Abstract
The primacy of glucose derived from photosynthesis as an existential source of chemical energy across plant and animal phyla is universally accepted as a core principle in the biological sciences. In mammalian cells, initial processing of glucose to triose phosphate intermediates takes place within the cytosolic glycolytic pathway and terminates with temporal transport of reducing equivalents derived from pyruvate metabolism by membrane-associated respiratory complexes in the mitochondrial matrix. The intra-mitochondrial availability of molecular oxygen as the ultimate electron acceptor drives the evolutionary fashioned chemiosmotic production of ATP as a high-efficiency biological process. The mechanistic bases of carcinogenesis have demonstrated profound alteration of normative mitochondrial function, notably dysregulated respiratory processes. Accordingly, the classic Warburg effect functionally links aerobic glycolysis, aberrant production and release of lactate, and metabolic down-regulation of mitochondrial oxidative processes with the carcinogenetic phenotype. We surmise, however, that aerobic fermentation by cancer cells may also represent a developmental re-emergence of an evolutionarily conserved early phenotype, which was “sidelined” with the emergence of mitochondrial oxidative phosphorylation as a primary mechanism for ATP production in normal cells. Regardless of state-dependent physiological status in mixed populations of cancer cells, it has been established that mitochondria are functionally linked to the initiation of cancer and its progression. Biochemical, molecular, and physiological differences in cancer cell mitochondria, notably mtDNA heteroplasmy and allele-specific expression of selected nuclear genes, may represent major focal points for novel targeting and elimination of cancer cells in metastatic disease afflicting human populations. To date, and despite considerable research efforts, the practical realization of advanced mitochondrial targeted therapies has not been forthcoming.
MeSH Keywords: Alleles, DNA, Glycolysis, Medical Oncology, Mitochondria
Background
Positive evolutionary pressure has engineered the conversion of endosymbiont bacterial precursors into two major classes of autonomous bio-energetic organelles known as mitochondria and chloroplasts. Operationally, mitochondria and chloroplasts contain membrane-bound integral protein respiratory and photosynthetic complexes mediating temporally entrained electron transport processes functionally linked to physiologically determined ATP production [1–3]. The biological exclusivities of these specialized anatomical structures indicate an invariant, error-free, set of internal regulatory controls functionally linked to a high degree of ATP pairing. In light of these existential requirements, selective evolutionary pairing of a multi-functional single-cell organism to a committed bio-energetic organelle has resulted in unilateral transfer of 98–99% of mitochondrial and chloroplast genes to nuclear DNA under the regulation of cellular transcription factors. A recent critical review has highlighted a binary regulatory system responsible for selective expression of genes contained within mitochondrial and chloroplast genomes [4]. Briefly, evolutionarily paired mitochondrial and chloroplast genomes are self-contained genetic systems that encode requisite catalytic subunits of respiratory and photosynthetic complexes and their regulatory proteins. The unifying principle responsible for reciprocal regulation of intra-organelle energy production is critically linked to maintenance of redox potential by electron transport through respiratory and photosynthetic complexes. In effect, restricted mitochondrial and chloroplast genomes co-localize genes encoding catalytic subunits with those encoding regulatory proteins driven by redox state. Accordingly, functional transformation of mitochondria and chloroplasts into high-efficiency bio-engines appears to be dependent on the veracity of ongoing gene expression within restricted metabolic or physiological demands.
