Abstract
Ivabradine (Corlanor) for heart failure: the first selective and specific If inhibitor
INTRODUCTION
Heart failure (HF) is a condition that results when the heart is unable to provide sufficient blood flow to meet the body’s metabolic demands. The current American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines define HF as a complex clinical syndrome that results from structural or functional impairment of ventricular filling or ejection of blood.1
HF is a major public health concern associated with a high prevalence and poor clinical outcomes. In the U.S., approximately 5 million people are affected, and more than 500,000 new cases are diagnosed each year.2,3 HF is the leading cause of hospitalization among adults older than 65 years of age.4 More than 1 million patients are hospitalized each year with a primary diagnosis of HF, accounting for a total Medicare expenditure exceeding $17 billion.4 Despite dramatic improvement in outcomes with medical treatment, admission rates after HF hospitalization remain high, with more than 50% of patients rehospitalized within six months of discharge.4,5
Any condition that leads to an alteration in left ventricular (LV) structure or function can predispose a patient to the development of HF.6 Coronary artery disease (CAD) is the predominant cause of HF.6 Approximately 50% of HF patients have a normal or preserved ejection fraction (EF) of 50% or greater.6 HF patients are broadly categorized into HF with a reduced EF (HFrEF; formerly systolic HF) or HF with a preserved EF (HFpEF; formerly diastolic HF). HFrEF is characterized by decreased myocardial contractility and inadequate emptying of the ventricle, whereas HFpEF is characterized by abnormal ventricular relaxation and impaired ventricular filling.6,7
The cardinal clinical symptoms of HF include dyspnea, fatigue, and signs of volume overload, such as peripheral edema and pulmonary rales.1 The New York Heart Association (NYHA) functional classification scheme (Table 1) is the most commonly used system for assessing the severity of functional limitations in patients with HF.8 Although it is difficult to predict prognosis in an individual, the development of symptomatic HF carries a poor prognosis.6 Patients who experience symptoms during moderate activity (NYHA class II) have an annual mortality rate of 5% to 10%, whereas patients with symptoms at rest (NYHA class IV) have an annual mortality rate of 30% to 70%.6
Table 1.
New York Heart Association (NYHA) Functional Classification8
| Class | Functional Capacity |
|---|---|
| I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, or dyspnea. |
| II | Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, or dyspnea. |
| III | Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea. |
| IV | Unable to perform any physical activities without discomfort. Symptoms of HF at rest. If physical activity is undertaken, discomfort increases. |
Pharmacological treatments of HF include angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), beta blockers, diuretics, aldosterone antagonists, hydralazine/nitrates, and digoxin. After undergoing “fast track” evaluation, ivabradine (Corlanor, Amgen) received Food and Drug Administration (FDA) approval in April 2015.9 Ivabradine is indicated to reduce the risk of hospitalization for worsening HF in patients with stable, symptomatic chronic HF with a left ventricular ejection fraction (LVEF) of 35% or less, who are in sinus rhythm with a resting heart rate of 70 beats per minute (bpm) or greater, and are either receiving maximally tolerated doses of beta blockers or have a contraindication to beta-blocker use.10 Amgen obtained the American rights to ivabradine from Servier, which has been marketing the drug in Europe for approximately 10 years.11
CHEMICAL AND PHYSICAL PROPERTIES
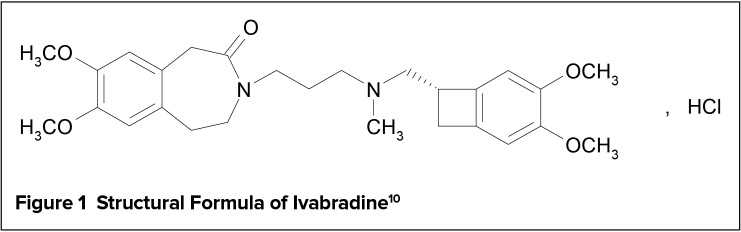
Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blocker with a chemical formula of 3-(3-{[((7S)-3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl] methyl amino} propyl)-1,3,4,5-tetrahydro-7,8dimethoxy-2H-3-benzazepin-2-one, hydrochloride.10 The structural formula of ivabradine is shown in Figure 1. It is formulated in strengths of 5 mg and 7.5 mg of ivabradine as the free drug equivalent.10 Ivabradine 5 mg is available as a salmon-colored, oval-shaped, film-coated tablet, marked with “5” on one face and bisected on the other face. The tablet is scored and can be divided into equal halves to provide a 2.5-mg dose.10 Ivabradine 7.5 mg is available as a salmon-colored, triangular-shaped tablet, debossed with “7.5” on one face and plain on the other face.10 Inactive ingredients include lactose monohydrate, maize starch, maltodextrin, magnesium stearate, colloidal silicon dioxide, hypromellose, titanium dioxide, glycerol, magnesium stearate, polyethylene glycol 6000, yellow iron oxide, and red iron oxide.10
Figure 1.
