Abstract
Background
African Americans have a predisposition to heightened systemic inflammation and a high prevalence of hypertension.
Objective
The purpose of this study was to evaluate the influence of interleukin-10 (IL-10) and laminar shear stress (LSS) on African American endothelial cells by measuring total endothelial nitric oxide synthase (eNOS) protein expression and its phosphorylated form (p-eNOS) at Serine 1177, and nitric oxide (NO) levels, in response to IL-10 incubation and high physiological levels of LSS, used as an in vitro mimetic for aerobic exercise training (AEXT).
Design
Human umbilical vein endothelial cells (HUVEC) from an African American donor were cultured. The experimental conditions included Static, Static with IL-10 Incubation, LSS at 20 dynes/cm2, and LSS at 20 dynes/cm2 with IL-10 Incubation. Western blotting was used to measure eNOS and p-eNOS protein expression in the cells. A modified Griess assay was used to measure NO metabolites in the cell culture media.
Results
There were significant increases in p-eNOS, eNOS, and NO in the LSS at 20 dynes/cm2 and LSS at 20 dynes/cm2 with IL-10 Incubation experimental conditions when compared to the Static experimental condition. There were no other statistically significant differences demonstrating that IL-10 did not have an additive effect on eNOS activity in our study.
Conclusion
The significant increases in p-eNOS, eNOS, and NO as a result of LSS in African American HUVECs suggest that AEXT may be a viable, nonpharmacologic method to improve vascular inflammation status and vasodilation, and thereby contribute to hypertension reduction in the African American population.
Keywords: African Americans, Endothelial Cells, Endothelial Dysfunction, Endothelial Nitric Oxide Synthase, Inflammation, Interleukin-10, Laminar Shear Stress, Nitric Oxide
Introduction
The preponderance of research on hypertension in ethnic populations supports the conclusion that African Americans have the highest prevalence of hypertension in the United States and in the world.1,2 Earlier onset and increased severity of this pathology in African Americans leads to higher rates of morbidity and mortality when compared with other ethnic groups.3,4 Hypertension has been linked to independent and interactive effects of multiple genetic and environmental factors.3 One of these factors is inflammation of the vessel wall, a systemic pathological mechanism that can cause endothelial dysfunction, which may be antecedent to hypertension.5,6
The high incidence of hypertension in African Americans may be attributed to their predisposition to heightened systemic inflammation.7-10 Long-term exposure of the endothelium to proinflammatory cytokines leads to endothelial dysfunction, which supports an environment favoring hypertension.11,12 It is hypothesized that the intravascular balance between pro- and anti-inflammation plays a crucial role as a determinant of endothelial health.13 Research data have demonstrated a positive association between hypertension and proinflammatory markers including C-reactive protein (CRP) and interleukin-6 (IL-6).5,6 In contrast, elevated circulating levels of the anti-inflammatory cytokine interleukin-10 (IL-10) have been associated with improved endothelial function.14,15 IL-10 potently inhibits proinflammatory cytokines such as IL-6 and tumor necrosis factor alpha (TNF-α), and the primary function of IL-10 seems to be to limit and ultimately terminate inflammatory responses.16,17 Studies have also demonstrated that IL-10 is contributory in the up-regulation of endothelial nitric oxide synthase (eNOS) and subsequent bioavailability of nitric oxide (NO), a well-documented facilitator of vascular dilation that is critical for normal endothelial function.15,18
Increased vascular shear stress during aerobic exercise has been associated with favorable endothelial adaptations.19-22 In addition, a high physiological level of laminar shear stress (LSS), used as an in vitro aerobic exercise mimetic, has been demonstrated to be important in protecting endothelial cells against inflammatory activation.7,21,23,24 We previously evaluated the influence of aerobic exercise training (AEXT) on inflammation and endothelial health and reported that AEXT elicited positive improvements in inflammation and endothelial function evident by decreasing plasma concentrations of IL-6, and increasing plasma concentration of IL-10 and NO in our African American cohort.25,26
Despite the high prevalence of hypertension and predisposition to heightened systemic inflammation in African Americans, a paucity of research exists on the influence of AEXT on inflammation and endothelial health as a preventive measure to reduce the risk for hypertension and cardiovascular disease (CVD) in this population. Furthermore, the influence of IL-10 in combination with LSS on endothelial cells of African Americans has not been examined. Therefore, the purpose of this study was to evaluate the influence of IL-10 and LSS on African American endothelial cells by measuring total eNOS protein expression and its activated, phosphorylated form (p-eNOS) at Serine 1177, as well as NO levels, in African American endothelial cells in response to 24 hours of IL-10 incubation, high physiological levels of LSS, and a combination of IL-10 incubation with LSS.
