Abstract
Background
In the past decades, a large number of randomized controlled trials (RCTs) on the efficacy of ligustrazine injection combined with conventional antianginal drugs for angina pectoris have been reported. However, these RCTs have not been evaluated in accordance with PRISMA systematic review standards. The aim of this study was to evaluate the efficacy of ligustrazine injection as adjunctive therapy for angina pectoris.
Material/Methods
The databases PubMed, Medline, Cochrane Library, Embase, Sino-Med, Wanfang Databases, Chinese Scientific Journal Database, Google Scholar, Chinese Biomedical Literature Database, China National Knowledge Infrastructure, and the Chinese Science Citation Database were searched for published RCTs. Meta-analysis was performed on the primary outcome measures, including the improvements of electrocardiography (ECG) and the reductions in angina symptoms. Sensitivity and subgroup analysis based on the M score (the refined Jadad scores) were also used to evaluate the effect of quality, sample size, and publication year of the included RCTs on the overall effect of ligustrazine injection.
Results
Eleven RCTs involving 870 patients with angina pectoris were selected in this study. Compared with conventional antianginal drugs alone, ligustrazine injection combined with antianginal drugs significantly increased the efficacy in symptom improvement (odds ratio [OR], 3.59; 95% confidence interval [CI]: 2.39 to 5.40) and in ECG improvement (OR, 3.42; 95% CI: 2.33 to 5.01). Sensitivity and subgroup analysis also confirmed that ligustrazine injection had better effect in the treatment of angina pectoris as adjunctive therapy.
Conclusions
The 11 eligible RCTs indicated that ligustrazine injection as adjunctive therapy was more effective than antianginal drugs alone. However, due to the low quality of included RCTs, more rigorously designed RCTs were still needed to verify the effects of ligustrazine injection as adjunctive therapy for angina pectoris.
MeSH Keywords: Angina Pectoris; Medicine, Chinese Traditional; Meta-Analysis as Topic
Background
Angina pectoris is triggered by decreased myocardial oxygen supply or increased myocardial oxygen demand [1]. An estimated 94.9 of every 100 000 urban residents die of coronary artery disease (CAD) in China and nearly 1 of every 6 deaths was due to CAD in the US in 2009 [2,3]. Chronic stable angina is the most prevalent manifestation of CAD, with an incidence of 500 000 new cases every year in the US [1]. Typical symptoms of angina pectoris are characterized by discomfort in various parts of the body, including the chest, jaw, shoulder, back, and arms. Angina pectoris elicited by exertion or emotional stress can be relieved by rest or nitroglycerin [4]. Conventional antianginal drugs (e.g., β-blockers, calcium antagonists, and nitrates) reduce angina attacks by lowering heart rate and blood pressure or enhancing coronary blood flow [5]. Despite the effectiveness of the antianginal drugs, episodes of angina pectoris may still persist or even worsen. In addition, most patients cannot tolerate the adverse effects of these antianginal drugs [6–8]. Therefore, a new treatment that has significant advantages in increasing the effects of conventional antianginal drugs is necessary. Recently, the benefits of traditional Chinese medicine (TCM) combined with the conventional antianginal drugs have attracted a growing and sustained interest from researchers and physicians.
Ligustrazine (2,3,5,6-tetramethylpyrazine, C8H12N2, Figure 1A) is an active ingredient from the plant Szechwan Lovage Rhizome. This plant has been widely used to treat cardiovascular disease, angina pectoris, and headache for hundreds of years in China as herbal medicine [9,10]. Owing to the impressive effects of ligustrazine in scavenging cytotoxic oxygen free radicals, promoting blood flow, and antiplatelet aggregation, various dosage forms based on ligustrazine are developed in China, such as injection, tablets, and capsule [11]. Ligustrazine injection is the most popular form among them and it is commercially available as ligustrazine hydrochloride (Figure 1B) (40 mg/10 mL) and ligustrazine phosphate (Figure 1C) (50 mg/10 mL). Ligustrazine injection ranges from 40 mg to 80 mg was usually used in the method of intravenous drip (diluted with 250–500 mL of isotonic normal saline or glucose solution; one time, once daily) [12]. In order to provide better efficacy in treating angina pectoris, ligustrazine injection is usually used in combination with conventional antianginal drugs in China.
Figure 1.
Structure of ligustrazine (A), ligustrazine hydrochloride (B) and ligustrazine phosphate (C).
The clinical studies on ligustrazine injection have been reported with potential positive results. However, neither systematic a review nor a meta-analysis on the therapeutic effect of ligustrazine injection as adjunctive therapy for angina pectoris has been reported so far. Therefore, this study offers a comprehensive and PRISMA-compliant systematic review with sensitivity and subgroup analysis for evaluating the efficacy of ligustrazine injection as adjunctive therapy in the treatment of angina pectoris [13].