Structural Formula of Ivabradine10
MECHANISM OF ACTION
Ivabradine is a heart-rate-lowering agent that acts by selectively and specifically inhibiting the cardiac pacemaker current (If), a mixed sodium-potassium inward current that controls the spontaneous diastolic depolarization in the sinoatrial (SA) node and hence regulates the heart rate.12,13 The molecular channel belongs to the HCN family.10 Inhibition of this channel disrupts If ion current flow, thereby prolonging diastolic depolarization, slowing firing in the SA node, and ultimately reducing the heart rate.14 The cardiac effects of ivabradine are specific to the SA node, and the drug has no effect on blood pressure, intracardiac conduction, myocardial contractility, or ventricular repolarization.10,13
Ivabradine also inhibits the retinal current (Ih), which has properties similar to that of cardiac If.15 Ih participates in the temporal resolution of the visual system by curtailing retinal responses to bright light stimuli.15 Under triggering circumstances, such as rapid changes in luminosity, partial inhibition of Ih may underlie the luminous phenomena (phosphenes) experienced by patients.10
PHARMACODYNAMICS
Ivabradine causes a dose-dependent reduction in heart rate. The magnitude of this reduction depends on the baseline heart rate; i.e., a greater reduction in heart rate occurs in subjects with a higher heart rate at baseline. At recommended doses, the reduction in heart rate is approximately 10 bpm whether the patient is at rest or exercising. The heart rate decreases almost linearly with increasing doses of ivabradine up to 15 mg to 20 mg twice daily. At higher doses, this effect has a tendency to plateau.10
Ivabradine does not exert negative inotropic effects. It increases the uncorrected QT interval with heart-rate slowing but does not prolong the corrected QT (QTc) interval.10
PHARMACOKINETICS
Ivabradine exhibits linear pharmacokinetics over an oral dosing range of 0.5 mg to 24 mg. After oral administration, the drug is rapidly and almost completely absorbed from the gastrointestinal tract. Peak plasma concentrations (Cmax) are reached in approximately one hour under fasting conditions. The absolute oral bioavailability of ivabradine is approximately 40% because of the first-pass effect in the liver and intestines. Food delays the absorption of ivabradine by approximately one hour and increases plasma exposure by 20% to 40%.10
Ivabradine is approximately 70% plasma protein-bound, and the volume of distribution at steady state is approximately 100 L.10
Ivabradine is metabolized predominantly in the liver and intestines by the cytochrome P450 (CYP) 3A4 enzyme. Therefore, potent inhibitors or inducers of CYP3A4 may have a significant effect on plasma concentrations of ivabradine. The major active metabolite is N-desmethyl ivabradine (S-18982), which circulates at concentrations of approximately 40% and is also metabolized by CYP3A4. Ivabradine has a distribution half-life of two hours and an effective half-life of approximately six hours.10
The total clearance of ivabradine is 24 L/h, and the renal clearance is approximately 4.2 L/h. Approximately 4% of an oral dose is excreted unchanged in urine. The excretion of metabolites occurs to similar extents via feces and urine.10
DOSAGE AND ADMINISTRATION
The recommended starting dosage of ivabradine is 5 mg twice daily, administered with food. After two weeks, the patient should be assessed, and dose adjustments should be made to achieve a resting heart rate of between 50 bpm and 60 bpm (Table 2). Thereafter, further dose adjustments (if necessary) should be based on the patient’s resting heart rate and tolerability. The maximum dosage of ivabradine is 7.5 mg twice daily.10
Table 2.
