Multiplanar MR imaging–guided endovascular navigation in an abdominal aortic phantom with the multicoil magnetically assisted remote-controlled catheter system is feasible at 1.5 T, improves at 3 T, and is comparable to x-ray guidance for a variety of vessels at 3 T.
Abstract
Purpose
To assess the feasibility of multiplanar vascular navigation with a new magnetically assisted remote-controlled (MARC) catheter with real-time magnetic resonance (MR) imaging at 1.5 T and 3 T and to compare it with standard x-ray guidance in simulated endovascular catheterization procedures.
Materials and Methods
A 1.6-mm–diameter custom clinical-grade microcatheter prototype with lithographed double-saddle coils at the distal tip was deflected with real-time MR imaging. Two inexperienced operators and two experienced operators catheterized anteroposterior (celiac, superior mesenteric, and inferior mesenteric arteries) and mediolateral (renal arteries) branch vessels in a cryogel abdominal aortic phantom. This was repeated with conventional x-ray fluoroscopy by using clinical catheters and guidewires. Mean procedure times and percentage success data were analyzed with linear mixed-effects regression.
Results
The MARC catheter tip was visible at 1.5 T and 3 T. Among inexperienced operators, MARC MR imaging guidance was not statistically different from x-ray guidance at 1.5 T (67% successful vessel selection turns with MR imaging vs 76% with x-ray guidance, P = .157) and at 3 T (75% successful turns with MR imaging vs 76% with x-ray guidance, P = .869). Experienced operators were more successful in catheterizing vessels with x-ray guidance (98% success within 60 seconds) than with 1.5-T (65%, P < .001) or 3-T (75%) MR imaging. Among inexperienced operators, mean procedure time was nearly equivalent by using MR imaging (31 seconds) and x-ray guidance (34 seconds, P = .436). Among experienced operators, catheterization was faster with x-ray guidance (20 seconds) compared with 1.5-T MR imaging (42 seconds, P < .001), but MARC guidance improved at 3 T (31 seconds). MARC MR imaging guidance at 3 T was not significantly different from x-ray guidance for the celiac (P = .755), superior mesenteric (P = .358), and inferior mesenteric (P = .065) arteries.
Conclusion
Multiplanar navigation with a new MARC catheter with real-time MR imaging at 1.5 T and 3 T is feasible and comparable to x-ray guidance for anteroposterior vessels at 3 T in a vascular phantom.
© RSNA, 2015
Online supplemental material is available for this article.
Introduction
Performing endovascular procedures with magnetic resonance (MR) imaging guidance is a key application in the growing field of interventional MR imaging (1–5). Exploiting the MR imaging environment to treat various diseases currently treated with x-ray fluoroscopic guidance can yield real-time physiological information, such as diffusion and perfusion, which augments intraprocedural decision making.
We previously demonstrated the navigation capabilities of a second-generation magnetically assisted remote-controlled (MARC) catheter at 1.5 T (6,7). Limitations of that catheter included (a) the ability to deflect in only one plane, (b) a solenoid coil tip that required the phantom to be oriented 90° to the MR imaging unit bore, and (c) the relatively large size of the catheter tip (2-mm diameter). In this study, we developed a third-generation MARC catheter with a double-saddle coil tip constructed with laser lithography to address the limitations of the previous catheter. The purpose of this study was to assess the feasibility of vascular navigation with a new MARC catheter with real-time MR imaging at 1.5 T and 3 T and to compare it with standard x-ray guidance in simulated endovascular catheterization procedures.
Materials and Methods
Design of the MARC Catheter
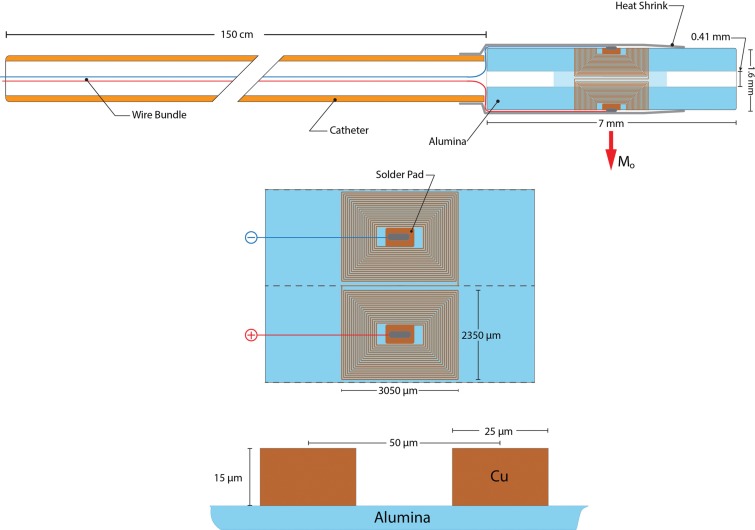
The MARC catheter prototype was constructed by using 150-cm-long 0.90-mm–diameter (2.9-F) custom catheters based on the clinical PX Slim (Penumbra, Alameda, Calif) neurovascular microcatheter but braided with nonmetallic polyether ether ketone fibers instead of standard metallic fibers. The catheter shaft was an in-kind donation from Penumbra; the authors who are not employees of or consultants for Penumbra had control of all data and information submitted for publication. Four 0.13-mm–diameter copper wires in the catheter lumen were connected to a double-saddle coil at the distal tip that was constructed by using a laser lithography method (8). With this method, a titanium and copper conductive seed coating is used in which a positive electrodeposited photoresist is patterned by using a 405-nm, 50-mW diode laser. Copper is electroplated into the developed pattern, after which the seed coating and remaining photoresist are chemically removed. We used this method to create saddle coil pairs consisting of 19 turns for each half of the coil pair (Fig 1). The final catheter tip consisted of two coil pairs oriented at 90° relative to each other. The tip was covered with heat shrink (Component Force, St Louis, Mo), resulting in a final distal catheter tip outer diameter of approximately 1.6 mm (5 F) (Fig 1). The copper wires were strung through the lumen and connected to a screened, fully shielded, twisted pair cable that was plugged into an MR imaging–compatible catheter controller cart.
