The power of traditional lesion mapping is limited when symptoms reflect network dysfunction. Boes et al. present a novel method that leverages normative human connectome data to link symptoms to lesion-associated networks. They validate the method in four lesion syndromes, linking subcortical lesions to cortical regions implicated in symptom expression.
Keywords: lesion network mapping, lesion networks, hallucination, central post-stroke pain, subcortical aphasia
The power of traditional lesion mapping is limited when symptoms reflect network dysfunction. Boes et al. present a novel method that leverages normative human connectome data to link symptoms to lesion-associated networks. They validate the method in four lesion syndromes, linking subcortical lesions to cortical regions implicated in symptom expression.
Abstract
A traditional and widely used approach for linking neurological symptoms to specific brain regions involves identifying overlap in lesion location across patients with similar symptoms, termed lesion mapping. This approach is powerful and broadly applicable, but has limitations when symptoms do not localize to a single region or stem from dysfunction in regions connected to the lesion site rather than the site itself. A newer approach sensitive to such network effects involves functional neuroimaging of patients, but this requires specialized brain scans beyond routine clinical data, making it less versatile and difficult to apply when symptoms are rare or transient. In this article we show that the traditional approach to lesion mapping can be expanded to incorporate network effects into symptom localization without the need for specialized neuroimaging of patients. Our approach involves three steps: (i) transferring the three-dimensional volume of a brain lesion onto a reference brain; (ii) assessing the intrinsic functional connectivity of the lesion volume with the rest of the brain using normative connectome data; and (iii) overlapping lesion-associated networks to identify regions common to a clinical syndrome. We first tested our approach in peduncular hallucinosis, a syndrome of visual hallucinations following subcortical lesions long hypothesized to be due to network effects on extrastriate visual cortex. While the lesions themselves were heterogeneously distributed with little overlap in lesion location, 22 of 23 lesions were negatively correlated with extrastriate visual cortex. This network overlap was specific compared to other subcortical lesions (P < 10−5) and relative to other cortical regions (P < 0.01). Next, we tested for generalizability of our technique by applying it to three additional lesion syndromes: central post-stroke pain, auditory hallucinosis, and subcortical aphasia. In each syndrome, heterogeneous lesions that themselves had little overlap showed significant network overlap in cortical areas previously implicated in symptom expression (P < 10−4). These results suggest that (i) heterogeneous lesions producing similar symptoms share functional connectivity to specific brain regions involved in symptom expression; and (ii) publically available human connectome data can be used to incorporate these network effects into traditional lesion mapping approaches. Because the current technique requires no specialized imaging of patients it may prove a versatile and broadly applicable approach for localizing neurological symptoms in the setting of brain lesions.
Introduction
There is a long tradition of understanding regional brain function by studying deficits that result from focal brain injury. If patients with similar symptoms have lesions that overlap in a specific brain region, one gains insight into the functional role of that region. Because this lesion mapping approach requires only a record of patient symptoms and the location of the lesion, it has proven broadly applicable across many neurological and psychiatric symptoms (Damasio and Damasio, 1989; Robinson, 1997; Ferro et al., 2010). Methodological improvements using statistics to identify critical sites of lesion overlap have further enhanced the utility of this approach (Frank et al., 1997; Bates et al., 2003; Rorden et al., 2007; Mah et al., 2014). However, traditional approaches to lesion mapping are limited by two important factors. First, similar symptoms may result from lesions in different locations, making localization to specific regions challenging (Chung et al., 2004; Vuilleumier, 2013; Corbetta et al., 2015). Second, symptoms may result from lesion-induced functional alterations in anatomically intact, connected brain regions (Feeney and Baron, 1986; He et al., 2007a; Honey and Sporns, 2008; Bartolomeo, 2011; Catani et al., 2012; Carrera and Tononi, 2014). The fact that lesions have remote functional effects has been appreciated for over a century (Brown-Sequard, 1875; Von Monakow and Harris, 1914); however, it has remained unclear how one might incorporate such effects into traditional lesion mapping.
One solution for localizing neurological symptoms that incorporates such network effects is to perform functional imaging on patient cohorts with brain lesions. This approach has firmly established the importance of remote network effects in symptom expression (Baron et al., 1986; Vuilleumier et al., 2004; Corbetta et al., 2005; Sobesky et al., 2005; He et al., 2007b; Kim et al., 2012) and recovery of function (Saur et al., 2006; Carter et al., 2012; Dijkhuizen et al., 2014). Unfortunately this requires specialized functional neuroimaging scans, which are not routinely collected for clinical purposes. Obtaining such data is especially difficult for rare symptoms, transient symptoms, or conditions that render brain scanning difficult. As such, many studies of lesion-induced neurological symptoms continue to rely solely on the site of the lesion for symptom localization (Corbetta et al., 2015).
In this article we determine whether one can incorporate the network effects of brain lesions into traditional lesion mapping without the need for specialized brain imaging of patients. Our method, termed lesion network mapping, leverages normative human connectome data to identify the distribution of regions likely to be functionally affected by a given brain lesion. For each lesion, a lesion-derived network is identified using resting state functional connectivity MRI, which examines correlations in spontaneous, low frequency fluctuations of the blood oxygen level-dependent signal (Fox and Raichle, 2007). In contrast to previous studies that collected resting state functional connectivity MRI in patients with brain lesions (He et al., 2007b; Carter et al., 2010; Wang et al., 2010; Park et al., 2011), we use a large normative resting state functional connectivity MRI database to identify regions likely to be affected by a brain lesion, without the need for specialized imaging of the patients.