Dose Adjustments of Ivabradine Based on Resting Heart Rate10
| Heart Rate | Dose Adjustment |
|---|---|
| Greater than 60 bpm | Increase dosage by 2.5 mg twice daily up to a maximum dosage of 7.5 mg twice daily. |
| 50 to 60 bpm | Maintain dosage. |
| Less than 50 bpm or signs and symptoms of bradycardia | Decrease dosage by 2.5 mg twice daily; if current dosage is 2.5 mg twice daily, discontinue therapy. |
bpm = beats per minute
Patients with a history of conduction defects or those in whom bradycardia could lead to hemodynamic compromise should be started at a dosage of 2.5 mg twice daily before the dose is increased based on heart rate. An initial dosage of 2.5 twice daily should also be considered in elderly patients (75 years of age or older).10
No dose adjustments are necessary in patients with mild-to-moderate hepatic impairment, but the use of ivabradine is contraindicated in patients with severe hepatic impairment. Although the effects of severe hepatic impairment on ivabradine metabolism have not been studied, increased systemic exposure is anticipated in these patients. No dose adjustments are required in patients with creatinine clearance values of 15 mL/min or greater. However, caution is advised in patients with severe renal impairment or end-stage renal failure.10
CLINICAL TRIALS
The safety and efficacy of ivabradine were assessed in three pivotal placebo-controlled studies: SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial); BEAUTIFUL (Morbidity-Mortality Evaluation of the If Inhibitor Ivabradine in Patients With Coronary Artery Disease and Left Ventricular Dys-function); and SIGNIFY (Study Assessing the Morbidity-Mortality Benefits of the If Inhibitor Ivabradine in Patients With Coronary Artery Disease).16–19
The SHIFT study enrolled patients with chronic HF and LV systolic dysfunction; the BEAUTIFUL study enrolled patients with both stable CAD and LV systolic dys-function; and the SIGNIFY study enrolled patients with stable CAD without overt HF and LV systolic dysfunction.17–19 The results of these trials demonstrated that an elevated heart rate is an important risk factor for ventricular remodeling in HF.16
SHIFT Trial
The randomized, double-blind, placebo-controlled SHIFT study was conducted to determine whether the addition of ivabradine to optimal medical therapy could reduce the occurrence of cardiovascular outcomes in patients with chronic HF and LV systolic dysfunction.17
