Abstract
Objective
To investigate the association between statin use, incident dementia, and Alzheimer disease (AD) in a prospective elderly African American cohort.
Design
Two stage design with a screening interview followed by a comprehensive in-home assessment conducted over an eight-year period. Diagnoses of incident AD and dementia were made by consensus. Statin use was collected at each evaluation. Measurements of low-density lipoprotein cholesterol (LDL), C-reactive protein (CRP) and APOE genotype were obtained from baseline blood samples. Logistic regression models were used to test the association of statin use on incident dementia and AD and its possible association with lipid and CRP levels.
Setting
Indianapolis, Indiana
Participants
From an original cohort of 2629 participants, a subsample of 974 African Americans aged >70 years with normal cognition, at least one follow up evaluation, complete statin information, and biomarker availability were included.
Main Outcome Measures
Incident dementia and incident AD.
Results
After controlling for age at diagnosis, sex, education level, presence of the APOE ε4 allele and history of stroke for the incident dementia model, baseline use of statins was associated with a significantly decreased risk of incident dementia (OR=.44, P=.029) and incident AD (OR=.40, P=.029). The significant effect of statin use on reduced AD risk and trend for dementia risk was found only for those participants who reported consistent use over the observational period (incident AD: P=.034; incident dementia: P=.061). Additional models found no significant interaction between baseline statin use, baseline LDL, or CRP level and incident dementia/AD.
Conclusions
Consistent use of statin medications during eight years of follow-up resulted in significantly reduced risk for incident AD and a trend toward reduced risk for incident dementia.
Keywords: Cohort Studies, Alzheimer Disease, Dementia, Hydroxymethylglutaryl-CoA Reductase Inhibitors, African Americans
Introduction
A recent American College of Cardiology/American Heart Association (ACC/AHA) report found consistent evidence supporting the use of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or “statins” for primary prevention as well as secondary prevention of atherosclerotic cardiovascular disease (ASCVD).1
The evidence for the use of statins to prevent dementia is less clear. Some individual studies from a variety of countries, including Europe, Asia, and North America, have reported an association between statin use and a reduced risk of dementia and Alzheimer disease (AD),2-6 as well as evidence of reduced AD-related neuropathological changes.7 Results from systematic reviews and meta-analyses, however, have been less positive. Neither the analysis conducted by Zhou et al8 nor the systematic review by Muangpaisan et al9 revealed any significant effect of statins on the risk for dementia or AD. A recent analysis by Wong et al did suggest that statins might provide a slight benefit in prevention of AD and dementia but noted considerable heterogeneity in individual studies.10
The putative mechanism for the beneficial effects of statins on dementia is also unclear. Reducing both LDL cholesterol levels and levels of CRP have been reported to be important mechanisms associated with decreasing the risk of cardiovascular events.11 In contrast, in most studies that report significant protective effects of statins on dementia the association does not appear to be related to changes in lipid levels.12-15
The incidence of AD and dementia has been reported to be higher in African Americans than in Caucasians16 as has the incidence of cardiovascular and cerebrovascular disease.17 Because cardiovascular disease and risk factors have also been implicated as risk factors for dementia,18 it suggests that statins may be particularly useful for dementia prevention in African Americans. The recent identification of the ABCA7 genetic variant as a risk factor for AD in African Americans, a genetic variant that affects lipid metabolism, also suggests that an exploration of lipid and inflammatory markers in this population, despite the previously reported negative findings, would be worthwhile.19
The Indianapolis-Ibadan dementia project, a comparative study involving elderly African Americans and Yoruba (Ibadan, Nigeria), provided an opportunity to study the effects of statin use in African Americans.20 Statins were seldom used in Yoruba. In a previous three-year prospective analysis of the effects of statin use on cognitive decline in African Americans, a significant protective effect was found between baseline statin use and cognitive decline three years later. However, only statin use at baseline conferred an advantage; no significant association with cognitive function was seen for later statin use.21 In this analysis, we now report on the effects of statin use on incident dementia and AD in this study population over an eight-year period. Measurements of lipid and inflammatory markers (LDL and CRP) were also included.