Cancer and Mitochondrial Coupling
Investigation into the mechanistic bases of cellular cancer progression has demonstrated profound alteration of normative mitochondrial function, notably dysregulated respiratory processes and maintenance of redox potential [5,6]. The classic theory promoted by Warburg [7,8] functionally links aerobic glycolysis, aberrant production and release of lactate, and metabolic down-regulation of mitochondrial oxidative processes with the carcinogenetic phenotype [9,10] (Figure 1). The primacy of cancer as a mitochondrial metabolic disease has been espoused, and markedly contrasts with widely held theories supporting the causative role of multiple somatic mutations in the etiology and persistence of many cancers. Ostensibly, this contention is supported by cytosolic transfer experiments whereby mitochondrial supplementation from non-malignant cells was observed to engender a significant arrest of carcinogenetic processes, including cellular proliferation [9,10]. In contrast, a parallel line of investigation involving horizontal transfer of competent mitochondria capable of normal respiration and oxidative phosphorylation to cancer cells demonstrates enhanced tumor-forming capabilities following mitochondrial acquisition [5]. A best-fit resolution of these observed effects involves the state-dependent polyclonal nature of tumor cells. A recent study demonstrates that a subpopulation of dormant pancreatic acinar tumor cells surviving oncogene ablation displays phenotypic characteristics of cancer stem cells with high levels of mitochondrial oxidative phosphorylation [6]. The subpopulation of surviving cancer stem cells displayed significant expression of nuclear mitochondrial genes functionally linked to strong mitochondrial respiration and decreased dependence on glycolysis for ATP production. In sum, it appears that a diversity of cellular/mitochondrial bioenergetics is functionally sorted according to metastatic profile, differentiation, or proliferative status within subpopulations of tumor cells.
Figure 1.
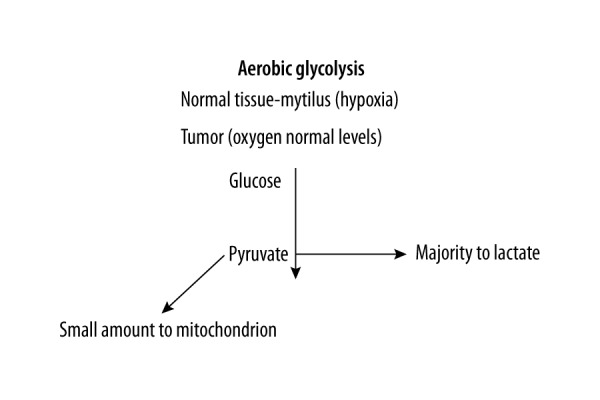
Normal cells rely on mitochondrial oxidative phosphorylation to meet their energy needs. The majority of cancer cells depend on aerobic glycolysis, e.g., Warburg effect. The Warburg effect confers on these cells the ability to undergo glucose fermentation in the presence of adequate oxygen levels. Interestingly, in normal healthy invertebrates, e.g., Mytilus edulis, some tissues can respire via this “Warburg” pathway [1]. This further indicates that mitochondria exhibit biochemical and functional variation, including the capacity for aerobic glycolysis [21]. In this regard, M. edulis has been well studied [22–25]. Thus, when hypoxia gives way to anoxia, an additional metabolic pathway is employed [26], allowing the animal to survive, especially since this animal is intertidal [1,20]. Further, the aerobic glycolysis hypothesis suggests most cancers rely on this pathway and, considering its origin or presence in simple animals, for example, mammalian cancers may represent a reversion to this low-energy – yielding pathway because its genomics are present in our cells.
The multimodal nature of mitochondrial bioenergetics within populations of cancer cells most certainly necessitates a higher order elucidation of molecular and biochemical events reflecting a developmental reiteration of ancient endosymbiotic recruitment of primitive bacteria for compartmentalization of ATP production. A resultant paradigm shift in tumor cell biology will incorporate synergistic mutations within nuclear and mitochondrial genomes to effectively predict the extent of horizontal mitochondrial transfer within metastatic foci to meet metabolic/bioenergetic demands. The expanded repertory of mitochondrial adaptations functionally linked to enhanced tumor progression will include morphological variants [11], alterations in TCA cycle substrate pools, and significant shifts in multi-enzyme-mediated regulatory processes [2,3,12]. For example, previous studies have demonstrated that pyruvate supplementation promotes proliferative effects within populations of cancer cells lacking viable mitochondrial electron transport chain function (as reviewed, [13]). Recently, it has been shown that pyruvate supplementation stimulates aspartate synthesis via enhancement of cytosolic aspartate aminotransferase expression [13]. The resultant enhancement of proliferative activity in cancer cells with impaired mitochondrial function by aspartate supplementation indicates that state-dependent aspartate production is a key metabolic event driving metastatic processes.