Figure 1:
Diagram of the catheter prototype.
Operating System and User Interface
The operating system (Figs 2, 3) consisted of a custom hardware control board placed within a shielded MR imaging–compatible cart with direct communication to a laptop computer (MacBook Air; Apple, Cupertino, Calif) via a Universal Serial Bus. The computer was running a custom software program designed in Laboratory Virtual Instrument Engineering Workbench (LabVIEW; National Instruments Corporation, Austin, Tex) to communicate with the catheter control system (9). Both the catheter prototype and a row of mounted foot pedals (Aquiline; Linemaster Switch, Woodstock, Conn) in the interventional MR imaging suite were connected to the MR imaging–compatible cart via screened, fully shielded, twisted pair cables to allow the operator to deliver current and maintain free use of the hands to push or pull the catheter in the craniocaudal axis parallel to the imager bore, while magnetically deflecting the catheter in the mediolateral or anteroposterior axes via foot pedal actuator. The foot pedal actuator was set to deliver ±300 mA to either or both tip coils to deflect the catheter superiorly, inferiorly, left, or right. Although in theory the catheter can be deflected up to 90° in any direction, in practice because of the mechanical properties of the catheter and tip, the limits of deflection experimentally have been up to 25° for single coil activation and up to 32° for dual coil activation for the specific prototype tested in this study.
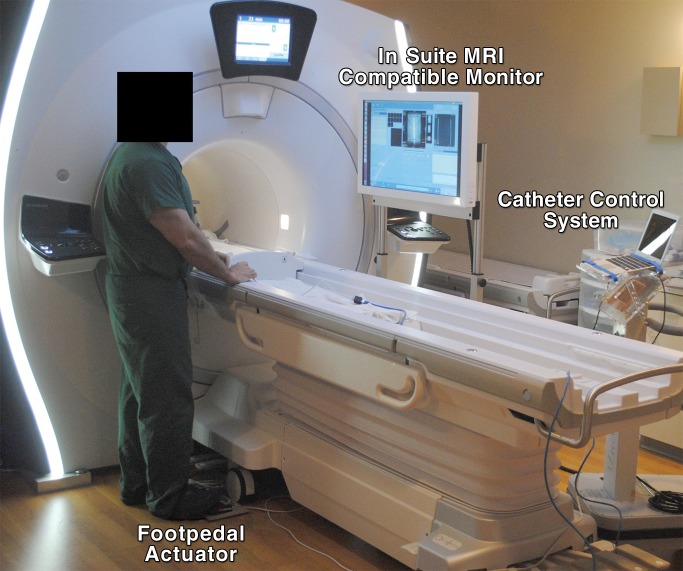
Figure 2:
Photograph of the navigation operating system and user interface.
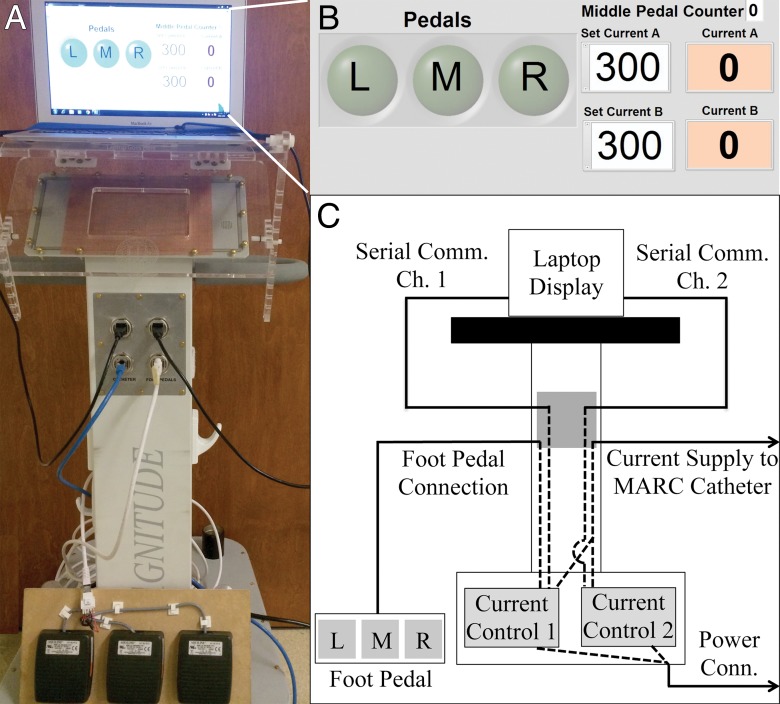
Figure 3:
A, Photograph of the catheter control system, with a laptop running the graphical user interface shown at the top and the foot pedal actuator shown at the bottom. B, Screen shot of the graphical user interface used to control current delivery to the catheter. On the left are indicators that are illuminated when the corresponding foot pedal is pressed. On the right are numerical fields where the user can enter the desired current value for each channel. C, Schematic depiction of the electrical connections between components inside and outside of the control system cart. The two printed circuit boards that are responsible for delivering electrical current to the MARC catheter are located at the bottom of the system. Dotted lines demonstrate connections made inside the cart, while solid lines demonstrate connections made outside the cart.