Methods
Participants
The Indianapolis-Ibadan Dementia Project is a longitudinal, prospective, community-based epidemiologic comparative study of rates and risk factors for dementia and Alzheimer disease in elderly African Americans living in Indianapolis, Indiana and Yoruba in Ibadan, Nigeria.20 This analysis is confined to participants from the Indianapolis cohort. In 1992, 2212 participants were enrolled using a random sampling of community-dwelling African Americans from 29 contiguous census tracts in Indianapolis in which African Americans represented 80% of the population in the 1990 US census. In 2001, an enrichment cohort of 1893 participants aged >70 years drawn from Medicare beneficiaries was added to the surviving original participants. Follow-up evaluations were conducted in both cohorts every two to three years at 2004, 2007 and 2009. The study was approved by the Indiana University Purdue University Indianapolis Institutional Review Board, and written informed consent was obtained from all participants prior to the study-related procedures.
Study Design
At each evaluation wave of the study, participants were assessed using a two-stage sampling design. The first stage of the assessment consisted of an in-home visit, during which time interviewers gathered: anthropometric measures (height, weight, and blood pressure); medical, social, and family history including comorbidities; medication use; and conducted the Community Screening Interview for Dementia (CSI-D). Interviews with the participants and informants were recorded. Participants were then stratified into good, intermediate, or poor performance groups according to CSI-D scores and/or a change in performance on previous evaluations. All of the poor performance group (100%), 75% of the intermediate performance group, and 2.5% of good performers were then invited to participate in the second stage of assessment.
Clinical Assessment and Diagnosis
The clinical assessment was conducted during a home visit by a specially trained research nurse and psychometrician. The assessment included the following: 1) a neuropsychological test battery adapted from the Consortium to Establish a Registry for AD (CERAD)22 (including measures of memory, language, attention, calculation, orientation, executive function, and visuospatial function). Normative values for this battery in this population had been developed in a separate study;23,24 2) a standardized neurological and physical examination and functional status review (The Clinician Home-based Interview to assess Function, or CHIF)25; and 3) a structured interview with a close relative based on an adaptation of the Cambridge Examination for Mental Disorders of the Elderly informant interview (CAMDEX).26
A consensus diagnostic conference by a group of clinicians that included the disciplines of geriatric psychiatry, neurology and neuropsychology was held in which all clinical assessment data were reviewed by the clinical team to reach an agreement on diagnosis. Diagnosis of dementia and subtype was made according to ICD-10 and DSM-IV-TR criteria.27,28 Participants diagnosed with dementia reached the endpoint of the study and were not included in additional follow-up evaluations.
Medications
Names of medications were recorded from the participants’ bottles at each evaluation by the interviewer. Medications were then classified into various categories including statins (simvastatin, atorvastatin, pravastatin, fluvastatin, cerivastatin, and lovastatin), and non-statin lipid-lowering agents (gemfibrozil, colestipol, and cholestyramine). Participants using only non-statin lipid lowering agents (n=25) were excluded from this analysis.
Laboratories
During the 2001 evaluation, blood samples were collected from participants who consented to blood collection. Biomarkers including low density lipoprotein (LDL) level, and CRP level were measured in plasma samples. In addition, the APOE genotype was also ascertained from this blood sample or from previous blood draws or filter cards. For this analysis, the APOE genotype was dichotomized into being an APOE ε4 carrier or not.
Statistical Analysis
To combine data from both the original and enrichment cohorts and to use biomarker information collected at the 2001 evaluation, we used the 2001 evaluation as the baseline of this analysis. Participants with incident dementia or AD were diagnosed by the consensus panel in 2004, 2007, or 2009. Those diagnosed with dementia in 2001 were excluded from this analysis. Participants with normal cognition consisted of those who were either diagnosed as normal or in the good performance group by screening at their last evaluation. Statin use over time was classified into three categories based upon use during all participating waves: continuous use, intermittent use, or no use.
T-tests for continuous variables and Fisher’s exact tests for categorical variables were used to compare those with incident dementia and incident AD with the participants with normal cognition on demographics and individual characteristics including comorbidities and laboratory values from 2001. They were also used to compare demographics, comorbidities, and laboratory values between participants included in this analysis with those who had normal cognition at baseline but were excluded from this analysis as well as between participants who used statins at baseline and those who did not. Variables that were significantly different between the analysis sample and the excluded participants were included in subsequent logistic regression models to ensure unbiased parameter estimates on the association between statin use and incident dementia/AD under the assumption that the probability of missing data depends on these demographic variables.29 We defined age at diagnosis as the age for a dementia diagnosis for those with incident dementia and as the age at the last evaluation for those with normal cognition. Since the participants in our cohorts were evaluated at predetermined times, age at dementia diagnosis is not a random variable and survival models cannot be appropriately applied. We chose to use logistic regression models as previous researchers have shown that the logistic model conditioning on age at diagnosis is more appropriate when time to event is not observed.30, 31
Logistic regression was used to test the association of statin use on incident dementia and AD compared to having normal cognition. Additional logistic regression models on incident dementia and AD included interactions between statin use at baseline and low baseline CRP (<2 mg/L) and elevated baseline LDL (>130mg/dL) levels in addition to the main effects for the biomarkers.11 Non-significant interactions were removed from the models. All models were adjusted for age at diagnosis, sex, years of education, and APOE ε4 carrier status. Comorbidities were also investigated in the logistic regression models and non-significant ones were removed.