Men and women 18 years of age or older who were in sinus rhythm and had resting heart rates of 70 bpm or greater, with stable symptomatic chronic HF (NYHA class II–IV) of four or more weeks’ duration, a previous admission to a hospital for worsening HF within the previous 12 months, and an LVEF of 35% or less were eligible for enrollment. Any cause of HF was allowed apart from congenital heart disease or primary severe valvular disease. The enrolled patients had to be on an optimized and stable clinical regimen for at least four weeks. The main exclusion criteria were myocardial infarction (MI) within the previous two months, atrioventricular (AV) pacing that was operative for 40% or more of the day, atrial fibrillation or flutter, and symptomatic hypotension.17
A total of 6,605 patients were enrolled. At baseline, approximately 49% of the patients were NYHA class II, 50% were NYHA class III, and 2% were NYHA class IV. HF was caused by ischemia in 68% of the patients. The mean LVEF was 29%. Most of the patients (89%) were taking beta blockers, with 26% receiving guideline-defined target daily doses. Most of the patients were also taking ACE inhibitors and/or ARBs (91%), diuretics (83%), and aldosterone antagonists (60%). Few of the patients had an implantable cardioverter-defibrillator or a cardiac resynchronization therapy device (3.2% and 1.1%, respectively).17
The patients received either ivabradine (n = 3,241) or placebo (n = 3,264). The starting dosage in both groups was 5 mg twice daily. This dosage was increased to 7.5 mg twice daily or decreased to 2.5 mg twice daily to maintain the resting heart rate at between 50 bpm and 60 bpm, as tolerated. The study’s primary endpoint was the composite of hospitalization for worsening HF or cardiovascular death. The median follow-up period was 22.9 months.17
The withdrawal rates were 21% in the ivabradine group and 19% in the placebo group (P = 0.017), although significantly fewer serious adverse events occurred among the ivabradine-treated patients than among those given placebo (P = 0.025). Bradycardia (both symptomatic and asymptomatic) also occurred more often in the ivabradine group than in the placebo group (P < 0.0001).17
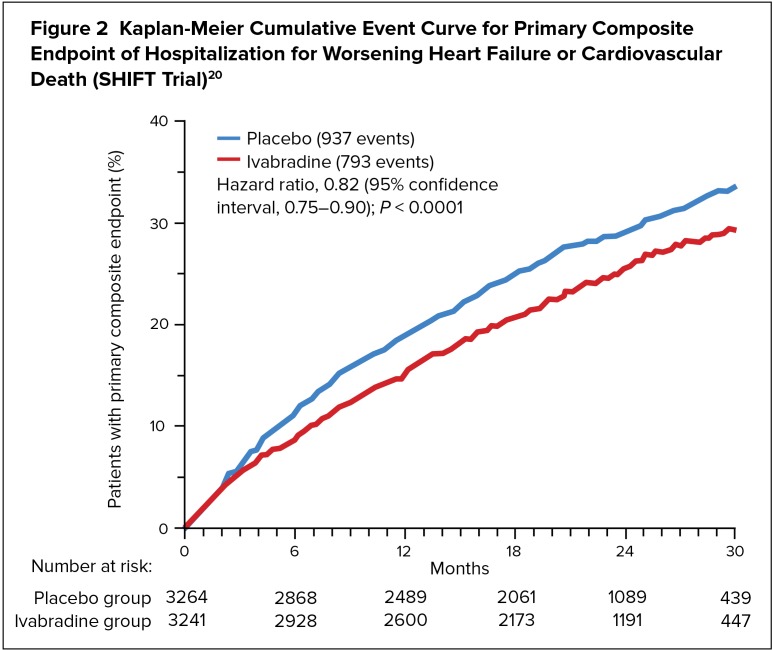
Ivabradine reduced the risk of the combined endpoint of hospitalization for worsening HF or cardiovascular death based on a time-to-event analysis (Figure 2). The primary endpoint was reached in 24% of the ivabradine group and in 29% of the placebo group (P < 0.0001). The treatment effect was driven mainly by hospital admissions for worsening HF, which occurred in 16% of ivabradine-treated patients and in 21% of those given placebo. Patients with heart rates higher than the median were at increased risk of an event and received greater event-reducing benefit from ivabradine than those with heart rates lower than the median. The rate of cardiovascular deaths was not significantly reduced in the ivabradine group, but the death rate due to HF dropped significantly (P = 0.014). All-cause hospital admissions were significantly reduced with ivabradine (P = 0.003), whereas all-cause deaths did not differ between the two groups.17
Figure 2.