Material and Methods
Eligibility criteria
The published RCTs were searched independently by 2 reviewers (HK, Shao and LG, Zhao). The participants with angina pectoris were examined according to the World Health Organization guideline [14]. Studies that met the following criteria were included: (a) Studies compared any antianginal drugs combined with or without ligustrazine injection (i.e., ligustrazine injection combined with antianginal drugs vs. antianginal drugs alone); (b) Duration of treatment was at least 2 weeks; (c) The sample size of studies was at least 50 patients; and (d) The primary outcome measures in RCTs were symptom improvement and ECG improvement.
Studies were excluded if they did not meet the criteria above and: (a) The RCTs involved animal, human cells or in vitro studies; (b) Studies did not include the symptom improvement and ECG improvement as the primary outcome measures; (c) Studies with same authors and similar content; and (d) The dosages of intervention in the treatment and control groups of included studies were not specifically stated.
Information sources
The databases PubMed, Medline, Cochrane Library, Embase, Sino-Med, Wanfang Databases, Chinese Scientific Journal Database, Google Scholar, Chinese Biomedical Literature Database, China National Knowledge Infrastructure and the Chinese Science Citation Database were independently searched and retrieved by 2 authors (HK, Shao and LG, Zhao). The latest search of databases was conducted on 29 May 2015.
Search strategies
The following terms were searched in separate or combined ways for English databases: ligustrazine injection, antianginal drugs, angina pectoris, cardiovascular diseases, coronary artery disease. The following terms were searched in separated or combined ways for Chinese databases: Chuanxiongqin zhusheye [ligustrazine injection], Chuanxiongqin [ligustrazine], guanxinbing [coronary artery disease], xinjiaotong [angina pectoris]. Moreover, the references listed in the selected articles were also searched to acquire additional papers related to this study.
Study selection
Two authors (HK. Shao and LG. Zhao) independently screened all titles and abstracts of clinical studies according to the eligibility criteria. Disagreement between the 2 authors was resolved by consensus.
Data collection process
One author (HK, Shao) extracted data from the included RCTs and then put them into Microsoft Excel. Another 2 authors (LG, Zhao and SQ, Liu) examined the accuracy of extracted data. Disagreements between authors were solved through discussion. The software Review Manager 5.0 was used to evaluate the extracted data.
Data items
Two authors (HK. Shao and LG. Zhao) independently extracted the following data items: (a) First author, publication year, and language of RCTs; (b) Characteristics of patients including age and sample size; (c) Intervention (dosages and duration); (d) Outcome measures; and (e) Incidence of adverse reactions.
Risk of bias in individual studies
The methodological quality of included RCTs was independently evaluated by 2 authors (HK. Shao and LG. Zhao) in reporting baseline comparison of participants, randomization methods, concealment of treatment allocation, blinding, and adverse event report according to M score (Table 1) [15].
Table 1.
M scale checklist.
| Question | Answer | Score |
|---|---|---|
| M1. Were the groups comparable? | Positive in comparability | 1 |
| Negative in comparability | 0 | |
| M2. Was the study described as randomized? | Randomization procedure was described and the procedure was appropriate | 2 |
| Randomization was mentioned without describing the procedure | 1 | |
| Randomization procedure was incorrect | −1 | |
| M3. Was the study described as blind? | Double blinding was described with a specific procedure | 2 |
| Double blinding was described without a specific procedure | 1 | |
| Single blinding was described with a specific procedure | 1 | |
| Single blinding was described without a specific procedure | 0.5 | |
| No blinding was described | 0 | |
| M4. Were withdrawals and dropouts described? | Counts and reasons of withdrawals and dropouts were reported | 1 |
| Only counts or reasons were reported | 0.5 | |
| No withdrawal or dropout was mentioned | 0 | |
| M5. Were the adverse effects described? | Counts and types of adverse effects were reported | 1 |
| Only counts or types of adverse effects were reported | 0.5 | |
| No adverse effect was mentioned | 0 |
Quality assessment
Two authors (HK, Shao and LG, Zhao) independently assessed the quality of included RCTs based on M scale (Studies with M scale >3 were considered to be moderate-quality RCTs). Any disagreement among the 2 authors was resolved by consensus.
Definitions of improvements of symptom and ECG
Improvement in symptom should reduce by at least 50% (i.e., basic improvement in angina symptoms) the frequency and duration of feeling angina chest pain. Improvement in ECG should achieve at least 0.05 mv at ST segment (i.e., basic improvements in ECG) in ECG during an exercise test, as mentioned in ACC/AHA guidelines [16].
Sensitivity and subgroup analysis
Sensitivity analysis was conducted to verify whether the overall efficacy of ligustrazine injection combined with antianginal drugs over antianginal drugs alone would be affected by the low quality of included RCTs. Subgroup analysis was conducted to evaluate the overall effects in subgroups based on sample sizes and publication year of the study.
Risk of bias across studies
The publication bias was assessed by funnel plots, Begg’s test, and Egger’s test with the software of STATA 12.0.