Phantom Design
In vitro navigation was tested quantitatively in a polyvinyl alcohol (Sevol Grade 165 PVA powder; Sekisui Specialty Chemicals America, Dallas, Tex) cryogel simplified abdominal aortic vascular phantom. The abdominal aorta phantom mold was created with Delrin rods (McMaster-Carr, Elmhurst, Ill) and provided physiologically relevant vessel trajectories and angles of the celiac artery (diameter, 8 mm; angle, 60° relative to the aorta), superior mesenteric artery (SMA) (diameter, 8 mm; angle, 50°), bilateral renal arteries (diameter, 6 mm; angle, 60°), and inferior mesenteric artery (IMA) (diameter, 6 mm; angle, 60°). The simulated IMA is larger than most human IMAs to facilitate catheterization. After removal of the Delrin rods, the phantom was placed in distilled water in a plastic bin with 0.5-inch vinyl tubing connected to the bin, and a nonferrous 15-F Check-Flo Performance Introducer (Cook, Bloomington, Ind) was inserted into the tubing to mimic vascular access.
Experiment Design
All interventionalists were given 5 minutes of proctored practice with the MARC catheter before they conducted navigation tests. In total, 200 turns were attempted with both MR imaging (200 turns at 1.5 T and 200 turns at 3 T) and x-ray guidance. Four operators, two experienced attending interventional neuroradiologists (S.W.H., with 13 years of endovascular experience; and D.L.C., with 9 years of experience) and two inexperienced operators that included a radiology resident physician (P.M., with 3 years of experience) and a medical student (A.D.L., with less than 1 year of experience), attempted navigation of the catheter into each branch vessel (10 attempts per branch for a total of 50 attempts by each operator). With magnetic assistance, the operator started at the celiac artery, then proceeded down inferiorly to catheterize the SMA, then the IMA, then returned rostrally from the IMA to the SMA to the celiac artery with sagittal MR imaging guidance. Selection of the renal arteries was performed with coronal MR imaging guidance, alternating between the right and left renal arteries. The guide catheter (custom pure plastic tubes with 16-F outer diameter and 12-F inner diameter) was parked at the origin of the next-most-distal branch point (eg, the guide catheter was placed at the celiac origin for attempts at microcatheterization of the SMA) for each attempt, and the phantom was oriented parallel to B0. The end point for each navigation was successful completion of turning into the branch and advancement to the edge of the phantom within 60 seconds. For successful attempts, the time was stopped once the catheter was advanced past the edge of the phantom (6 cm lateral to the branch origin from the aorta). If the catheter tip did not reach the edge of the branch vessel within 60 seconds, the trial was scored as a failure.
All 1.5-T MR imaging experiments were performed while viewing an in-room monitor (Philips examination room display, Cleveland, Ohio) that displayed real-time imaging by using a single-section balanced steady-state free precession sequence on a clinical MR imaging unit (Achieva; Philips). The pulse sequence parameters were repetition time msec/echo time msec, 3.2/1.1; field of view, 28 × 28 cm2; reconstructed image matrix, 224 × 224; in-plane resolution, 1.25 × 1.25 mm2; flip angle, 60°; frames per second, 1.6; section thickness, 10 mm; and specific absorption rate, 2.8 W/kg.
MARC experiments were also performed in a 3-T clinical MR imaging unit by using a balanced steady-state free precession sequence (GE Discovery 750w; Milwaukee, Wis) (in-room monitor provided by Nordic NeuroLab, Milwaukee, Wis). The pulse sequence parameters were 3.3/1.5; field of view, 31 × 31 cm2; reconstructed image matrix, 256 × 256; in-plane resolution, 1.21 × 1.21 mm2; flip angle, 30°; frames per second, 2.3; section thickness, 10 mm; and specific absorption rate, 3.44 W/kg.
Control navigation experiments were performed in the abdominal aortic phantom by using C-arm x-ray fluoroscopic guidance (OEC 9600; GE Medical Systems) with a clinically standard 110-cm 4-F UCSF3 Super Torque Catheter (Cordis, Miami Lakes, Fla) and a 150-cm-long 0.035-inch–diameter angled stiff-type Glidewire (Terumo, Somerset, NJ) instead of a MARC catheter.