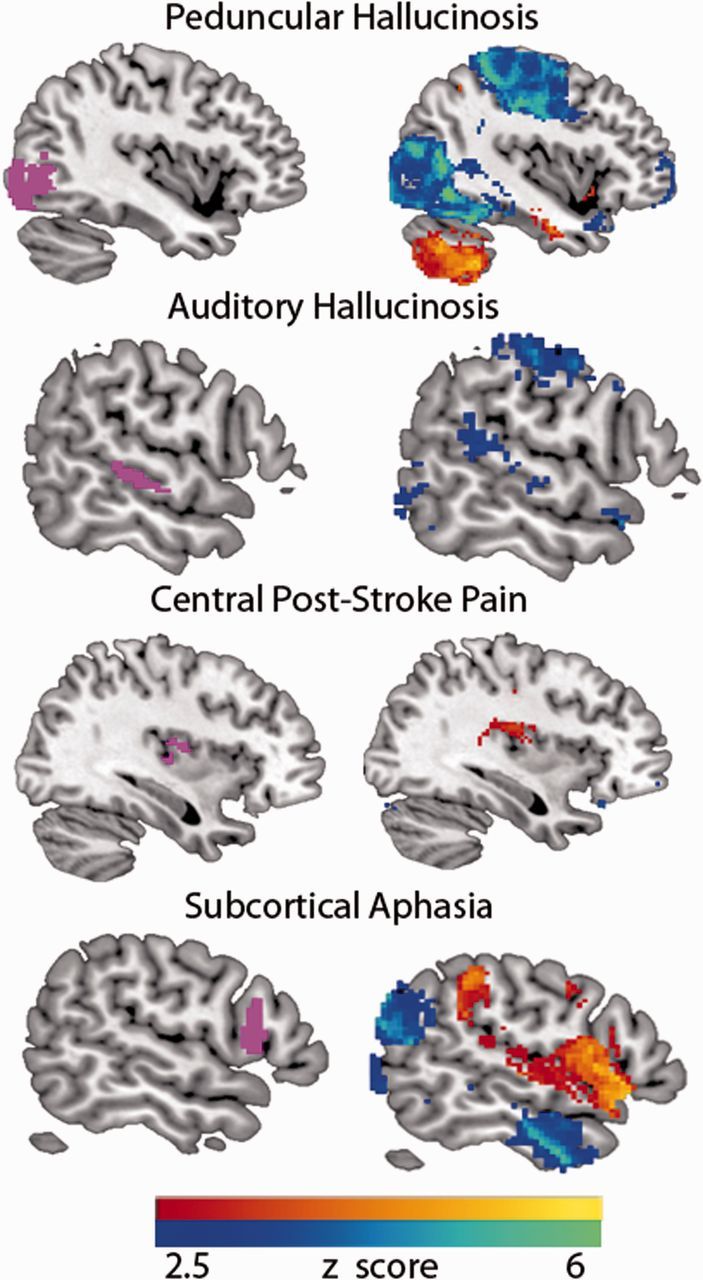
To demonstrate the utility of the approach we test two main hypotheses: (i) lesions that cause similar symptoms but occur in different locations will show overlap in network connectivity; and (ii) sites of network overlap will occur specifically in regions implicated in symptom expression. We start with peduncular hallucinosis, a neurological syndrome in which lesion-induced network effects are thought to play a pivotal role in generating symptoms. Peduncular hallucinosis is characterized by vivid, dynamic, well-formed visual hallucinations following a lesion to the pons, midbrain, or thalamus (Lhermitte, 1922; De Morsier, 1935; McKee et al., 1990; Risser and Powell, 1993; Manford and Andermann, 1998; Mocellin et al., 2006; Benke, 2006). Why visual hallucinations result from these lesions in non-visual structures remains unknown, but a ‘release’ of cortical activity in the extrastriate visual cortex, a region active during visual hallucinations, is thought to occur (Dunn et al., 1983; Asaad and Shapiro, 1986; Ffytche et al., 1998; Manford and Andermann, 1998; Adachi et al., 2000; Vetrugno et al., 2009). Peduncular hallucinosis thus serves as an ideal test of lesion network mapping. Specifically, lesion localization is heterogeneous, symptoms are hypothesized to result from distributed network effects, and there is a clear a priori hypothesis regarding what remote site should be involved in symptom generation.
A priori hypotheses regarding sites of remote network effects in other stroke syndromes are not as clear. However, utility outside peduncular hallucinosis is required to show that our technique is broadly applicable. We therefore identified three additional syndromes in which reasonable predictions regarding network effects could be made: auditory hallucinosis, with network effects in the superior temporal gyrus (Griffiths, 2000; Allen et al., 2008; Kumar et al., 2014), central post-stroke pain, with network effects in the posterior insula (Garcia-Larrea, 2012; Garcia-Larrea and Peyron, 2013), and subcortical expressive aphasia, with network effects in Broca’s area (Nadeau and Crosson, 1997; Crosson, 2013).
Materials and methods
We focus here on the methodological details for the analysis of peduncular hallucinosis and provide details for the other stroke syndromes in the online Supplementary material.
Cases of peduncular hallucinosis were identified from either local cases seen by the authors or from the existing literature. Consent was obtained for the local cases according to the Declaration of Helsinki and the study was approved by the Partners Human Subjects Institutional Review Board. Cases from the literature were identified through a systematic search of pubmed.org with search terms of ‘peduncular hallucinosis’ or ‘Lhermitte’s hallucinosis’, and citations from each selected article were cross-referenced. The search was performed in August 2012 and limited to articles in English, although an exception was made for the historical French articles (Lhermitte, 1922; Van Bogaert, 1927). Inclusion criteria included patients with predominantly visual hallucinations presumed to have been caused by a focal intraparenchymal lesion restricted to the brainstem or diencephalon, as demonstrated by imaging or anatomic examination. Exclusion criteria included: (i) co-occurring cortical lesions; (ii) lesions of the direct visual pathway; (iii) extrinsic compression injuries without a clearly delineated intra-parenchymal lesion; (iv) the presence of obvious competing aetiologies for the hallucinations (e.g. a patient with comorbid psychosis or prior hallucinations from psychiatric disease, alcoholism, drug abuse or a suspected pharmacologic or metabolic cause); or (v) poor image resolution such that lesion boundaries could not be delineated.
We found 23 cases of peduncular hallucinosis with identifiable causative brain lesions (mean age 61 ± 19 years, range 17–85). This included three original cases from our centre and 20 cases from the existing literature. Details of these cases are provided in Supplementary Table 1 and Supplementary Fig. 1.
Brain lesions were mapped by hand onto a standard template brain from FSL (MNI152 brain, 1 mm × 1 mm, http://fsl.fmrib.ox.ac.uk/fsldownloads/) using lesion mapping software (MRIcron http://www.mccauslandcenter.sc.edu/mricro/mricron/). Lesions from local cases were mapped in 3D using simultaneous axial, coronal and sagittal views. Lesions from published figures were traced in the 2D plane(s) in which they were displayed, using neuroanatomical landmarks to accurately transfer the lesion location onto the template brain. To identify areas of lesion overlap, 2D lesions from figures were extended by 2 mm perpendicular to the plane in which they were displayed to more closely approximate natural 3D lesion contours. A 2 mm extension was selected because it can easily be replicated by others and it conservatively balances the risk of creating spurious sites of overlap versus missing sites of overlap relative to the actual 3D lesion shape. A more liberal lesion extension of 4 mm was also included for comparison. All lesions were mapped true to their laterality and areas of overlap were displayed using MRIcron.
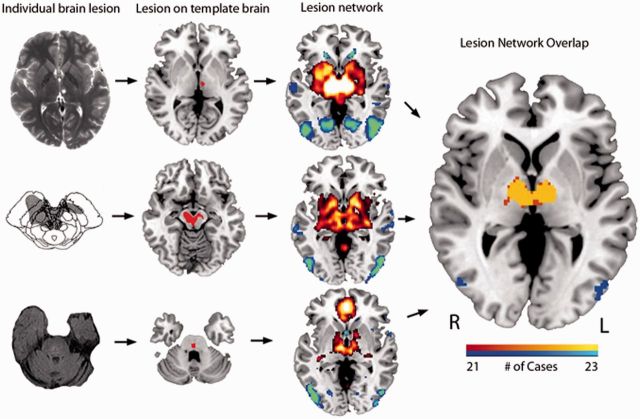
Investigation of the networks associated with peduncular hallucinosis lesions involved three steps: (i) the volume of each of the 23 lesions was transferred to a reference brain; (ii) the lesion volume was used as a seed region of interest in a resting state functional connectivity MRI analysis that used normative data; and (iii) the resulting network associated with each lesion volume was thresholded and overlaid across lesions to identify common sites of network overlap (Fig. 1).