Methods
The experiment consisted of the following four conditions: Static, Static with IL-10 Incubation, LSS at 20 dynes/cm2, and LSS at 20 dynes/cm2 with IL-10 Incubation. Human umbilical vein endothelial cells (HUVEC) from an African American donor were obtained from Lonza (Walkersville, Md.) and preserved in liquid nitrogen until time of culture. Experiments were conducted with HUVECs between passages 4-7. HUVECs were cultured in phenol red-free M199 media supplemented with 20% fetal bovine serum and endothelial cell growth supplement and grown in 10% gelatin-coated dishes. It has been demonstrated that the addition of phenol red to media could potentially interfere with nitrate and nitrite measurements, and that measurement of NO metabolites (NOx) is most reliable when levels are quantified from cells that have been cultured in phenol red-free media.27,28 Cells were maintained at 37oC (98.6oF) and 5% CO2 in tissue culture dishes and were examined daily for confluency and morphology observation. LSS was applied when cells reached 95%-100% confluency. The culture dishes were exposed to the four experimental conditions for a period of 24 hours. Recombinant human IL-10 was reconstituted and diluted to a final concentration of 2.0 ng/mL in the complete cell media as recommended (Sigma-Aldrich; SRP3071; St. Louis, Missouri). This dose was used for the incubation of cells in the tissue culture dishes that were included in the IL-10 experimental conditions.
Laminar Shear Stress
Confluent HUVECs grown in 100-mm tissue culture dishes were exposed for a period of 24 hours to LSS at 20 dynes/cm2using a rotating Teflon cone (cone and plate viscometer, 0.5 degree cone angle). Shear stress experiments were conducted in a cell incubator. Immediately following LSS application, both the static and LSS culture dishes were harvested for protein analysis. Radio-Immunoprecipitation Assay (RIPA) Buffer, protease inhibitor, and phosphatase inhibitor were used to enable cell lysis and to stabilize the protein solution in order to measure protein concentration in the cell lysate. Phenylmethylsulfonyl fluoride protease inhibitor was freshly added. Briefly, cells were washed twice with cold Dulbecco’s phosphate buffered saline. A 300 µL volume of the RIPA cocktail was added and a rubber scraper was used to harvest adherent cells. Cell lysates were collected and centrifuged at 16,000 g for 20 minutes at 4oC (39.2oF). The top one-third portion of supernatant was collected and stored at -80oC (-112oF) until time of assay. A Bradford protein assay using Bio-Rad protein reagent was conducted to measure the protein concentration. A 5X sodium dodecyl sulfate (SDS) solution was made, and the protein-SDS samples were boiled for 3 minutes at 95oC (203oF) and frozen at -80oC (-112oF) until use.
Western Blotting
Levels of eNOS, p-eNOS, and alpha tubulin (α-tubulin) were analyzed by Western blotting with mouse monoclonal anti-eNOS (BD Biosciences; 610296; San Jose, Calif.), mouse monoclonal anti-p-eNOS; s1177 (BD Biosciences; 612392; San Jose, Calif.), and mouse monoclonal anti-α-tubulin (Sigma-Aldrich; T9026; St. Louis, Missouri) antibodies, respectively. Alpha tubulin antibody was used as the loading control. Proteins were separated by SDS-Polyacrylamide Gel Electrophoresis on 10% gels and electrotransferred to polyvinylidene difluroide immobilion transfer membranes. Membranes were incubated overnight with a primary antibody at 4oC (39.2oF). After washing and incubating with a secondary antibody conjugated with horseradish peroxidase, total protein was detected by chemiluminescence. Band densitometry analyses were completed using ImageJ software (National Institutes of Health; Bethesda, Md.).
Nitric Oxide Assay
Cell culture supernatant was collected from the four experimental conditions and stored at -80oC (-112 oF) until the time of the assay. On the day of assay, all samples were centrifuged to remove particulates at 16,000g for 20 minutes at 4oC (39.2oF) and then ultrafiltered through a 10,000 molecular weight cut-off filter by micro-centrifuge at 14,000 g for 20 minutes at 4oC (39.2oF). Concentrations of NOx (nitrite/nitrate) in the cell culture supernatant were determined using an assay based on the enzymatic conversion of nitrate to nitrite by nitrate reductase, followed by colorimetric detection of nitrite as an azo dye product of the Griess reaction (R&D Systems; Minneapolis, Minn.). The intra-assay CV value was 1.8%.