Statistical Analysis
Mean procedure times were determined and presented as means ± standard error of the mean. Percentage branch catheterization success was reported. A linear mixed-effects regression analysis was used to compare mean procedure times and percentage success. The model included a random effect for each interventionalist that performed the procedure. A P value of less than .05 indicated a significant difference. Statistical analyses were performed by using Stata version 13 software (StataCorp, College Station, Tex). The statistical power was computed for both the percentage success comparisons and the mean procedure time comparisons between x-ray guidance and MR imaging. When comparing data pooled from both experienced and inexperienced interventionalists (n = 200), a difference of 12% in success rate could be detected with 81% power. When comparing the individual data sets (ie, only inexperienced or only experienced interventionalists, n = 100), a difference of 18% in success rate could be detected with 81% power. For procedure times, a difference of 5.6 seconds and 8.0 seconds could be detected with 80% power for the pooled data and individual data sets, respectively. Percentage success power calculations assumed a baseline success rate of 85%, while procedure time power calculations assumed a standard deviation of 20 seconds. These values were taken from results obtained in a previous study with the MARC catheter (7). All statistical powers were computed by using two-sided models with α = .05. The statistical powers computed for the percentage success comparisons included continuity correction (10).
Results
Catheter Deflection and Visualization
The MARC catheter tip was visible with MR imaging in the phantom while activated for magnetic guidance by using ±300 mA of electric current. The catheter tip could be deflected in four planes, depending on the channel setting and the current polarity (positive or negative) for each tip coil (Fig 4). Visualization of the catheter tip allowed the users to navigate the catheter into targeted vessels with MR imaging guidance (Fig 5) (Movies 1, 2 [online]).
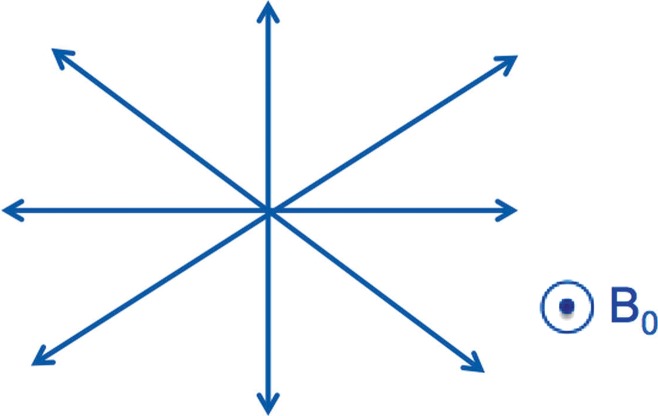
Figure 4:
Diagram shows that the catheter tip could be deflected in four orthogonal directions, depending on the channel setting and the current polarity (positive or negative).
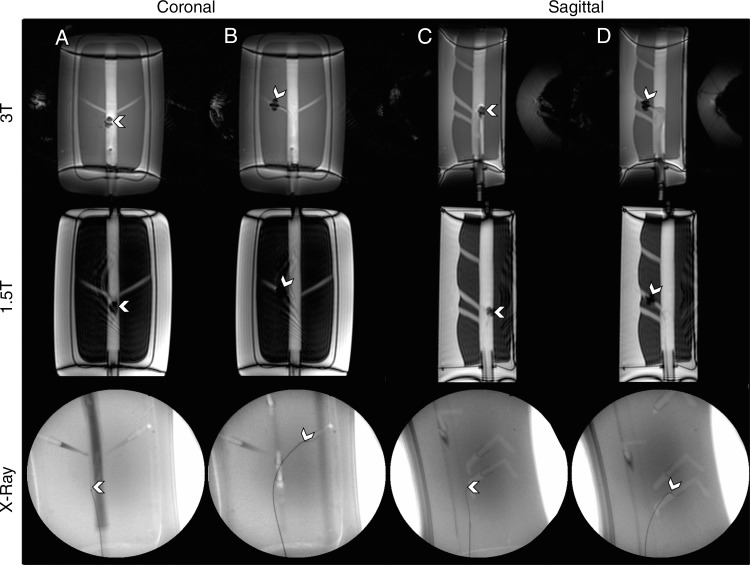
Figure 5:
Images demonstrate navigation experiments in the abdominal aorta phantom (white arrowhead indicates the catheter tip). A, Navigation is shown in the coronal plane of the renal arteries with 3-T and 1.5-T MR imaging and x-ray guidance. B, The tip of the microcatheter is deflected into the renal arteries. C, Navigation is shown in the sagittal plane with 3-T and 1.5-T MR imaging and x-ray guidance. D, The tip of the microcatheter is deflected into the SMA.
Movie 1.
Real-time MARC catheter navigation at 3 T by using a coronal imaging plane to target the simulated left renal artery in the phantom. The video playback has not been altered and demonstrates the actual frame rate experienced by the interventionalist.
Movie 2.
Real-time MARC catheter navigation at 3 T by using a sagittal imaging plane to target the simulated SMA in the phantom. The video playback has not been altered and demonstrates the actual frame rate experienced by the interventionalist.