Results
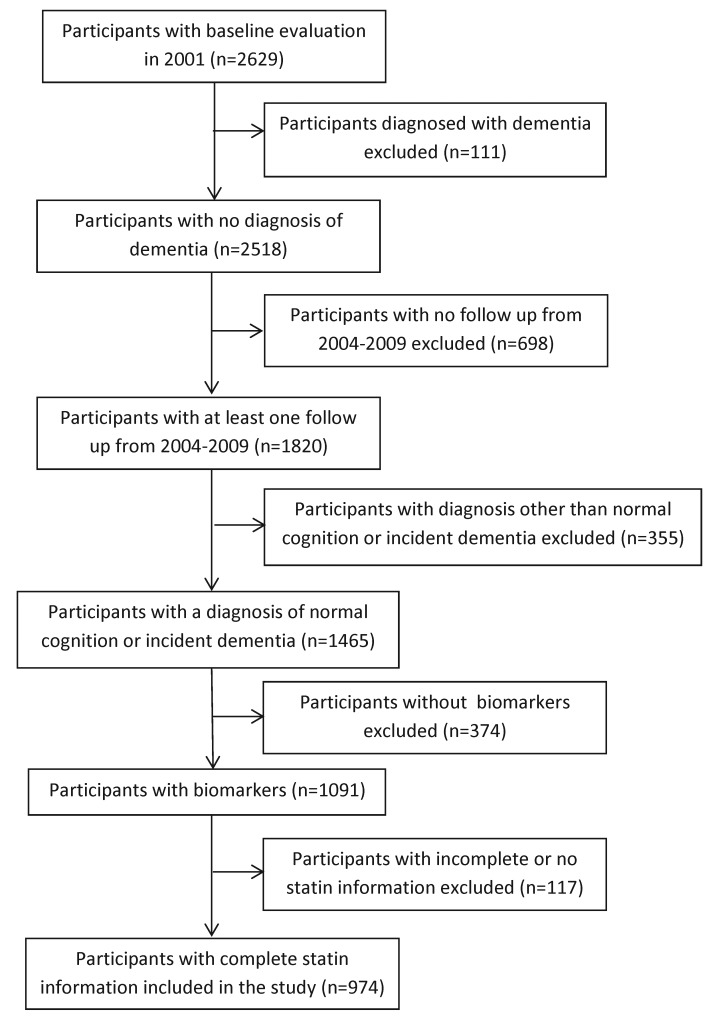
From the original 1992 and 2001 cohorts a total of 2629 participants underwent baseline evaluation in 2001. Of these, 111 were diagnosed with dementia at this evaluation and excluded from this analysis. There were 1820 participants who had at least one follow-up evaluation from 2004 to 2009, of which 1465 either had incident dementia or were in the normal cognition group. Of these, 1091 participants had provided blood samples for the APOE genotype and 974 had statin use information at all participating waves (Figure 1). Approximately 41% of statin users at baseline were taking simvastatin, 42% atorvastatin, 9% pravastatin, 5% fluvastatin, 2% cerivastatin, and 2% lovastatin.
Figure 1. Enrollment flowchart.
The 974 participants in our study were more likely to be female (69.7% vs 65.6%; P=.0496), younger (76.6 vs 78.9 years; P<.0001), and had more years of education (11.6 vs 10.8 years; P<.0001) than the 1544 subjects with normal cognition with baseline evaluations excluded from the study. Age, sex, and years of education were included in subsequent logistic models for incident dementia/AD to minimize potential bias from missing data.
Over the course of the study, 65 (6.7%) participants were diagnosed with dementia (27 in 2004, 20 in 2007 and 18 in 2009), 56 of whom with AD (22 in 2004, 20 in 2007 and 14 in 2009). The normal cognition group consisted of 909 participants (199 in 2004, 141 in 2007 and 569 in 2009). The average length of follow-up was 6.0 (SD=2.1) years with a range from 1.9-8.6 years. The average time to dementia diagnosis was 5.1 years (SD=2.1), the average time to AD diagnosis was also 5.1 years (SD=2.0), while the average length of follow up for the normal cognition participants was 6.1 years (SD=2.1).