Figure 1.
Lesion network mapping method. Twenty-three lesions resulting in peduncular hallucinosis were identified, three of which are illustrated here (column 1) and mapped to a reference brain (column 2). The brain network associated with each lesion was identified using resting state functional connectivity from a large cohort of normal subjects (column 3). Positive correlations with the lesion are shown in hot colours while negative correlations (anticorrelations) are shown in cool colours. These lesion-based networks were overlapped to identify networks common to at least 21 of 23 lesions (right). The image in column 1 row 2 was reprinted with permission from John Wiley & Sons.
For Step 2 the full 3D lesion location was used as the seed region of interest for local cases, while a 2D slice or slices (i.e. non-expanded) were used for the previously published peduncular hallucinosis cases. The blood oxygen level-dependent signal for each lesion was an average of all voxels contained in the lesion volume. The resting state functional connectivity MRI data set included 98 healthy right-handed subjects (48 male subjects, age 22 ± 3.2 years), part of a larger publically available data set (Buckner et al., 2014). Full methodological and processing details for the normative resting state functional connectivity MRI data set are available (Fox et al., 2012). Briefly, subjects completed one or more resting state functional connectivity MRI scans during which they were asked to rest in the scanner with their eyes open. Resting state functional connectivity MRI data were processed in accordance with the strategy of Fox et al. (2005) as implemented in Van Dijk et al. (2010), including global signal regression (Fox et al., 2009a).
Each of the 23 individual lesion-seeded resting state functional connectivity MRI network maps was thresholded at a t-value of ±4.25 (P < 0.00005, uncorrected) (Fox et al., 2012). After applying this statistical threshold, the resulting 23 binarized resting state functional connectivity MRI network maps were overlapped to identify regions of shared positive and negative correlation, masked using a whole-brain template.
A priori region of interest
An a priori region of interest covering the predicted location of network overlap in peduncular hallucinosis was selected from the Harvard Oxford Atlas distributed with FSL (lateral occipital cortex, inferior division, threshold of 50) (Desikan et al., 2006). This region was selected because it provided the best fit for the coordinates and Brodmann areas previously identified in the generation of release hallucinations (Ffytche et al., 1998; Adachi et al., 2000; Kazui et al., 2009; Vetrugno et al., 2009).
Anatomical specificity and statistical analysis
In addition to identifying sites of network overlap we also sought to determine if lesion-based network results were specific to the actual lesion locations and not due to limitations in functional MRI spatial resolution, such that any subcortical lesion in the brainstem or thalamus could produce similar findings. To address this question we repeated the lesion network mapping with the same 23 peduncular hallucinosis lesion masks in terms of volume, but randomized the location to anywhere within the brainstem or thalamus, repeated on 100 iterations. The inter-lesion distance and degree of lesion overlap was kept similar to that of the original lesions (lesion overlap of 6 ± 2). Lesion volume was converted to a cube with automated morphing of the lesion shape to ensure that all voxels fell within the brainstem/thalamus mask.
Network results from the actual lesions were compared to that of the randomized lesions using a voxel-wise Liebermeister test (Rorden et al., 2007). This statistical approach is commonly referred to as voxel-based lesion symptom mapping and can identify voxels significantly more likely to relate to a particular lesion-induced symptom (Bates et al., 2003). The difference here is that we apply it towards lesion networks rather than just lesion locations. Voxels affected in <10% of cases were ignored and the resulting Z-maps were thresholded at a false discovery rate (FDR)-corrected P < 0.05 (Rorden et al., 2007). This analysis was performed using non-parametric mapping software (http://www.mccauslandcenter.sc.edu/mricro/npm/).
The voxel-wise Liebermeister test was used to assess whether (i) network overlap from actual lesions is greater than that of randomized lesions within the a priori region of interest; and (ii) network results preferentially localize to the a priori cortical region of interest relative to other cortical regions. The latter analysis compared the average voxel intensity (Z-score resulting from the voxel-wise Liebermeister test) in the a priori cortical region of interest to that of all other cortical areas from the Harvard Oxford Atlas (45 other regions, with right and left sides considered separately).
Cluster algorithm
For both the lesion and lesion network mapping, coordinates of local maxima were identified using the cluster algorithm in FSL (Oxford, UK, minimum cluster size of two voxels, 15 local maxima per cluster, minimum distance between maxima of 10 mm). For the lesion analysis, clustering was performed on the lesion overlap image. For the lesion networks, clustering was performed on the Z-score maps resulting from the voxel-wise Liebermeister test comparing actual to randomized lesion networks.
Addressing possible confounds
Global signal regression
There is concern that global signal regression confounds the ability to interpret anticorrelations (Fox et al., 2009a; Murphy et al., 2009). To ensure that our results were not dependent on a specific processing technique we repeated the analysis using an alternative method, anatomical CompCor (Behzadi et al., 2007) implemented in the Conn toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012), which is described in the Supplementary material.
Age
Age can impact the strength of functional connectivity (Ferreira and Busatto, 2013). Our analysis included patients with brain lesions that were older than the control cohort from which the normative functional connectivity MRI data were derived (61 ± 18.7 versus 22 ± 3.2). We therefore repeated the analysis using functional connectivity data from a healthy older adult cohort (n = 56, age 70.3 ± 4.4). These data were derived from the Harvard Brain Aging Study and details regarding processing methods are published elsewhere (Schultz et al., 2014).
2D versus 3D lesions
We performed a quantitative comparison of the network results from the local cases in which 3D lesions were used for the functional connectivity analysis, relative to a single 2D slice taken from the centre of the lesion. This analysis was undertaken to assess the validity of using 2D slices to represent 3D lesions, which was done for the literature-derived lesions. Spatial correlation coefficient was used to quantify the similarity between network results.
Additional lesion syndromes
The same methods used in the primary analysis of peduncular hallucinosis were repeated for three additional syndromes (details provided in the online Supplementary material). Finally, using lesion and lesion network data from all four conditions, a between-group analysis was performed using a voxel-wise Liebermeister test to assess whether the lesions and\or lesion networks could segregate between lesion syndromes (e.g. peduncular hallucinosis lesions and lesion networks compared to the other three conditions as ‘controls’).
Results
Application in peduncular hallucinosis
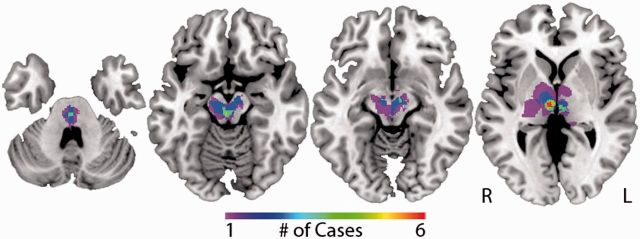
Following the traditional approach for relating symptoms to brain lesions, each lesion was mapped to a reference brain and sites of lesion overlap were identified. Of 23 lesions, the maximum overlap was only six cases (26%), indicating marked heterogeneity in lesion location (Fig. 2). The site of maximum overlap was the right central thalamus (intralaminar and paramedian nuclei, n = 6). Sites of maximum overlap were similar when extending the 2D lesions by 4 mm rather than 2 mm.