Navigation Success
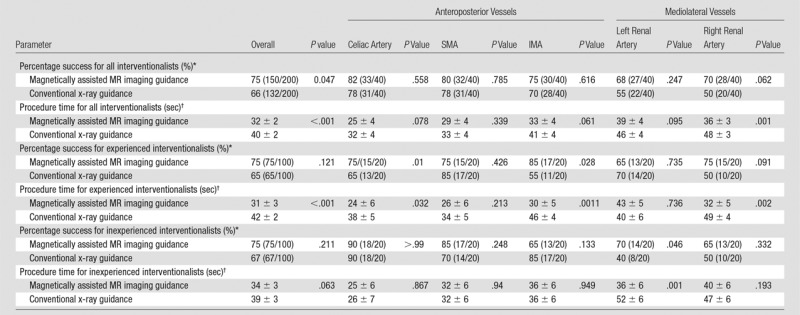
For all four interventionalists combined, 132 (66%) of 200 turns were completed successfully within 60 seconds with MR imaging guidance at 1.5 T versus 174 (87%) of 200 turns with x-ray guidance (P < .001). Overall mean procedure time was longer with MR imaging guidance at 1.5 T (40 seconds ± 2) than for x-ray guidance (26 seconds ± 3) (P < .001) (Table 1). At 3 T, for all interventionalists combined, success rate and navigation times improved as compared with 1.5 T but were still not equivalent to those with x-ray guidance. Specifically, 150 (75%) of 200 turns were completed successfully with MR imaging guidance at 3 T versus 174 (87%) of 200 turns with x-ray guidance (P = .001). Mean procedure time for MR imaging guidance at 3 T was 32 seconds ± 2 versus 26 seconds ± 3 for x-ray guidance (P = .001) (Table 1).
Table 1.
Navigation Success Rate and Time for MR Imaging vs X-ray Guidance
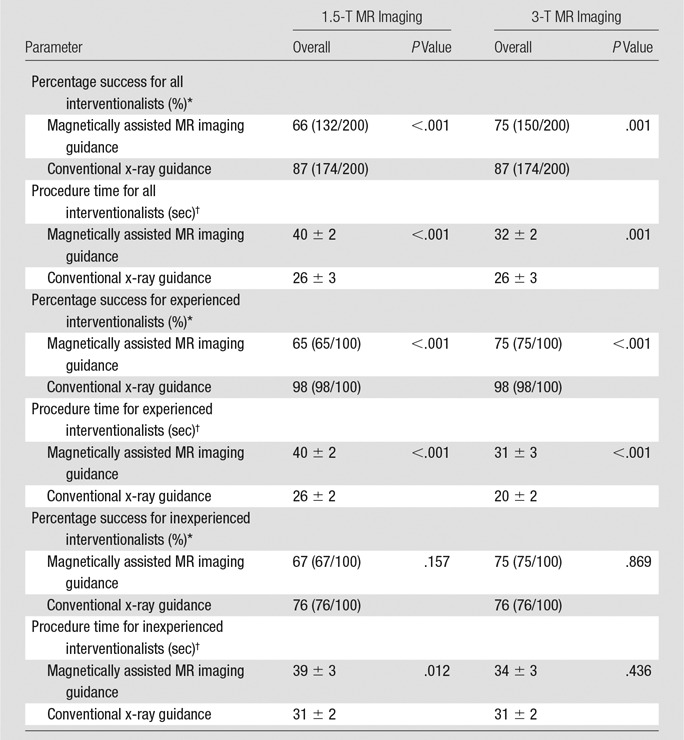
*Data are the percentage of success, with the numerator and denominator in parentheses.
†Procedure time data are presented in seconds as mean ± standard error of the mean.
For the two inexperienced interventionalists (50 turns each), 67 (67%) of 100 selective catheterizations were completed successfully with MR imaging guidance at 1.5 T versus 76 (76%) of 100 turns completed successfully with x-ray guidance (P = .157). There was no significant difference in mean procedure time between MR imaging at 1.5 T and x-ray guidance (39 seconds ± 3 vs 31 seconds ± 2, respectively; P = .259) (Table 1). Catheterization rates and navigation times again improved with 3-T MR imaging; in fact, 3-T MR imaging and x-ray guidance (75% vs 76% successful turns, 34 seconds ± 3 vs 31 seconds ± 2, respectively; P = .869) were equivalent (Table 1).
For the two experienced interventionalists, 65 (65%) of 100 selective catheterizations were completed successfully with MR imaging guidance at 1.5 T versus 98 (98%) of 100 turns completed successfully with x-ray guidance (P < .001). X-ray guidance was faster compared with MR imaging at 1.5 T (20 seconds ± 2 vs 42 seconds ± 2, respectively; P < .001) (Table 1). Catheterization success rate and navigation times improved with 3-T MR imaging for experienced interventionalists, but x-ray guidance was still used more successfully and faster than 3-T MR imaging guidance (98% vs 75% successful turns, respectively, P < .001; 20 seconds ± 2 vs 31 seconds ± 3, respectively; P < .001) (Table 1).
Results stratified by branch vessels are presented in Tables 2 and 3. For both inexperienced and experienced interventionalists, the renal arteries were the most difficult to catheterize on the basis of both success rate and mean procedure time. At 3 T, renal artery catheterization improved (both success rate and mean procedure time) but was still more challenging than visceral artery (celiac artery, SMA, and IMA) catheterization. Direct comparison between 1.5 T and 3 T demonstrates that overall and for most vessels, MARC navigation at 3 T was more successful and faster (Table 4).
Table 2.