Statistical analysis
All analyses were performed with RevMan 5.0. For continuous outcome variables, standard mean difference was used with 95% confidence interval (CI). For dichotomous outcome variables, odds ratio (OR) was given with 95% CI. If OR <1, it indicated a lower risk for treatment group than control group. If OR >1, it showed that a greater risk for treatment group than control group. Statistical heterogeneity was measured by using the chi-squared test and I2 statistic. Significant difference for heterogeneity test was considered when P<0.05 or I2 statistic >50% [17–19]. The random-effects model was used to analyze the pooled effects when heterogeneity was significantly different, otherwise the fixed-effects model was used. The Z test was used to compare the overall effects of treatment group and control group, and differences were considered to be statistically significant when P<0.05. Results are expressed as pooled odds ratios (OR (95% confidence intervals, CIs)). The Mantel-Haenszel method with fixed-effects model was used to calculate the pooled OR and 95% CI considering the small trials, high event rates, and dichotomous outcome variable in this study.
Results
Study selection
The selection process is described in Figure 2. We initially identified 767 potential records, including 266 articles from CNKI, 176 articles from Wanfang data, 77 articles from VIP, 116 articles from PubMed, and 132 articles from Medline, Cochrane Library, Embase, Sino-Med, Google Scholar, Chinese Biomedical Literature Database, and Chinese Science Citation Database. After screening the titles and abstracts, 721 articles were excluded because a large number of records from 3 Chinese databases (CNKI, VIP, and Wanfang) were duplication and a large quantity of RCTs did not meet the inclusion criteria. Full texts of 46 articles were retrieved for further identification according to the pre-defined eligibility criteria; 11 studies were finally selected for quality assessment and meta-analysis.
Figure 2.

The process of study selection. CNKI is China National Knowledge Infrastructure; WF is WangFang data; VIP is Chinese Scientific Journal Database; Other resoures is Medline, Cochrane Library, Embase, Sino-Med, Google Scholar, Chinese Biomedical Literature Database and the Chinese Science Citation Database. LSI is ligustrazine injection.
Study characteristics
The characteristics of 11 RCTs are summarized in the Supplementary Table 1. The included RCTs were all published in Chinese journals between the 2000 and 2015. The sample size of RCTs ranged from 50 to 120. Eleven RCTs with 870 patients (436 patients in treatment group) were included in this study [20–30]. The duration of treatment ranged from 14 to 28 days. Ten RCTs reported symptoms changes as the outcome measures and 7 studies reported ECG changes. The antianginal drugs used in the control groups included nitroglycerin, metoprolol, aspirin, isosorbide dinitrate, isosorbide-5-mononitrate, and simvastatin. Based on the drugs used in the control groups, the dosage of ligustrazine injection used in treatment group ranged from 80 to 240 mg daily.
Risk of bias in individual studies
The M scale (between -1 and 7 points) was used to measure the methodological quality of the included studies. The results of quality assessment were provided in Table 2. Five RCTs scored 3 and 6 RCTs scored 4 according to M scale, indicating the poor methodological quality of most RCTs.