Figure 2.
Traditional lesion mapping results – peduncular hallucinosis. Areas of overlap among 23 peduncular hallucinosis lesions are shown (from left to right) in the pontine tegmentum, paramedian mesencephalic tegmentum, substantia nigra pars reticulata and intralaminar/paramedian thalamus. The colour scale indicates the number of overlapping lesions. The location of all lesions, additional slices of the lesion overlap, and coordinates of lesion overlap are available (Supplementary Fig. 1 and 2, and Supplementary Table 4).
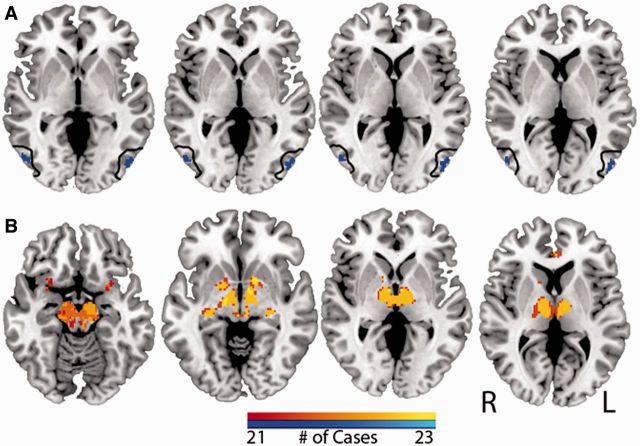
Analogous to viewing overlap at the lesion sites we next assessed overlap in lesion-based resting state networks. In contrast with the low overlap in lesion location (26%), overlap in lesion-based networks was high (>90%) for both positive and negatively correlated networks. Twenty-two of 23 lesions had a significant network anticorrelation with the extrastriate visual cortex within the region of interest defined a priori (Fig. 3A). Twenty-one of these lesions overlapped at the same location within this region of interest and a 22nd case had significant anticorrelation within this region, but at a site that did not overlap with the other 21. Using a slightly lower threshold, overlap in anticorrelated networks included regions in auditory and somatosensory association cortex (Supplementary Fig. 3), which is of interest given that hallucinations in peduncular hallucinosis can be multimodal (Caplan, 1980; McKee et al., 1990; Benke, 2006).
Figure 3.
Lesion network mapping results – peduncular hallucinosis. Regions of common network overlap in at least 21 of 23 cases with negative correlation (top, in cool colours) and positive correlation (bottom, in warm colours) are displayed. Note significant anticorrelation in extrastriate visual cortex, within the a priori region of interest (outlined in black). The colour scale indicates the number of cases with common overlap. MNI coordinates of axial slices shown are, left to right: top −2, 0, 2, 4, bottom −13, −8, 0, 6. Additional brain slices are provided (Supplementary Fig. 3).
Anatomical specificity
Next, we evaluated the specificity of the primary finding, that lesions causing peduncular hallucinosis show network anticorrelation with extrastriate visual cortex. We compared network overlap in the a priori region of interest from actual lesions relative to randomized lesions using a voxel-wise Liebermeister test. This showed significantly stronger network results for the actual lesions, with a peak level of significance of P < 10−5 which withstood correction for false discovery rate (<1%). Comparison of average voxel intensity from the cortical region of interest relative to all other cortical regions showed that these network findings were specific to the a priori region of interest (P < 0.01).
Excluding confounds
The finding of anticorrelation in the extrastriate visual cortex was present after re-analysing the data using an alternative algorithm that avoids global signal regression (Supplementary Fig. 4) (Whitfield-Gabrieli and Nieto-Castanon, 2012). When repeating the analysis using an older adult cohort that more closely matched the age of peduncular hallucinosis patients the extrastriate anticorrelation was present irrespective of age (Supplementary Fig. 4). Finally, networks resulting from 2D versus 3D lesions were nearly identical, with a spatial correlation coefficient of 0.96 (Supplementary Fig. 5), supporting the validity of the lesion networks derived from the literature.
Additional lesion syndromes
To determine whether lesion network mapping is generalizable beyond the application shown for peduncular hallucinosis we applied the technique to three additional clinical syndromes: auditory hallucinosis, central post-stroke pain, and subcortical expressive aphasia. As in peduncular hallucinosis, there were relatively low levels of overlap in lesion location [auditory hallucinosis 3/15 (20%), central post-stroke pain, 6/23 (26%), and subcortical expressive aphasia 5/12 (42%)] (Fig. 4, coordinates in Supplementary Table 6). However, lesion-based networks for each syndrome showed a high degree of overlap in the cortical region of interest hypothesized to be involved in symptom expression: superior temporal gyrus in auditory hallucinosis (88%), posterior insula in central post-stroke pain (78%), and Broca’s area in subcortical expressive aphasia (100%). For all four conditions, network overlap for actual lesions significantly exceeded network overlap from randomized lesions within the a priori region of interest (P < 10−4). Moreover, lesion networks localized to the a priori region of interest more than other cortical regions for each syndrome (P < 0.05) (Fig. 5). These results were consistent across different statistical approaches including the voxel-wise Liebermeister test (Fig. 5), a simple t-test (Supplementary Table 9), and a subtraction analysis (Supplementary Table 10 and Supplementary Fig. 6).
Figure 4.
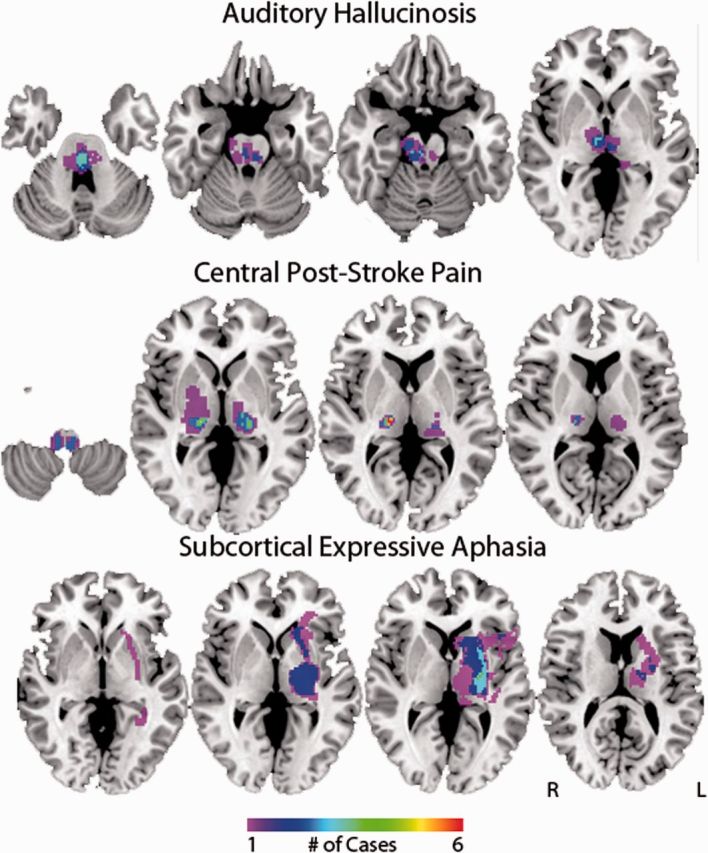
Lesion mapping results for other syndromes. Areas of lesion overlap are shown for auditory hallucinosis, central post-stroke pain, and subcortical aphasia. The colour scale indicates the number of overlapping lesions. The coordinates of lesion overlap sites are available (Supplementary Table 6).
