high throughput screen for inhibitors of the oncogenic transcription factor activator protein-1 (AP-1) was applied to the NCI repository of natural product extracts. The liphophilic extract of the plant Nothospondias staudtii (Simaroubaceae) displayed significant AP-1 inhibition. Bioassay-guided fractionation of the extract lead to a new quassinoid named nothospondin (1), and the known compound glaucarubinone (2). The structure of 1 was elucidated by spectroscopic methods. Compounds 1 and 2 showed potent, dose-dependent AP-1 inhibition at noncytotoxic concentrations.
Keywords: Quassinoid, Nothospondias staudtii, Simaroubaceae, Transcription factor, AP-1
Oncogenic transcription factors help regulate the transcription of numerous genes associated with cell growth and cell cycle progression, and an increase in their transcriptional activity is required to support the transformation and unrestrained growth of cancer cells. Activator protein-1 (AP-1) is a transcription factor that resides within the nucleus and regulates various cellular processes including differentiation, proliferation, and apoptosis.1 AP-1 is activated by signal transduction through the mitogen activated protein kinase (MAPK) cascade that can be induced by extracellular stimuli such as growth factors or cytokines.2 Carcinogenesis is a multi-step process comprised of tumor initiation, promotion, and progression. Activation of AP-1 is required for tumor promotion, and elevated AP-1 activity is believed to play a pivotal role in tumor progression.3 Since the rate limiting stages of carcinogenesis occur during tumor promotion and progression, small molecule inhibitors of AP-1 function could serve as potential therapeutic agents. AP-1 exists as a dimer of Jun proteins (c-Jun, JunB, JunD) or as a heterodimer of Jun and Fos proteins, and dimerization is required for activation and DNA binding.4 Inhibition of AP-1 by the dominant negative c-Jun mutant TAM67 prevented cellular transformation but it did not significantly diminish in vitro cell survival, and it effectively blocked tumorigenesis in vivo.5 AP-1 inhibitors that can mimic the effect of TAM67 could have utility for the prevention and treatment of cancer. As part of an ongoing molecularly targeted discovery effort, extracts from the NCI Natural Products Repository were screened in a high throughput assay for AP-1 inhibitors.6 The lipophilic extract of the tropical plant Nothospondias staudtii (Simaroubaceae),7,8 collected in Cameroon, showed potent inhibition of AP-1 activity. Bioactivity-directed fractionation of this extract afforded a new quassinoid,9 nothospondin A (1), and glaucarubinone (2).10
Nothospondin (1, Fig. 1, Table 1)11 provided a [M+H]+ ion at m/z 393.1927 in the HREIMS consistent with a molecular formula of C21H28O7. Analysis of the 13C and gHSQC NMR spectra revealed that the 21 carbon resonances consisted of 4 methyls, 2 methylenes, 7 methines, 7 quaternary carbons, and one methoxyl group. An oxymethine doublet assigned to H-2 (δH 4.71) showed HMBC correlations with a ketone at C-1 (δC 209.1) and an oxymethine at C-3 (δC 83.7), and it had a 8.7 Hz coupling to H-3 (δH 2.93) that indicated a diaxial orientation for these two protons. Assignment of the H3-18 doublet (δH 1.04) was based on COSY coupling to H-4 and HMBC correlations with C-3, C-4 (δC 34.7), and C-5 (δC 41.6). Substitution of H3-19 (δH 1.46) on C-10 was established by HMBC correlations with C-1, C-5, and C-10 (δC 48.1). These data and COSY correlations between H-3/H-4 (δH 1.96) and H-4/H-5 (δH 1.30) were consistent with the structure proposed for the A ring. Assignment of ring B was facilitated by the HMBC correlations of H-9 (δH 3.15) with C-8 (δC 36.9), C-10, C-17 (δC 23.2), and C-19 (δC 15.6). In addition, HMBC correlations of H-7 (δH 4.29) with C-5 and C-9 (δC 47.5) and COSY correlations between H-5/H-6b (δH 2.10) and H-6b/H-7 unambiguously established the connectivity from C-5 through C-7. Ring C was revealed by a carbonyl at δC 190.8 (C-11) consistent with a conjugated ketone and HMBC correlations from H-9 to C-11, from H-14 (δH 2.42) to C-12 (δC 148.4), C-13 (δC 140.5), and C-20 (δC 16.0), and from the methoxyl group (δH 3.67) to C-12. Finally, the presence of a six-membered lactone ring that encompassed an ester carbonyl at C-16 (δC 169.0) linked to the oxygen at C-7 was deduced from the low field shift of H-7 (δH 4.29) and HMBC correlations from H-15a (δH 2.55) to C-13, C-14 (δC 47.3), and C-16, and from H-15b (δH 2.97) to C-8, C-14, and C-16. The relative stereochemistry of 1 was established from a series of selective 1D ROESY experiments. Irradiation of H3-19 produced ROESY enhancements in H-2, H-4, H-6a (δH 1.82) and H3-17 (δH 1.20) that indicated these groups are located at the top (β) face of the molecule (Fig. 2). In addition, H3-17 had ROESY interactions with H-7 and H-14 that established these protons as β as well. Substituents on the bottom (α) face of 1 were defined by ROESY interactions between H3-18/H-3, H3-18/H-6b and H-5/H-9. These data allowed the structural and relative configurational assignment of nothospondin (1) as a new tetracyclic quassinoid. The identity of compound 2 was established by comparison of its 1H and 13C data with published values for glaucarubinone.10
Figure 1.