Statistical Analyses
Data are expressed as mean ± standard error of the mean. One-way analysis of variance followed by post-hoc testing with Fisher’s least significant difference were used to assess differences across the four experimental conditions. Statistical significance was set at P<.05. All statistical analyses were performed using SPSS version 21.0 (SPSS Inc.; Chicago, Ill.).
Results
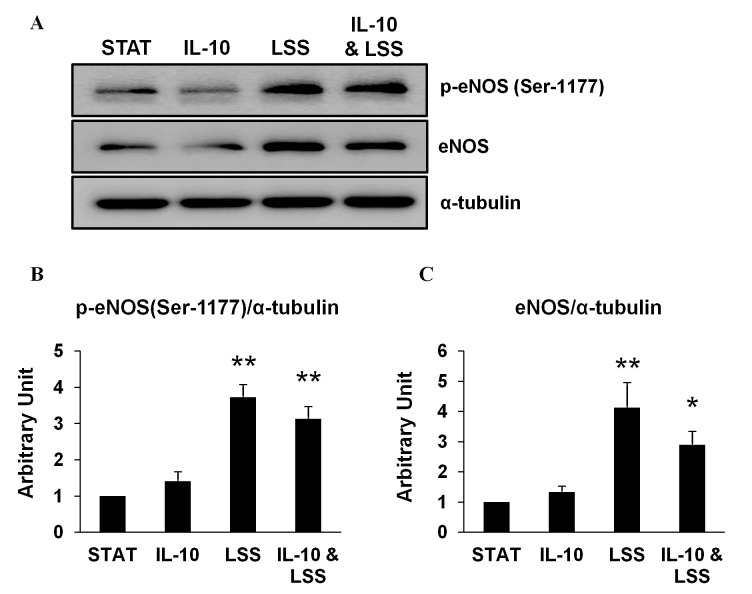
Western blotting experiments were conducted to measure total eNOS protein expression and its phosphorylated form (p-eNOS) at Serine 1177 of the cells in the four experimental conditions with α-tubulin used as the loading control. Protein expression levels of both eNOS and p-eNOS were significantly increased in the LSS at 20 dynes/cm2 and LSS at 20 dynes/cm2 with IL-10 Incubation experimental conditions when compared to the Static experimental condition. There were no significant differences between the Static with IL-10 Incubation and the Static condition or the LSS at 20 dynes/cm2 with IL-10 Incubation and the LSS at 20 dynes/cm2 condition. These results are presented in Figure 1.
Figure 1. (A) Western blotting results from three independent experiments of phosphorylated endothelial nitric oxide synthase (p-eNOS) at Serine 1177 (Ser-1177) and endothelial nitric oxide synthase (eNOS) normalized to alpha tubulin (α-tubulin) for the four experimental conditions. STAT, Static; IL-10, Static with Interleukin-10 (IL-10) Incubation; LSS, Laminar shear stress (LSS) at 20 dynes/cm2; IL-10 & LSS, LSS at 20 dynes/cm2 with IL-10 Incubation. (B) The bar graph depicts the results from densitometry analyses from western blotting experiments of p-eNOS at Ser-1177 normalized to α-tubulin for the four experimental conditions. (C) The bar graph depicts the results from densitometry analyses from western blotting experiments of eNOS normalized to α-tubulin for the four experimental conditions. Bars are expressed as mean ± standard error of the mean. *Denotes significant difference from STAT; P<.05. **Denotes significant differences from STAT; P<.01.
Total concentration of NOx, including nitrite and nitrate, was measured in cell culture supernatant, and concentration levels were determined using an assay based on the enzymatic conversion of nitrate to nitrite by nitrate reductase. NOx concentration levels were significantly higher in the LSS at 20 dynes/cm2 and LSS at 20 dynes/cm2 with IL-10 Incubation experimental conditions when compared with the Static experimental condition. There were no significant differences in the Static with IL-10 Incubation compared to the Static experimental condition or the LSS at 20 dynes/cm2 with IL-10 Incubation compared to the LSS at 20 dynes/cm2 experimental condition. The results are presented in Figure 2.