Figure 5.
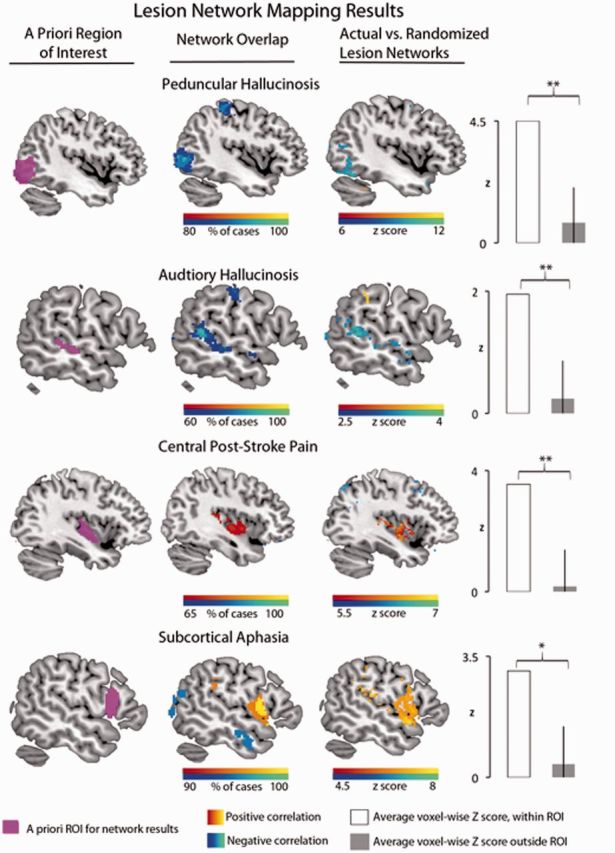
Lesion network mapping results: summary of main findings. Column 1 shows the hypothesized site of network overlap for each lesion syndrome. Column 2 shows the network overlap results, with positive correlations displayed in warmer colours and negative correlations in cooler colours. Column 3 shows the results of the voxel-wise Liebermeister test that compared network overlap from actual lesions relative to that of randomized lesions. The bar graph on the far right shows quantitative data supporting the specificity of the network overlap in the a priori cortical region of interest relative to all other cortical regions, derived from the voxel-wise Liebermeiseter results. Coordinates of findings in column 3 and additional regions are available (Supplementary Table 7). *P ≤ 0.05, **P ≤ 0.01. ROI = region of interest.
In addition to testing our a priori hypotheses we also noted significant network results in other regions outside the a priori region of interest. This includes the peduncular hallucinosis lesions being positively correlated with the lateral geniculate nucleus (Fig. 6A), subcortical aphasia lesions being positively correlated with the right lateral cerebellum in a region previously implicated in language (Fig. 6B) (Stoodley and Schmahmann, 2009), and central post-stroke pain lesions being positively correlated with the anterior cingulate cortex/medial prefrontal cortex, a node of the pain matrix identified in a meta-analysis of central pain functional MRI studies (Fig. 6C) (Friebel et al., 2011).
Figure 6.
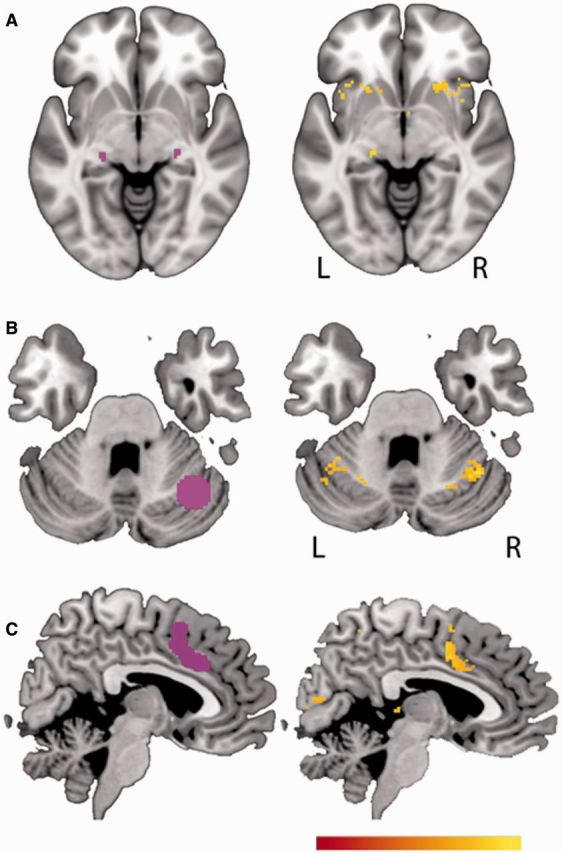
Unexpected findings from lesion network mapping. (A) The lateral geniculate nucleus is on the left, taken from the Julich Histological Atlas (Bürgel et al., 2006), and the image on the right shows areas that are significantly positively correlated with peduncular hallucinosis lesions. (B) The lateral cerebellum language area is shown on the left, as identified in a meta-analysis of functional MRI studies, represented as a sphere at MNI coordinate 35, 62, 28 (Stoodley and Schmahmann, 2009). The image on the right shows areas positively correlated with subcortical aphasia lesions. (C) A node of the pain matrix on the left, as identified from a meta-analysis of functional MRI studies (Friebel et al., 2011). The image on the right shows a cortical region that is significantly positively correlated with central post-stroke pain lesions. All displayed voxels represent Z-scores from a voxel-wise Liebermeister test, significant at a false discovery rate of 5% or greater. The colour bar minimum and maximum values show Z-scores of 7–9 for A, 3.5–5 for B, and 4–6 for C. Peak coordinates of these sites are available (Supplementary Table 7).
Lastly, we assessed whether lesion networks could segregate between lesion syndromes better than the lesions themselves. Using lesions alone there were no voxels that significantly associated with one lesion syndrome compared to the other three. In contrast, comparison of lesion networks showed voxels significantly associated with each individual lesion syndrome using the same statistical threshold (Fig. 7). Further, voxels significantly associated with each syndrome were located within the a priori cortical region of interest.
Figure 7.
Between-syndrome lesion network mapping results. Voxel-based lesion-symptom mapping of the lesions did not segregate between lesion syndromes using a false discovery rate of 5%. In contrast, applying the same statistical approach there were voxels that segregated between lesion syndromes. The colour scale denotes a voxel-wise Z-score from a Leibermeister test; 2.5 is statistically significant with a false discovery rate of 5%; 6 is significant at both a false discovery rate <1% and at P < 0.01 after applying Bonferroni correction for multiple comparisons.