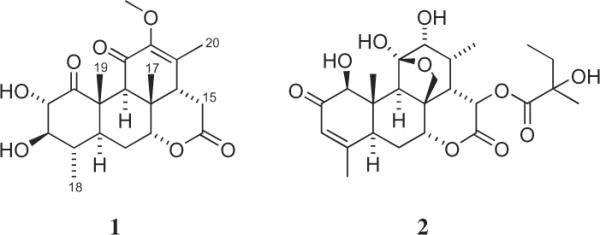
Structure of nothospondin (1) and glaucarubinone (2).
Table 1.
NMR data (CDCl3) for nothospondin (1)a
| No. | δ C | δH (J in Hz) | gHMBC |
|---|---|---|---|
| 1 | 209.1 | ||
| 2 | 76.9 | 4.71 d (8.7) | 1, 3, 19 |
| 3 | 83.7 | 2.93 dd (10.1, 8.7) | 2, 4, 18 |
| 4 | 34.7 | 1.96 m | 3, 5, 18 |
| 5 | 41.6 | 1.30 m | 19 |
| 6a | 25.7 | 1.82 ddd (14.8, 12.4, 2.1) | 5, 10 |
| 6b | 2.10 dt (14.8, 3.6) | 4, 5, 8, 10 | |
| 7 | 82.1 | 4.29 brt (2.9) | 5, 9 |
| 8 | 36.9 | ||
| 9 | 47.5 | 3.15 s | 1, 7, 8, 10, 11, 17, 18 |
| 10 | 48.1 | ||
| 11 | 190.8 | ||
| 12 | 148.4 | ||
| 13 | 140.5 | ||
| 14 | 47.3 | 2.42 dd (12.1, 7.0) | 8, 9, 12, 13, 17, 20 |
| 15a | 31.7 | 2.55 dd (18.6, 12.1) | 12, 13, 14 |
| 15b | 2.97 dd (18.6, 7.0) | 8, 9, 16 | |
| 16 | 169.0 | ||
| 17 | 23.2 | 1.20 s | 7, 8, 9, 14 |
| 18 | 14.9 | 1.04 d (6.5) | 3, 4, 5 |
| 19 | 15.6 | 1.46 s | 1, 5, 9, 10 |
| 20 | 16.0 | 1.89 s | 12, 13, 14 |
| OCH3 | 60.0 | 3.67 s | 12 |
Data were acquired with a Bruker Avance 111 600 MHz spectrometer; chemical shifts are in ppm and referenced to the residual solvent signal; J in Hz; HMBC correlations are from the proton(s) stated to the indicated carbon.
Figure 2.
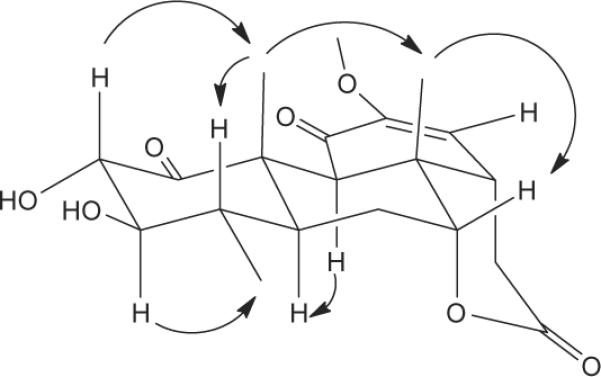
Key ROESY correlations for nothospondin (1).
The AP-1 inhibitory activity of compounds 1 and 2 was determined by a β-lactamase driven reporter assay using fluorescence resonance energy transfer (FRET) technology, followed by an XTT assay to test for cytotoxicity.12 Glaucarubinone (2) showed the most potent AP-1 inhibition with an EC50 of 0.13 μM and it was noncytotoxic at a high-test concentration of 80 μM. Nothospondin (1) was less potent against AP-1 (EC50 1.49 μM) and it showed some cytotoxicity (IC50 approximately 10 μM). The potent AP-1 inhibitory activity of 2, which has an ether bridge between C-17 and C-11, was consistent with a prior structure–activity study in which all of the AP-1 active quassinoids had C-17/C-11 or C-17/C-13 ether bridges.13 Nothospondin (1) is the first quassinoid without an ether link that can inhibit AP-1, albeit at significantly reduced potency.