Table 2.
Quality measures of the included studies.
| Study | M1 | M2 | M3 | M4 | M5 | M Score |
|---|---|---|---|---|---|---|
| Da MF, 2008 | 1 | 1 | 0 | 1 | 1 | 4 |
| Ji ZJ, 2009 | 1 | 1 | 0 | 1 | 1 | 4 |
| Li Y, 2010 | 1 | 1 | 0 | 1 | 0 | 3 |
| Liao JQ, 2006 | 1 | 1 | 0 | 1 | 1 | 4 |
| Liu YJ, 2009 | 1 | 1 | 0 | 1 | 1 | 4 |
| Luo B, 2007 | 1 | 0 | 0 | 1 | 1 | 3 |
| Sun DN, 2007 | 1 | 1 | 0 | 1 | 0 | 3 |
| Wang QZ, 2008 | 1 | 1 | 0 | 1 | 1 | 4 |
| Wang XY, 2008 | 1 | 1 | 0 | 1 | 1 | 4 |
| Yang JX, 2008 | 1 | 1 | 0 | 1 | 0 | 3 |
| Yao MJ, 2009 | 1 | 1 | 0 | 1 | 0 | 3 |
Results of individual studies and their synthesis
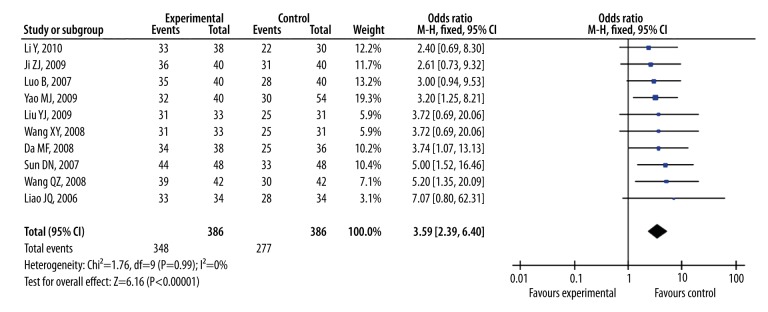
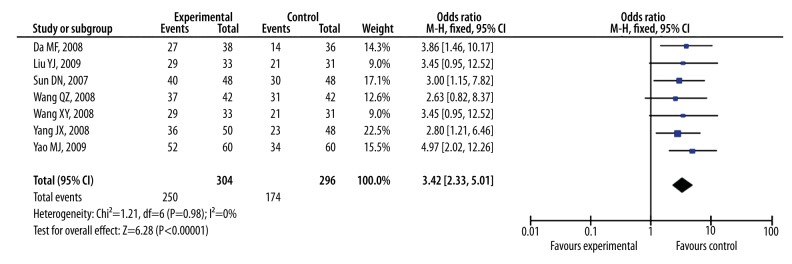
As shown in Figure 3, the Pooled odds ratio of symptoms was 3.59 (95% CI, 2.39 to 5.40, Z=6.16, P<0.00001) with no heterogeneity (I2=0%; χ2=1.76; df=9, P=0.99) among the 10 studies with symptomatic improvements as outcome measure. Figure 4 showed that the Pooled odds ratio of ECG was 3.42 (95% CI, 2.33 to 5.01, Z=6.28, P<0.00001) with no heterogeneity (I2=0%; χ2=1.21; df=6, P=0.98) among the 7 studies with ECG as outcome measure. The Pooled odds ratios of symptoms and ECG indicated that ligustrazine injection as adjunctive therapy for angina pectoris was more effective than antianginal drugs alone.
Figure 3.
The forest plot of outcome measure symptoms.
Figure 4.
The forest plot of outcome measure ECG.
Risk of bias across studies
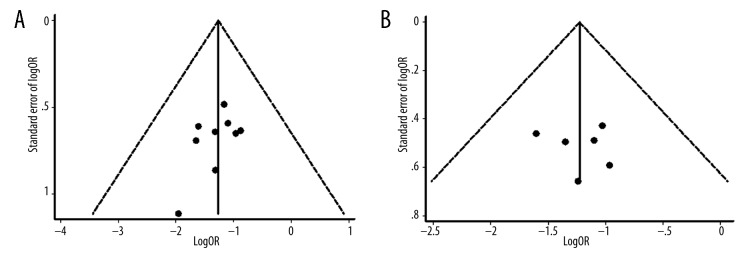
As shown in Figure 5, no obvious asymmetry was observed in the funnel plots for symptoms and ECG. Begg’s test (symptoms: Z=0.98, P=0.325; ECG: Z=0.15, P=0.881) and Egger’s test (symptoms: t=−1.45, P=0.185; ECG: t=0.23, P=0.827) also indicated that there was no statistically significant publication bias.
Figure 5.
Funnel plots of the included studies with symptomatic data (A); Funnel plots of the included studies with ECG data (B).
Adverse events
One RCT reported ligustrazine injection as adjunctive therapy could cause discomfort in stomach for 1 patient. Respectively, 3 RCTs reported that there were 2 patients suffering from headache both in treatment group and control group. The rest of studies did not report the adverse events.
Subgroup and sensitivity analysis
Based on the characteristics of RCTs including publication year (before or after 1 January 2008) and sample size (≤70 or >70), subgroup analysis was performed and the results were shown in Tables 3 and 4. No significant difference was found between the overall odds ratios of subgroups, indicating that the efficacies were consistently stable among all included studies.
Table 3.
Sensitivity and subgroups analysis based on symptoms.
| Group | No. of studies | No. of participants | OR | 95% CI | Z | P(effect) | I2 | χ2 | P(het) | |
|---|---|---|---|---|---|---|---|---|---|---|
| M score | ≤3 | 4 | 338 | 3.31 | 1.91, 5.76 | 4.24 | <0.0001 | 0% | 0.75 | 0.86 |
| >3 | 6 | 434 | 3.93 | 2.15, 7.19 | 4.46 | <0.00001 | 0% | 0.85 | 0.97 | |
| Sample Size | ≤70 | 3 | 200 | 3.45 | 1.42, 8.42 | 2.27 | 0.006 | 0% | 0.75 | 0.69 |
| >70 | 7 | 572 | 3.63 | 2.30, 5.74 | 5.52 | <0.00001 | 0% | 0.98 | 0.99 | |
| Publication year | ≤2008 | 6 | 436 | 4.24 | 2.43, 7.39 | 5.09 | <0.00001 | 0% | 0.78 | 0.98 |
| >2008 | 4 | 306 | 2.92 | 1.60, 5.34 | 3.49 | 0.0005 | 0% | 0.24 | 0.97 |
Table 4.