Navigation according to Vessel Type for 1.5-T MR Imaging versus X-ray Guidance
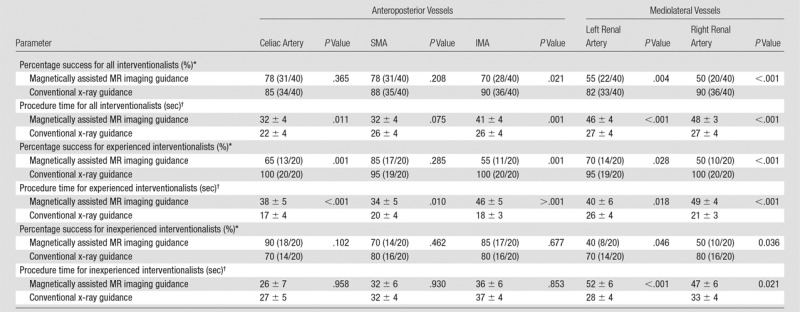
*Data are the percentage of success, with the numerator and denominator in parentheses.
†Procedure time data are presented in seconds as mean ± standard error of the mean.
Table 3.
Navigation according to Vessel Type for 3-T MR Imaging versus X-ray Guidance
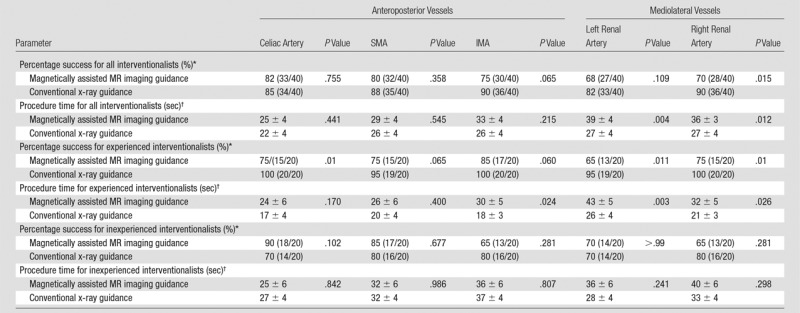
*Data are the percentage of success, with the numerator and denominator in parentheses.
†Procedure time data are presented in seconds as mean ± standard error of the mean.
Table 4.
Navigation Success Rate and Time for 1.5-T versus 3-T MR Imaging
*Data are the percentage of success, with the numerator and denominator in parentheses.
†Procedure time data are presented in seconds as mean ± standard error of the mean.
Discussion
The third-generation dual-axis prototype MARC catheter demonstrates progress toward creating a clinically relevant endovascular catheter that is safe and visible at MR imaging, with augmented steering capabilities. When fully developed, such a system would enable interventionalists to make treatment decisions based on real-time MR imaging–based physiological information (not available with current x-ray guidance), while still navigating as fast as with clinically standard x-ray guidance. This is especially pertinent to the treatment of acute ischemic stroke, since, currently, many treatment decisions are made on the basis of noninvasive imaging (eg, computed tomography [CT], perfusion MR imaging, or diffusion MR imaging) performed before an endovascular intervention is started. Intraprocedurally, x-ray fluoroscopy provides vascular luminography, not real-time tissue-level physiological information, such as diffusion or perfusion. It is currently not ideal to perform multiple flat-panel CT perfusion studies in the angiography suite, given the relatively high doses of x-ray radiation involved and the lack of validation of such techniques. The ability to perform stroke interventions with real-time MR imaging guidance would allow diffusion-weighted imaging sequences to be performed multiple times during an intervention, thus allowing differentiation of viable from nonviable brain tissue at any point during the procedure and therefore helping to guide the interventionalist as to which occluded arteries should be revascularized (to improve blood flow to living brain tissue) and which occluded arteries are more safely left occluded (those supplying dead brain tissue that may be more likely to hemorrhage if reperfused).
Furthermore, radiation from medical imaging has come under increasing scrutiny from the medical community and the lay press. Patients are increasingly concerned about the radiation doses they receive and in some cases may delay or defer nonemergency image-guided procedures, resulting in an overall greater risk to themselves (11–13). Successful development of endovascular catheters for the MR imaging environment would eliminate the risks associated with ionizing radiation to both patient and operator. Although relatively higher doses of x-ray radiation are justifiable during interventions for treatment of severe acute conditions like ischemic stroke and brain aneurysm rupture, many patients undergo multiple catheterization procedures either acutely (eg, to treat cerebral vasospasm after brain aneurysm rupture) (14) or over a lifetime (eg, to follow up previously treated aneurysms). Patients destined for multiple angiograms, as well as young patients who are particularly vulnerable to ionizing radiation, would thus benefit most from MR imaging–guided endovascular interventions made possible by an efficient MR imaging catheterization system.
The development of our third-generation catheter addresses limitations of our previous catheter (7). First, instead of only deflecting in one plane, the double-saddle coil tip now allows deflection in multiple planes. Second, the new catheter is no longer limited by its orientation relative to the main B0 magnetic field of the MR imaging unit. Previously, phantoms had to be placed with the primary axis at 90° to the magnetic field (ie, transverse across the bore of the imaging unit) to produce deflection of a catheter with a solenoidal coil at its tip. The current double-saddle coil catheter produces magnetic moments that create left-right and anterior-posterior deflection, thus permitting phantoms, animals, or eventually human subjects to be placed into the MR imaging unit along the long axis of the bore. Third, laser lithography has permitted further miniaturization of our catheter tips, which is especially important for future applications in the smaller vessels of the brain, liver, and heart. Laser lithography has enabled fabrication of multiple coil tips that are both precise and consistent, allowing predictable catheter tip deflections and equations that describe correlation between applied current and catheter tip movements (15–18).