Figure 2. Total nitric oxide metabolite (NOx) concentrations from three independent samples of cell culture supernatant exposed to the four experimental conditions: STAT, Static; IL-10, Static with Interleukin-10 (IL-10) Incubation; LSS, Laminar shear stress (LSS) at 20 dynes/cm2; IL-10 & LSS, LSS at 20 dynes/cm2 with IL-10 Incubation. Bars are expressed as mean ± standard error of the mean. *Denotes significant differences from STAT; P<.05.
Discussion
Research studies conducted on HUVECs have demonstrated that African American HUVECs have increased systemic inflammation, oxidative stress, and subsequent endothelial dysfunction when compared to Caucasian HUVECs.7-10 It has been well-documented in the literature that oxidative stress and inflammation often occur simultaneously and have been linked to endothelial dysfunction and hypertension.29-31 Elucidating the mechanisms relating to inflammation, endothelial dysfunction, and hypertension may be beneficial in developing preventive measures in reducing the CVD risk burden among the African American population.
We have previously reported that African American endothelial cells had significantly greater levels of IL-6 protein expression and produced greater amounts of IL-6 in response to TNF-α, an inflammatory cytokine.8 In addition, it was demonstrated that, compared with Caucasian endothelial cells, African American endothelial cells had significantly greater protein expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, the principal source of reactive oxygen species in endothelial cells.9 The findings from our studies suggest a heightened inflammatory and oxidative stress status in African American endothelial cells. Therefore, an intervention that can diminish this condition before endothelial dysfunction develops to the point where it manifests clinically may be very important.
A pivotal function of the endothelium altered by inflammation is NO-mediated regulation of vessel tone and blood flow, and this modification includes the reduction in the bioavailability of NO that impairs relaxation and contributes to endothelial dysfunction.11,31,32 Research studies have demonstrated that NO exerts an anti-inflammatory influence by protecting endothelial cells against inflammatory activation.33,34 In a study conducted on ethnic differences in endothelial function, the authors concluded that apparently healthy African Americans have impaired endothelial vasoreactivity when compared with apparently healthy Caucasians, and this disparity may be related to the increased inflammatory state demonstrated in African Americans.35 This further emphasizes the importance of interventions for the African American population that target the bioavailability of NO, which has been demonstrated to be critically important for vasodilation and subsequent endothelial health.
Research data obtained from cell culture studies have demonstrated the beneficial effects of exercise on vascular health, which have been attributed to the increased exercise-induced shear stress.7,21,23,24 The effect of LSS on cultured African American HUVECs has previously been reported, and significant improvements were demonstrated in eNOS protein expression and NO concentrations subsequent to high levels of LSS.7 The results of our study demonstrate a similar outcome with significant increases in eNOS and p-eNOS protein expression, as well as concentrations of NO, subsequent to LSS; however, IL-10 did not provide an additive beneficial effect, as was originally hypothesized.
Other studies have examined the effect of IL-10 on eNOS protein expression and concentrations of NO. In a study conducted on mice, the authors reported that IL-10 exerted its anti-inflammatory influence by inhibiting the in vivo and in vitro adverse effects of TNF-α on the endothelium of the murine aorta by restoring the eNOS protein expression that was reduced by TNF-α.15 This study also showed that IL-10 without the presence of TNF-α had no effect on eNOS expression.15 An additional study was conducted on HUVECs pre-incubated with TNF-α, and it was demonstrated that eNOS protein expression and NO production were increased in the cells subsequent to incubation with IL-10.18 The authors concluded that in the presence of a pro-inflammatory stimulus, increased eNOS protein expression was mediated by the anti-inflammatory effect of IL-10.18 An important commonality in both of these studies, that should be noted, is that IL-10 exerted its anti-inflammatory influence by increasing eNOS protein expression and NO in the presence of TNF-α, an inflammatory cytokine. These findings complement other in vivo studies which demonstrated that IL-10 exerted its anti-inflammatory effects in human subjects with diseases in which higher levels of inflammation are manifested.