Table 1 shows the demographics and baseline characteristics of the participants both overall and by incident dementia status. The majority (69.7%) of the participants were women. The participants with dementia or AD were older at baseline and at diagnosis but significantly less educated than the participants with normal cognition. APOE ε4 carriers were also over-represented in the group with dementia (55.4%, P<.0001 vs normal cognition) and the group with AD (58.9%, P<.0001 vs normal cognition) compared with the group with normal cognition (30.0%). Participants with incident AD were significantly less likely to be taking statins either at baseline or over the duration of the study than the normal participants (P=.0433 and P=.0256, respectively). This was also true of the participants with incident dementia compared with those with normal cognition but significance was only met for the baseline use (P=.0369 at baseline and P=.0926 over time). There were no significant differences of LDL and CRP levels between the groups.
Table 1. Demographic and baseline information on the 974 participants with APOE and statin information both overall and by diagnostic group.
| Overall (N=974) | Incident Dementia (n=65) | Incident AD (n=56) | Normal Cognition (n=909) | Pa | Pb | |
| Age at baseline, mean (SD) | 76.6 (4.9) | 78.7 (5.5) | 79.0 (5.6) | 76.4 (4.8) | .0003 | .0001 |
| Age at diagnosis, mean (SD) | 82.6 (5.0) | 83.8 (5.6) | 84.1 (5.7) | 82.5 (4.9) | .0519 | .0238 |
| Female, n (%) | 679 (69.7) | 48 (73.8) | 40 (71.4) | 631 (69.4) | .4883 | .8812 |
| Years of education, mean (SD) | 11.6 (2.5) | 10.6 (3.0) | 10.6 (3.0) | 11.6 (2.5) | .0085 | .0116 |
| ApoE Ɛ4 carrier, n (%) | 309 (31.7) | 36 (55.4) | 33 (58.9) | 273 (30.0) | <.0001 | <.0001 |
| Baseline LDL, mean, mg/dL (SD) | (n=824) 112.1 (32.7) | (n=61) 113.4 (29.4) | (n=53) 114.5 (29.1) | (n=763) 112.0 (32.9) | .7470 | .5917 |
| Baseline CRP, mean, mg/dL (SD) | (n=819) 11.8 (21.6) | (n=60) 10.3 (16.5) | (n=52) 9.5 (16.9) | (n=759) 11.9 (22.0) | .4699 | .3262 |
| Baseline LDL >130 mg/dL, n(%) | (n=824) 216 (26.2) | (n=61) 15 (24.6) | (n=53) 14 (26.4) | (n=763) 201 (26.3) | .8800 | 1.0000 |
| Baseline CRP ≤2 mg/dL, n (%) | (n=819) 213 (26.0) | (n=60) 19 (31.7) | (n=52) 18 (34.6) | (n=759) 194 (25.6) | .2889 | .1906 |
| Baseline statin use | 244 (25.1%) | 9 (13.8) | 7 (12.5) | 235 (25.9) | .0369 | .0256 |
| Statin use over all participating waves, n (%) | .0926 | .0433 | ||||
| Continuous use | 150 (15.4) | 5 (7.7) | 3 (5.4) | 145 (16.0) | ||
| Intermittent use | 322 (33.1) | 19 (29.2) | 17 (30.4) | 303 (33.3) | ||
| Did not use | 502 (51.5) | 41 (63.1) | 36 (64.3) | 461 (50.7) | ||
a. P comparing incident dementia with normal cognition. b. P comparing incident AD with normal cognition.
A separate analysis was conducted comparing statin users with non-users. In this analysis the participants reporting statin use at baseline had a mean baseline LDL of 99.4 mg/dL (SD=29.6; n=210) and a mean baseline CRP of 13.1 mg/L (SD=29.1; n=208). For those not taking statins at baseline, the mean baseline LDL was 116.4 mg/dL (SD=32.5; n=614) and the mean baseline CRP was 11.4 mg/L (SD=18.4; n=611). The levels were not significantly different between the baseline statin users and non-users for CRP (P=.4403) but the LDL levels were significantly lower in the statin users (P<.0001). There was no difference in the rates of low CRP (≤2) between those taking statins at baseline and those who did not (27.9% vs 25.4%; P=.4660). However, significantly fewer baseline statin users had high baseline LDL (>130 mg/dL) than those who did not take the medications (15.7% vs.29.8% P<.0001). Cognitive scores at baseline were not different between statin users (mean=69.7, SD=6.0) and non-users (mean=69.5, SD=6.7, P=.7947). (data not shown)
Table 2 shows the presence of baseline comorbidities by either self or informant report in each of the groups. The rates of comorbidities were similar between those with incident dementia and AD compared with the participants with normal cognition (P>.05 for all).