Discussion
Here we demonstrate that (i) lesion sites that produce similar neurological symptoms but occur in different locations show overlap in their functional connectivity networks; (ii) this overlap occurs in regions hypothesized a priori to be involved in symptom expression; and (iii) these findings hold true across lesion syndromes. Together, these findings suggest that human connectome data can be used to incorporate network effects of brain lesions into symptom localization. Because this technique does not require advanced neuroimaging of patients, it may prove broadly applicable towards understanding the neural correlates of symptom expression across a variety of neurological and psychiatric syndromes.
First we demonstrate utility in a syndrome long hypothesized to be due to remote network effects, linking brainstem and thalamus lesions in peduncular hallucinosis to cortical areas implicated in visual release hallucinations. Next, we show generalizability of the technique by applying it to three additional disorders: auditory hallucinosis, central post-stroke pain, and subcortical expressive aphasia. Below we discuss how the current technique may help address limitations of traditional lesion mapping, offers complimentary information relative to functional imaging in patients, and provides unique insights into the lesion syndromes investigated here.
Augmenting the traditional approach
The lesion network mapping approach described in the present paper addresses two limitations of traditional lesion mapping: (i) it can allow for heterogeneously distributed lesions resulting in the same clinical syndrome to be grouped into a single unifying network; and (ii) it can link lesions to remote brain regions with a more direct or more easily recognized role in the behavioural expression of the lesion. This was demonstrated for each syndrome studied. For example, peduncular hallucinosis lesions had low levels of overlap that spanned multiple regions, but almost every lesion localized to the same networks. Moreover, sites of lesion overlap that did occur in peduncular hallucinosis were not in visual areas, leaving it unclear how these sites related to the symptom of visual hallucinations. In contrast, network overlap localized specifically to the extrastriate visual cortex, a region clearly implicated in visual hallucinations based on prior functional neuroimaging of patients (Dunn et al., 1983; Asaad and Shapiro, 1986; Ffytche et al., 1998; Manford and Andermann, 1998; Adachi et al., 2000; Vetrugno et al., 2009).
Complimenting functional neuroimaging of patients
Functional brain imaging in patients has been used to relate symptoms to the network effects of brain lesions (Baron et al., 1986; Vuilleumier et al., 2004; Corbetta et al., 2005; Sobesky et al., 2005; He et al., 2007a; Kim et al., 2012). While similar in motivation, that approach is different and complimentary to the technique presented here. The most obvious difference is that that approach requires functional neuroimaging data to be collected on patients while the current approach does not. While there is clear value to direct measurement of neurophysiological effects in symptomatic patients, there is also value to increased versatility. The present technique can be applied to almost any neurological syndrome based solely on lesion location. Second, post-lesion functional neuroimaging is not able to investigate the physiology or connectivity of the lesion location itself, as this tissue has been destroyed by the lesion. This contrasts with our current technique, which investigates properties of the lesion location based on a cohort of intact subjects. Finally, functional neuroimaging abnormalities in patients likely represent a combination of direct lesion-induced functional changes and secondary compensatory responses (Grefkes and Fink, 2014). Our technique based on connectivity alone might isolate or at least emphasize the direct lesion-induced functional changes. In fact, combining the two techniques may prove a powerful approach for differentiating direct versus compensatory processes (see discussion on central post-stroke pain below). The current method may also be used to identify a priori regions of interest in which to investigate the network effects of lesion patients undergoing functional imaging.
The current study joins a limited number of other studies that have begun to leverage normative human connectome databases to predict network effects in patients. Examples include predicting: cortical atrophy in stroke patients (Kuceyeski et al., 2014), atrophy progression in patients with neurodegenerative disease (Seeley et al., 2009; Zhou et al., 2012), effects of focal brain stimulation (Fox et al., 2014), and lesion-induced connectivity changes based on computational modelling (Honey and Sporns, 2008; Alstott et al., 2009). Because network effects of brain lesions can impact prognosis (Gratton et al., 2012; Lim et al., 2014; Warren et al., 2014) or be used to guide therapy (Grefkes and Fink, 2014), these approaches may represent clinical applications of the human connectome project (Van Essen et al., 2013).
Positive versus negative correlations
The current results suggest that heterogeneous lesions causing similar symptoms share functional connectivity to specific areas implicated in symptom expression. However, in some cases this shared functional connectivity was based on positive correlations, while in other cases it was based on negative correlations. An important question is whether the sign of the functional connectivity predicts what type of remote functional effect will occur. For example, we observed an anticorrelated relationship between subcortical regions involved in release hallucinations and the cortical regions hypothesized to be ‘released.’ This includes extrastriate visual cortex in visual hallucinations and superior temporal gyrus in auditory hallucinations. Hypermetabolism has been demonstrated previously in both cortical regions in association with hallucinations (Ffytche et al., 1998; Adachi et al., 2000; Griffiths, 2000; Allen et al., 2008; Kazui et al., 2009; Vetrugno et al., 2009; Kumar et al., 2014), raising the possibility that sites of anticorrelation predict sites of post-lesion hyperactivity. Although there remains debate regarding the appropriate interpretation of anticorrelated brain networks, the finding that lesion sites are anticorrelated with cortical regions that become hyperactive following the lesion suggests that anticorrelations may reflect causal functional interactions (Fox et al., 2005, 2009b; Murphy et al., 2009; Carbonell et al., 2011; Chai et al., 2012).
If negative correlation relates to post-lesion hyperperfusion, one would predict that positive correlation would relate to post-lesion hypoperfusion. Consistent with this notion, positive network connectivity between lesion location and language areas in subcortical aphasia corresponds to post-lesion hypoperfusion previously observed in these areas (de Boissezon et al., 2005; Choi et al., 2007; Kim et al., 2012). In contrast, post-stroke pain appears to deviate from this rule. Prior studies of central post-stroke pain have shown increased activity in the insula and anterior cingulate cortex (Peyron et al., 2004, 2013; Ducreux et al., 2006), yet lesion network mapping showed positive correlation to these areas (Figs 5 and 6C). One possible interpretation is that insula and anterior cingulate hypermetabolism seen in central post-stroke pain is not a direct effect of the lesion on these brain areas, but is the result of reorganization and neuroplasticity in these regions. Such an interpretation would be consistent with the observation that central post-stroke pain has a delay in symptom onset of weeks or months after the injury (Klit et al., 2009), unlike other syndromes studied here. This could suggest that lesion network mapping predicts which remote brain areas are most likely to undergo compensation and reorganization over an extended time course, a process that likely differs from the immediate effects of the lesion on these same areas. Such a hypothesis requires further validation.