In CVD patients with elevated plasma CRP levels, IL-10 has been associated with improved vasoreactivity and a more favorable prognosis, providing some evidence for the importance of IL-10 in endothelial health.13,36 In addition, several studies have previously examined the effect of AEXT on circulating levels of IL-10 in subjects with type 2 diabetes and patients with CVD, and reported a significant increase in IL-10 subsequent to an AEXT intervention.37-39 Furthermore, a review conducted by Batista et al on the role of TNF-α and IL-10 on the anti-inflammatory effect of AEXT included heart failure patients who exhibited elevated baseline levels of TNF-α.37 The authors concluded that the anti-inflammatory effect induced by AEXT seemed to be primarily mediated by IL-10.37
In our study, the protein expression of p-eNOS and eNOS, as well as NOx concentration, were measured in HUVECs that were incubated with IL-10; however, the cultured cells were not previously exposed to any inflammatory medium, such as TNF-α. A future direction for an in vitro study may be to pre-incubate the cultured cells with TNF-α and subsequently measure eNOS protein expression and NOx concentration under the same four experimental conditions as our study.
Conclusion
The results of our study are novel because the effect of IL-10 incubation on protein expression of p-eNOS and eNOS in African American HUVECs has not been previously investigated. The primary findings of our study conducted on HUVECs suggest that IL-10 did not have an additive effect on total eNOS and p-eNOS. Although IL-10 had little effect in our study, p-eNOS and eNOS protein expression, as well as NO concentrations, were demonstrated to be significantly increased as a result of LSS in African American HUVECs. Therefore, AEXT may be a viable, non-pharmacologic method to improve vascular inflammation status and vasodilation, and thereby contribute to reducing hypertension and CVD risk in African Americans.
Acknowledgments
This work was supported by the American Heart Association Scientist Development under Grant 12SDG12070327; NIH/NHLBI under Grant RO1 [HL085497].
Statement of Human Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399-410. 10.1161/01.cir.0000442015.53336.12 [DOI] [PubMed] [Google Scholar]
- 2. Gillespie CD, Hurvitz KA; Centers for Disease Control and Prevention (CDC) . Prevalence of hypertension and controlled hypertension - United States, 2007-2010. MMWR Surveill Summ. 2013;62(suppl 3):144-148. [PubMed] [Google Scholar]
- 3. . Ferdinand KC. Management of high blood pressure in African Americans and the 2010 ISHIB consensus statement: meeting an unmet need. J Clin Hypertens (Greenwich). Volume 12. United States. 2010. p 237-239. [DOI] [PMC free article] [PubMed]
- 4. Rooks RN, Simonsick EM, Klesges LM, Newman AB, Ayonayon HN, Harris TB. Racial disparities in health care access and cardiovascular disease indicators in Black and White older adults in the Health ABC Study. J Aging Health. 2008;20(6):599-614. 10.1177/0898264308321023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17(4):223-230. 10.1038/sj.jhh.1001537 [DOI] [PubMed] [Google Scholar]
- 6. Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006;12(13):1623-1635. 10.2174/138161206776843313 [DOI] [PubMed] [Google Scholar]
- 7. Brown MD, Feairheller DL. Are there race-dependent endothelial cell responses to exercise? Exerc Sport Sci Rev. 2013;41(1):44-54. 10.1097/JES.0b013e318279cbbd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown MD, Feairheller DL, Thakkar S, Veerabhadrappa P, Park JY. Racial differences in tumor necrosis factor-α-induced endothelial microparticles and interleukin-6 production. Vasc Health Risk Manag. 2011;7:541-550. 10.2147/VHRM.S22930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feairheller DL, Park JY, Sturgeon KM, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4(1):32-37. 10.1111/j.1752-8062.2011.00264.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511-2517. 10.1161/01.CIR.0000129087.81352.7A [DOI] [PubMed] [Google Scholar]
- 11. Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006;16(1):15-20. 10.1016/j.tcm.2005.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desideri G, Ferri C. Endothelial activation. Sliding door to atherosclerosis. Curr Pharm Des. 2005;11(17):2163-2175. 10.2174/1381612054367382 [DOI] [PubMed] [Google Scholar]
- 13. Fichtlscherer S, Breuer S, Heeschen C, Dimmeler S, Zeiher AM. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J Am Coll Cardiol. 2004;44(1):44-49. 10.1016/j.jacc.2004.02.054 [DOI] [PubMed] [Google Scholar]