Kaplan-Meier Cumulative Event Curve for Primary Composite Endpoint of Hospitalization for Worsening Heart Failure or Cardiovascular Death (SHIFT Trial)20
BEAUTIFUL Trial
BEAUTIFUL was a randomized, double-blind, placebo-controlled study designed to determine whether lowering the heart rate with ivabradine could reduce cardiovascular morbidity and death in patients with CAD and LV systolic dysfunction.18
Eligible patients were 55 years of age or older (18 years of age or older if diabetic) with CAD, an LVEF of less than 40%, and a resting heart rate of 60 bpm or greater. All of the patients had stable symptoms of HF and/or angina for at least three months and had received conventional cardiovascular medications at stable doses for at least one month.18
At baseline, most of the patients were NYHA class II (61.4%) or class III (23.2%), and none was class IV. In addition, most of the patients (87%) were receiving beta blockers in addition to study drugs.18
A total of 10,917 patients were randomly assigned to receive either ivabradine (n = 5,479) or placebo (n = 5,438) at an initial dosage of 5 mg twice daily, which was increased to the target dosage of 7.5 mg twice daily depending on the patient’s resting heart rate and tolerability. The study’s primary endpoint was a composite of hospitalization for acute MI, hospitalization for new-onset or worsening HF, or cardiovascular death. The median follow-up period was 19 months.18
Ivabradine did not significantly reduce the study’s primary composite endpoint. However, in a prespecified subgroup of patients with heart rates of 70 bpm or greater, ivabradine significantly reduced hospital admissions for fatal or nonfatal MI (P = 0.001) and coronary revascularization (P = 0.016) (secondary endpoints).18
SIGNIFY Trial
SIGNIFY was a randomized, double-blind, placebo-controlled study designed to determine whether treatment with ivabradine could reduce cardiovascular events and mortality in patients with stable CAD but without clinically evident HF (NYHA class I). Eligible patients were 55 years of age or older with stable CAD, an LVEF of more than 40%, a baseline heart rate of 70 bpm or greater, and at least one additional cardiovascular risk factor. Eligible patients were also receiving optimized background treatment for stable CAD.19
A total of 19,102 patients were randomly assigned to receive ivabradine (n = 9,550) or placebo (n = 9,552). The patients were started at an initial dosage of 7.5 mg twice daily, except for those 75 years of age or older, who were started at 5 mg twice daily. Treatment was titrated to as much as 10 mg twice daily or reduced to 5 mg twice daily to achieve a target heart rate of 60 bpm. The study’s primary endpoint was a composite of nonfatal MI or cardiovascular death. The median follow-up period was 27.8 months.19
Among patients who had stable CAD without clinical HF, ivabradine did not significantly reduce the primary composite endpoint, but there were significant differences between ivabradine and placebo in the incidence of death from cardiovascular causes and nonfatal MI. Ivabradine was associated with a significant (P = 0.02) increase in the incidence of the primary endpoint among patients with activity-limiting angina but not among those without activity-limiting angina. The incidence of bradycardia was higher with ivabradine than with placebo (18% versus 2.3%; P < 0.001).19
DRUG INTERACTIONS
CYP-Based Interactions
Ivabradine is primarily metabolized by CYP3A4. The concomitant use of CYP3A4 inhibitors increases ivabradine plasma concentrations, which may exacerbate bradycardia and conduction disturbances. Potent CYP3A4 inhibitors that are contra-indicated when using ivabradine include azole antifungals (e.g., itraconazole), macrolide antibiotics (e.g., clarithromycin and telithromycin), human immuno-deficiency virus (HIV) protease inhibitors (e.g., nelfinavir), and nefazodone. Moderate CYP3A4 inhibitors that should be avoided include diltiazem, verapamil, and grapefruit juice. Moreover, concomitant use of CYP3A4 inducers reduces ivabradine plasma concentrations and may require clinicians to increase the dose of ivabradine. The concomitant use of CYP3A4 inducers, such as St. John’s wort, rifampicin, barbiturates, and phenytoin, should be restricted.10