Sensitivity and subgroups analysis based on ECG.
| Group | No. of studies | No. of participants | OR | 95% CI | Z | P(effect) | I2 | χ2 | P(het) | |
|---|---|---|---|---|---|---|---|---|---|---|
| M score | ≤3 | 3 | 314 | 3.47 | 2.08, 5.80 | 4.74 | <0.00001 | 0% | 0.95 | 0.62 |
| >3 | 4 | 286 | 3.35 | 1.88, 5.96 | 4.11 | <0.0001 | 0% | 0.25 | 0.97 | |
| Sample Size | ≤70 | 2 | 162 | 2.98 | 1.48, 6.01 | 3.05 | 0.002 | 0% | 0.07 | 0.79 |
| >70 | 5 | 438 | 3.62 | 2.28, 5.72 | 5.49 | <0.00001 | 0% | 0.94 | 0.92 | |
| Publication year | ≤2008 | 5 | 416 | 3.09 | 1.97, 4.85 | 4.91 | <0.00001 | 0% | 0.36 | 0.99 |
| >2008 | 2 | 184 | 4.41 | 2.11, 9.23 | 3.94 | <0.0001 | 0% | 0.21 | 0.65 |
Sensitivity analysis based on M scale was conducted in this study. When the lower quality studies (M score ≤3) were excluded, the odd ratio by symptom increased from 3.59 to 3.93 (95%, 2.15–7.19, Z=4.46, P<0.00001) with no heterogeneity (Chi2=0.85, P=0.97, I2=0%). The odd ratio by ECG decreased from 3.42 to 3.35 (95%, 1.88–5.96, Z=4.11, P<0.0001) with no heterogeneity (Chi2=0.25, P=0.97; I2=0%). However, the above results showed that no significant difference happened in odds ratios for symptoms (Table 3) and ECG (Table 4). It had statistical significance (the 95% CI did not contain the value 1) after all low quality studies were excluded during the process of sensitivity analysis. In addition, no obvious heterogeneity was found from the results of sensitivity analysis.
Discussion
Analysis of effectiveness
In recent years, Chinese medicine as adjunctive or alternative medicine for angina pectoris has attracted widely interests because conventional antianginal drugs might sometimes fail to treat angina pectoris. As one of the most popular medicine in China, ligustrazine injection had played a very important role in treating angina pectoris. Although there are many studies about ligustrazine injection, neither systematic review nor meta-analysis has been performed on ligustrazine injection as adjunctive therapy for angina pectoris so far. Therefore, in this study, we firstly provided a comprehensive and PRISMA-compliant systematic review with sensitivity and subgroup analysis for evaluating the efficacy of ligustrazine injection as adjunctive therapy in the treatment of angina pectoris. PRISMA Checklist was provided in Supplementary Table 2. The results from the 11 included RCTs suggested that ligustrazine injection as adjunctive therapy for angina pectoris was more effective than conventional antianginal drugs alone. Sensitivity and subgroup analysis conducted on M scale, sample size and year of publication also consistently proved that ligustrazine injection as adjunctive therapy has superior effects in the treatment of angina pectoris. Moreover, no publication bias was found in this study.
Limitations
Some limitations existed in this study. The low quality of RCTs included in this study was the major limitation. Only half of RCTs scored 3 or above according to the M scale. Apart from this, the poor design of clinical trials in the RCTs was also an important limitation. The included RCTs were not strictly designed according to the golden standard. Allocation concealment and blinding were not clearly described in majority of the RCTs. Besides, this research had the following defects: (1) All included studies came from mainland China and were published in Chinese. Moreover, no multi-center/country clinical randomized controlled trial was found in this study. (2) The different dosage of ligustrazine injection used in the treatment group might lead to heterogeneity among the included RCTs. (3) Duration of treatment was relatively short as most duration of RCTs was 2 weeks. Therefore, further larger scale, higher quality and rigorously designed RCTs were still needed to confirm the efficacy of ligustrazine injection as an adjunctive therapy in the treatment of angina pectoris.
Implications for further research
At present, ligustrazine injection as adjunctive therapies are not well known to most Western physicians and its efficacy in treating angina pectoris has not been evaluated. This study could potentially help physicians manage the problem of drug tolerance and side effects occurred in their patients with angina pectoris.
This study found that a majority of the RCTs on ligustrazine injection had poor methodological quality. The description of randomization methods and concealment of allocation procedures was not provided in many RCTs. Problems existed in blinding of the patients and researchers were prevalent. In addition, placebo-controlled design was rarely adopted in their study. In some studies, the researchers were not blinded to treatment allocation when they assessed the treatment outcome. The low quality of RCTs included in this study indicated that many researchers might not be aware of the crucial components of RCTs. Therefore, future RCTs about ligustrazine injection should comply with the following recommendations: (1) Researchers should have received some formal educations about clinical trial design when they conducted RCTs; (2) Researchers should register clinical trials and publish their reports in a standard trial registration platforms; (3) The researchers should understand the rationale for selecting different types of control groups; (4) The CONSORT 2010 checklist should be adopted when the researchers reported the RCTs about ligustrazine injection. In general, further RCTs with good methodology were still required to accurately verify the efficacy of ligustrazine injection as adjunctive therapy in the treatment of angina pectoris.