- 14. Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31(11):2534-2542. 10.1161/ATVBAHA.111.233262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zemse SM, Chiao CW, Hilgers RH, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am J Physiol Heart Circ Physiol. 2010;299(4):H1160-H1167. 10.1152/ajpheart.00763.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21(5):331-344. 10.1016/j.cytogfr.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 17. Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R713-R719. 10.1152/ajpregu.00712.2009 [DOI] [PubMed] [Google Scholar]
- 18. Cattaruzza M, Słodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J Biol Chem. 2003;278(39):37874-37880. 10.1074/jbc.M301670200 [DOI] [PubMed] [Google Scholar]
- 19. Johnson BD, Mather KJ, Wallace JP. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med. 2011;16(5):365-377. 10.1177/1358863X11422109 [DOI] [PubMed] [Google Scholar]
- 20. Luk TH, Dai YL, Siu CW, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19(4):830-839. 10.1177/1741826711415679 [DOI] [PubMed] [Google Scholar]
- 21. . Newcomer SC, Thijssen DH, Green DJ Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. J Appl Physiol (1985). 2011;111(1):311-320. [DOI] [PubMed]
- 22. Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312-318. 10.1161/HYPERTENSIONAHA.109.146282 [DOI] [PubMed] [Google Scholar]
- 23. Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J. 2009;73(11):1983-1992. 10.1253/circj.CJ-09-0583 [DOI] [PubMed] [Google Scholar]
- 24. Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9(1):27-41. 10.1152/physiolgenomics.00075.2001 [DOI] [PubMed] [Google Scholar]
- 25. Feairheller DL, Diaz KM, Kashem MA, et al. Effects of moderate aerobic exercise training on vascular health and blood pressure in African Americans. J Clin Hypertens (Greenwich). 2014;16(7):504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. . Babbitt DM, Diaz KM, Feairheller DL, et al. Endothelial activation microparticles and inflammation status improve with exercise training in african americans. Int J Hypertens. 2013;2013:538017. 10.1155/2013/538017 [DOI] [PMC free article] [PubMed]
- 27. Boo YC, Tressel SL, Jo H. An improved method to measure nitrate/nitrite with an NO-selective electrochemical sensor. Nitric Oxide. 2007;16(2):306-312. 10.1016/j.niox.2006.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gooch KJ, Frangos JA. Flow- and bradykinin-induced nitric oxide production by endothelial cells is independent of membrane potential. Am J Physiol. 1996;270(2 Pt 1):C546-C551. [DOI] [PubMed] [Google Scholar]
- 29. Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71(2):247-258. 10.1016/j.cardiores.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez-Mañas L, El-Assar M, Vallejo S, et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8(3):226-238. 10.1111/j.1474-9726.2009.00466.x [DOI] [PubMed] [Google Scholar]
- 31. Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64(1):172-178. 10.1016/j.cardiores.2004.06.020 [DOI] [PubMed] [Google Scholar]
- 32. Trepels T, Zeiher AM, Fichtlscherer S. The endothelium and inflammation. Endothelium. 2006;13(6):423-429. 10.1080/10623320601061862 [DOI] [PubMed] [Google Scholar]
- 33. Desjardins F, Balligand JL. Nitric oxide-dependent endothelial function and cardiovascular disease. Acta Clin Belg. 2006;61(6):326-334. 10.1179/acb.2006.052 [DOI] [PubMed] [Google Scholar]
- 34. Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88(9):877-887. 10.1161/hh0901.090440 [DOI] [PubMed] [Google Scholar]
- 35. Marchesi S, Lupattelli G, Sensini A, et al. Racial difference in endothelial function: role of the infective burden. Atherosclerosis. 2007;191(1):227-234. 10.1016/j.atherosclerosis.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 36. Heeschen C, Dimmeler S, Hamm CW, et al. ; CAPTURE Study Investigators . Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003;107(16):2109-2114. 10.1161/01.CIR.0000065232.57371.25 [DOI] [PubMed] [Google Scholar]
- 37. Batista ML Jr, Lopes RD, Seelaender MC, Lopes AC, Lopes AC. Anti-inflammatory effect of physical training in heart failure: role of TNF-alpha and IL-10. Arq Bras Cardiol. 2009;93(6):643-651, 692-700. [PubMed] [Google Scholar]
- 38. Ribeiro F, Alves AJ, Teixeira M, et al. Exercise training increases interleukin-10 after an acute myocardial infarction: a randomised clinical trial. Int J Sports Med. 2012;33(3):192-198. 10.1055/s-0031-1297959 [DOI] [PubMed] [Google Scholar]
- 39. Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100(1):93-99. 10.1016/j.ijcard.2004.08.073 [DOI] [PubMed] [Google Scholar]