Beyond a priori regions of interest
To validate our current approach, we focused on a priori regions of interest already implicated in the symptom of interest; however, interesting findings were also observed outside these regions. One example was the positive network overlap of peducular hallucinosis lesions in the lateral geniculate nucleus (Fig. 6A). This finding raises the possibility that visual hallucinations stemming from insult to the direct visual pathway, variably termed cortical release hallucinations or Charles Bonnet syndrome, share both clinical features (Mocellin et al., 2006) and similar network localization to peduncular hallicinosis. Another example from subcortical aphasia was positive network overlap in the right lateral cerebellum (Fig. 6B). This finding fits well with an emerging literature on a role for the cerebellum in language, which includes cerebellum lesions causing aphasia (Mariën et al., 1996, 2000; Fabbro et al., 2000; Stoodley and Schmahmann, 2009). Together, these findings suggest that our technique is capable of generating new unexpected findings and insights, not just confirming existing hypotheses.
Limitations and conclusions
There are several limitations to the present work, some of which provide important avenues for further research. First, the goal of the present study was to provide validation of a technique for incorporating the network effects of brain lesions into symptom localization, not to provide a definitive explanation for four syndromes with historically challenging and controversial brain–behaviour correlations. Second, lesion network mapping appears capable of identifying which regions are likely to be functionally affected by a lesion, but further work is needed to determine whether we can predict how these regions will be affected. There are dynamic compensatory mechanisms that unfold after a lesion occurs and predicting these effects is likely to require further work combining the present technique with longitudinal post-lesion imaging of patients. Third, the present analysis focused on shared network overlap across a group of lesions. It remains unknown whether individual differences in lesion-based networks relate to individual differences in symptoms. Finally, there are limitations in MRI resolution such that localizing results to specific brainstem or thalamic nuclei is difficult. However, resting state functional connectivity MRI has been used to study subcortical connectivity in these areas (Zhang et al., 2010; Lu et al., 2011) and the results of our specificity analyses suggest that anatomical resolution with the current data is sufficient to accurately resolve cortical connections of subcortical lesion sites. Aside from the limitation imposed by MRI resolution there are inherent limitations to the anatomical accuracy of lesion mapping when voxels are analysed independently. To circumvent this limitation, future studies may incorporate multivariate approaches to lesion inference mapping, as recently introduced (Mah et al., 2014). Finally, the interpretation of lesion networks in and adjacent to the lesions themselves is challenging using the current approach, which is why we focused here on cortical sites of network overlap derived from subcortical lesions.
In conclusion, the current article links heterogeneous subcortical lesions to cortical areas implicated in symptom generation across four separate conditions. We anticipate that lesion network mapping will be an important addition to lesion methodology, expanding localization of symptoms from a focus on lesion sites to lesion networks. Finally, such localization may facilitate tailored modulation of connected networks using techniques like non-invasive brain stimulation with the therapeutic aim of alleviating clinical symptoms.
Supplementary Material
Acknowledgements
We thank Randy Buckner and the Brain Genomics Superstruct Project for contributing data and analysis tools. We thank Drs Schultz, Chhatwal, and Sperling for sharing functional connectivity data from a cohort of older healthy adults and Dr Friebel for sharing the results of a pain meta-analysis.
M.D.F. is listed as inventor on submitted or issued patents on guiding neurological interventions with fMRI. A.P.L serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Neosync, and Novavision, and is listed as inventor in issued patents and patent applications on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI).
Funding
A.D.B. was supported by National Institute of Health \ National Institute of Neurologial Disorders and Stroke (Grant number 5R25NS065743-05) and the Sidney R. Baer, Jr Foundation. H.L. was supported by National Institute of Health \ National Institute of Neurologial Disorders and Stroke (Grant number K25NS069805) and the Brain & Behavior Research Foundation NARSAD Young Investigator grant. M.D.F. was supported by National Institute of Health \ National Institute of Neurologial Disorders and Stroke (Grant numbers R25NS065743, K23NS083741), and the American Brain Foundation. Work on this study was also supported by grants from the National Institute of Health \ National Center for Research Resources: Harvard Clinical and Translational Science Center (Grant number UL1 RR025758). The National Institute of Health \ National Institute on Aging funded the data collection for the older cohort used in this study (Harvard Aging Brain Study, Grant number P01AG036694).
Supplementary material
Supplementary material is available at Brain online.
References
- Adachi N, Watanabe T, Matsuda H, Onuma T. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci 2000; 54: 157–62. [DOI] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 2008; 32: 175–91. [DOI] [PubMed] [Google Scholar]
- Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol 2009; 5: e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry 1986; 143: 1088–97. [DOI] [PubMed] [Google Scholar]
- Baron JC, D’Antona R, Serdaru M, Pantano P, Bousser MG, Samson Y. Cortical hypometabolism after a thalamic lesion in man: positron tomography study [in French]. Rev Neurol (Paris) 1986; 142: 465–74. [PubMed] [Google Scholar]
- Bartolomeo P. The quest for the ‘critical lesion site’ in cognitive deficits: problems and perspectives. Cortex 2011; 47: 1010–12. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003; 6: 448–50. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007; 37: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke T. Peduncular hallucinosis: a syndrome of impaired reality monitoring. J Neurol 2006; 253: 1561–71. [DOI] [PubMed] [Google Scholar]
- Brown-Séquard CE. Séance du 18 décembre. C R Soc Biol. 1875; 424. [Google Scholar]
- Buckner RL, Roffman JL, Smoller JW. Brain Genomics Superstruct Project (GSP). Harvard Dataverse, V10; 2014. 10.7910/DVN/25833. [DOI] [Google Scholar]
- Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 2006; 29: 1092–105. [DOI] [PubMed] [Google Scholar]
- Caplan LR. ‘Top of the basilar’ syndrome. Neurology 1980; 30: 72–9. [DOI] [PubMed] [Google Scholar]
- Carbonell F, Bellec P, Shmuel A. Global and system-specific resting-state fMRI fluctuations are uncorrelated: principal component analysis reveals anti-correlated networks. Brain Connect 2011; 1: 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Tononi G. Diaschisis: past, present, future. Brain 2014; 137: 2408–22. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev S V, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 2010; 67: 365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage 2012; 62: 2271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, et al. Beyond cortical localization in clinico-anatomical correlation. Cortex 2012; 48: 1262–87. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage 2012; 59: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Lee KH, Na DL, Byun HS, Lee SJ, Kim H, et al. Subcortical aphasia after striatocapsular infarction: quantitative analysis of brain perfusion SPECT using statistical parametric mapping and a statistical probabilistic anatomic map. J Nucl Med 2007; 48: 194–200. [PubMed] [Google Scholar]
- Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical-radiological correlation. J Neurol 2004; 251: 725–9. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 2005; 8: 1603–10. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron 2015; 85: 927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B. Thalamic mechanisms in language: a reconsideration based on recent findings and concepts. Brain Lang 2013; 126: 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- de Boissezon X, Démonet J-F, Puel M, Marie N, Raboyeau G, Albucher J-F, et al. Subcortical aphasia: a longitudinal PET study. Stroke 2005; 36: 1467–73. [DOI] [PubMed] [Google Scholar]