Table 2. Baseline comorbidities: Overall and by diagnostic group, n (%).
| Overall (N=974) | Incident Dementia (n=65) | Incident AD (n=56) | Normal Cognition (n=909) | Pa | Pb | |
| Diabetes | (n=966) 277 (28.7) | 18 (27.7) | 16 (28.6) | (n=901) 259 (28.7) | 1.0000 | 1.0000 |
| Cancer | (n=961) 147 (15.3) | (n=64) 7 (10.9) | (n=55) 5 (9.1) | (n=897) 140 (15.6) | .3727 | .2463 |
| Depression | (n=965) 91 (9.4) | (n=63) 6 (9.5) | (n=54) 6 (11.1) | (n=902) 85 (9.4) | 1.0000 | .6337 |
| Coronary Heart Disease | (n=973) 305 (31.3) | 16 (24.6) | 15 (26.8) | (n=908) 289 (31.8) | .2687 | .4631 |
| Hypertensionc | (n=962) 744 (77.3) | 48 (73.8) | 41 (73.2) | (n=897) 696 (77.6) | .5390 | .5100 |
| Stroke | (n=970) 122 (12.6) | (n=64) 13 (20.3) | (n=55) 9 (16.4) | (n=906) 109 (12.0) | .0757 | .3941 |
a. P comparing incident dementia with normal cognition. b. P comparing incident AD with normal cognition. c. By self or informant report or medication use in 2001.
Table 3 shows the results from the final logistic regression models on the association of statin use and incident dementia or AD using two models.
Table 3. Results from logistic regression models on incident dementia/AD by statin use.
| Incident Dementia, N=974 | Incident AD, N=965 | |||||||
| OR | 95% CI | P | OR | 95% CI | P | |||
| Model 1 | ||||||||
| Age at diagnosis | 1.05 | 1.00-1.10 | .0578 | 1.06 | 1.01-1.12 | .0319 | ||
| Female | 1.22 | .68-2.21 | .5083 | 1.10 | .59-2.03 | .7679 | ||
| Years of education | .87 | .80-.96 | .0049 | .87 | .79-.96 | .0072 | ||
| ApoE ε4 carrier | 3.11 | 1.84-5.25 | <.0001 | 3.66 | 2.08-6.42 | <.0001 | ||
| History of stroke | 2.06 | 1.05-4.02 | .347 | N/A | N/A | N/A | ||
| Statin use at baseline | .44 | .21-.92 | .0286 | .40 | .18-.91 | .0291 | ||
| Model 2 | ||||||||
| Age at diagnosis | 1.05 | 1.00-1.11 | .0541 | 1.06 | 1.01-1.12 | .0309 | ||
| Female | 1.17 | .65-2.12 | .6005 | 1.04 | .56-1.92 | .9135 | ||
| Years of education | .88 | .80-.97 | .0073 | .88 | .80-.97 | .0117 | ||
| ApoE ε4 carrier | 3.08 | 1.82-5.21 | <.0001 | 3.65 | 2.08-6.40 | <.0001 | ||
| History of stroke | 2.00 | 1.03-3.90 | .0412 | N/A | N/A | N/A | ||
| Statin use over timea | ||||||||
| Continuous use vs never used | .40 | .15-1.04 | .0609 | .27 | .08-.90 | .0336 | ||
| Intermittent use vs never used | .72 | .40-1.28 | .2644 | .73 | .40-1.34 | .3113 | ||
a. Statin use is classified based upon all participating waves for each subject from 2001-2009.
In model 1, after controlling for age at diagnosis, sex, education level, the presence of the APOE ε4 allele, and history of stroke for the incident dementia model, statin use at baseline was significantly associated with a decreased risk of both incident dementia (P=.0286) and incident AD (P=.0291). Baseline inflammatory status (CRP≥2 mg/dL) (P=.6947 for dementia and P=.3808 for AD) or baseline high LDL levels (>130 mg/dL) (P=.3433 for dementia and P=.5319 for AD) were not associated with incident dementia or AD in the baseline statin models.