A key recent study has demonstrated significant sequence heterogeneity, termed heteroplasmy, in the mtDNA of normal human cells [14]. Although the frequency of mtDNA heteroplasmy was observed to vary across different cell types from the same individual, it was found that cancer cells contained extensive heteroplasmic mutations of mtDNA that could be associated with carcinogenic processes. Accordingly, it appears that the normal expression of multiple mtDNA genotypes in individual human cell types undergoes significant dysregulation, resulting in an altered metabolically compromised phenotype characteristic of cancer cells [15]. It has also been observed that recurrent heteroplasmic mutations occur within or in very close proximity to genetic loci that regulate mtDNA replication, suggesting that carcinogenic processes may be functionally linked to alterations in the replication dynamics of the mutated mtDNA genome [16]. Finally, another important 2015 study has linked allele-specific expression of nuclear DNA with mtDNA heteroplasmy with functional impairment in bioenergetics and an apparent positive selection for reduced mitochondrial function [17]. The overall results and conclusions of the study provide supportive evidence for positive selection processes driving heteroplasmic mtDNA mutations in tumor progression.
Conclusions
In summary, biochemical and molecular differences in cancer cell mitochondria indicate multiple potential therapeutic targets for treatment of major metastatic diseases [1,9,10, 18–20]. An evolutionary perspective on the diversity of mitochondrial expression will certainly enhance translational comprehension of the critical role mitochondria play in tumor progression. The potential cellular burden of oxygen toxicity functionally linked to cytochrome C release and initiation of apoptotic processes justifies a pro-hypoxic respiratory state favoring ongoing glycolytic activity according to parameters outlined by the classic Warburg effect [9,10]. Accordingly, a very early trigger in glycolytic-driven cancer progression may be hypoxia-induced stress responses with resultant down-regulation of mitochondrial oxidative phosphorylation [20]. Conversely, it has become apparent that rapidly proliferating cancer stem cells require highly efficient mitochondrial electron transport processes driven by enhanced respiratory activities. The theoretical and practical translational endpoints of these findings will be directed at the development of novel anticancer agents directed at multiple targets within diverse classes of tumor cell mitochondria.
Footnotes
Source of support: Departmental sources
References
- 1.Stefano GB, Mantione KJ, Casares FM, Kream RM. Anaerobically functioning mitochondria: Evolutionary perspective on modulation of energy metabolism in Mytilus edulis. Invertebrate Survival Journal. 2015;12:22–28. [Google Scholar]
- 2.Snyder C, Stefano GB. Mitochondria and chloroplasts shared in animal and plant tissues: Significance of communication. Med Sci Monit. 2015;21:1507–11. doi: 10.12659/MSM.894481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefano GB, Snyder C, Kream RM. Mitochondria, chloroplasts in animal and plant cells: Significance of conformational matching. Med Sci Monit. 2015;21:2064–69. doi: 10.12659/MSM.894758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen JF. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc Natl Acad Sci USA. 2015;112:10231–38. doi: 10.1073/pnas.1500012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MV, Dong L, Neuzil J. Mitochondrial DNA in tumor initiation, progression, and metastasis: Role of horizontal mtDNA transfer. Cancer Res. 2015;75(16):3203–8. doi: 10.1158/0008-5472.CAN-15-0859. [DOI] [PubMed] [Google Scholar]
- 6.Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–32. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburg O, Geissler AW, Lorenz S. On growth of cancer cells in media in which glucose is replaced by galactose. Hoppe Seylers Z Physiol Chem. 1967;348(12):1686–87. [in German] [PubMed] [Google Scholar]