- De Morsier G. Pathogenie de I’hallucinosepedonculaire: & pro- pos d'un nouveau cas. Rev Neurol 1935; 64: 606–8. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Zaharchuk G, Otte WM. Assessment and modulation of resting-state neural networks after stroke. Curr Opin Neurol 2014; 27: 637–43. [DOI] [PubMed] [Google Scholar]
- Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain 2006; 129: 963–76. [DOI] [PubMed] [Google Scholar]
- Dunn DW, Weisberg LA, Nadell J. Peduncular hallucinations caused by brainstem compression. Neurology 1983; 33: 1360–1. [DOI] [PubMed] [Google Scholar]
- Fabbro F, Moretti R, Bava A. Language impairments in patients with cerebellar lesions. J. Neurolinguistics 2000; 13: 173–88. [Google Scholar]
- Feeney DM, Baron JC. Diaschisis. Stroke 1986; 17: 817–30. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev 2013; 37: 384–400. [DOI] [PubMed] [Google Scholar]
- Ferro JM, Martins IP, Caeiro L. Behavioral neurology of stroke. In: Brainin M, Heiss W-D, editors. Textbook of stroke medicine. Cambridge, UK: Cambridge University Press; 2010. p. 178–202. [Google Scholar]
- Ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci 1998; 1: 738–42. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano a. M, Pascual-Leone a. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA 2014; 111: E4367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012; 72: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8: 700–11. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005; 102: 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 2009a; 101: 3270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 2009b; 101: 3270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage 1997; 5: 13–30. [DOI] [PubMed] [Google Scholar]
- Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage 2011; 58: 1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain 2013; 154: S29–43. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L. The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol Clin 2012; 42: 299–313. [DOI] [PubMed] [Google Scholar]
- Gratton C, Nomura EM, Perez F, D’Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J Cogn Neurosci 2012; 24: 1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol 2014; 13: 206–16. [DOI] [PubMed] [Google Scholar]
- Griffiths TD. Musical hallucinosis in acquired deafness: phenomenology and brain substrate. Brain 2000; 123: 2065–76. [DOI] [PubMed] [Google Scholar]
- He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol 2007a; 20: 655–60. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007b; 53: 905–18. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 2008; 29: 802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazui H, Ishii R, Yoshida T, Ikezawa K, Takaya M, Tokunaga H, et al. Neuroimaging studies in patients with Charles Bonnet Syndrome. Psychogeriatrics 2009; 9: 77–84. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim HS, An Y-S. Statistical mapping analysis of brain metabolism in patients with subcortical aphasia after intracerebral hemorrhage: a pilot study of F-18 FDG PET images. Yonsei Med J 2012; 53: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 2009; 8: 857–68. [DOI] [PubMed] [Google Scholar]
- Kuceyeski A, Kamel H, Navi BB, Raj A, Iadecola C. Predicting future brain tissue loss from white matter connectivity disruption in ischemic stroke. Stroke 2014; 45: 717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sedley W, Barnes GR, Teki S, Friston KJ, Griffiths TD. A brain basis for musical hallucinations. Cortex 2014; 52: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermitte J. Syndrome de la calotte du pedoncule cerebral. Les troubles psycho-sensorielsdans les lesions du mesodphale. Rev Neurol 1922; 38: 1359–65. [Google Scholar]
- Lim JS, Kim N, Jang MU, Han MK, Kim S, Baek MJ, et al. Cortical hubs and subcortical cholinergic pathways as neural substrates of poststroke dementia. Stroke 2014; 45: 1069–76. [DOI] [PubMed] [Google Scholar]
- Lu J, Liu H, Zhang M, Wang D, Cao Y, Ma Q, et al. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci 2011; 31: 15065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah Y, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain 2014; 137: 2522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain 1998; 121 (Pt 1): 1819–40. [DOI] [PubMed] [Google Scholar]
- Mariën P, Engelborghs S, Pickut BA, De Deyn PP. Aphasia following cerebellar damage: fact or fallacy? J. Neurolinguistics 2000; 13: 145–71. [Google Scholar]
- Mariën P, Saerens J, Nanhoe R, Moens E, Nagels G, Pickut BA, et al. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. J Neurol Sci 1996; 144: 34–43. [DOI] [PubMed] [Google Scholar]
- McKee AC, Levine DN, Kowall NW, Richardson EP. Peduncular hallucinosis associated with isolated infarction of the substantia nigra pars reticulata. Ann Neurol 1990; 27: 500–4. [DOI] [PubMed] [Google Scholar]
- Mocellin R, Walterfang M, Velakoulis D. Neuropsychiatry of complex visual hallucinations. Aust N Z J Psychiatry 2006; 40: 742–51. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 2009; 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang 1997; 58: 355–402; discussion 418–23. [DOI] [PubMed] [Google Scholar]
- Park C, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 2011; 42: 1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Faillenot I, Pomares FB, Le Bars D, Garcia-Larrea L, Laurent B. Mechanical allodynia in neuropathic pain. Where are the brain representations located? A positron emission tomography (PET) study. Eur J Pain 2013; 17: 1327–37. [DOI] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, et al. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology 2004; 63: 1838–46. [DOI] [PubMed] [Google Scholar]
- Risser AH, Powell FC. Lhermitte’s peduncular hallucinosis. In: 45th Annual Meeting of the American Academy of Neurology, New York; 1993. [Google Scholar]
- Robinson RG. Neuropsychiatric consequences of stroke. Annu Rev Med 1997; 48: 217–29. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007; 19: 1081–8. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain 2006; 129: 1371–84. [DOI] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Huijbers W, Hedden T, van Dijk KRA, McLaren DG, et al. Template based rotation: a method for functional connectivity analysis with a priori templates. Neuroimage 2014; 102 (Pt 2): 620–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky J, Thiel A, Ghaemi M, Hilker RH, Rudolf J, Jacobs AH, et al. Crossed cerebellar diaschisis in acute human stroke: a PET study of serial changes and response to supratentorial reperfusion. J Cereb Blood Flow Metab 2005; 25: 1685–91. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 2009; 44: 489–501. [DOI] [PubMed] [Google Scholar]
- Van Bogaert L. L’Hallucinose pedonculaire. Rev Neurol 1927; 1927: 608–17. [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 2010; 103: 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU-Minn human connectome project: an overview. Neuroimage 2013; 80: 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrugno R, Vella A, Mascalchi M, Alessandria M, D’Angelo R, Gallassi R, et al. Peduncular hallucinosis: a polysomnographic and spect study of a patient and efficacy of serotonergic therapy. Sleep Med 2009; 10: 1158–60. [DOI] [PubMed] [Google Scholar]
- Von Monakow C, Harris G. Die Lokalisation im Grosshirn: Undder Abbau der Funktion durch kortikale Herde. Wiesbaden: Bergmann: In: Pribam KH, editor. Brain and behavior I: Mood states and mind. Baltimore: Penguin 1969; 1914. p. 27–36. [Google Scholar]
- Vuilleumier P. Mapping the functional neuroanatomy of spatial neglect and human parietal lobe functions: progress and challenges. Ann N Y Acad Sci 2013; 1296: 50–74. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci 2004; 7: 1271–8. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain 2010; 133: 1224–38. [DOI] [PubMed] [Google Scholar]
- Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, Sun H, et al. Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci USA 2014; 111: 14247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012; 2: 125–41. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex 2010; 20: 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73: 1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.