Interactions between baseline statin use with both baseline LDL and CRP levels were not significant (P>.45).
In model 2, after controlling for age at diagnosis, sex, education level, the presence of the APOE ε4 allele, and history of stroke for the incident dementia model, continuous use of statins since baseline was associated with a significantly decreased risk of incident AD (P=.0336) and a trend toward a decreased risk of incident dementia (P=.0609) compared with those who had never taken statins. Intermittent use of statins did not confer a significant reduction in risk of either incident dementia (P=.2644) or for incident AD (P=.3113) compared with non-users.
When the analysis is confined to only those participants aged >80 years in 2001, there were a total of 24 incident dementia cases, 23 of whom were diagnosed with AD and 189 in the normal cognition group. The trend toward reduced risk for incident dementia/AD associated with baseline statin use remained (incident dementia: OR=.17, 95% CI .02-1.32, P=.0897; incident AD: OR=.19, 95% CI .03-1.50, P=.1154) but the significance was lost most likely due to the small numbers included.
Discussion
In this analysis, baseline exposure to statin medications in this elderly African American population was associated with a significantly lower risk both for incident AD (P=.0291) and for incident dementia (P=.0286). This result is consistent with findings from our previous report on statin use and cognitive decline21 and with the results from previous studies,2,3 one of which included African Americans.32
When statin use over time was incorporated into our model, however, the significant effect of statin use on reduced AD risk and the trend for reduction in dementia risk was found only for those participants who reported consistent use over the observational period. This result differs from the finding of our previous analysis where the significant effects on cognitive decline remained only for those participants who had discontinued statin use over the study period.
The explanation for this discrepancy is not clear. There have been sufficient individual reports of temporary memory loss with statin use to stimulate a recent FDA warning.33 However, there is little evidence in the literature of persistent cognitive impairment associated with statin use although the issue with high doses of statins remains unresolved.34
Our current analysis took place over a longer period of time, eight years vs three years in our previous analysis, and involved different outcomes, dementia and AD rather than cognitive decline. The observational period for studies that have reported significant protective effects for statin use for incident dementia/AD have ranged from three to 14 years.2-6
Longer exposure to statin use has also been associated with better outcomes.35 Wong et al in their meta-analysis have suggested a useful metric to compare studies might be measuring lag period, ie, the time between the first exposure to the drug and the diagnostic outcome.10 In a nationwide data survey in Taiwan, Wu et al reported a significant protective effect for individuals who took statins for more than 830 days.36 In our study, for the continuous statin users, the average time from baseline until diagnosis was 5.8 years.
Two studies37, 38 have reported an age-associated effect of statin use and incident dementia with the protective effect occurring only in participants aged <80 years. In our study, the average age at baseline was 76.6 years (SD 4.9 years) and the average age at diagnosis was 82.6 years (SD 5.0 years). When our analysis was confined to participants >80 years, the trend toward reduced risk for incident AD/dementia for statin users remained (OR for dementia=.17, P=.09; OR for AD=.19, P=.12) but the significance was lost most likely due to the smaller numbers included in this analysis.
Concerns about prescription bias that might lead to reverse causality have been raised, that is, statins are less likely to be prescribed in patients who have cognitive defects.32 However, in our study the baseline cognitive scores between statin users and non-users were almost identical (statin users mean=69.7, SD=6.0, non-users mean=69.5, SD=6.7, P=.7947).
Previous reports have indicated that reducing lipids39 and controlling inflammation40 may be important pathways in reducing the risk of dementia. Consequently, we included in our models measurements of lipids (LDL levels) and indicators of inflammation (CRP levels). However, we found no relationship between baseline LDL and CRP levels and the protective effects of statins. This finding is consistent with our previous analysis using cognitive decline as an outcome, and is similar to the reports from other studies that have found a significant protective effect for statin use and incident dementia or AD but no association with serum lipid levels,12-15 although one study did report a beneficial effect for statins only in participants with elevated lipid levels.41 There have been no published reports on the association of CRP levels with the protective effects of statins on incident dementia but one article reported that treatment with atorvastatin did not have any significant effect on CRP levels in patients with AD.42 Statins are known to have multiple physiologic effects in addition to their ability to reduce serum cholesterol levels including antioxidant, antinitrosative, and immunomodulatory properties.43 It is possible that one or more of these pathways may be involved in its effect on dementia.