This systematic review was more reliable than previous reviews on ligustrazine injection. The previous reviews had the following defects: (1) Non-randomized controlled trials were included in their search strategy; (2) The combination use of other medicines in both treatment and control groups was allowed in their study selection; (3) Sensitivity and subgroup analysis were not provided in their evidence evaluation; (4) Their performance did not follow the PRISMA requirements. On the contrary, this systematic review and meta-analysis included only the RCTs comparing the efficacy of ligustrazine injection combined with conventional antianginal drugs with antianginal drugs alone. Moreover, in order to avoid possible publication biases, subgroup and sensitivity analysis were performed in this study.
In summary, the overall result from the eleven RCTs indicated that the efficacy of ligustrazine injection as adjunctive therapy was more effective than conventional antianginal drugs alone for angina pectoris. Due to the low quality of the included RCTs, more rigorously designed RCTs were still needed to verify the efficacy of ligustrazine injection as adjunctive therapy for angina pectoris.
Conclusions
This study showed that ligustrazine injection combined with conventional antianginal drugs was more effective than conventional antianginal drugs alone in treating angina pectoris. Moreover, sensitivity and subgroup analysis conducted on M scale, sample size, and years of publication also consistently corroborated that ligustrazine injection as adjunctive therapy had superior effect in the treatment of angina pectoris. However, more rigorously designed RCTs were still needed to accurately verify the efficacy of ligustrazine injection as an adjunctive therapy for angina pectoris.
Supplementary materials
Supplementary Table 1.
Summary of the included studies evaluating the adjunctive therapy of ligustrazine injection in treating angina.
| Study | Year | Country | Age | Intervention | Sample | Follow-up (day) | Outcome measures | |
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | |||||||
| Da MF | 2008 | China | 41–83 | Ligustrazine injection 80 mg/d, Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | 74 | 14 | SYM, ECG |
| Ji ZJ | 2009 | China | 29–52 | Ligustrazine injection 100 mg/d, Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d, Simvastatin 40 mg/d, Isosorbide-5-mononitrate 20 mg/d | Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d, Simvastatin 40 mg/d, Isosorbide-5-mononitrate 20 mg/d | 80 | 14 | SYM |
| Li Y | 2010 | China | 42–76 | Ligustrazine injection 120 mg/d, Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | 68 | 14 | SYM |
| Liao JQ | 2006 | China | 46–71 | Ligustrazine injection 120 mg/d, Nitroglycerin 50 mg/d, Aspirin 150 mg/d | Nitroglycerin 50 mg/d, Aspirin 150 mg/d | 68 | 14 | SYM |
| Liu YJ | 2009 | China | 42–70 | Ligustrazine injection 150 mg/d, Isosorbide-5-mononitrate 60 mg/d | Isosorbide-5-mononitrate 60 mg/d | 97 | 28 | SYM, ECG |
| Luo B | 2007 | China | 44–78 | Ligustrazine injection 100 mg/d, Nitroglycerin 10 mg/d, Aspirin 75 mg/d | Nitroglycerin 10 mg/d, Aspirin 75 mg/d | 80 | 14 | SYM |
| Sun DN | 2007 | China | 48–80 | Ligustrazine injection 120–200 mg/d, Isosorbide dinitrate 30 mg/d, Nitroglycerin 0.3–0.6 mg/d, Aspirin 100 mg/d | Isosorbide dinitrate 30 mg/d, Nitroglycerin 0.3–0.6 mg/d, Aspirin 100 mg/d | 96 | 21 | SYM, ECG |
| Wang QZ | 2008 | China | 48–80 | Ligustrazine injection 80 mg/d, Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | 84 | 14 | SYM, ECG |
| Wang XY | 2008 | China | 44–79 | Ligustrazine injection 150 mg/d, Isosorbide-5-mononitrate 60 mg/d | Isosorbide-5-mononitrate 60 mg/d | 64 | 14 | SYM, ECG |
| Yang JX | 2008 | China | 46–78 | Ligustrazine injection 100 mg/d, Isosorbide dinitrate 30 mg/d, Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | Isosorbide dinitrate 30 mg/d, Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | 50 | 14 | ECG |
| Yao MJ | 2009 | China | 43–74 | Ligustrazine injection 240 mg/d, Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d | Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d | 120 | 15 | SYM, ECG |
Supplementary Table 2.