Negative Chronotropes
The concomitant use of drugs that slow the heart rate (e.g., digoxin, amiodarone, and beta blockers) may enhance the bradycardic effect of ivabradine. It is important to monitor the heart rate in patients receiving ivabradine along with other negative chronotropes.10
Interaction With Pacemakers
Ivabradine acts by reducing the heart rate to a target of 50 to 60 bpm. Therefore, the use of ivabradine is not recommended in patients with demand pacemakers set to a rate of 60 bpm or greater.10
CONTRAINDICATIONS
Ivabradine is contraindicated in patients with acute decompensated HF, severe hepatic impairment, blood pressure below 90/50 mm Hg, a resting heart rate below 60 bpm prior to treatment, or pacemaker dependence (i.e., the patient’s heart rate is maintained solely by the pacemaker). In addition, ivabradine is contraindicated in patients with sick sinus syndrome, sinoatrial block, or third-degree AV block unless a functioning demand pacemaker is present. As noted previously, the concomitant use of ivabradine and potent CYP3A4 inhibitors is also contraindicated.10
ADVERSE EVENTS
In the SHIFT study, the most common adverse events included bradycardia (10% for ivabradine versus 2.2% for placebo), hypertension (8.9% versus 7.8%), atrial fibrillation (8.3% versus 6.6%), and phosphenes (2.8% versus 0.5%). The following adverse events have been reported during post-approval use: syncope, hypo-tension, angioedema, erythema, rash, pruritus, urticaria, vertigo, diplopia, and visual impairment.10
WARNINGS AND PRECAUTIONS
Fetal Toxicity
Based on findings in animal studies, ivabradine may cause fetal toxicity when administered to pregnant women. The FDA-required medication guide for ivabradine recommends that women of reproductive potential use effective contraception when taking ivabradine. Women who require treatment during pregnancy should be closely monitored, especially during the first trimester, for destabilization of HF, which could potentially result from slowing of the heart rate caused by ivabradine. Pregnant women with chronic HF should also be monitored for preterm birth.10
Atrial Fibrillation
Ivabradine increases the risk of atrial fibrillation. In the SHIFT study, the rate of atrial fibrillation was 5.0% in patients treated with ivabradine compared with 3.9% in those given placebo.17 Clinicians must regularly monitor cardiac rhythm during treatment with ivabradine, and the drug should be discontinued if atrial fibrillation develops.10
Bradycardia and Conduction Disturbances
Ivabradine may cause bradycardia, sinus arrest, and heart block. Risk factors for bradycardia include sinus node dysfunction, conduction defects (e.g., first- or second-degree AV block or bundle branch block), ventricular dys-synchrony, and the use of other negative chronotropes, such as digoxin, diltiazem, verapamil, and amiodarone. The concurrent use of verapamil or diltiazem will increase ivabradine exposure, and these drugs may contribute to lowering of the heart rate. For these reasons, verapamil and diltiazem should be avoided. The use of ivabradine in patients with second-degree AV block is also contraindicated, unless a functioning demand pacemaker is present.10
Visual Function
Phosphenes (transiently enhanced brightness in a limited area of the field, halos, image decomposition, colored bright lights, or multiple images) may occur during treatment with ivabradine. These phenomena are usually triggered by sudden variations in light intensity. Phosphenes typically appear within the first two months of therapy. Most cases are mild to moderate in severity and usually resolve spontaneously during or after treatment.10
P&T CONSIDERATIONS
Ivabradine is dispensed with an FDA-approved patient medication guide.10 The average wholesale price is $450 for one month of therapy.21
Because a critical unmet need remains for patients who do not respond adequately to current HF therapies, ivabradine offers an attractive alternative for lowering the heart rate. In view of the established clinical efficacy of ivabradine and its favorable safety profile, pure heart-rate lowering via If inhibition with this agent appears to offer a feasible means to reduce HF hospitalizations and readmissions.