The MARC system is relatively easy to use. Experienced and inexperienced interventionalists were only given 5 minutes to practice and were able to implement this technology effectively. Previous testing of another magnetic navigation system required up to 6 months of nonclinical training in magnetic navigation prior to participation in a validation study (19). Our results demonstrate that the MARC catheter can be used among interventionalists with a wide range of training experience, which is important when introducing a new technology into clinical practice. Furthermore, our catheter system was successful in an abdominal aortic phantom with diameters and angles of branch vessels that are anatomically relevant.
Although x-ray guidance was faster and more successful than magnetically assisted MR imaging guidance among experienced interventionalists, the latter was still effective, especially at 3 T. Given that experienced interventionalists operate with x-ray navigation every day in clinical practice, it comes as no surprise that they were efficient at using x-ray guidance. Furthermore, to mimic the real-world environment, interventionalists used angled-tip catheters with x-ray guidance, thus making navigation easier compared with using straight catheters (as was done in magnetically assisted experiments).
Navigation with MR imaging was feasible at both 1.5 T and 3 T, and there was a trend toward it being more successful at 3 T. At a higher magnetic field strength, there was better visualization of the catheter tip. Additionally, at a higher magnetic strength, the catheter tip is deflected more strongly for the same amount of current. Use of 3-T MR imaging clinical systems is increasingly popular because of increased signal-to-noise ratio and spatial resolution and quicker imaging times (20). Furthermore, we previously demonstrated experimentally that there is less resonant radiofrequency heating of a variety of endovascular catheters at 3 T compared with 1.5 T (21).
Our study was limited in its statistical power by a small number of operators (a total of four); thus, we cannot claim that MR imaging–guided catheterization is equivalent to x-ray–guided navigation. Limitations of the MARC catheter system included navigating right-left into the renal arteries. This may be secondary to the small diameter of the renal ostia and the 60° oblique angle of the vessels, making navigation more difficult. Furthermore, to navigate into the renal arteries, a thick coronal plane had to be used to visualize the full course of the renal arteries without it running out of the imaging plane. Although this third-generation catheter tip was smaller than our previous catheters (7), susceptibility at the catheter tip during current activation can obscure navigation into smaller vessels. Continued improvement with laser lithography and thin heat shrink will help us further miniaturize the catheters, allowing for future use in smaller vessels, such as cerebral and coronary arteries. Finally, although our double-saddle microcoil tip demonstrated successful navigation, in future clinical practice, interventionalists will likely require a variety of catheters in their armamentarium. Possibilities include catheters with a variety of microcoil tips (solenoid or saddle) with which the interventionalists can change channels to activate each. Alternatively, a variety of different catheters, each with different microcoil tips, can be produced and exchanged (over guide wires safe for use with MR imaging that are currently being developed) (22), as is done in clinical practice with x-ray fluoroscopy.
In conclusion, MR imaging–guided navigation in an abdominal aortic phantom with the third-generation MARC catheter system is feasible at 1.5 T, improves at 3 T, and is comparable to x-ray guidance for a variety of vessels at 3 T. Furthermore, this technology can be used by operators of different skill levels. Future in vivo studies with MR imaging guidance will be needed to evaluate the MARC catheter system in simulated clinical environments, such as ischemic stroke thrombolysis and tumor embolization.
Advances in Knowledge
■ In an abdominal aortic phantom among inexperienced operators, magnetically assisted remote-controlled (MARC) MR imaging guidance was equivalent to x-ray guidance at 1.5 T (67% successful vessel selection turns with MR imaging vs 76% with x-ray guidance, P = .157) and at 3 T (75% with MR imaging vs 76% with x-ray guidance, P = .869).
■ Mean procedure time among the inexperienced group was equivalent by using MR imaging (31 seconds) and x-ray guidance (34 seconds, P = .436).
■ Experienced operators were more successful in catheterizing vessels with x-ray guidance (98% success within 60 seconds) than with 1.5-T (65% success, P < .001) or 3-T (75% success) MR imaging.
■ In the experienced group, catheterization was faster with x-ray guidance (20 seconds) compared with 1.5-T MR guidance (42 seconds, P < .001), but MARC guidance improved at 3 T (31 seconds).
■ MARC MR imaging guidance at 3 T was not significantly different from x-ray guidance for the celiac (P = .755), superior mesenteric (P = .358), and inferior mesenteric (P = .065) arteries.
Acknowledgments
Acknowledgments
We acknowledge the kind help and assistance of Dave Barry, BS, and Andrew Chu, BS, of Penumba for building catheter shafts for these experiments and Cindy Maskeny, RN, of Penumbra for facilitating the transfer of catheter materials. We also thank Vince Malba, PhD, and Lee Evans, BS, of Lawrence Livermore National Laboratory for their insights on coil materials, design, and engineering.
Received November 14, 2014; revision requested December 29; revision received January 23, 2015; accepted February 19; final version accepted April 6.
Funding: This research was supported by the National Institutes of Health (grants T32EB001631 and R01EB012031).