Checklist of items to include when reporting a systematic review or meta-analysis.
| Section/topic | # | Checklist item | Section &/or figure reported in |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Title |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | Abstract |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Introduction |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | Introduction |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | No |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | Methods-Eligibility criteria |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Methods-Information sources |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeate | Methods-Search strategies |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Methods-Study selection |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Methods-Data collection process |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | Methods-Data items |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Methods-Risk of bias in individual studies |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | Methods-Statistical analysis |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | Methods-Statistical analysis |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | Methods-Risk of bias across studies |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | Methods-Sensitivity and subgroup analysis |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Results-Literature Study selection |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | Results-Study characteristics |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Results-Risk of bias within studies |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Result-Results of individual studies and their synthesis |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Result-Results of individual studies and their synthesis |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15 | Result-Risk of bias across studies |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | Result-Sensitivity and subgroup analysis |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | Discussion-Summary of evidence |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | Discussion-Limitations |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | Discussion-Conclusions |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | Funding |
Footnotes
Source of support: This study was supported by Shenzhen Science and Technology Plan Project (no. JCYJ20140414100411116); Natural Science Foundation of Guangdong Province, China (no. 2015A030310454), and Hubei Province Health and Family Planning Scientific Research Project (no. WJ2015MB290)
References
- 1.Tobin KJ. Stable angina pectoris: what does the current clinical evidence tell us? J Am Osteopath Assoc. 2010;110(7):364–70. [PubMed] [Google Scholar]
- 2.National Center for Cardiovascular Diseases, China (NCCD) Report on cardiovascular diseases in China (2011) Beijing, China: Encyclopedia of China Publishing House; 2012. pp. 7–12. [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127(1):6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27(11):1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 5.Marzilli M, Klein WW. Efficacy and tolerability of trimetazidine in stable angina: a meta-analysis of randomized, double-blind, controlled trials. Coron Artery Dis. 2003;14(2):171–79. doi: 10.1097/00019501-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Holubkov R, Laskey WK, Haviland A, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J. 2002;144(5):826–33. doi: 10.1067/mhj.2002.125505. [DOI] [PubMed] [Google Scholar]
- 7.Hueb W, Soares PR, Gersh BJ, et al. The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol. 2004;43(10):1743–51. doi: 10.1016/j.jacc.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 8.Aldakkak M, Stowe DF, Camara AKS. Safety and efficacy of ranolazine for the treatment of chronic angina pectoris. Clin Med Insights Ther. 2013;2103(5):1–14. doi: 10.4137/CMT.S7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ju XD, Deng M, Ao YF, et al. The protective effect of tetramethylpyrazine on cartilage explants and chondrocytes. J Ethnopharmacol. 2010;132(2):414–20. doi: 10.1016/j.jep.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Xu Y, Shen Y, et al. Tetramethylpyrazine potentiates arsenic trioxide activity against HL-60 cell lines. Braz J Med Biol Res. 2012;45(3):187–96. doi: 10.1590/SO100-879X2012007500017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, Xiong Y, Cheng F, et al. Effect of Ligustrazine on ischemia-reperfusion injury in murine kidney. Transplant Proc. 2004;36(7):1949–51. doi: 10.1016/j.transproceed.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Wei LJ, Marasini N, Li G, et al. Development of ligustrazine-loaded lipid emulsion: Formulation optimization, characterization and biodistribution. Int J Pharm. 2012;437(1–2):203–12. doi: 10.1016/j.ijpharm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomenclature and Criteria for Diagnosis of Ischemic Heart Disease. Report of the joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979;59(3):607–9. doi: 10.1161/01.cir.59.3.607. [DOI] [PubMed] [Google Scholar]
- 15.Jia YL, Huang FY, Zhang SK, Leung SW. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. Int J Cardiol. 2012;157(3):330–40. doi: 10.1016/j.ijcard.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina) J Am Coll Cardiol. 1999;33(7):2092–97. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Zhu J. Linear reduction in thyroid cancer risk by oral contraceptive use: a dose-response meta-analysis of prospective cohort studies. Hum Reprod. 2015;30(9):2234–40. doi: 10.1093/humrep/dev160. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Wang Z, Zhu J, et al. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73(7):409–25. doi: 10.1093/nutrit/nuv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Zhu J, Prokop LJ, et al. Pharmacologic therapy of diabetes and overall cancer risk and mortality: A meta-analysis of 265 studies. Sci Rep. 2015;5:10147. doi: 10.1038/srep10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da MF. The clinical curative effect observation of ligustrazine in the treatment of unstable angina pectoris. Qinghai Med J. 2008;38(10):69–70. [Google Scholar]
- 21.Ji ZJ, Ji KG, Wang GL. Clinical observation on unstable angina treated by isosorbide mononitrate combined with tetramethylpyrazine phosphate. Chin J Integr Tradit West Med. 2009;7(12):1394–95. [Google Scholar]
- 22.Li Y. To explore the clinical experience of diagnosis and treatment of traditional Chinese medicine in unstable angina pectoris. Chin J Mod Drug Appl. 2010;4(9):113–14. [Google Scholar]
- 23.Liao JQ, Luo XW. The effects of ligustrazine in the treatment of unstable angina pectoris. Med Inf. 2006;19(7):1242–43. [Google Scholar]
- 24.Liu YJ, Liu TJ. Clinical observation of 97 cases in treating stable angina pectoris. China J Chin Mater Med. 2009;7(1):41–42. [Google Scholar]
- 25.Luo B. The effects of ligustrazine injection in treating angina effect. Chin J Rural Med Pharm. 2007;14(9):45. [Google Scholar]
- 26.Sun DN. Analysis of therapeutic effect of Chuanxiongqin glucose injection in curing unstable angina. J Pract Med Techn. 2007;14(11):1431–32. [Google Scholar]
- 27.Wang XY. Observation on the effect of isosorbide mononitrate in combination with tetramethylpyrazine on angina pectoris due to coronary heart disease. China Trop Med. 2008;8(8):1353–54. [Google Scholar]
- 28.Yang JS. Clinical observation on 50 cases of ligustrazine phosphate in treatment of angina pectoris of coronary heart disease. J Jilin Med Coll. 2008;29(5):272. [Google Scholar]
- 29.Yao MJ. Observation of the curative effect of ligustrazine combined with isosorbide mononitrate in the treatment of angina pectoris. Chin J Integr Tradit West Med. 2009;18(22):2666. [Google Scholar]
- 30.Wang QZ. Clinical study on ligustrazine hydrochloride injection in treatment of unstable angina pectoris. Herald Med. 2008;5(32):29–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Summary of the included studies evaluating the adjunctive therapy of ligustrazine injection in treating angina.