CONCLUSION
The resting heart rate plays a pivotal role in the pathophysiology of HF. Until recently, the two main types of heart-rate-limiting agents were beta blockers and nondihydropyridine calcium-channel blockers. Ivabradine is the only agent shown to clinically lower the heart rate without negative inotropism or effects on conduction and contractility.22,23 As a result, ivabradine is not associated with the adverse events typically encountered with other bradycardic agents.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somberg JC, Molnar J. The pharmacologic treatment of heart failure. Am J Ther. 2004;11:480–488. doi: 10.1097/01.mjt.0000094281.81391.7f. [DOI] [PubMed] [Google Scholar]
- 4.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Torgdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 6.Mann DL, Chakinala M. Heart failure: pathophysiology and diagnosis. In: Kasper D, Fauci A, Hauser S, et al., editors. Harrison’s Principles of Internal Medicine. 19th ed. New York: McGraw-Hill; 2015. Available at: http://accessmedicine.mhmedical.com.jerome.stjohns.edu:81/content.aspx?bookid=1130&Sectionid=79742466. Accessed April 23, 2015. [Google Scholar]
- 7.Bhuiyan T, Maurer MS. Heart failure with preserved ejection fraction: persistent diagnosis, therapeutic enigma. Curr Cardiovasc Risk Rep. 2011;5:440–449. doi: 10.1007/s12170-011-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Heart Association Classes of heart failure. Sep 30, 2015. Available at: http://www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp. Accessed April 24, 2015.
- 9.Food and Drug Administration FDA approves Corlanor to treat heart failure. Apr 15, 2015. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm442978.htm. Accessed April 24, 2015.
- 10.Corlanor (ivabradine tablets) prescribing information. Thousand Oaks, California: Amgen; Apr, 2015. Available at: http://pi.amgen.com/united_states/corlanor/corlanor_pi.pdf. Accessed April 24, 2015. [Google Scholar]
- 11.Amgen FDA grants Amgen priority review designation for ivabradine for the treatment of chronic heart failure. Aug 27, 2014. Available at: http://www.amgen.com/media/media_pr_detail.jsp?releaseID=1961392. Accessed April 25, 2015.
- 12.DiFrancesco D, Camm JA. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–1765. doi: 10.2165/00003495-200464160-00003. [DOI] [PubMed] [Google Scholar]
- 13.Sulfi S, Timmis AD. Ivabradine: the first selective sinus node I(f) channel inhibitor in the treatment of stable angina. Int J Clin Pract. 2006;60:222–228. doi: 10.1111/j.1742-1241.2006.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nawarskas JJ, Bowman BN, Anderson JR. Ivabradine: a unique and intriguing medication for treating cardiovascular disease. Cardio Rev. 2015;23:201–211. doi: 10.1097/CRD.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 15.Urbanek I, Kaczmarek K, Cygankiewicz I, et al. Risk-benefit assessment of ivabradine in the treatment of chronic heart failure. Drug Healthc Patient Saf. 2014;6:47–54. doi: 10.2147/DHPS.S43275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari R, Fox K. The role of heart rate may differ according to pathophysiology setting: from SHIFT to SIGNIFY. Eur Heart J. 2015;36:2042–2046. doi: 10.1093/eurheartj/ehv150. [DOI] [PubMed] [Google Scholar]
- 17.Swedberg K, Komajda M, Böhn M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 18.Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dys-function (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 19.Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 20.Amgen Ivabridine clinical fact sheet. Available at: http://www.corlanorhcp.com/_pdf/IvabradineClinicalFactSheet.pdf. Accessed October 29, 2015.
- 21.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; 2015. Available at: http://sites.truvenhealth.com/red-book/index.html. Accessed November 16, 2015. [Google Scholar]
- 22.Rushworth GF, Lambrakis P, Leslie SJ. Ivabradine: a new rate-limiting therapy for coronary artery disease and heart failure. Ther Adv Drug Saf. 2011;2:19–28. doi: 10.1177/2042098610393209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lupi A, Rognoni A, Cavallino C, et al. Ivabradine for treatment of coronary artery disease: from last chance resort to mainstem of a reasoned therapy. Cardiovasc Hematol Agents Med Chem. 2015;13:4–9. doi: 10.2174/1871525713666141218162102. [DOI] [PubMed] [Google Scholar]