- 8.Warburg O, Gawehn K, Geissler AW, et al. Experiments on Anaerobiosis of Cancer Cells. Klin Wochenschr. 1965;43:289–93. doi: 10.1007/BF01485244. [in German] [DOI] [PubMed] [Google Scholar]
- 9.Seyfried TN. Cancer as a mitochondrial metabolic disease. Front Cell Dev Biol. 2015;3:43. doi: 10.3389/fcell.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyfried TN, Flores R, Poff AM, et al. Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Lett. 2015;356(2 Pt A):289–300. doi: 10.1016/j.canlet.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Stefano GB, Cadet P, Scharrer B. Stimulatory effects of opioid neuropeptides on locomotory activity and conformational changes in invertebrate and human immunocytes: Evidence for a subtype of delta receptor. Proc Natl Acad Sci USA. 1989;86:6307–11. doi: 10.1073/pnas.86.16.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefano GB. Conformational matching: a possible evolutionary force in the evolvement of signal systems. In: Stefano GB, editor. CRC Handbook of comparative opioid and related neuropeptide mechanisms. Boca Raton: CRC Press Inc; 1986. pp. 271–77. [Google Scholar]
- 13.Birsoy K, Wang T, Chen WW, et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–51. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Wu J, Dressman DC, et al. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature. 2010;464(7288):610–14. doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greaves LC, Reeve AK, Taylor RW, Turnbull DM. Mitochondrial DNA and disease. J Pathol. 2012;226(2):274–86. doi: 10.1002/path.3028. [DOI] [PubMed] [Google Scholar]
- 16.Samuels DC, Li C, Li B, et al. Recurrent tissue-specific mtDNA mutations are common in humans. PLoS Genet. 2013;9(11):e1003929. doi: 10.1371/journal.pgen.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Schroder R, Ni S, Madea B, et al. Extensive tissue-related and allele-related mtDNA heteroplasmy suggests positive selection for somatic mutations. Proc Natl Acad Sci USA. 2015;112(8):2491–96. doi: 10.1073/pnas.1419651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kream RM, Stefano GB. Endogenous morphine and nitric oxide coupled regulation of mitochondrial processes. Med Sci Monit. 2009;15(12):RA263–68. [PubMed] [Google Scholar]
- 19.Kream RM, Stefano GB. Interactive effects of endogenous morphine, nitric oxide, and ethanol on mitochondrial processes. Arch Med Sci. 2010;6(5):658–62. doi: 10.5114/aoms.2010.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefano GB, Kream RM. Hypoxia defined as a common culprit/initiation factor in mitochondrial-mediated proinflammatory processes. Med Sci Monit. 2015;21:1478–84. doi: 10.12659/MSM.894437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller M, Mentel M, van Hellemond JJ, et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev. 2012;76(2):444–95. doi: 10.1128/MMBR.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doeller JE, Grieshaber MK, Kraus DW. Chemolithoheterotrophy in a metazoan tissue: thiosulfate production matches ATP demand in ciliated mussel gills. J Exp Biol. 2001;204(Pt 21):3755–64. doi: 10.1242/jeb.204.21.3755. [DOI] [PubMed] [Google Scholar]
- 23.Doeller JE, Kraus DW, Shick JM, Gnaiger E. Heat flux, oxygen flux, and mitochondrial redox state as a function of oxygen availability and ciliary activity in excised gills of Mytilus edulis. J Exp Zool. 1993;265(1):1–8. doi: 10.1002/jez.1402650102. [DOI] [PubMed] [Google Scholar]
- 24.Mantena SK, Vaughn DP, Andringa KK, et al. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417(1):183–93. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor KM, Gracey AY. High-resolution analysis of metabolic cycles in the intertidal mussel Mytilus californianus. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R103–11. doi: 10.1152/ajpregu.00453.2011. [DOI] [PubMed] [Google Scholar]
- 26.Woo S, Jeon HY, Kim SR, Yum S. Differentially displayed genes with oxygen depletion stress and transcriptional responses in the marine mussel, Mytilus galloprovincialis. Comp Biochem Physiol Part D Genomics Proteomics. 2011;6(4):348–56. doi: 10.1016/j.cbd.2011.07.003. [DOI] [PubMed] [Google Scholar]