Disclosures of Conflicts of Interest: P.M. disclosed no relevant relationships. P.L. Activities related to the present article: authors received nonfinancial support from Penumbra in the form of the donation of catheter prototypes. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. A.D.L. disclosed no relevant relationships. D.L.C. disclosed no relevant relationships. A.J.M. disclosed no relevant relationships. B.R.H.T. disclosed no relevant relationships. R.L.A. disclosed no relevant relationships. M.S. disclosed no relevant relationships. M.W.W. disclosed no relevant relationships. S.W.H. Activities related to the present article: authors received the provision of substrate catheters from Penumbra, as well as manufacturing assistance for tested catheters. Activities not related to the present article: author received payment from Medina Medical and ChemoFilter for board membership and payment from Silk Road Medical for consultancy; institution received grants from Stryker neurovascular and MicroVention Terumo; institution received payment for patents pending on unrelated technology; author received payment from Penumbra for development of educational presentations; author received payment from Medina Medical and ChemoFilter in the form of stock and/or stock options. Other relationships: disclosed no relevant relationships.
Abbreviations:
- IMA
- inferior mesenteric artery
- MARC
- magnetically assisted remote-controlled
- SMA
- superior mesenteric artery
References
- 1.Bell JA, Saikus CE, Ratnayaka K, et al. A deflectable guiding catheter for real-time MRI-guided interventions. J Magn Reson Imaging 2012;35(4):908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosselin FP, Lalande V, Martel S. Characterization of the deflections of a catheter steered using a magnetic resonance imaging system. Med Phys 2011;38(9):4994–5002. [DOI] [PubMed] [Google Scholar]
- 3.Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J 2013;34(5):380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt EJ, Mallozzi RP, Thiagalingam A, et al. Electroanatomic mapping and radiofrequency ablation of porcine left atria and atrioventricular nodes using magnetic resonance catheter tracking. Circ Arrhythm Electrophysiol 2009;2(6):695–704. [DOI] [PubMed] [Google Scholar]
- 5.Tse Z, Dumoulin C, Watkins R, et al. MRI guided electrophysiological intervention with a voltage-based electro-anatomic mapping system. J Cardiovasc Magn Reson 2012;14(Suppl 1):P206. [Google Scholar]
- 6.Arenson RLH, Hassenzahl WV, Roberts TPLUS. Patent no. 6,304,769 B1. Washington, DC: U.S. Patent and Trademark Office, 2001. [Google Scholar]
- 7.Losey AD, Lillaney P, Martin AJ, et al. Magnetically assisted remote-controlled endovascular catheter for interventional MR imaging: in vitro navigation at 1.5 T versus x-ray fluoroscopy. Radiology 2014;271(3):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillaney P, Malba V, Evans L, et al. Catheters for interventional MR: LaserLathe fabrication of micro-coils for remote catheter tip deflection [abstr]. In: Proceedings of the Twenty-First Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: Internal Society for Magnetic Resonance in Medicine, 2013; 0472. [Google Scholar]
- 9.Sincic RS, Caton CJ, Lillaney P, et al. System architecture for a magnetically guided endovascular microcatheter. Biomed Microdevices 2014;16(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenth RV. Some practical guidelines for effective sample size determination. Am Stat 2001;55(3):187–193. [Google Scholar]
- 11.Hendee WR, O’Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology 2012;264(2):312–321. [DOI] [PubMed] [Google Scholar]
- 12.National Council on Radiation Protection and Measurements . Ionizing Radiation Exposure of the Population of the United States. NCRP Report No. 160. Bethesda, Md: National Council on Radiation Protection and Measurements, 2009. [Google Scholar]
- 13.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 14.Jun P, Ko NU, English JD, et al. Endovascular treatment of medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2010;31(10):1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Settecase F, Sussman MS, Wilson MW, et al. Magnetically-assisted remote control (MARC) steering of endovascular catheters for interventional MRI: a model for deflection and design implications. Med Phys 2007;34(8):3135–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MW, Martin AB, Lillaney P, et al. Magnetic catheter manipulation in the interventional MR imaging environment. J Vasc Interv Radiol 2013;24(6):885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetts SW, Saeed M, Martin A, et al. Magnetically-assisted remote controlled microcatheter tip deflection under magnetic resonance imaging. J Vis Exp 2013;(74). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillaney P, Caton C, Martin AJ, et al. Comparing deflection measurements of a magnetically steerable catheter using optical imaging and MRI. Med Phys 2014;41(2):022305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiemann M, Killmann R, Kleen M, Abolmaali N, Finney J, Vogl TJ. Vascular guide wire navigation with a magnetic guidance system: experimental results in a phantom. Radiology 2004;232(2):475–481. [DOI] [PubMed] [Google Scholar]
- 20.Blamire AM. The technology of MRI—the next 10 years? Br J Radiol 2008;81(968):601–617. [DOI] [PubMed] [Google Scholar]
- 21.Losey AD, Lillaney P, Martin AJ, et al. Safety of retained microcatheters: an evaluation of radiofrequency heating in endovascular microcatheters with nitinol, tungsten, and polyetheretherketone braiding at 1.5 T and 3 T. J Neurointerv Surg 2014;6(4):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidewires Marvis MR. http://www.marvistech.com/technology-products/mr-guidewires.html. Accessed October 20, 2014.