Study Limitations
Since the participants of this study were limited to African Americans (who, as a group, tend to have a higher prevalence of cardiovascular comorbidities), the findings are not necessarily generalizable to other ethnic populations.
In order to control for the effects of APOE ε4 and investigate the relationship between statin effects on lipids, only participants with blood samples were included in this analysis. The study participants were more likely to be female, younger, and had more years of education than those excluded from the study. These variables were included in the subsequent models for incident dementia/AD to minimize potential bias.
Use of statins is likely to be associated with hyperlipidemia. Levels of LDLs and CRP were not measured prior to statin use. Thus the beneficial effects of statins in reducing lipid levels may have been underestimated. LDL and CRP levels were only measured once. In elderly populations these levels are likely to fluctuate and therefore the possible effects of these fluctuations on dementia risk could not be measured.
Although medication usage was checked in person by querying the participants and examining pill bottles, there remains the possibility that the ascertainment of consistency of medication compliance was not always accurate. Medication dosage was not recorded.
Observational studies by design rely on an analysis of the association between putative risk factors and outcomes.44 Determining causality from these associations is difficult, thus limiting their ability to determine the mechanisms of drug action.
Conclusions
In this cohort, the prevalence both of vascular disease and vascular risk factors such as hypertension was high. The results of this study would suggest that clinicians treating elderly African Americans at high risk for vascular disease in accordance with the ACA/AHA recommendations, regardless of lipid levels, would have the additional benefit of reducing the risk of dementia in this population. Whether or not there is any additional advantage in treating elderly African Americans at lower risk for vascular disease remains an open question. Given the limitations of observational studies and the concerns about the potential adverse effects of high dosage use, a prospective randomized control study of the use of statins particularly in relatively healthy older individuals in preventing dementia, although difficult to design, may be worthwhile.
Acknowledgments
Supported by NIA grants R01-AG009956 and P30 AG10133.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 2. Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5(1):20. 10.1186/1741-7015-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71(5):344-350. 10.1212/01.wnl.0000319647.15752.7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horsdal HT, Olesen AV, Gasse C, Sørensen HT, Green RC, Johnsen SP. Use of statins and risk of hospitalization with dementia: a Danish population-based case-control study. Alzheimer Dis Assoc Disord. 2009;23(1):18-22. 10.1097/WAD.0b013e318180f55b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis. 2012;21(6):436-444. 10.1016/j.jstrokecerebrovasdis.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masse I, Bordet R, Deplanque D, et al. Lipid lowering agents are associated with a slower cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(12):1624-1629. 10.1136/jnnp.2005.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69(9):878-885. 10.1212/01.wnl.0000277657.95487.1c [DOI] [PubMed] [Google Scholar]
- 8. Zhou B, Teramukai S, Fukushima M. Prevention and treatment of dementia or Alzheimer’s disease by statins: a meta-analysis. Dement Geriatr Cogn Disord. 2007;23(3):194-201. 10.1159/000099037 [DOI] [PubMed] [Google Scholar]
- 9. Muangpaisan W, Brayne C; Alzheimer’s Society Vascular Dementia Systematic Review Group . Systematic review of statins for the prevention of vascular dementia or dementia. Geriatr Gerontol Int. 2010;10(2):199-208. [DOI] [PubMed] [Google Scholar]
- 10. Wong WB, Lin VW, Boudreau D, Devine EB. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22(4):345-358. 10.1002/pds.3381 [DOI] [PubMed] [Google Scholar]
- 11. Quist-Paulsen P. Statins and inflammation: an update. Curr Opin Cardiol. 2010;25(4):399-405. 10.1097/HCO.0b013e3283398e53 [DOI] [PubMed] [Google Scholar]
- 12. Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631. 10.1016/S0140-6736(00)03155-X [DOI] [PubMed] [Google Scholar]
- 13. Beydoun MA, Beason-Held LL, Kitner-Triolo MH, et al. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health. 2011;65(11):949-957. 10.1136/jech.2009.100826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dufouil C, Richard F, Fiévet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64(9):1531-1538. 10.1212/01.WNL.0000160114.42643.31 [DOI] [PubMed] [Google Scholar]
- 15. Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59(3):378-384. 10.1001/archneur.59.3.378 [DOI] [PubMed] [Google Scholar]