| Study | Year | Country | Age | Intervention | Sample | Follow-up (day) | Outcome measures | |
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | |||||||
| Da MF | 2008 | China | 41–83 | Ligustrazine injection 80 mg/d, Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | 74 | 14 | SYM, ECG |
| Ji ZJ | 2009 | China | 29–52 | Ligustrazine injection 100 mg/d, Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d, Simvastatin 40 mg/d, Isosorbide-5-mononitrate 20 mg/d | Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d, Simvastatin 40 mg/d, Isosorbide-5-mononitrate 20 mg/d | 80 | 14 | SYM |
| Li Y | 2010 | China | 42–76 | Ligustrazine injection 120 mg/d, Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | Nitroglycerin 10–15 mg/d, Aspirin 100 mg/d, Metoprolol 50 mg/d | 68 | 14 | SYM |
| Liao JQ | 2006 | China | 46–71 | Ligustrazine injection 120 mg/d, Nitroglycerin 50 mg/d, Aspirin 150 mg/d | Nitroglycerin 50 mg/d, Aspirin 150 mg/d | 68 | 14 | SYM |
| Liu YJ | 2009 | China | 42–70 | Ligustrazine injection 150 mg/d, Isosorbide-5-mononitrate 60 mg/d | Isosorbide-5-mononitrate 60 mg/d | 97 | 28 | SYM, ECG |
| Luo B | 2007 | China | 44–78 | Ligustrazine injection 100 mg/d, Nitroglycerin 10 mg/d, Aspirin 75 mg/d | Nitroglycerin 10 mg/d, Aspirin 75 mg/d | 80 | 14 | SYM |
| Sun DN | 2007 | China | 48–80 | Ligustrazine injection 120–200 mg/d, Isosorbide dinitrate 30 mg/d, Nitroglycerin 0.3–0.6 mg/d, Aspirin 100 mg/d | Isosorbide dinitrate 30 mg/d, Nitroglycerin 0.3–0.6 mg/d, Aspirin 100 mg/d | 96 | 21 | SYM, ECG |
| Wang QZ | 2008 | China | 48–80 | Ligustrazine injection 80 mg/d, Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | 84 | 14 | SYM, ECG |
| Wang XY | 2008 | China | 44–79 | Ligustrazine injection 150 mg/d, Isosorbide-5-mononitrate 60 mg/d | Isosorbide-5-mononitrate 60 mg/d | 64 | 14 | SYM, ECG |
| Yang JX | 2008 | China | 46–78 | Ligustrazine injection 100 mg/d, Isosorbide dinitrate 30 mg/d, Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | Isosorbide dinitrate 30 mg/d, Aspirin 75 mg/d, Nitroglycerin 0.3–0.6 mg/d | 50 | 14 | ECG |
| Yao MJ | 2009 | China | 43–74 | Ligustrazine injection 240 mg/d, Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d | Aspirin 100 mg/d, Isosorbide dinitrate 30 mg/d | 120 | 15 | SYM, ECG |
Supplementary Table 2.
Checklist of items to include when reporting a systematic review or meta-analysis.
| Section/topic | # | Checklist item | Section &/or figure reported in |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Title |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | Abstract |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Introduction |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | Introduction |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | No |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | Methods-Eligibility criteria |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Methods-Information sources |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeate | Methods-Search strategies |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Methods-Study selection |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Methods-Data collection process |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | Methods-Data items |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Methods-Risk of bias in individual studies |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | Methods-Statistical analysis |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | Methods-Statistical analysis |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | Methods-Risk of bias across studies |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | Methods-Sensitivity and subgroup analysis |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Results-Literature Study selection |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | Results-Study characteristics |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Results-Risk of bias within studies |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Result-Results of individual studies and their synthesis |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Result-Results of individual studies and their synthesis |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15 | Result-Risk of bias across studies |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | Result-Sensitivity and subgroup analysis |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | Discussion-Summary of evidence |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | Discussion-Limitations |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | Discussion-Conclusions |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | Funding |