Acknowledgments
We thank D. Newman (NCI) and T. McCloud (SAIC-Frederick) for the plant extract and M. Dyba and S. Terasova (Biophysics Resource, SBL, NCI-Frederick) for assistance with the HRLCMS studies. This research was supported in part by the Intramural Research Program of NIH, NCI, CCR. This project was also funded in part with Federal funds from the NCI, NIH, under Contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
References and notes
- 1.Weinstein IB. Cancer Res. 1988;48:4135. [PubMed] [Google Scholar]
- 2.Matthew CP, Colburn NH, Young MR. Curr. Cancer Drug Targets. 2007;7:317. doi: 10.2174/156800907780809723. [DOI] [PubMed] [Google Scholar]
- 3.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Proc. Natl. Acad. Sci. U.S.A. 1994;91:609. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young MR, Li J-J, Rincórn M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn NH. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9827. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young MR, Farrell L, Lambert P, Awasthi P, Colburn NH. Mol. Carcinog. 2002;34:72. doi: 10.1002/mc.10050. [DOI] [PubMed] [Google Scholar]
- 6.Ruocco KM, Goncharova EI, Young MR, Colburn NH, McMahon JB, Henrich CJ. J. Biomol. Screen. 2007;12:133. doi: 10.1177/1087057106296686. [DOI] [PubMed] [Google Scholar]
- 7.Owoyele BV, Olaleye SB, Oke JM, Elegbe RA. Niger. J. Physiol. Sci. 2004;19:102. [Google Scholar]
- 8.Clayton JW, Fernando ES, Soltis PS, Soltis DG. Int. J. Plant Sci. 2007;168:1325. [Google Scholar]
- 9.Plant material, extraction, and isolation: Nothospondias staudtii Engl. plant material was collected from a secondary forest in Ikiliwindi, Southwest Province, Cameroon (9°29′E, 4°45′N) on March 15, 1987 by Etuge Martin and Duncan Thomas. The specimen was identified by L. Ake Assix and the collection number was Q66P343. A voucher sample is deposited at the Missouri Botanical Garden in St. Louis, Missouri, USA. The ground stem bark of the plant (616 g) was sequentially extracted with CH2Cl2–MeOH (1:1) and 100% MeOH, using the standard NCI natural products extraction protocol,14 to yield 11.3 g of crude extract. A 1 g aliquot of this extract was fractionated on a diol column eluting sequentially with hexane (fraction A, 121 mg), CH2Cl2 (fraction B, 185 mg), EtOAc (fraction C, 141.6 mg) and MeOH (fraction D, 337 mg). Fractions B and C showed potent AP-1 inhibition and further separation of fraction B by size exclusion chromatography on Sephadex LH-20 with hexane–CH2Cl2–MeOH (2:5:1) afforded compounds 1 (3.5 mg) and 2 (2.7 mg).
- 10.Usami Y, Nakagawa-Goto K, Lang J-Y, Kim Y, Goto M, Sakurai N, Taniguchi M, Akiyama T, Morris-Natschke SL, Bastow KF, Cragg G, Newman DJ, Fujitake M, Takeya K, Hung M-C, Lee E, Lee K-H. J. Nat. Prod. 2010;73:1553. doi: 10.1021/np100406d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nothospondin (1): Yellow amorphous solid; (c 0.021, MeOH); UV (MeOH) λmax (ε) 207 (8815), 244 (5064) nm; IR (thin film) 3444 (br), 2849, 1731, 1682, 1639, 1462, 1379, 1240, 1093, 1032, 896, 730 cm−1; 1H NMR (600 MHz) and 13C NMR (150 MHz) data, see Table 1; HRESIMS m/z (M+H)+ 393.1927 (C21H29O7 requires 393.1908).
- 12.AP-1 assay: Inhibition of AP-1 activity by compounds 1 and 2 was assessed using a FRET based β-lactamase reporter assay that was described previously.6 In brief, AP-1-bla HEK293T cells that stably express a plasmid containing β-lactamase under the control of an AP-1 promoter were maintained in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% heat deactivated dialyzed fetal bovine serum (FBS), penicillin–streptomycin, nonessential amino acids, HEPES, sodium pyruvate and blasticidin. The cells at 70% confluency were trypsinized, resuspended in media that did not have blasticidin, seeded into 384-well assay plates (40 μL) and incubated (37 °C) for 18 h. DMSO solutions of the test materials were diluted with media and then added to the cell culture wells. The cells were then incubated at 37 °C for 1 h, treated with TPA to induce AP-1 activity, and incubated for an additional 18 h. β-Lactamase activity was quantified with the LiveBLAzer FRET substrate and the fluorescence was read on a Tecan Safire fluorescent plate reader (409 nm excitation and 460 nm emission, followed by 409 nm excitation and 530 nm emission). The β-lactamase activity was calculated from the ratio of emission at 460 nm to the emission at 530 nm.
- 13.Beutler JA, Kang M-I, Robert F, Clemant J, Pelletier J, Colburn NH, McKee TC, Goncharova EI, McMahon JB, Henrich CJ. J. Nat. Prod. 2009;72:503. doi: 10.1021/np800732n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCloud TG. Molecules. 2010;15:4526. doi: 10.3390/molecules15074526. [DOI] [PMC free article] [PubMed] [Google Scholar]