- 16. Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49-56. 10.1212/WNL.56.1.49 [DOI] [PubMed] [Google Scholar]
- 17. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23(1):1-10. 10.1097/WAD.0b013e318187541c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reitz C, Jun G, Naj A, et al. ; Alzheimer Disease Genetics Consortium . Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4,and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483-1492. 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer’s disease and dementia in two communities: nigerian Africans and African Americans. Am J Psychiatry. 1995;152(10):1485-1492. 10.1176/ajp.152.10.1485 [DOI] [PubMed] [Google Scholar]
- 21. Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69(19):1873-1880. 10.1212/01.wnl.0000279333.77404.d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159-1165. 10.1212/WNL.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 23. Unverzagt F, Hall K, Torke A, et al. . Effects of age, education, and gender on CERAD neuropsychological test performance in an African-American sample. Clin Neuropsychol. 1996;10(2):180-190. 10.1080/13854049608406679 [DOI] [Google Scholar]
- 24. Guruje O, Unverzargt FW, Osuntokun BO, et al. The CERAD Neuropsychological Test Battery: norms from a Yoruba-speaking Nigerian sample. West Afr J Med. 1995;14(1):29-33. [PubMed] [Google Scholar]
- 25. Hendrie HC, Lane KA, Ogunniyi A, et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. Int Psychogeriatr. 2006;18(4):653-666. 10.1017/S104161020500308X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roth M, Tym E, Mountjoy CQ, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149(6):698-709. 10.1192/bjp.149.6.698 [DOI] [PubMed] [Google Scholar]
- 27. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition, Rev.Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 28. American Psychiatric Association Press ICD-10. The International Statistical Classification of Diseases and Related Health Problems: 1 and 2; Report No.: V. 3. 1992.
- 29. Little RJA. Rubin, Donald B. Statistical analysis with missing data. 2nd ed Hoboken, N.J.: Wiley; 2002, 10.1002/9781119013563. [DOI] [Google Scholar]
- 30. Efron B. Logistic regression, survival analysis, and the Kaplan-Meier Curve. J Am Stat Assoc. 1988;83(402):414-425. 10.1080/01621459.1988.10478612 [DOI] [Google Scholar]
- 31. D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9(12):1501-1515. 10.1002/sim.4780091214 [DOI] [PubMed] [Google Scholar]
- 32. Green RC, McNagny SE, Jayakumar P, Cupples LA, Benke K, Farrer LA; MIRAGE Study Group . Statin use and the risk of Alzheimer’s disease: the MIRAGE study. Alzheimers Dement. 2006;2(2):96-103. 10.1016/j.jalz.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 33. US Food and Drug Administration FDA Expands Advice on Statin Risks [online]. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm293330.htm. Accessed June 6.
- 34. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159(10):688-697. 10.7326/0003-4819-159-10-201311190-00007 [DOI] [PubMed] [Google Scholar]
- 35. Bernick C, Katz R, Smith NL, et al. ; Cardiovascular Health Study Collaborative Research Group . Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005;65(9):1388-1394. 10.1212/01.wnl.0000182897.18229.ec [DOI] [PubMed] [Google Scholar]
- 36. Wu CK, Yang YH, Lin TT, et al. Statin use reduces the risk of dementia in elderly patients: a nationwide data survey and propensity analysis. J Intern Med. 2015;277(3):343-352. 10.1111/joim.12262 [DOI] [PubMed] [Google Scholar]
- 37. Li G, Shofer JB, Rhew IC, et al. Age-varying association between statin use and incident Alzheimer’s disease. J Am Geriatr Soc. 2010;58(7):1311-1317. 10.1111/j.1532-5415.2010.02906.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rockwood K, Kirkland S, Hogan DB, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59(2):223-227. 10.1001/archneur.59.2.223 [DOI] [PubMed] [Google Scholar]
- 39. Notkola IL, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17(1):14-20. 10.1159/000026149 [DOI] [PubMed] [Google Scholar]
- 40. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61(5):668-672. 10.1001/archneur.61.5.668 [DOI] [PubMed] [Google Scholar]
- 41. Zigman WB, Schupf N, Jenkins EC, Urv TK, Tycko B, Silverman W. Cholesterol level, statin use and Alzheimer’s disease in adults with Down syndrome. Neurosci Lett. 2007;416(3):279-284. 10.1016/j.neulet.2007.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stankovic G, Sparks DL. Change in circulating C-reactive protein is not associated with atorvastatin treatment in Alzheimer’s disease. Neurol Res. 2006;28(6):621-624. 10.1179/016164106X130452 [DOI] [PubMed] [Google Scholar]
- 43. Butterfield DA, Barone E, Mancuso C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res. 2011;64(3):180-186. 10.1016/j.phrs.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]